Abstract
Background and aims
Hyperuricaemia can lead to gout and is associated with an increased risk of cardiometabolic disease. We aimed to investigate the prevalence of hyperuricaemia and its related factors in Chinese children and adolescents.
Methods
We pooled data from 11 population-based studies comprising 54,580 participants aged 3–19 years. The sex- and age-standardized prevalence of hyperuricaemia was estimated overall and by sex, age, weight status, geographic region and survey year.
Results
Serum uric acid (SUA) increased gradually from 3 to 11 years with no significant sex difference, and then increased dramatically during 11–15 years. The estimated overall prevalence of hyperuricaemia was 23.3% (26.6% in boys and 19.8% in girls, p < .001). The prevalence increased with growing age (3.7, 9.8, 15.8, 35.5 and 31.7% among children aged 3–5, 6–8, 9–11, 12–15 and 16–19 years, respectively, p for trend < .001) and with increasing weight status (18.2, 37.6, 50.6 and 64.5% among children with non-overweight, overweight, obesity and extreme obesity, respectively, p for trend < .001). The prevalence was higher in North than in South (24.2 vs. 19.7%, p < .001), and increased markedly from 16.7% during 2009–2015 to 24.8% during 2016–2019. In multivariable regression analyses, sex, age, obesity, region and survey year were independently associated with odds of hyperuricaemia.
Conclusions
The prevalence of hyperuricaemia in Chinese children and adolescents is unexpectedly high. The findings suggest an urgent need to implement effective interventions to reduce risk of hyperuricaemia in Chinese youths.
Question: What is the prevalence of hyperuricaemia in Chinese children and adolescents?
Findings: In this large pooled cross-sectional study comprising >50,000 children and adolescents aged 3–19 years, we found that the prevalence of hyperuricaemia was high in overall population and subgroups of sex, age, obesity, region and survey year.
Meaning: Our findings indicate that hyperuricaemia is an important health problem in Chinese children and adolescents, and effective intervention strategies are needed to reduce its burden.
KEY MESSAGES
1. Introduction
Serum uric acid (SUA) is the ultimate metabolite of purine. Hyperuricaemia is caused by urine metabolism disorder that is the interaction result of many factors including high purine diet and obesity [Citation1]. There is convincing evidence that hyperuricaemia is an established risk factor for gout and nephrolithiasis [Citation2]. Several longitudinal studies have shown that elevated SUA levels are associated with increased risks of future renal dysfunction [Citation3], type 2 diabetes [Citation4] and adverse cardiovascular outcomes [Citation5].
With the rapid socio-economic development of China in recent decades, purine-abundant diets and physical inactivity have become increasingly common [Citation6,Citation7], which may lead to increasing prevalence of hyperuricaemia [Citation8]. A meta-analysis of adults showed that the prevalence of hyperuricaemia was 13.3% in mainland China during 2000–2014 [Citation9]. However, the prevalence of hyperuricaemia among Chinese children and adolescents is poorly characterized. Previous cross-sectional studies have demonstrated that the prevalence of hyperuricaemia in Chinese youths varied across surveys ranging from 10.1 to 25.4% [Citation10–19], and most of these studies are limited to certain area, small sample size, specific age period or inconsistent diagnostic criteria. Therefore, it is particularly important to obtain national prevalence and related factors, which can help to improve health awareness and formulate appropriate public health policies. In this study, we aimed to estimate the prevalence of hyperuricaemia and identify its related factors in Chinese children and adolescents during 2009–2019 using pooled sample from 11 population-based studies.
2. Methods
2.1. Study population
We pooled sample of 58,993 children and adolescents from 11 population-based studies conducted during 2009–2019. Detailed information of these studies has been previously published [Citation10–20]. These studies involved 14 provinces or municipalities and the sample size ranged from 509 to 21,602. Participants were recruited from communities in six studies, schools in three studies or health examination centres in two studies (). Data on demographic characteristics, anthropometric parameters, socioeconomics and SUA were obtained from each study. We finally included a total of 54,580 participants for analysis after excluding those who: 1) had missing data for sex or age (n = 901); 2) were aged < 3 years (2987) or > 19 years (n = 525); or 3) had genetic diseases or acute or serious chronic diseases (Supplemental Figure 1). Each study had been approved by their respective Institutional Ethics Review Board.
