Abstract
Background
To quantitatively synthesize evidence from prospective observational studies regarding the mean levels of circulating adiponectin in patients with gestational diabetes mellitus (GDM) and the association between adiponectin levels and GDM risk.
Methods
PubMed, EMBASE and Web of Science were searched from their inception until November 8th, 2022, for nested case-control studies and cohort studies. Random-effect models were applied to the synthesized effect sizes. The difference in circulating adiponectin levels between the GDM and control groups was measured using the pooled standardized mean difference (SMD) and 95% confidence interval (CI). The relationship between circulating adiponectin levels and GDM risk was examined using the combined odds ratio (OR) and 95% CI. Subgroup analyses were performed according to the study continent, GDM risk in the study population, study design, gestational weeks of circulating adiponectin detection, GDM diagnostic criteria, and study quality. Sensitivity and cumulative analyses were performed to evaluate the stability of the meta-analysis. Publication bias was assessed by funnel plots and Egger’s test.
Results
The 28 studies included 13 cohort studies and 15 nested case-control studies, containing 12,256 pregnant women in total. The mean adiponectin level in GDM patients was significantly lower than in controls (SMD = −1.514, 95% CI = −2.400 to −0.628, p = .001, I2 = 99%). The risk of GDM was significantly decreased among pregnant women with increasing levels of circulating adiponectin (OR = 0.368, 95% CI = 0.271–0.500, p < .001, I2=83%). There were no significant differences between the subgroups.
Conclusions
Our findings indicate that increasing circulating adiponectin levels were inversely associated with the risk of GDM. Given the inherent heterogeneity and publication bias of the included studies, further well-designed large-scale prospective cohort or intervention studies are needed to confirm our finding.
KEY MESSAGES
Increasing circulating adiponectin levels in the first to the second trimester could decrease the risk of incident GDM.
1. Introduction
Gestational diabetes mellitus (GDM) is defined as glucose intolerance first found in pregnancy, which affects more than 16.7% of pregnant women worldwide [Citation1]. GDM is one of the most common pregnancy-related complications and has been proven to increase the risk of adverse pregnancy outcomes such as caesarean section, preterm birth, large for gestational age, and macrosomia [Citation2]. In addition, GDM not only increases the long-term risk of cardiovascular disease (CVD), type 2 diabetes (T2DM), and dyslipidemia in mothers but is also associated with metabolic syndrome, impaired glucose tolerance, and neurodevelopmental abnormalities in children [Citation3–8]. It is well known that insulin resistance (IR) and pancreatic β-cells dysfunction play an important role in the development of GDM [Citation9,Citation10]. However, the exact etiology of GDM remains unclear. Hence, it is important to identify risk factors or biomarkers for the disease.
Adiponectin, a 30 kDa collagen-like protein product solely secreted by adipocytes, has been proven to have antidiabetic properties as a hormone mainly by participating in insulin signaling and gluconeogenesis [Citation11]. A previous systematic review and meta-analysis of prospective studies also revealed that higher plasma adiponectin levels are associated with a lower risk of T2DM, with a dose-response relationship in the general population [Citation12]. Another meta-analysis performed on pregnant women showed that measurement of circulating adiponectin before pregnancy or early pregnancy could improve the identification accuracy of pregnant women at high risk for GDM [Citation13]. Furthermore, several epidemiological studies from pregnant women also reported plasma or serum adiponectin levels in GDM patients. They investigated the relationship between circulating adiponectin and GDM, but the conclusions were inconsistent [Citation14–17]. Hence, we performed this systematic review and meta-analysis to determine the difference in circulating adiponectin levels between GDM and normal pregnant women and to examine the relationship between circulating adiponectin levels and the risk of GDM.
2. Methods
2.1. Search strategy
This systematic review and meta-analysis was registered at the PROSPERO (ID: CRD42022332382) and performed according to the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) statement [Citation18]. Two researchers conducted the systematic literature search independently from PubMed, EMBASE and Web of Science from their inception until November 8th, 2022. The following search strategies were performed: (1) (‘adiponectin’ [Mesh term] or ‘adiponectin’ [title/abstract]) AND (‘Diabetes, Gestational’ [Mesh term] OR ‘gestational diabetes’ [title/abstract] OR ‘gestational diabetes mellitus’ [title/abstract] OR ‘GDM’ [title/abstract]) in PubMed; (2) ((adiponectin):ti,ab,kw) AND ((GDM):ti,ab,kw OR (‘gestational diabetes’):ti,ab,kw OR (‘gestational diabetes mellitus’):ti,ab,kw OR (‘pregnancy induced diabetes’):ti,ab,kw) in EMBASE; (3) ‘adiponectin’ AND (‘gestational diabetes’ OR ‘gestational diabetes mellitus’ OR ‘GDM’ or ‘pregnancy-induced diabetes’) in Web of Science. In addition, potential references from identified articles and reviews were also searched. No language or geographic area restrictions were applied. Endnote 20 software was used for studies’ management.
