Abstract
Background: The aim of this study was to explore factors affecting cardiorespiratory fitness in males and females with different body mass index (BMI).
Methods: The National Health and Nutrition Examination Survey 1999–2004 data were used for this retrospective study. Estimated maximal oxygen uptake (VO2max) is surrogate for cardiorespiratory fitness (CRF). Univariate and multivariate linear regression analyses were performed to explore whether study variables were associated with estimated VO2max stratified by gender and BMI categories.
Results: A total of 3292 subjects 20–49 years of age were included in the analysis. CRF significantly decreased as BMI increased in both females and males. Ethnic difference was found in normal BMI in both genders and obese females; homocysteine was significantly negatively associated with estimated VO2max, as was total cholesterol. Obese male subjects with diabetes had a lower estimated VO2max than those without diabetes, and C-reactive protein (CRP) level and vitamin B12 level were significantly negatively associated with CRF. Female subjects with diabetes had higher estimated VO2max than those without diabetes. Folate was significantly positively correlated with estimated VO2max, whereas CRP was negatively correlated in obese female.
Conclusions: There are different predictors of CRF in males and females, and in individuals with different BMI.
Different BMI classes are associated with different predictors of cardiorespiratory fitness.
Indicators of cardiorespiratory fitness differ between sexes.
Key messages
Introduction
Cardiovascular diseases (CVDs) are among the leading cause for adult deaths globally. An estimated 17.5 million people died from CVDs in 2012, representing 31% of all global deaths (Citation1). The economic burden of CVDs is of great concern; in the United States $1 of every $6 spent on healthcare is for the treatment of CVDs (Citation2). Estimates also suggest that CVDs will continue to dominate mortality trends in the future (Citation3). This makes preventive care for CVDs one of the top priority health issues in the world.
The anticipated increase in CVDs has been attributed to risk factors such as diabetes, hypertension, hypercholesterolemia, and obesity (Citation4), unhealthy diet, physical inactivity, tobacco use, harmful use of alcohol, and low socioeconomic status. Homocysteine, a suspected etiological factor for atherosclerosis, is associated with an increased risk of CVD (Citation5,Citation6). C-reactive protein (CRP) is considered one of the best measures of the body’s response to inflammation, and has been confirmed as an independent predictor of future cardiovascular events (Citation7,Citation8). Fat deposition in the android region is also associated with an increased risk of CVD (Citation9,Citation10). These factors associated with CVD are also associated with mortality and can be categorized into three dimensions: biological (e.g., diabetes, CRP); behavioral (e.g., smoking, physical activity); and socioeconomic (e.g., poverty) (Citation11).
Fortunately, CVDs are preventable, and factors protective against CVDs have been identified. Cardiorespiratory fitness (CRF), a measure of the body’s capacity to transport and utilize oxygen, is associated with a lower risk of CVDs (Citation12–14). Folate, involved in carbohydrate metabolism, can reduce plasma homocysteine levels, and thus indirectly protect against CVDs (Citation15). Hemoglobin, an oxygen-transport metalloprotein, impacts maximal oxygen uptake and thus CRF (Citation16).
The period from 20 to 49 years of age is considered the “prime of our life,” and the main working population in developed countries. The epidemiology report from the Framingham Heart Study revealed that age-specific CVD incidence rates are substantially lower in women than men in younger and middle-aged groups (Citation17). CRF is considered to be an effective predictor for CVDs, and CRF has been found to vary in different populations and with different lifestyle factors. For example, non-Hispanic black adults as a group have lower cardiovascular fitness than other major race groups in the United States (Citation18,Citation19), moderate activity and fitness, but not sedentary time, are independently associated with cardiovascular risk in the United States adults (Citation20), and CRF, independent of fatness, may have beneficial effects on lipid profiles in girls and on lipid profiles, insulin metabolism, and inflammation levels among boys in the United States (Citation21). Despite considerable study in this area, there is insufficient evidence available to characterize contributors to CRF in males and females, and in individuals with different body mass index (BMI).
The role of CRF, behavior, and body composition with respect to health is a topic of great importance as we move toward a better understanding of the role that physiological and metabolic factors and the physical, social and cultural environments have on the health of our populations. Thus, the purpose of this study was to explore factors affecting CRF in males and females with different BMI.
