?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The presence of chalcogen bonds in native proteins was investigated on a non-redundant and high-resolution (≤ 1 Angstrom) set of protein crystal structures deposited in the Protein Data Bank. It was observed that about one half of the sulfur atoms of methionines and disulfide bridges from chalcogen bonds with nucleophiles (oxygen and sulfur atoms, and aromatic rings). This suggests that chalcogen bonds are a non-bonding interaction important for protein stability. Quite numerous chalcogen bonds involve water molecules. Interestingly, in the case of disulfide bridges, chalcogen bonds have a marked tendency to occur along the S-S bond extension rather than along the C-S bond extension. Additionally, it has been observed that closer residues have a higher probability of being connected by a chalcogen bonds, while the secondary structure of the two residues connected by a chalcogen bond do not correlate with its formation.
Communicated by Ramaswamy H. Sarma
Introduction
A variety of non-covalent interactions contribute to protein stability and flexibility (Karshikoff, Citation2021), and one of them, the chalcogen bond (Chb) (Aakeroy et al., Citation2019), has not yet been investigated in depth in macromolecular structures.
It is the attractive interaction between nucleophiles and the heavy elements of group 16 (chalcogens; sulfur, selenium and tellurium), which might be due to several factors. In the intermolecular subunit C-Ch…Nu, where Ch is a chalcogen and Nu is a nucleophile, an electrostatic attraction can be caused by the positively charged region found along the prolongation of the C-Ch bond; additionally, a n→σ* orbital delocalization between the nucleophile lone pair and the anti-bonding orbital of C-Ch may occur (Pascoe et al., Citation2017). The strongest Chbs may be as strong as conventional H-bonds (Pascoe et al., Citation2017).
Its importance in determining molecular and crystal structures of organic and inorganic materials is well documented (Ho, Citation2015). However, less it is known about Chbs in proteins, where sulfur is the only chalcogen natively present.
Sulfur-based Chbs are expected to be weaker that selenium and tellurium-based Chbs (Scheiner, Citation2021) and, in effect, no Chbs were observed in the first report on sulfur-based Chbs in proteins—a survey of methionine-nucleophile interaction in a small data set of protein crystal structures determined at room temperature (Carugo, Citation1999). The growth of the Protein Data Bank (Berman et al., Citation2000; Bernstein et al., Citation1977), the improving resolution of protein crystal structures (Blakeley et al., Citation2015), and the successful development of low temperature diffraction data collection techniques (Pflugrath, Citation2004) allowed to get some evidence of sulfur-based Chbs later (Iwaoka et al., Citation2002; Pal & Chakrabarti, Citation2001) and recently stronger selenium-based Chbs have been described in proteins where methionines were substituted by seleniomethionines (Carugo et al., Citation2021).
It has also been shown that protein-ligand recognition may involve Chbs (Beno et al., Citation2015; Fick et al., Citation2016; Koebel et al., Citation2016; Kříž et al., Citation2018; Mitchell, Citation2017), that Chb plays a role in the mechanism of inhibition of maltase glucoamylase by salacinol and katalanol (Galmés et al., Citation2020), and that the activity of ebselen is enhanced by Chb formation (Daolio et al., Citation2020). The ability of sulfur atoms of Cys and Met in proteins of forming Chbs has also been verified with statistical surveys and molecular orbital calculations (Iwaoka et al., Citation2002; Junming et al., Citation2011).
Several types of sulfur atoms can be found in proteins, the sulfur atom of cysteine sidechains, the sulfur atom of methionine side-chains, and the sulfur atoms of disulfide bridges. They may form Chbs with other sulfur atoms, with oxygen atoms and with aromatic rings.
A recent survey of Chbs in protein structures deposited in the Protein Data Bank (Berman et al., Citation2000; Bernstein et al., Citation1977) has been recently published (Biswal et al., Citation2022). It was based on a redundant set of about 13000 crystal structures refined at a resolution of at least 1.5 Å and found about 1700 S…O Chbs and very few Se…O and Se…S Chbs—presumably by including any type of interactions: protein-protein, protein-ligand, and ligand-ligand. Moreover, quantum chemistry calculations showed that, in some selected examples, the energy of these Chbs is limited to few Kcal/mol (Biswal et al., Citation2022).