2.2. Study selection criteria
We performed a two-step study selection program. First, two independent researchers screened for titles and abstracts to identify relevant studies by the following criteria: (1) observational studies, (2) investigated the association between serum or plasma adiponectin and GDM, (3) measured the circulating adiponectin levels before the GDM diagnosis. Reviews, letters, protocol, animal studies or in vitrol studies were excluded in the current step. Subsequently, studies identified by the first step were reviewed through full texts in the second step. Articles were excluded in this step if the information reported is insufficient to obtain the standard mean difference (SMD), adjusted odds ratio (OR) and their 95% confidence interval (CI). When more than one articles were from the same population, we only included the most relevant study in the meta-analysis. Any dispute of the two researchers were resolved by discussing with the third researcher.
2.3. Data extraction
Two researchers independently extracted data from included studies using a predesigned standard ized data form. Relevant information included the first author’s name, year of publication, country of the study, study design, sample size, GDM diagnostic approach, GDM risk of study participants, gestation weeks (GW) of circulating adiponectin detection, adiponectin levels and units, adjusted OR with 95% CIs, and adjusted or matched variables.
2.4. Quality assessment
The methodological quality of included studies was assessed by two researchers independently using Newcastle-Ottawa-Scale (NOS) [Citation19]. Briefly, the NOS including 8 items from three domain (selection, comparability, and outcomes of interest) with a range of 0–9 stars. We considered a study with ≥ 7 stars as a high-quality study [Citation20].
2.5. Statistical analysis
The SMD and 95% CI were summarized to measure the difference of circulating adiponectin level between the GDM and normal glucose tolerance (NGT) group. If the adiponectin level was expressed as median with interquartile range, the mean and SD were converted by the equations from Luo et al. and Wan et al. [Citation21,Citation22]. Additionally, the pooled OR and 95% CI were also calculated to summarized the association between circulating adiponectin and developing GDM. When heterogeneities were detected by the p-value < .05 in the Cochran Q test or the I2 statistics > 50%, the DerSimonian and Laird random effects model was adopted to calculate pooled effect size and presented as forest plots with 95% CI [Citation23,Citation24]. Otherwise, the inverse variance fixed effect model was selected [Citation25]. Subgroup analyses were performed on the basis of study continent, GDM risk in the study population, study design, GW of circulating adiponectin detection, GDM diagnostic criteria and study quality. Sensitivity and cumulative analyses were conducted to evaluate the stability of this meta-analysis. Publication bias was assessed by funnel plots and Egger’s test. All data analyses were performed using R software (version 4.2.1).
3. Results
3.1. Literature search
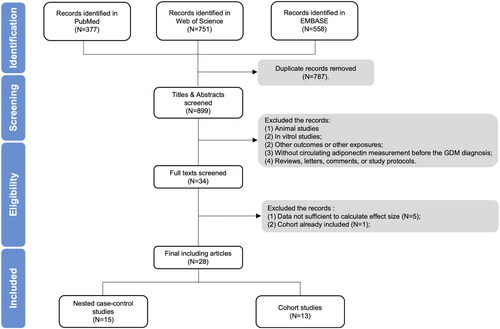
The process of studies selection was illustrated in . Briefly, 1686 relevant literature were identified by the initial search from PubMed, EMBASE and Web of Science. After excluded 787 duplicate records, titles and abstracts were screening with 865 articles removed. This left 34 papers for full-text review, of which 6 were excluded: data not sufficient to calculate SMD or adjusted OR in five studies [Citation26–30], while the cohort in one study already been included [Citation31]. Finally, a total of 28 articles were included in the meta-analysis [Citation14–17,Citation32–55].