Materials and methods
Data source
Data from the National Health and Nutrition Examination Survey (NHANES) collected from 1999 to 2004 were used for this analysis. The NHANES program began in the early 1960s, and has been conducted as a series of surveys focusing on different population groups and health topics. The sample for the NHANES survey is selected to represent the United States population of all ages. Tools and methods used for testing and data collection in the NHANES program have been well-validated (Citation22–25). Further information about background, design and operation is available on the NHANES website (http://wwwn.cdc.gov/nchs/nhanes).
Study population
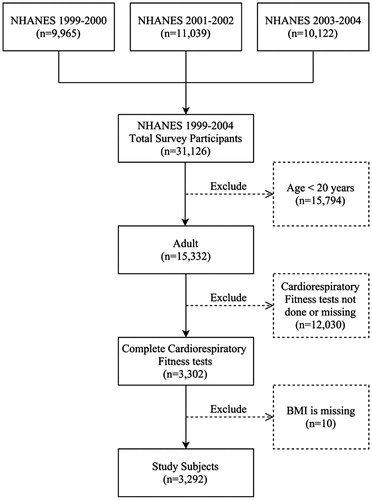
During the period from 1999 to 2004, NHANES CRF data were collected through means of submaximal treadmill testing for subjects 12–49 years of age. We excluded subjects <20 years of age because some important covariates were not available for this age group. As BMI was a major comparison criterion in this study, subjects with missing BMI data were also excluded. A flow diagram of the selection process is shown in . A total of 31,126 participants who completed the interview and examination at a mobile exam center (MEC) were included in the NHANES survey during the period from 1999 to 2004. Of these, 3302 participants 20 years of age or older completed the CRF test. Ten participants with missing BMI information were excluded, and a total of 3292 participants were included in this study.
Study variables
Cardiorespiratory fitness
The estimated maximal oxygen uptake (VO2max) (ml/kg/min) was the dependent variable in this study. In NHANES, CRF was evaluated through a submaximal exercise test. The protocol included a 2-minute warm-up, two 3-minute exercise stages, and a 2-minute cool-down period. Heart rate was monitored continuously, and was recorded at the end of warm-up, each exercise stage, and each minute of recovery. At the end of the warm-up and each exercise stage, participants were asked to rate their perceived exertion using the Borg scale. The VO2max was estimated by extrapolation using measured heart rate responses to known levels of exercise workloads, assuming the relation between heart rate and oxygen consumption is linear during exercise. All technicians who performed the testing were trained by exercise physiologists from the Cooper Institute, were monitored twice a year for competence in performing the testing, and participated in an annual retraining session to assure quality control. Participants were excluded from this component based on medical conditions, medications, physical limitations, limits on heart rate and blood pressure, and irregular heart rates. Detailed descriptions of the protocol are provided in the NHANES Cardiovascular Fitness Procedure Manual (Citation26).
BMI
BMI was calculated as body weight (kg) divided by height (m) squared (kg/m2). Height and weight were all measured in the survey. According to the World Health Organization (WHO) classification, we further divide participants based on BMI: underweight, BMI <18.5 kg/m2; normal weight, BMI = 18.5–24.9 kg/m2; overweight, BMI = 25.0–29.9 kg/m2; obese, BMI ≥30 kg/m2.
Socioeconomic dimension
Age, sex, and race/ethnicity were obtained from the NHANES database. Poverty income ratio (PIR) data, an indicator of family income to poverty threshold, were collected from the interview record.