Table 1. Characteristics of included studies.
2.2. Measurements
Weight and height were measured twice with lightweight clothing and no shoes in all studies. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in metres. SUA was measured using the automatic analyser based on the enzymatic colorimetric method in all studies.
2.3. Definitions
Weight status for children aged 3–19 years were classified as non-overweight, overweight and obesity according to sex- and age-specific BMI cut-off values recommended by the International Obesity Task Force (IOTF) [Citation21]. Extreme obesity was defined according to 1.2 times of BMI cut-off values for obesity [Citation22]. For those ≥18 years, overweight and obesity were defined using cut-off points of 25 and 30 kg/m2, respectively [Citation23]. Hyperuricaemia was defined as SUA > 420 μmol/L (7 mg/dL) in boys and >360 μmol/L (6 mg/dL) in girls [Citation24].
2.4. Statistical analysis
The prevalence rates were sex- and age-standardized according to the Sixth National Census. We estimated the prevalence in overall population and among subgroups of sex, age period, weight status, survey year and region. The current guideline for the diagnosis and management of hyperuricaemia and gout in China recommends that urate-lowering drugs should be used immediately regardless of the symptoms if SUA ≥ 540 μmol/L [Citation25]. Thus, we also estimated the prevalence of children with SUA ≥ 540 μmol/L. Chi-square tests were used to compare the prevalence among subgroups. Multivariable logistic regression analyses were used to identify related factors associated with risk of hyperuricaemia. We stratified China into North and South by Qinling–Huaihe Line (longitude 104°15′E − 120°21′E and latitude 32°18′N − 34°05′N) to investigate whether the prevalence of hyperuricaemia varies across regions (Supplemental Table 1).
All data analyses were performed by IBM SPSS Statistics for Windows version 23.0 (IBMCorp., Armonk, NY). A two-sided p value <.05 was considered statistically significant.
3. Results
3.1. Characteristics of the participants
A total of 54,580 children and adolescents (boys, 52.9%) aged 3–19 years were included (). Participants aged 12–15 years accounted for 21.9%, and those aged 16–19 years accounted for 37.9%. The prevalence of overweight and obesity was 15.3 and 6.8%, respectively. Most participants were from the North (77.4%) and surveyed during 2016–2019 (81.1%).
Table 2. Characteristics of study population.
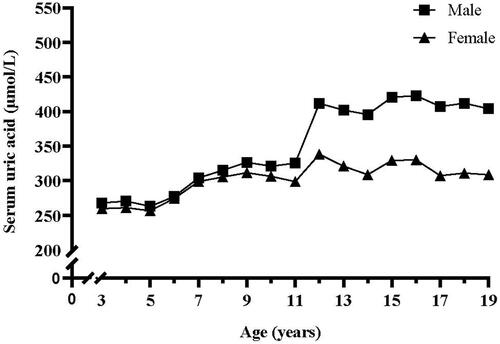
3.2. Sex- and age-specific level of serum uric acid
presents the change curves of SUA during 3–19 years by sex. Boys vs. girls showed similar change patterns. SUA increased gradually from 3 to 11 years with no significant sex difference; then SUA increased dramatically around 11–15 years when SUA reached adult levels, but boys had a more rapid increase during this period; from 15 to 19 years, SUA remained almost unchanged for both sexes, but boys had significantly higher SUA levels than girls.