The present manuscript analyses Chbs between protein sulfur atoms and protein nucleophiles (including water, which can be considered to be part of the native protein molecule (Carugo, Citation2017)), excluding any interaction with small molecules—ligands, inhibitors or other exogenous small molecules that may be fortuitously present. Moreover, the attention is focused only on crystal structures refined at ultra-high resolution (better than or equal to 1.0 Å) and with redundancy reduced to 40% sequence identity. In this way, it should be possible to get a statistical evidence about the frequency of occurrence of Chbs and, consequently, on their importance in protein folding, in a well-controlled, non-redundant, and high-quality ensemble of protein crystal structures.
The attention is focused on sulfur atoms of methionine side chains and of disulfide bridges. Sulfur atoms of cysteine side-chains are not considered here, since the thiol group –SH may be involved in both Chbs and hydrogen bonds, which may be difficult to discriminate from each other, since the position of the thiol hydrogen atoms, which are acidic and rotatable, is usually unknown, even at extremely high resolution. The analysis of cysteine Chbs is therefore not reported in the present manuscript since it requires the development of different tools and strategies.
All protein oxygen and sulfur atoms and all water oxygen atoms can behave as nucleophiles in Chbs. Additionally, all aromatic rings –which play a relevant role in protein folding by forming stacked substructures where two or more rings are close to each other (Lobanov et al., Citation2021) or by behaving as hydrogen acceptor in hydrogen bonds (Newberry & Raines, Citation2019)—can be nucleophiles attracted by chalcogen atoms (Scheiner, Citation2022).
Methods
Data
All data were taken from the Protein Data Bank (Berman et al., Citation2000; Bernstein et al., Citation1977). In order to assemble a high quality and non-redundant subset of data, the attention was focused on high-resolution crystal structures determined in the same temperature range. A constrain on resolution is necessary to ensure that atom positions have been determined with sufficiently high accuracy and a constrain on temperature is necessary to ensure that all structures have been determined in similar physico-chemical conditions. For this reason, (i) all protein X-ray crystal structures determined in the 90-110 K temperature range and refined at at least 1.0 Å resolution were retained—multi-model refinements, Cα-only structures, and nucleic acid structures were discarded—and (ii) the redundancy was reduced to an upper pairwise percentage of sequence identity of 40% with CD-HIT (Fu et al., Citation2012). Removing protein chains with less than 50 amino acids eventually resulted in a set of 336 structures ().
Table 1. Observed and expected percentages of oxygen atoms in Chbs formed by methionines and disulfide bridges.
Relative positions of chalcogen and nucleophile
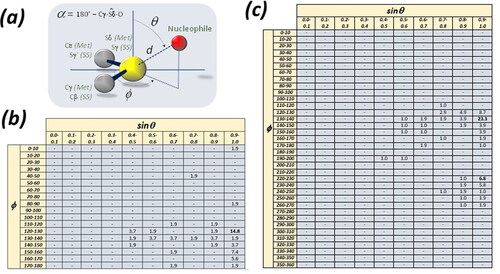
The sulfur atom belonging to a methionine or to a disulfide bridge was put at the origin of a spherical coordinate system (). The three spherical coordinates (d, ϕ and θ) were defined as it follows—here the methionine atom names are used but the same definitions may be done by using the disulfide-bridge atom names: (i) d is the Euclidean distance between the sulfur atom Sδ and the nucleophile; (ii) the polar angle θ is defined by the normal to the Cγ-Sδ-Cε plane passing through atom Sδ and the Sδ-nucleophile vector; (iii) the azimuthal angle ϕ is defined by bisector of the Cγ–Sδ − Cε angle and the vector from Sδ and the projection of the nucleophilic atom on the plane defined by Cγ, Sδ, and Cε.
Figure 1. (a) Schematic view of a contact between a nucleophile and a sulfur atom belonging to a methionine (Met) or to a disulfide bridge (SS). The three spherical coordinates (d, ϕ and θ) are indicated together with the angle α. (b) Distribution of the nucleophiles around the sulfur atom of methionine (the upper limit of f is limited to 180° because of the C2v symmetry of the C-S-C molecular moiety). (c) Distribution of the nucleophiles around the sulfur atom of disulfide bridges.
For methionine, the azimuthal angle ϕ was constrained in the 0-180° range, and polar angle θ in the 0-90° range, because of the local, intrinsic C2v symmetry of the Cγ–Sδ − Cε molecular moiety. For disulfide bonds, each sulfur atom was in turn put at the center of the system, the polar angle θ was constrained in the 0-90° range and no constrains were adopted for the azimuthal angle ϕ, since the symmetry of the Cβ–Sγ − Cγ’ (Cs) is lower than that of the Cγ–Sδ − Cε molecular moiety of methionine (C2v).