Biological dimension
The presence of diabetes mellitus was ascertained by the question “Have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” and the results dichotomized. Hypertension was also dichotomized, and defined by the question “Have you ever been told by a doctor or other health professional that you had hypertension, also called high blood pressure?” or by asking if the participant was taking a prescription medication to lower blood pressure. In NHANES, total homocysteine in plasma was measured by the Abbott homocysteine assay on an Abbott AxSym analyzer. Serum folate was measured using the Bio-Rad Laboratories Quantaphase II Folate/Vitamin B12 radioassay kit. CRP was quantified by latex-enhanced nephelometry using a Behring nephelometer. Total cholesterol was measured enzymatically in serum or plasma in a series of coupled reactions that hydrolyze cholesteryl esters and oxidize the 3-OH group of cholesterol. The method used to determine hemoglobin level was based on the Beckman Coulter method of counting and sizing, in combination with an automatic diluting and mixing device for sample processing, and a single beam photometer for hemoglobinometry. Serum Vitamin B12 was measured by the Bio-Rad Quantaphase II radioimmunoassay (RIA) kit (Bio-Rad Laboratories, Hercules, CA). Detailed specimen collection, processing instructions, and laboratory methods are discussed in the NHANES Laboratory/Medical Technologists Procedures Manual (LPM) (Citation26).
Behavioral dimension
Alcohol intake was ascertained by the question “In any one year, have you had at least 12 drinks of any type of alcoholic beverage?”, and dichotomized. For our analysis, current smokers were those who had smoked at least 100 cigarettes during their lifetime and reported smoking either every day or some days. Former smokers were those who reported smoking at least 100 cigarettes during their lifetime, but currently did not smoke. Never-smokers were those who reported never having smoked cigarettes during their lifetime.
Statistical analyses
Data of demographic and basic characteristics were expressed as mean ± standard error (SE) for continuous variables, or unweighted counts (weighted %) for categorical variables. Chi-square test was conducted to examine the differences in categorical variables stratified by gender and BMI categories, and differences in continuous variables were examined using the Complex Samples General Linear Model (CSGLM).
Univariate and multivariate linear regression analyses were performed to explore whether these study variables were associated with estimated VO2max (surrogate of CRF) stratified by gender and BMI categories. Variables with a p-value <.05 by univariate analysis were selected and input into the multivariate logistic regression model.
All analyses included special sample weights (2/3 × WTMEC4YR [4 year MEC exam weight) for the 1999–2002 data set; 1/3 × WTMEC2YR [2 year MEC exam weight] for the 2003–2004 data set, stratum, and primary sampling units (PSU) per recommendations from National Center for Health Statistics (NCHS), to address oversampling, non-response, non-coverage, and to provide nationally representative estimates (Citation27,Citation28). All statistic assessments were two-sided and evaluated at the 0.05 level of significance. Statistical analyses were performed by IBM SPSS statistical software version 22 for Windows (IBM Corp., Armonk, NY).
Results
Characteristics of study subjects
Of the 3292 participants, 1720 (52.1%) were males and 1572 (47.9%) were females, and the overall mean age was 33.92 years. Using the NHANES sample weight formulae, the analytic sample size was equivalent to a population-based sample size of 60,821,464 participants. Basic characteristics of the study subjects according to the BMI and stratified by gender are shown in .
Table 1. Subject characteristics (unweighted n = 3292, weighted n = 60,821,464).
The mean estimated VO2max was significantly higher in males than in females, and it significantly decreased as BMI increased in both males and females (both, p < .001). The mean age significantly increased as BMI increased in both males (p < .001) and females (p = .002). Obese male and female subjects had a higher incidence of diabetes and hypertension than other subjects in other BMI categories (both, p < .05). CRP and total cholesterol increased with increased BMI in both males and females (both, p < .05); however, folate decreased with increased BMI in both males and females (both, p < .05). Vitamin B12 level decreased with increased BMI in males (p = .012).
Smoking was significantly different among BMI categories in males (p = .025), while there were no differences in females among BMI categories (p = .214). Current smoking was most frequently noted in underweight males. Alcohol intake was significantly different among BMI categories in both males and females (both, p < .05).
Linear regression analysis of estimated VO2max predictors in males stratified by BMI category
In linear regression analysis, the group of underweight subjects was affected by the number of samples and the design-based covariance matrix was singular, resulting mode was not estimated. For normal BMI subjects, univariate linear regression analysis indicated age, ethnicity, homocysteine level, and total cholesterol level were significantly associated with estimated VO2max. These significant variables were input into the multivariate linear regression model, which showed that ethnicity was significantly associated with estimated VO2max; Hispanic and other races had lower CRF than non-Hispanic Caucasians (coefficient = −3.021, p = .037; coefficient = −5.721, p = .002, respectively). Homocysteine was significantly negatively associated with estimated VO2max (coefficient = −.347, p = .002) ().