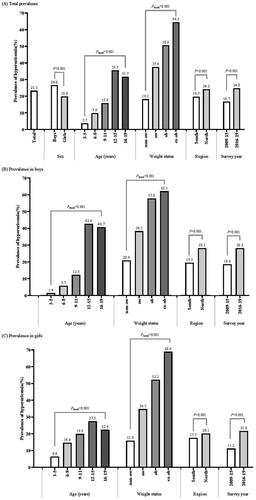
3.3. Prevalence of hyperuricaemia
The overall estimated prevalence of hyperuricaemia was 23.3% (26.6% in boys and 19.8% in girls, p < .001). The prevalence of hyperuricaemia gradually increased with age and peaked at 12–15 years old (35.5%). Compared with non-overweight children, the prevalence of hyperuricaemia in overweight children (37.6%), obese children (50.6%) and extremely obese children (64.5%) was significantly higher (p for trend < .001). The prevalence in the North was higher than that in the South (24.2 vs. 19.7%, p < .001) and increased significantly over the past decade from 16.7% in 2009–2015 to 24.8% in 2016–2019 (p < .001). Similar trends were found among subgroups of sex ( and Supplemental Table 2) and region (Supplemental Table 3). Of note, 2.9% of children and adolescents had a SUA level of ≥ 540 μmol/L (boys, 5.0%; girls, 0.5%) (Supplemental Table 4).
3.4. Related factors of hyperuricaemia
In the overall population, after adjustment for covariates, boys vs. girls conferred an increased odds of hyperuricaemia (OR = 1.54, 95% CI = 1.47–1.61). Increasing age was associated with increased odds of hyperuricaemia, and the ORs and 95% CIs were 4.05 (3.77–4.35) for 12–15 years and 3.65 (3.41–3.91) for 16–19 years, respectively. The odds of hyperuricaemia increased with obesity severity, and the ORs and 95% CIs were 2.99 (2.82–3.16) for overweight, 5.40 (4.93–5.91) for obesity and 9.76 (8.03–11.87) for extreme obesity, respectively. Moreover, the odds of hyperuricaemia were higher in the North than South, higher in 2016–2019 than 2009–2015 (). Similar results were found in both boys and girls.
Table 3. Odds ratios (95% confidence intervals) for hyperuricaemia associated with related factors.
4. Discussion
This large pooled cross-sectional study reported the overall prevalence and related factors of hyperuricaemia in Chinese children and adolescents aged 3–19 years and found high prevalence in the overall population and subgroups. Moreover, sex, age, weight status, region and survey year were independent factors related to risk of hyperuricaemia.
Previous studies have demonstrated that the prevalence of hyperuricaemia in children and adolescents varied across countries. A nationally representative subsample from 2013 to 2016 US National Health and Nutrition Examination Survey showed that the weighted prevalence of hyperuricaemia was 16.56% among adolescents aged 12–19 years [Citation26]. Another cross-sectional survey using data from the 7th Korea National Health and Nutrition Examination Survey (2016–2017) reported that the prevalence of hyperuricaemia was 9.4% (male, 8.4%; female, 10.5%) among children aged 10–18 years [Citation27]. In contrast, our study found that the prevalence in Chinese children and adolescents was 23.3%, which was much higher than that in other countries. This difference may be due to difference in ethnic, obesity epidemic, eating habits and lifestyle [Citation28,Citation29]. Of note, we found that 2.9% of children had a SUA level of higher than 540 μmol/L (Supplemental Table 4), which means they need to receive uric acid-lowering therapy according to Chinese clinical guidelines [Citation25]. Our findings suggest that the high prevalence of hyperuricaemia in children and adolescents in China has become a public health problem that cannot be ignored.
The increasing consumption of high purine diet may be one of the reasons explaining the high prevalence of hyperuricaemia among Chinese youths observed in our study. In the past several decades, rapid urbanization in China has led to changes from traditional to Western diets characterized by a large proportion of animal source foods with high purine [Citation30]. Fructose is a popular dietary ingredient and widely used in sugar-sweetened beverages, and there is compelling evidence that fructose-containing sweeteners and drinks are associated with increased risk of hyperuricaemia by stimulating the catabolism of adenine nucleotides [Citation31]. The consumption of sugar-sweetened beverages has progressively increased more than tenfold from 2003 to 2014 in children and adolescents [Citation32]. In parallel with the trend of these dietary factors, we found that the prevalence of hyperuricaemia increased from 16.7% during 2009–2015 to 24.8% during 2016–2019. Unfortunately, data on diet are unavailable in our study and the effect of diet needs to be explored further. Several randomized clinical trial studies have demonstrated that low-purine diet and DASH diet can effectively lower SUA among participants with hyperuricaemia [Citation33], but few studies have focussed on diet intervention on child hyperuricaemia. Thus, future studies are required to explore the feasibility of dietary intervention strategies for children with hyperuricaemia.