Moreover, the α angle, necessary for the identification of Chbs, was computed as it is pictured in .
Chb detection
Three types of nucleophiles present in hydrated proteins were considered: oxygen atoms, sulfur atoms, and aromatic rings, for which the center of the ring was considered the nucleophile position. They form a Chb with a sulfur atom if the distance d is sufficiently short and the angle α is sufficiently narrow (). The following threshold values were used: d ≤ 3.4 Å, if the nucleophile is an oxygen atom, d ≤ 3.7 Å if it is a sulfur atom, and d ≤ 3.8 Å if it is the center of an aromatic ring; and α ≤ 25° independently of the type of nucleophile.
The thresholds on the distance d were defined as d = H + K + L, where H is the van der Waals radius of sulfur (1.80 Å (Bondi, Citation1964)), K is the wan der Waals radius of the nucleophile − 1.80 Å for sulfur, 1.50 Å for oxygen, and for the aromatic rings K is one half of the van der Waals distance between stacked aromatic rings (3.4-3.6 Å) (Dahl et al., Citation1994) - and L is a slight increase to account for the minor accuracy of the positional coordinates in macromolecular structures relative to small molecule structures (the average positional standard error can be estimated to be close to 0.02 Å (Kumar et al., Citation2015) in the crystal structures examined here and the estimated standard error on interatomic distances is close to 0.04 Å (Giacovazzo et al., Citation2002)). L is slightly larger for the aromatic rings (0.2 Å) than for sulfur and oxygen atoms (0.1 Å) since their electric negative charge is more diffuse.
According to these threshold values on the distance d and the angle a, a chalcogen bond is expected to be associated with ϕ values close to 120-130°, and θ values close to 90°.
Miscellaneous
Solvent accessible surface areas (SASA) were computed with NACCESS (Hubbard & Thornton, Citation1993), which computes the relative SASA, too, which is defined as
where SASAmax is the SASA of the same residue R computed in the tripeptide G-R-G in extended conformation. SASAmax is a reference value, considered to be the maximal possible SASA for residue R. Consequently, it is expected that 0 ≤ relative SASA ≤ 100. In reality, values slightly larger than 100 are seldom observed, depending on the conformation of the R side-chain.
While absolute SASAs are dimensional (Å2), relative SASAs are obviously dimensionless.
Secondary structures were assigned with STRIDE (Heinig & Frishman, Citation2004). Only three secondary structure types were considered: helices (STRIDE labels H, G and I), extended (E, B and b), and loops (C and L). In case of conformationally disordered atoms, only the first conformation was considered.
The distance between the two residues that form a Chb was defined as the absolute difference D between the positions of the two residues i and j
Alternatively, the distance D was normalized by the length (nres) of the protein chain as
Results and discussion
Directionality of the chbs
The distributions of nucleophile atoms around sulfur atoms of methionine and disulfide bridges are shown in . The sinθ range was divided into 10 equal spaces from 0.0 to 1.0, and the ϕ range was divided into 18 equal spaces from 0.0° to 180° in the case of methionines and in 36 equal spaces from 0.0° to 360° in the case of disulfide-bridges—as described above, the different treatment of the ϕ range is dues to the different symmetries of the C-S-C unit of a methionine (C2v) and of the C-S-S unit of a disulfide-bridge (Cs). In this way, the space around the methionine sulfur atom is divided into 180 equal bi-dimensional bins and the space around the disulfide-bridge into 360 equal bi-dimensional bins. In case of uniform distribution, 0.6% of observations are expected in each bin in and 0.3% in .
show the percentage of observations in each bi-dimensional bin. For simplicity, only bins with percentages of observations higher than those expected in case of uniform distribution are shown in these Figures.
Clearly, the nucleophiles tend to cluster in well refined regions. The most frequently occupied bin of is defined by 0.9 < sinθ < 1.0 and 120° < ϕ < 130° and about one third of the observations are at sinθ > 0.8 and at 110° < ϕ < 150°. This clearly indicates that the nucleophile atoms tend to approach the sulfur atom along the direction opposite to the carbon atom covalently bound to the sulfur atom.
Similar considerations can be done about . Here there are two clusters, one larger at 0.9 < sinθ < 1.0 and 130° < ϕ < 140°, and one smaller at 0.9 < sinθ < 1.0 and 220° < ϕ < 230°. Nearly one half of the observations are at 0.8 < sinθ < 1.0 and 120° < ϕ < 150° and nearly 20% of them are at 0.8 < sinθ < 1.0 and 220° < ϕ < 250°. This indicates that the nucleophile atoms tend to be in the direction opposite to the C-S and S-S covalent bonds and more often opposite to the S-S covalent bond.