Table 2. Linear regression analysis of estimated VO2max predictors in male stratified by BMI categories.
For overweight subjects, univariate linear regression revealed that ethnicity, smoking, homocysteine level, and total cholesterol level were significantly associated with estimated VO2max. Multivariate linear regression analysis indicated that smoking was significantly associated with estimated VO2max; former and current smokers had better CRF than never-smokers (coefficient = 1.656, p = .038; coefficient = 2.583, p = .025, respectively). Homocysteine and total cholesterol were significantly negatively associated with estimated VO2max (coefficient = −.304, p = .001; coefficient = −.812, p = .010, respectively).
For obese subjects, ethnicity, diabetes, CRP level, and vitamin B12 level were found to be significant in the univariate model, and input into the multivariate linear regression model. The results showed that DM patient have lower CRF (coefficient = −3.986, p = .017). CRP and vitamin B12 level were significantly negatively associated with estimated VO2max (coefficient = −2.970, p = .019; coefficient = −0.007, p = .006, respectively).
Linear regression analysis of estimated VO2max predictors in females stratified by BMI category
Result of regression analysis on underweight subjects are not shown here due to low sample sizes and the covariance matrix is singular. For normal BMI subjects, age, ethnicity, diabetes, homocysteine level, and vitamin B12 level were significantly associated with estimated VO2max by univariate linear regression analysis. Multivariate linear regression analysis of these significant factors revealed that non-Hispanic black subjects had a significantly lower estimated VO2max than non-Hispanic Caucasians (coefficient = −2.136, p = .010). Subjects with diabetes had a significantly higher estimated VO2max than those without diabetes (coefficient = 10.126, p = .001). Vitamin B12 level was significantly positively correlated with estimated VO2max (coefficient = .0002, p < .001), whereas homocysteine level was negatively correlated (coefficient = −.527, p = .001) ().
Table 3. Linear regression analysis of estimated VO2max predictors in female stratified by BMI categories.
For overweight subjects, age, ethnicity, diabetes, smoking, alcohol intake, total cholesterol level, and hemoglobin level were significantly associated with estimated VO2max by univariate linear regression analysis. Multivariate linear regression analysis indicated that alcohol intake was significantly associated with estimated VO2max; current alcohol intake had a significantly higher estimated VO2max than never alcohol intake. Hemoglobin was significantly correlated with estimated VO2max (coefficient = .954, p = .001), whereas total cholesterol was negatively correlated (coefficient = −1.081, p = .010).
For obese subjects, ethnicity, folate, CRP, and hemoglobin were significantly associated with estimated VO2max by univariate linear regression analysis. Multivariate linear regression indicated that ethnicity was significantly associated with estimated VO2max, in which non-Hispanic black and other races had a significantly lower estimated VO2max than non-Hispanic Caucasians (coefficient = −2.402, p = .009; coefficient = −3.740, p = .018, respectively). In addition, folate was significantly positively correlated with estimated VO2max (coefficient = .013, p < .001), whereas CRP was negatively correlated (coefficient = −1.797, p = .008).
Discussion
The present study revealed modifiable risk factors of cardiovascular fittness among different BMI goups in males and females. Importantly, factors associated with cardiovascular fittness different among males and females, and among BMI groups. BMI is simple to determine, and these data may allow clinicians to identify crucial contributors to the CRF, and provide effective interventions.
The potential mechanisms contributing to the observed gender/BMI disparity in CRF outcomes may include differences in socioeconomics, biological characteristics, and behavioral and psychosocial factors. We found the frequency of obesity increased with aging in both genders. However, after controlling for confounders age was inversely associated with CRF only in normal BMI males and females, although statistical significance was not reached. Similar findings have been described in other studies; a decline in VO2max of about 1.6% per year has been reported in both men and women (Citation29,Citation30). In women, the possible mechanism may be loss of estrogen with advancing age (Citation31). Age-related changes in BMI are affected by a variety of factors such as physical activity, menopausal status, nutrition, and disease (Citation32). Interestingly, in the obese population age is not a key factor related to CRF. Further research is needed to clarify possible mechanism.