Consistent with previous findings, our study found that obesity was associated with increased risk of hyperuricaemia in children and adolescents. Previous studies have reported that the prevalence of overweight and obesity increased from 17.1% in 2010 to 22.5% in 2014 [Citation34]. In addition, the prevalence of obesity in children in the north is significantly higher than that in the south [Citation35], and similar results were found in our study (23.3% in the north vs. 11.7% in the south) (Supplemental Table 5). The regional difference in obesity prevalence may partly explain the finding that hyperuricaemia was more prevalent in North. Obesity is thought to be the cause of hyperinsulinemia, and the physiological mechanisms mainly involve dysregulation of lipid, insulin resistance, inflammation and adipokines imbalance [Citation36–38]. Therefore, maintaining the ideal weight or guiding obese children to lose weight is conducive to reduce the risk of hyperuricaemia.
In this study, we found that SUA experienced a gradual increase before 11 years for both sexes. SUA underwent a rapid increase around puberty with boys showing a more rapid increase, and thus sex difference begins to appear. One previous study of children aged 1–19 years in Taiwan reported similar results [Citation39]. The underlying mechanisms for sex difference in SUA levels remain unclear, but this physiological difference may be partially explained by the action of sex hormones, such as oestrogen on the xanthine oxidase activity and the renal excretion of uric acid [Citation40]. Muscle tissue is a major site of de novo purine production in the body, and muscle mass increase more rapidly in boys than in girls during puberty [Citation41]. Thus, the difference in muscle mass between boys and girls may be another potential explanation. Sex- and age-specific reference intervals for SUA have been established in US children and adolescents to deal with the fluctuation of SUA during childhood [Citation42]. Given race/ethnic difference in SUA levels, it is of great importance to establish sex- and age-specific references for Chinese children and adolescents in the future study.
The major strength of our study is that this large mixed sample comprising >50,000 children and adolescents aged 3–19 years, allowing for stratified analyses by multiple factors. All studies applied rigorous quality control procedures, including standardized anthropometric and SUA measurement. However, several limitations should be noted. First, our participants were predominantly from the North and recruited during 2016–2019, and the number of participants from south region and before 2016 were small. Second, unhealthy lifestyle including diet and physical activity is an established risk factor for hyperuricaemia; however, data on lifestyle are unavailable in our study. Third, we used adult SUA cut-points for defining hyperuricaemia, which may be not appropriate for children and adolescents. Further study is necessary to establish age- and sex- specific SUA cut-points in children and adolescents.
5. Conclusion
In this large pooled sample in China, the prevalence of hyperuricaemia is unexpectedly high in children and adolescents aged 3–19 years. Sex, age and obesity are important risk factors of hyperuricaemia. These findings have important public health implications for implementing effective interventions to reduce the risk of hyperuricaemia in Chinese youths.
Ethical approval
Written informed consent had been obtained from parents and children in each study. Each study had been approved by their respective Institutional Ethics Review Board.
Author contributions
CL, YY and JM designed the study protocol and pooled data. JR and PY conducted all analyses, interpreted the results, drafted and revised the manuscript. JL, BC, NL, HZ, HB, XC, HL, CZ, QW and HW provided data. All authors critically reviewed and revised the manuscript.
Supplemental Material
Download MS Word (50.2 KB)Acknowledgements
The authors thank all the the following researchers for sharing their valuable data selflessly: Minghua Jiang, Wenzhou Medical University; Jingyan Yang, General Hospital of Tianjin Medical University; Ping Wang, The First People's Hospital of Jinzhong; Yan Li, Kunming Medical University; Meihong Ma, Huidong County People's Hospital; Lihua Yu, Department of Central Laboratory, Qingdao Navy Special Service Sanatorium of Jinzhong; including those do not list in this article (Minghua Jiang, Jingyan Yang, Ping Wang, Yan Li, Meihong Ma, and Lihua Yu). We also thank all the parents and children participated in this study.
