Abstract
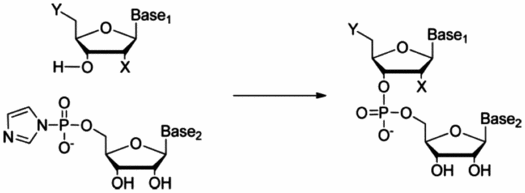
A synthesis has been developed, providing nucleotide dimers comprising natural or unnatural nucleoside residues. A ribonucleoside 5′-phosphorimidazolide is added to a nucleoside adsorbed on montmorillonite at neutral pH with the absence of protecting groups. Approximately, 30% of the imidazolide is converted into each 2′-5′ dimer and 3′-5′ dimer with the rest hydrolyzed to the 5′-monophosphate. Experiments with many combinations have suggested the limits to which this method may be applied, including heterochiral and chimeric syntheses. This greener chemistry has enabled the synthesis of dimers from activated nucleotides themselves, activated nucleotides with nucleosides, and activated nucleotides with nucleotide 5′-monophosphates. Both homo- and heterochiral combinations of reagents have been tried. The montmorillonite-catalyzed oligomerization of 5′-activated nucleotides leads to oligomers up to 50 residues in length (Huang & Ferris, 2007) using the excellent catalyst Volclay®. However, all oligomers must necessarily begin as dimers, so we considered it important to study in detail the formation of these products under prebiotic conditions. Then, a meaningful comparison could be drawn between our syntheses and the formation of long oligomers that is part of our studies of the origins of life. In the synthesis of trimers from these dimers, we looked for alternative synthetic methods via a 5′-phosphate dimer with activated nucleotides as well as 5′-hydroxy nucleotide dimers with the same reactant. The method has shown promise in targeting trimer synthesis and the procedure lends itself to the development of combinatorial libraries. The use of enzymatic hydrolysis has played a crucial role in this work, facilitating product identity across the spectrum of products prepared. The yields of the corresponding homochiral and heterochiral dimers from A and U will require careful modeling of the reactants in their interactions with both the clay and one another to locate the source of the similarities and differences. The lack of reactivity of arabino- and xylo-nucleosides also poses interesting structural, modeling, and origins of life issues. Results with clays that catalyze long oligomer formation only poorly reveal that they too catalyze these dimer syntheses, albeit less well than Volclay.®
This research was supported by NASA Astrobiology Institute grant NNA09DA80A.
References
- Huang , W. and Ferris , J. P. 2007 . One-step, regioselective synthesis of up to 50-mers of RNA oligomers by montmorillonite catalysis . Journal of the American Chemical Society , 128 : 8914 – 8919 .
- Joshi , P. C. , Aldersley , M. F. , Zagorevskii , D. V. and Ferris , J. P. 2012 . Nucleosides, Nucleotides and Nucleic Acids , 31 : 536 – 566 .