In our sample, higher socioeconomic status (family PIR) was associated with lower BMI, but only among women (overweight men tend to come from wealthy families). Previous study has demonstrated that the effects of socioeconomic status on obesity are different for men and women due to dietary habits, leisure time physical activity, and perceived life control (Citation33), potentially translating into a different CRF.
Homocysteine level has been found to be inversely associated with CRF in women in data from NHANES 1999 to 2002 (Citation34). In the current study, in both genders homocysteine was inversely associated with CRF. Previous study showed that protein homocysteinylation is a possible mechanism of homocysteine-related protein damage, and may thus result in impaired cardiovascular fitness and function (Citation34). However, the study found homocysteine levels were inversely associated with cardiovascular fitness in women, but not in men (the difference between their study and ours was sample size (1999–2002 vs 1999–2004).
In addition, we found folate and hemoglobin levels were positive associated with CRF. Potential mechanisms of antioxidant actions, lowering of homocysteine levels, or direct interactions with the enzyme endothelial nitric oxide (NO) synthase have been proposed (Citation35).
Numerous studies have indicated an inverse relationship between CRF and diabetes (Citation36,Citation37). Evidence from several previous studies also indicated that higher fitness is associated with a 40–70% lower risk for type 2 diabetes in men (Citation38,Citation39). Obesity is related to inflammation and fat metabolism, which leads to insulin resistance (Citation40,Citation41). Our results were similar to those of prior studies, but only in obese males. This conflicting finding may be due to the weaknesses of cross-sectional studies. In this cross-sectional observation study, we were not able to discern temporality of comorbid diabetes mellitus and physical fitness training. The association awaits further longitudinal research to clarify the causal influence. Unexpectedly, we found DM was positively related to CRF in normal BMI females. A possible explanation is that diabetic patients who maintain normal BMI may put more effort towards a healthy diet and enhanced physical activity, potentially translating into improvement in CRF.
Similar to other studies, we found that CRP level was inversely related to CRF in obese males. A proposed explanation is that visceral fat accumulation and its cross-interaction with inflammatory cells results in systemic inflammation and subsequent increase in CRF (Citation42). For overweight men, CRF declined with increasing total cholesterol level. Studies have suggested that improving CRF may delay the onset of dyslipidemia that commonly occurrs with aging in men by up to 15 years (Citation43).
Interestingly, our results suggest that alcohol intake can improve CRF in overweight females. Gender differences of the relationship between alcohol intake and cardiovascular health have been reported (Citation44,Citation45). The beneficial effect is likely due to alcohol’s favorable pleiotropic effects on lipids, adhesion molecules, platelet activation, and oxidative stress (Citation46).
Our findings regarding the relation between smoking and CRF are in contrast to those of other studies (Citation47). Adaptive theory may provide a possible explanation. Smokers have lower aerobic capacity and, thus, less oxygen supply while they are exercising. Smoking also requires an additional energetic cost, which is caused by greater respiratory muscle work and pulse rate (Citation48). However, once the body adapts to a hypoxic status, the relation between heart rate and oxygen consumption may be altered (CRF was estimated by extrapolation using measured heart rate responses to known levels of exercise workloads). In addition, smokers were defined as those who had smoked at least 100 cigarettes during their lifetime in the present study, and no further quantification was done.
It is well-accepted that improvement in the CRF can be achieved by participating in regular physical activities (Citation49–51). Previous study observed that increased time spent watching television was significantly associated with obesity for both boys and girls; this relationship has been widely recognized and likely mediated by corresponding decreased physical activity (Citation52,Citation53). Some types of physical activity, such as low intensity or limited to a small amount of tissue, may lead to significant local changes important for metabolic health. Adaptation specific to the working muscle has been illustrated in a number of studies of one-legged exercise training, which utilizes a small fraction of whole body muscle mass. Results of these studies showed local adaptations in trained muscle such as increased capillarization, muscle glycogen content, mitochondrial activity, insulin sensitivity, and transcription of metabolic genes (Citation54–57). Physical activity is an important issue when investigating CRF; however, the physical activity questionnaire in NHANES was only given to participants 12–15 years of age from 1999 to 2004. Our study was limited to those 20 or more years of age, so physical activity was not studied.