Disclosure statement
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Stanhope KL. Sugar consumption, metabolic disease and obesity: the state of the controversy. Crit Rev Clin Lab Sci. 2016;1608–67.
- Li X, Meng X, Timofeeva M, et al. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and mendelian randomisation studies. BMJ. 2017;j2376.
- Srivastava A, Kaze AD, McMullan CJ, et al. Uric acid and the risks of kidney failure and death in individuals with CKD. Am J Kidney Dis. 2018;54:362–370.
- Su H, Liu T, Li Y, et al. Serum uric acid and its change with the risk of type 2 diabetes: a prospective study in China. Prim Care Diabetes. 2021;15(1):1002–1006.
- Kleber ME, Delgado G, Grammer TB, et al. Uric acid and cardiovascular events: a Mendelian randomization study. J Am Soc Nephrol. 2015;26(11):2831–2838.
- Gong P, Liang S, Carlton EJ, et al. Urbanisation and health in China. Lancet. 2012;379(9818):843–852.
- Liu L, Lou S, Xu K, et al. Relationship between lifestyle choices and hyperuricemia in chinese men and women. Clin Rheumatol. 2013;32(2):233–239.
- Kuo CF, Grainge MJ, Zhang W, et al. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11(11):649–662.
- Liu R, Han C, Wu D, et al. Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: a systematic review and Meta-Analysis. Biomed Res Int. 2015;2015:762820.
- Bo H, Yang QY, Liu GL, et al. Analysis of serum uric acid in school-age children. J Appl Clin Pcdiatr. 2011;26(11):853–855.
- Chen XC, Kou YM, Gu XN, et al. Distribution of serum uric acid and risk factors of hyperuricemia in children and adolescents in Tangshan. Chin Gen Pract. 2019;22(26):3227–3232.
- Liu HT, Wen BZ, Zhang YH, et al. Detection rate and related factors of hyperuricemia in 808 children aged from 10 to 11 years. Chin J Health Manage. 2016;10(06):463–466.
- Zhuang JY, Xu BH, Duan BH, et al. Association between serum uric acid level and cardiovascular metabolism risk factors in adolescents. Chin J Sch Health. 2016;37(03):425–427.
- Li N, Zhang S, Li W, et al. Prevalence of hyperuricemia and its related risk factors among preschool children from China. Sci Rep. 2017;7(1):9448.
- Zhang CH, Li Y, Xiong XL, et al. Correlation of hyperuricemia and BMI among male freshmen in Yunnan universities. Mod Prev Med. 2016;43(17):3182–3184.
- Lu J, Sun W, Cui L, et al. A cross-sectional study on uric acid levels among Chinese adolescents. Pediatr Nephrol. 2020;35(3):441–446.
- Chen B, Shu KY, Li MM, et al. Establishment of reference range of serum uric acid for children in Wenzhou region. Zhejiang Med. 2020;42(12):1307–1309.
- Wu Q, Zhou H, Zhou BB, et al. Serum uric acid level and its correlation among adolescents in Hanjiang district of Yangzhou city. Chin J Rheumatol. 2019;23(11):731–734.
- Ye PY, Zhao XY, Yan YK, et al. Association between hyperuricemia and incidence risk for cardiometabolic abnormity in children. Chin J Epidemiol. 2021;42(03):21–27.
- Popkin BM, Du S, Zhai F, et al. Cohort profile: the China health and nutrition Survey-monitoring and understanding socio-economic and health change in China, 1989–2011. Int J Epidemiol. 2010;39(6):1435–1440.
- Cole TJ, Bellizzi MC, Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240–1243.
- Koebnick C, Smith N, Coleman KJ, et al. Prevalence of extreme obesity in a multiethnic cohort of children and adolescents. J Pediatr. 2010;157(1):26–31 e2.