These observations complement and enrich those reported earlier and clearly indicate that Chbs tend to be highly directional (Biswal et al., Citation2022). In , a superposition of all nucleophilic atoms on the same framework of methionine or disulfide bond is reported.
Frequency of the chbs
Chbs are numerous too, in a statistical perspective.
In the 336 crystal structures examined here there are 185 methionines that form 44 Chbs and 227 disulfide bridges that form 53 Chbs (Table S2). This implies that about 25% of the methionines and of the disulfide bridges form Chbs.
Table 2. Observed and expected percentages of observations of secondary structure types in Chbs formed by methionines and disulfide bridges.
Most of the Chbs are with oxygen atoms − 89% of those involving methionine and 91% of those involving disulfide bridges. For this reason, the statistical analyses described below are limited to the Chbs between protein sulfur and oxygen atoms.
It is clear that although Chb are considered weak interactions, they occur frequently in proteins, indicating that are utilized by Nature to regulate the physiological stability of proteins.
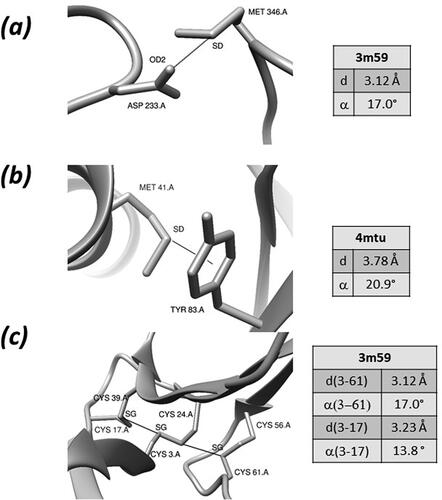
Few examples of Chbs are shown in . In the manganese peroxidase from Phanerochaete chrysosporium, a sulfur-oxygen Chb is formed between the side-chains of Met 346 and Asp 233 () (Sundaramoorthy et al., Citation2010). A Chb with an aromatic nucleophile is formed in Anaerotignum propionicum beta-alanyl-CoA:ammonia lyase between the sidechains of Net 41 and Tyr 83 () (Heine et al., Citation2014). Two sulfur-sulfur Chb are formed by Cys 3 and Cys17, and by Cys 24 and Cys 61 in bucandin, a toxin isolated from the Malayan krait () (Kuhn et al., Citation2000).
Nucleophiles
Although it is impossible to classify the nucleophilic atoms according to numerous types of residues they belong, because of the paucity of data, it is possible to classify them according to their chemical nature. Five types of oxygen atoms were considered: main-chain carbonyl, side-chain hydroxyl (Ser OG, Thr OG1 and Tyr OH), side-chain amide (Asn OD1 and Gln OE1), side-chain carboxylate (Asp OD1 and OD2, and Glu OE1 and OE2), and water.
shows the observed percentages of these oxygen atom types in Chbs formed by methionines and disulfide bridges. These values can be compared with the expected percentages, which are the frequencies of observation of the nucleophiles—for example, in the structures examined here that have Chbs, 34.7% of the nucleophilic oxygen atoms belong to main-chain carbonyls and, consequently, 34.7% of the Chbs are expected to involve main-chain carbonyl oxygens.”
Chbs formed by methionines and disulfide bridges show similar trends. In both cases, main-chain carbonyl oxygen atoms are observed more frequently than expected and water oxygen atoms are observed less frequently than expected. However, the differences are more marked in Chbs formed by disulfide bridges than in Chbs formed by methionines. Amongst the side-chain oxygen atoms, charged oxygen atoms of carboxylates are observed slightly less frequently than expected and hydroxyl and amide oxygen atoms more frequently than expected.
The small frequency of charged oxygen atoms is somewhat surprising and may not indicate that these atoms are reluctant to form Chbs but prefer other interactions, such as, for example, salt bridges. In addition, methionines and disulfide bridges tend to be far from negatively charged oxygen atoms: while the former are usually poorly accessible to the solvent, the latter are usually found on the surface of the protein.
The low frequency of water molecules as nucleophiles must depend on the fact that both methionines and disulfide bridges then to be little accessible to the solvent. This low frequency is therefore compensated by protein oxygen atoms.
Solvent accessibility
The analysis of the solvent accessible solvent areas (SASA) agrees with the observations reported in the previous paragraph.