The strengths of this study are the large sample size, and that the findings open the opportunity to begin looking at sex and BMI-based interventions to improve cardiovascular fitness, and reduce the risk of CVD. However, there are a number of limitations that need to be taken into consideration. The study was cross-sectional rather than longitudinal, and thus causality cannot be established. We only have baseline data on fitness, other exposures, and weight, so we do not know if changes in any of these variables occurred during follow-up or if potential changes may have influenced the results. Further longitudinal study is need. However, planned follow-up times would need to be sufficiently long to enable relationships to emerge. Participants were excluded from CRF testing based on medical conditions, medications, physical limitations, limits on heart rate and blood pressure, and irregular heart rates. As a result, a healthier and more fit sample was included in the present study. It has been found that the exclusion rate for the fitness test increased with age (Citation50). The youth and relatively good health of our sample might also explain why our findings are discordant with previous studies. Future study should compare or target these excluded populations for further investigation. In addition, the CRF test is an applied test, and is confounded by the motivation of the participants and by the instructions and encouragement provided by the examiner. Smoking was not quantified, and neither physical activity nor body fat was included as variables in the study because both variables were not included in the 1999–2002 and 2003–2004 datasets. For example, questions about hours of television watched or hours at the computer only applied to respondents in the 1999–2002 data set. Lastly, self-reporting bias was not taken into account.
In conclusion, different BMI classes are associated with different predictors of CRF, and the relationships are independent between sexes. Clinicians and healthcare professionals can target the crucial factors based on a patients BMI and sex to reduce the risk of CVDs.
Disclosure statement
The authors report no conflicts of interest.
References
- World Health Organization. Cardiovascular diseases; [cited 2016 Mar 10]. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/.
- Centers for Disease Control. Taking on the Nation's Leading Killers. 2010; [cited 2016 Mar 10]. Available from: http://www.cdc.gov/dhdsp/docs/dhdsp_factsheet.pdf.
- World Health Organization. World health statistics 2009; [cited 2016 Mar 10]. Available from: http://www.who.int/gho/publications/world_health_statistics/EN_WHS09_Full.pdf.
- Lemieux I, Lamarche B, Couillard C, Pascot A, Cantin B, Bergeron J, et al. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Arch Intern Med. 2001;161:2685–92.
- Wierzbicki AS. Homocysteine and cardiovascular disease: a review of the evidence. Diab Vasc Dis Res. 2007;4:143–50.
- Warren CJ. Emergent cardiovascular risk factor: homocysteine. Prog Cardiovasc Nurs. 2002;17:35–41.
- Ridker PM. C-reactive protein: eighty years from discovery to emergence as a major risk marker for cardiovascular disease. Clin Chem. 2009;55:209–15.
- Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:483–95.
- Samsell L, Regier M, Walton C, Cottrell L. Importance of android/gynoid fat ratio in predicting metabolic and cardiovascular disease risk in normal weight as well as overweight and obese children. J Obes. 2014;2014:846578.
- Okosun IS, Seale JP, Lyn R. Commingling effect of gynoid and android fat patterns on cardiometabolic dysregulation in normal weight American adults. Nutr Diabetes. 2015;5:e155.
- Loprinzi PD, Davis RE. Psycho-socioeconomic bio-behavioral associations on all-cause mortality: cohort study. Health Promot Perspect. 2016;6:66–70.
- Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–35.
- Duncan GE. Exercise, fitness, and cardiovascular disease risk in type 2 diabetes and the metabolic syndrome. Curr Diab Rep. 2006;6:29–35.
- Myers J, McAuley P, Lavie CJ, Despres JP, Arena R, Kokkinos P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: their independent and interwoven importance to health status. Prog Cardiovasc Dis. 2015;57:306–14.
- Molloy AM, Scott JM. Folates and prevention of disease. Public Health Nutr. 2001;4:601–09.
- Cureton K, Bishop P, Hutchinson P, Newland H, Vickery S, Zwiren L. Sex difference in maximal oxygen uptake. Effect of equating haemoglobin concentration. Eur J Appl Physiol Occup Physiol. 1986;54:656–60.