- Physical status: the use and interpretation of anthropometry Report of a WHO expert committee. World Health Organ Tech Rep Ser. 1995;854:1–452.
- WS/T 560-2017 dietary guide for hyperuricemia and gout patients. Beijing, China: China Standard Press; 2017.
- Chinese Society of Endocrinology, Chinese Medical Association. Guideline for the diagnosis and management of hyperuricemia and gout in China (2019). Chin J Endocrinol Metab. 2020;36(01):1–13.
- Wei Y, Zhu J, Wetzstein SA. Plasma and water fluoride levels and hyperuricemia among adolescents: a cross-sectional study of a nationally representative sample of the United States for 2013–2016. Ecotoxicol Environ Saf. 2021;208:111670.
- Lee JH. Prevalence of hyperuricemia and its association with metabolic syndrome and cardiometabolic risk factors in Korean children and adolescents: analysis based on the 2016-2017 Korea national health and nutrition examination survey. Korean J Pediatr. 2019;62(8):317–323.
- Yu X, Zhu C, Zhang H, et al. Association between urbanisation and the risk of hyperuricaemia among Chinese adults: a cross-sectional study from the China Health and nutrition survey (CHNS). BMJ Open. 2021;11(3):e044905.
- Miao Z, Li C, Chen Y, et al. Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China. J Rheumatol. 2008;35(9):1859–1864.
- Howard AG, Attard SM, Herring AH, et al. Socioeconomic gradients in the westernization of diet in China over 20 years. SSM Popul Health. 2021;16:100943.
- Zhang C, Li L, Zhang Y, et al. Recent advances in fructose intake and risk of hyperuricemia. Biomed Pharmacother. 2020;131:110795.
- Ma GS. Report on the consumption of Sugar-Sweetened beverages (SSBs) of children in China. Beijing, China: China Population Publishing House; 2018.
- Juraschek SP, Yokose C, McCormick N, et al. Effects of dietary patterns on serum urate: results from a randomized trial of the effects of diet on hypertension. Arthritis Rheumatol. 2021;73(6):1014–1020.
- Dong Y, Ma Y, Dong B, et al. Geographical variation and urban-rural disparity of overweight and obesity in chinese school-aged children between 2010 and 2014: two successive national cross-sectional surveys. BMJ Open. 2019;9(4):e025559.
- Zhang L, Chen J, Zhang J, et al. Regional disparities in obesity among a heterogeneous population of chinese children and adolescents. JAMA Netw Open. 2021;4(10):e2131040.
- Zhang T, Zhang H, Li Y, et al. Long-term impact of temporal sequence from childhood obesity to hyperinsulinemia on adult metabolic syndrome and diabetes: the Bogalusa heart study. Sci Rep. 2017;7:43422.
- Johnson RJ, Titte S, Cade JR, et al. Uric acid, evolution and primitive cultures. Semin Nephrol. 2005;25(1):3–8.
- Katsiki N, Mantzoros C, Mikhailidis DP. Adiponectin, lipids and atherosclerosis. Curr Opin Lipidol. 2017;28(4):347–354.
- Hsia SH, Chou IJ, Kuo CF, et al. Survival impact of serum uric acid levels in children and adolescents. Rheumatol Int. 2013;33(11):2797–2802.
- Budhiraja R, Kayyali US, Karamsetty M, et al. Estrogen modulates xanthine dehydrogenase/xanthine oxidase activity by a receptor-independent mechanism. Antioxid Redox Signal. 2003;5(6):705–711.
- Alvim RO, Siqueira JH, Zaniqueli D, et al. Influence of muscle mass on the serum uric acid levels in children and adolescents. Nutr Metab Cardiovasc Dis. 2020;30(2):300–305.
- Clifford SM, Bunker AM, Jacobsen JR, et al. Age and gender specific pediatric reference intervals for aldolase, amylase, ceruloplasmin, creatine kinase, pancreatic amylase, prealbumin, and uric acid. Clin Chim Acta. 2011;412(9–10):788–790.