Since methionines and disulfide bridges have different numbers of atoms and different dimensions, the relative SASAs computed by Naccess (Hubbard & Thornton, Citation1993) were used in addition to the SASAs (see Methods for details).
For disulfide bridges, the SASA and relative SASA was the average value of the SASA and relative SASA of the two oxidized cysteines.
On average, SASA of the disulfide bridges that form Chbs (13.0 ± 2.1 Å2) is lower than the SASA of the methionines that form Chbs (24.4 ± 2.1). The relative SASA of the disulfide bridges that form Chbs (9.7 ± 1.6) is lower than the relative SASA of the methionines that form Chbs (16.1 ± 3.6).
This agrees with the observation that disulfide bonds have a smaller tendency to form Chbs with water than methionines (see ). If they tend to be less accessible to the solvent, they are less likely to interact with water molecules through Chbs, too. The fact that water oxygen atoms are more frequent in Chbs formed by methionines is thus due, at least partially, to the larger exposure of methionines.
Secondary structures
The attention was focused on observed and expected frequencies of secondary structure types in Chbs formed by methionines and disulfide bridges. In a previous report it was observed that about one half of the nucleophiles are in loops, one fourth in helices and one forth in strands (Biswal et al., Citation2022). Here, the secondary structure of both chalcogen and nucleophile have been considered. For disulfide bridges, which are formed by two residues, I selected the secondary structure of the cysteine whose sulfur atom forms the Chb and I ignored the secondary of the second cysteine.
The expected frequencies were calculated from the frequencies of each type of chalcogen and nucleophile secondary structure in the Chb-containing protein structures examined in this manuscript. For example, if 40% of the chalcogens are in helices and 20% of the nucleophiles are in helices, 8% of the Chbs are expected between a helical chalcogen and a helical nucleophile (100 × 0.40 × 0.20). Since it is impossible to discriminate the expected frequency of a pair of secondary structures ss1-ss2 from the frequency of ss2-ss1, it was imposed that both observed and expected frequencies be symmetrical (ss1-ss2 = ss2-ss1). Expected and observed frequencies, shown in .
Observed frequencies match quite well the expectations, with some deviations. For example, Chbs where both chalcogen and nucleophile belong to residues that adopt a helical conformation are less frequent than expected both in methionine and disulfide bridge Chbs. This is somewhat surprising, since the propensities to adopt a helical conformation are opposite for methionine and cysteine (Costantini et al., Citation2006), the former having a marked tendency to be observed in helices and the latter having a marked tendency not to be observed in helices.
Although it is reasonable to assume that these observations are only preliminary findings, which need to be confirmed by further studies on larger data sets, it seems that the secondary structure of residues linked by a Chb plays a minor role in Chb formation.
Positions along the sequence
A further important question is the determination of the distance D, defined as the absolute difference between the positions of the two residues that form a Chb (see Methods).
The average D values are 57 ± 9 and 24 ± 6 for Chb formed by methionines and disulfide bridges, respectively. It would seem, therefore that D is quite smaller for disulfide bridges.
However, the average values of the distances DNs, normalized by the chain length (see Methods), are 19 ± 3 and 15 ± 3 for Chb formed by methionines and disulfide bridges, respectively. The two average DN values are thus very similar, suggesting that there are not substantial differences between the two Chbs.
The average DNs, whose minimum possible value is 1 and maximum tends to 100, are quite low. This indicates that, as expected because of elementary conformational entropy considerations, closer residues have a higher probability of being connected by a Chb.
Conclusions
This work was motivated by the need to obtain an overview of Chbs in native proteins, excluding exogenous ligands or artificial amino acids, such as selenomethionines, but including water molecules, the presence of which is essential for protein stability. Therefore, the attention was focused on sulfur atoms of methionines and disulfide bridges—cysteine sulfur atoms will be examined separately, since they may form both Chbs and hydrogen bonds. Several nucleophilic atoms are contained in proteins, like main- and side-chain oxygen atoms, side-chains sulfur atoms, and aromatic rings.
It was observed that Chbs are frequent. About one fourth of the methionines and one fourth of the disulfide bridges form Chbs. Interestingly, in the case of disulfide bridges, Chbs have a marked tendency to occur along the S-S bond extension rather than along the C-S bond extension.
The most frequently observed nucleophile is oxygen, especially from the backbone. This is not surprising, since it is also much more frequent in proteins than sulfur or aromatic rings. Interestingly, several Chbs are formed with water molecules, less frequently with disulfide bridges and more frequently with methionines, which are slightly more accessible to the solvent than disulfide bridges, too.