- D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53.
- Duncan GE, Li SM, Zhou XH. Cardiovascular fitness among U.S. adults: NHANES 1999-2000 and 2001-2002. Med Sci Sports Exerc. 2005;37:1324–28.
- Sanders LF, Duncan GE. Population-based reference standards for cardiovascular fitness among U.S. adults: NHANES 1999–2000 and 2001–2002. Med Sci Sports Exerc. 2006;38:701–07.
- van der Velde JH, Savelberg HH, Schaper NC, Koster A. Moderate activity and fitness, not sedentary time, are independently associated with cardio-metabolic risk in U.S. adults aged 18–49. Int J Environ Res Public Health. 2015;12:2330–43.
- Kwon S, Burns TL, Janz K. Associations of cardiorespiratory fitness and fatness with cardiovascular risk factors among adolescents: the NHANES 1999-2002. J Phys Act Health. 2010;7:746–53.
- Astrand PO, Ryhming I. A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during sub-maximal work. J Appl Physiol. 1954;7:218–21.
- Pate RR, Wang CY, Dowda M, Farrell SW, O'Neill JR. Cardiorespiratory fitness levels among US youth 12 to 19 years of age: findings from the 1999–2002 National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2006;160:1005–12.
- Quinart S, Mougin F, Simon-Rigaud ML, Nicolet-Guénat M, Nègre V, Regnard J. Evaluation of cardiorespiratory fitness using three field tests in obese adolescents: validity, sensitivity and prediction of peak VO2. J Sci Med Sport. 2014;17:521–5.
- Bennett H, Parfitt G, Davison K, Eston R. Validity of submaximal step tests to estimate maximal oxygen uptake in healthy adults. Sports Med. 2016;46:737–50.
- National Health and Nutrition Examination Survey (NHANES). Cardiovascular fitness procedures manual. January 2005; [cited 2006 Sep 12]. Available from: https://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/CV.pdf.
- National Health and Nutrition Examination Survey. 2003–2004 Data documentation, codebook, and frequencies. Cardiovascular fitness. Analytical notes. 2009; [cited 2016 Sep 20]. Available from http://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/CVX_C.htm#Analytic_Notes.
- Kim Y, Park S, Kim NS, Lee BK. Inappropriate survey design analysis of the Korean National Health and Nutrition Examination Survey may produce biased results. J Prev Med Public Health. 2013; 46:96–104.
- Hakola L, Komulainen P, Hassinen M, Savonen K, Litmanen H, Lakka TA, et al. Cardiorespiratory fitness in aging men and women: the DR's EXTRA study. Scand J Med Sci Sports. 2011; 21:679–87.
- Baur DM, Christophi CA, Cook EF, Kales SN. Age-related decline in cardiorespiratory fitness among career firefighters: modification by physical activity and adiposity. J Obes. 2012;2012:710903.
- Parker BA, Kalasky MJ, Proctor DN. Evidence for sex differences in cardiovascular aging and adaptive responses to physical activity. Eur J Appl Physiol. 2010;110:235–46.
- Tian S, Morio B, Denis J, Mioche L. Age-related changes in segmental body composition by ethnicity and history of weight change across the adult lifespan. Int J Environ Res Public Health. 2016;13:821.
- Merino Ventosa M, Urbanos-Garrido RM. Disentangling effects of socioeconomic status on obesity: a cross-sectional study of the Spanish adult population. Econ Hum Biol. 2016;22:216–24.
- Kuo HK, Yen CJ, Bean JF. Levels of homocysteine are inversely associated with cardiovascular fitness in women, but not in men: data from the National Health and Nutrition Examination Survey 1999–2002. J Intern Med. 2005;258:328–35.
- Verhaar MC, Stroes E, Rabelink TJ. Folates and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002;22:6–13.
- Zaccardi F, O'Donovan G, Webb DR, Yates T, Kurl S, Khunti K, et al. Cardiorespiratory fitness and risk of type 2 diabetes mellitus: a 23-year cohort study and a meta-analysis of prospective studies. Atherosclerosis. 2015;243:131–37.