The above observations have been performed on a small sample of protein crystal structures refined at very high resolution (≤ 1 Å). This resolution cutoff ensures that atomic positions are reliable. However, their estimated standard errors, computed according to (Kumar et al., Citation2015), are close to 0.02 Å, a value that is too large to monitor and compare additional stereochemical features, like for example differences between side-chain and main-chain oxygen atoms. Better data are needed for analyzing these details. Unfortunately, it is not possible to be certain that very high-resolution experimental data, obtained by X-ray, electron or neutron diffraction or cryo-electron microscopy, will enter the Protein Data Bank in the near future, as most of the attention is currently focused on artificial intelligence predictions (Jumper et al., Citation2021; Kleywegt & Velankar, Citation2022; Tunyasuvunakool et al., Citation2021; Varadi et al., Citation2022). The future is likely to be interesting and surprising.
Supplemental Material
Download MS Word (151.9 KB)Acknowledgements
J. B. Lully and K. Djinović are gratefully acknowledged with constant support and kind hospitality in Vienna, respectively.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Funding
The author(s) reported there is no funding associated with the work featured in this article.
Data availability statement
No data were produced in this study. All data used in this study are freely available at the Protein Data Bank (www.rcsb.org).
References
- Aakeroy, C. B., Bryce, D. L., Desiraju, G. R., Frontera, A., Legon, A. C., Nicotra, F., Rissanen, K., Scheiner, S., Terraneo, G., Metrangolo, P., & Resnati, G. (2019). Definition of the chalcogen bond (IUPAC Recommendations 2019). Pure and Applied Chemistry, 91(11), 1889–1892. https://doi.org/10.1515/pac-2018-0713
- Beno, B. R., Yeung, K.-S., Bartberger, M. D., Pennington, L. D., & Meanwell, N. A. (2015). A survey of the role of noncovalent sulfur interactions in drug design. Journal of Medicinal Chemistry, 58(11), 4383–4438. https://doi.org/10.1021/jm501853m
- Berman, H. M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T. N., & Weissig, H. (2000). The protein data bank. Nucleic Acids Research, 28(1), 235–242. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10592235. https://doi.org/10.1093/nar/28.1.235
- Bernstein, F. C., Koetzle, T. F., Williams, G. J., Meyer, E. F., Brice, M. D., Rodgers, J. R., Kennard, O., Shimanouchi, T., & Tasumi, M. (1977). The Protein Data Bank: a computer-based archival file for macromolecular structures. Journal of Molecular Biology, 112(3), 535–542. https://doi.org/10.1016/S0022-2836(77)80200-3
- Biswal, H. S., Sahu, A. K., Galmés, B., Frontera, A., & Chopra, D. (2022). Se…O/S and S…O chalcogen bonds in small molecules and proteins: a combined CSD and PDB study. ChemBioChem. 23(2), e202100498. https://doi.org/10.1002/cbic.202100498
- Blakeley, M. P., Hasnain, S. S., & Antonyuk, S. V. (2015). Sub-atomic resolution X-ray crystallography and neutron crystallography: promise, challenges and potential. IUCrJ, 2(Pt 4), 464–474. https://doi.org/10.1107/S2052252515011239
- Bondi, A. (1964). van der Waals volumes and radii. The Journal of Physical Chemistry, 68(3), 441–451. https://doi.org/10.1021/j100785a001
- Carugo, O. (1999). Stereochemistry of the interaction between methionine sulfur and the protein core. Biological Chemistry, 380(4), 495–498. https://doi.org/10.1515/BC.1999.064
- Carugo, O. (2017). Protein hydration: Investigation of globular protein crystal structures. International Journal of Biological Macromolecules, 99, 160–165. https://doi.org/10.1016/j.ijbiomac.2017.02.073
- Carugo, O., Resnati, G., & Metrangolo, P. (2021). Chalcogen bonds involving selenium in protein structures. ACS Chemical Biology, 16(9), 1622–1627. https://doi.org/10.1021/acschembio.1c00441
- Costantini, S., Colonna, G., & Facchiano, A. M. (2006). Amino acid propensities for secondary structures are influenced by the protein structural class. Biochemical and Biophysical Research Communications, 342(2), 441–451. https://doi.org/10.1016/j.bbrc.2006.01.159
- Dahl, T., Kozma, D., Ács, M., Weidlein, J., Schnöckel, H., Paulsen, G. B., Nielsen, R. I., Olsen, C. E., Pedersen, C., & Stidsen, C. E. (1994). The nature of stacking interactions between organic molecules elucidated by analysis of crystal structures. Acta Chemica Scandinavica, 48, 95–106. https://doi.org/10.3891/acta.chem.scand.48-0095
- Daolio, A., Scilabra, P., Di Pietro, M. E., Resnati, C., Rissanen, K., & Resnati, G. (2020). Binding motif of ebselen in solution: chalcogen and hydrogen bonds team up. New Journal of Chemistry, 44(47), 20697–20703. https://doi.org/10.1039/D0NJ04647G
- Fick, R. J., Kroner, G. M., Nepal, B., Magnani, R., Horowitz, S., & Houtz, R. L. (2016). Oxygen chalcogenbonding mediates adomet recognition in the lysine methyltransfer-ase SET7/9. ACS Chem Biol, 11, 748-754.
- Fu, L., Niu, B., Zhu, Z., Wu, S., & Li, W. (2012). CD-HIT: accelerated for clustering the next generation sequencing data. Bioinformatics (Oxford, England), 28(23), 3150–3152. https://doi.org/10.1093/bioinformatics/bts565
- Galmés, B., Juan-Bals, A., Frontera, A., & Resnati, G. (2020). Charge-assisted chalcogen bonds: csd and dft analyses and biological implication in glucosidase inhibitors. Chemistry (Weinheim an Der Bergstrasse, Germany), 26(20), 4599–4606. https://doi.org/10.1002/chem.201905498
- Giacovazzo, C., Monaco, H. L., Artioli, G., Viterbo, D., Ferraris, G., & Gilli, G. (2002). Fundamentals of crystallography. Oxford University Press.
- Heine, A., Herrmann, G., Selmer, T., Terwesten, F., Buckel, W., & Reuter, K. (2014). High resolution crystal structure of Clostridium propionicum beta-alanyl-CoA:ammonia lyase, a new member of the “hot dog fold” protein superfamily. Proteins, 82(9), 2041–2053. https://doi.org/10.1002/prot.24557
- Heinig, M., & Frishman, D. (2004). STRIDE: A web server for secondary structure assignment from known atomic coordinates of proteins. Nucleic Acids Research, 32(Web Server issue), w500–2. https://doi.org/10.1093/nar/gkh429
- Ho, P. S. (2015). Biomolecular halogen bonds. In P. Metrangolo, G. Resnati (Eds.), Halogen bonding I: Impact on material chemistry and life science (pp. 241–276). Springer.
- Hubbard, S. J., & Thornton, J. M. (1993). NACCESS. Department of Biochemistry and Molecular Biology, University College London.
- Iwaoka, M., Takemoto, S., Okada, M., & Tomoda, S. (2002). Weak nonbonded S ≥ X (X = O, N, and S) interactions in proteins. Statistical and theoretical studies. Bulletin of the Chemical Society of Japan, 75(7), 1611–1625. https://doi.org/10.1246/bcsj.75.1611
- Jumper, J., Evans, R., Pritzel, A., Green, T., Figurnov, M., Ronneberger, O., Tunyasuvunakool, K., Bates, R., Žídek, A., Potapenko, A., Bridgland, A., Meyer, C., Kohl, S. A. A., Ballard, A. J., Cowie, A., Romera-Paredes, B., Nikolov, S., Jain, R., Adler, J., … Hassabis, D. (2021). Highly accurate protein structure prediction with AlphaFold. Nature, 596(7873), 583–589. https://doi.org/10.1038/s41586-021-03819-2
- Junming, L., Yunxiang, L., Subin, Y., & Weiliang, Z. (2011). Theoretical and crystallographic data investigationsof noncovalent S…O interactions. Structural Chemistry, 22(4), 757–763. https://doi.org/10.1007/s11224-011-9751-x
- Karshikoff, A. (2021). Non-covalent interactions in proteins (2nd ed.). World Scientific.