- Juraschek SP, Blaha MJ, Blumenthal RS, Brawner C, Qureshi W, Keteyian SJ, et al. Cardiorespiratory fitness and incident diabetes: the FIT (Henry Ford ExercIse Testing) project. Diabetes Care. 2015;38:1075–81.
- Lynch J, Helmrich SP, Lakka TA, Kaplan GA, Cohen RD, Salonen R, et al. Moderately intense physical activities and high levels of cardiorespiratory fitness reduce the risk of non-insulin-dependent diabetes mellitus in middle-aged men. Arch Intern Med. 1996;156:1307–14.
- Sawada SS, Lee IM, Muto T, Matuszaki K, Blair SN. Cardiorespiratory fitness and the incidence of type 2 diabetes: prospective study of Japanese men. Diabetes Care. 2003;26:2918–22.
- Hu G, Lakka TA, Kilpelainen TO, Tuomilehto J. Epidemiological studies of exercise in diabetes prevention. Appl Physiol Nutr Metab. 2007;32:583–95.
- Holt HB, Wild SH, Wareham N, Ekelund U, Umpleby M, Shojaee-Moradie F, et al. Differential effects of fatness, fitness and physical activity energy expenditure on whole-body, liver and fat insulin sensitivity. Diabetologia. 2007;50:1698–06.
- Meguro S, Ishibashi M, Takei I. (The significance of high sensitive C reactive protein as a risk factor for cardiovascular diseases). Rinsho Byori. 2012;60:356–61. (Article in Japanese).
- Park YM, Sui X, Liu J, Zhou H, Kokkinos PF, Lavie CJ, et al. The effect of cardiorespiratory fitness on age-related lipids and lipoproteins. J Am Coll Cardiol. 2015;65:2091–100.
- Fernandez-Sola J, Nicolas-Arfelis JM. Gender differences in alcoholic cardiomyopathy. J Gend Specif Med. 2002;5:41–47.
- Nolen-Hoeksema S, Hilt L. Possible contributors to the gender differences in alcohol use and problems. J Gen Psychol. 2006;133:357–74.
- Carnevale R, Nocella C. Alcohol and cardiovascular disease: still unresolved underlying mechanisms. Vascul Pharmacol. 2012; 57:69–71.
- de Borba AT, Jost RT, Gass R, Nedel FB, Cardoso DM, Pohl HH, et al. The influence of active and passive smoking on the cardiorespiratory fitness of adults. Multidiscip Respir Med. 2014;9:34.
- Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med. 1976;295:573–77.
- Laye MJ, Nielsen MB, Hansen LS, Knudsen T, Pedersen BK. Physical activity enhances metabolic fitness independently of cardiorespiratory fitness in marathon runners. Dis Markers. 2015;2015:806418.
- Wang CY, Haskell WL, Farrell SW, Lamonte MJ, Blair SN, Curtin LR, et al. Cardiorespiratory fitness levels among US adults 20–49 years of age: findings from the 1999–2004 National Health and Nutrition Examination Survey. Am J Epidemiol. 2010;171:426–35.
- DeFina LF, Haskell WL, Willis BL, Barlow CE3, Finley CE, Levine BD, et al. Physical activity versus cardiorespiratory fitness: two (partly) distinct components of cardiovascular health?. Prog Cardiovasc Dis. 2015;57:324–29.
- Andersen RE, Crespo CJ, Bartlett SJ, Cheskin LJ, Pratt M. Relationship of physical activity and television watching with body weight and level of fatness among children: results from the Third National Health and Nutrition Examination Survey. JAMA. 1998;279:938–42.
- Gortmaker SL, Must A, Sobol AM, Peterson K, Colditz GA, Dietz WH. Television viewing as a cause of increasing obesity among children in the United States, 1986–1990. Arch Pediatr Adolesc Med. 1996;150:356–62.
- Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B. Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J Physiol. 1993;469:459–78.
- Pilegaard H, Saltin B, Neufer DP. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–58.
- Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279:E806–14.
- Dela F, Larsen JJ, Mikines KJ, Ploug T, Petersen LN, Galbo H. Insulin-stimulated muscle glucose clearance in patients with NIDDM. Effects of one-legged physical training . Diabetes. 1995;44:1010–20.