- Kleywegt, G. J., & Velankar, S. (2022). The scientific impact of accurate protein-structure prediction methods is being felt already, but how might they affect the work and careers of structural biologists? IUCrJ, 9(Pt 4), 399–400. https://doi.org/10.1107/S2052252522005802
- Koebel, M. R., Cooper, A., Schmadeke, G., Jeon, S., Narayan, M., Sirimulla, S., & S-O, S.-N. (2016). Sulfur bonding interactions in protein-ligand complexes: empirical considerations and scoring function. Journal of Chemical Information and Modeling, 56(12), 2298–2309. https://doi.org/10.1021/acs.jcim.6b00236
- Kříž, K., Fanfrlík, J., & Lepšík, M. (2018). Chalcogen bonding in protein-ligand complexes: PDB survey and quantim mechanical calculations. Chemphyschem: A European Journal of Chemical Physics and Physical Chemistry, 19(19), 2540–2548. https://doi.org/10.1002/cphc.201800409
- Kuhn, P., Deacon, A. M., Comoso, S., Rajaseger, G., Kini, R. M., Usón, I., & Kolatkar, P. R. (2000). The atomic resolution structure of bucandin, a novel toxin isolated from the Malayan krait, determined by direct methods. Acta Crystallographica. Section D, Biological Crystallography, 56(Pt 11), 1401–1407. https://doi.org/10.1107/s0907444900011501
- Kumar, K. S. D., Gurusaran, M., Satheesh, S. N., Radha, P., Pavithra, S., Thulaa Tharshan, K. P. S., Helliwell, J. R., & Sekar, K. (2015). Online_DPI: a web server to calculate the diffraction precision index for a protein structure. Journal of Applied Crystallography, 48(3), 939–942. https://doi.org/10.1107/S1600576715006287
- Lobanov, M. Y., Pereyaslavets, L. B., Likhachev, I. V., Matkarimov, B. T., & Galzitskaya, O. V. (2021). Is there an advantageous arrangement of aromatic residues in proteins? Statistical analysis of aromatic interactions in globular proteins. Computational and Structural Biotechnology Journal, 19, 5960–5968. https://doi.org/10.1016/j.csbj.2021.10.036
- Mitchell, M. O. (2017). Discovering protein-ligand chalcogen bonding in the protein data bank using endocyclic sulfur-containing heterocycles as ligand search subsets. Journal of Molecular Modeling, 23, 287.
- Newberry, R. W., & Raines, R. T. (2019). Secondary forces in protein folding. ACS Chemical Biology, 14(8), 1677–1686. https://doi.org/10.1021/acschembio.9b00339
- Pal, D., & Chakrabarti, P. (2001). Non-hydrogen bond interactions involving the methionine sulfur atom. Journal of Biomolecular Structure and Dynamics, 1, 115–128.
- Pascoe, D. J., Ling, K. B., & Cockroft, S. L. (2017). The origin of chalcogen-bonding interactions. Journal of the American Chemical Society, 139(42), 15160–15167. https://doi.org/10.1021/jacs.7b08511
- Pflugrath, J. W. (2004). Macromolecular cryocrystallography–methods for cooling and mounting protein crystals at cryogenic temperatures. Methods (San Diego, CA), 34(3), 415–423. https://doi.org/10.1016/j.ymeth.2004.03.032
- Scheiner, S. (2021). Participation od S and Se in hydrogen and chalcogen bonds. CrystEngComm, 23(39), 6821–6837. https://doi.org/10.1039/D1CE01046H
- Scheiner, S. (2022). Various sorts of chalcogen bonds formed by an aromatic system. The Journal of Physical Chemistry. A, 126(25), 4025–4035. https://doi.org/10.1021/acs.jpca.2c02451
- Sundaramoorthy, M., Gold, M. H., & Poulos, T. L. (2010). Ultrahigh (0.93A) resolution structure of manganese peroxidase from Phanerochaete chrysosporium: implications for the catalytic mechanism. Journal of Inorganic Biochemistry, 104(6), 683–690. https://doi.org/10.1016/j.jinorgbio.2010.02.011
- Tunyasuvunakool, K., Adler, J., Wu, Z., Green, T., Zielinski, M., Žídek, A., Bridgland, A., Cowie, A., Meyer, C., Laydon, A., Velankar, S., Kleywegt, G. J., Bateman, A., Evans, R., Pritzel, A., Figurnov, M., Ronneberger, O., Bates, R., Kohl, S. A. A., … Hassabis, D. (2021). Highly accurate protein structure prediction for the human proteome. Nature, 596(7873), 590–596. https://doi.org/10.1038/s41586-021-03828-1
- Varadi, M., Anyango, S., Deshpande, M., Nair, S., Natassia, C., Yordanova, G., Yuan, D., Stroe, O., Wood, G., Laydon, A., Žídek, A., Green, T., Tunyasuvunakool, K., Petersen, S., Jumper, J., Clancy, E., Green, R., Vora, A., Lutfi, M., … Velankar, S. (2022). AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Research, 50(D1), D439–D444. https://doi.org/10.1093/nar/gkab1061