3.2. Study characteristics
Detailed characteristics of the included 28 studies were shown in . The year of publication ranged from 2004 to 2022. These 28 studies performed in 14 different countries, covering four continents: 7 in Asia [Citation15,Citation17,Citation36,Citation41,Citation43,Citation47,Citation54], 12 in Europe [Citation14,Citation16,Citation33,Citation34,Citation38,Citation44–46,Citation48,Citation49,Citation51,Citation52], 6 in North America [Citation35,Citation39,Citation40,Citation42,Citation53,Citation55] and 3 in Oceania [Citation32,Citation37,Citation50]. The sample size of included studies ranged from 28 to 2590. A total of 12,256 pregnant women involved in the meta-analysis, of which 2422 suffered from GDM. In addition, 6 studies included pregnant women with high GDM risk [Citation32,Citation33,Citation38,Citation44,Citation46,Citation52], 20 studies involved pregnant women with low GDM risk [Citation14–17,Citation34–37,Citation39–43,Citation45,Citation47,Citation49,Citation50,Citation53–55], and remaining 2 studies recruited pregnant women with both high and low GDM risk [Citation48,Citation51]. There were 13 cohort studies [Citation16,Citation17,Citation32,Citation33,Citation38,Citation39,Citation41,Citation42,Citation44,Citation46,Citation51,Citation52,Citation54], 11 matched nested-case control studies [Citation14,Citation15,Citation34,Citation35,Citation37,Citation40,Citation45,Citation48–50,Citation55] and 4 non-matched nested-case control studies [Citation36,Citation43,Citation47,Citation53]. Blood samples collected in these 28 studies ranged from 6 to 20 weeks of gestation. Among them, 18 studies assayed circulating adiponectin in the first trimester [Citation14,Citation16,Citation17,Citation34,Citation35,Citation37–41,Citation43,Citation45–51], and 10 assayed in the first to the second trimester [Citation15,Citation32,Citation33,Citation36,Citation42,Citation44,Citation52–55]. The 1-step GDM diagnostic approach were adopted in 15 studies [Citation14–17,Citation32,Citation33,Citation37–39,Citation43,Citation44,Citation46,Citation51,Citation52,Citation54], while the other 13 studies used a 2-step approach [Citation34–36,Citation40–42,Citation45,Citation47–50,Citation53,Citation55]. In addition, the SMD of circulating adiponectin between the GDM group and NGT group could be calculated from 22 papers [Citation14–17,Citation32,Citation34,Citation36–43,Citation45–50,Citation52,Citation54], and the adjusted OR of adiponectin were reported by 15 articles [Citation14,Citation15,Citation32,Citation33,Citation35,Citation39–42,Citation44,Citation51–55]. The methodological quality assessment of included studies according to NOS was showed in , and 21 studies were high-quality [Citation15,Citation17,Citation32,Citation33,Citation35–37,Citation39–44,Citation46,Citation47,Citation49,Citation51–55].
Table 1. Characteristics of included studies.
Table 2. Methodological quality of studies included in meta-analysis.
3.3. Different of circulating adiponectin levels between GDM and NGT
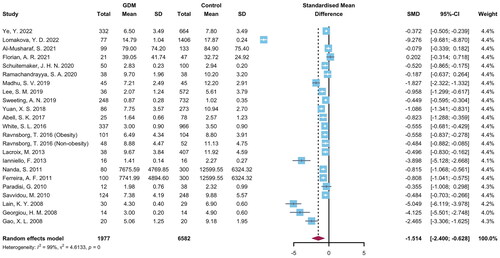
The individual studies and pooled results were shown in . The SMD between GDM and NGT was evaluated in 8559 pregnant women in 22 studies and ranged from −9.276 to 0.202. The synthesized results revealed that the mean level of adiponectin in GDM was significantly lower than those in NGT pregnant women (SMD = −1.514, 95% CI = −2.400 to −0.628, p = .001, I2 = 99%). Sensitivity analysis showed the corresponding pooled SMD ranged from −1.103 to −1.593, which were not significantly changed after each study omitted (Supplementary Figure 1). The publication bias was observed by Begg’s test (p = .003) but not Egger’s test (p = .085) (Supplementary Figure 2).
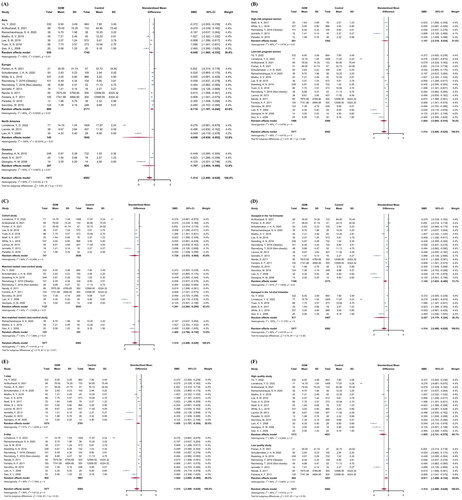
Subgroup analyses were performed in . There was no significant diversity between different continents (Asia: SMD = −0.948, 95% CI = −1.565 to −0.332; Europe: SMD = −0.710, 95% CI = −1.181 to −0.240; North America: SMD = −4.938, 95% CI = −9.930 to 0.053; Oceania: SMD = −1.707, 95% CI = −3.904 to 0.490; p among subgroups = .308), GDM risk of study population (high-risk women: SMD = −1.147, 95% CI = −2.318 to 0.024; low-risk women: SMD = −1.604, 95% CI = −2.693 to −0.516; p among subgroups = .575), study design (cohort study: SMD = −1.724, 95% CI = −3.512 to 0.064; matched nested case-control study: SMD = −1.281, 95% CI = −2.264 to −0.299; non-matched nested case-control study: SMD = −1.462, 95% CI = −2.794 to −0.130; p among subgroups = .909), GW of adiponectin detection (1st trimester: SMD = −1.145, 95% CI = −1.823 to −0.466; 1st to 2nd trimester: SMD = −2.427, 95% CI = −5.179 to −0.324; p among subgroups = .375), GDM diagnostic approach (1-step approach: SMD = −1.055, 95% CI = −1.757 to −0.354; 2-step approach: SMD = −1.945, 95% CI = −3.602 to −0.289; p among subgroups = .332), and methodological quality (high quality studies: SMD = −1.855, 95% CI = −3.131 to −0.579; low quality studies: SMD = −0.811, 95% CI = −1.489 to −0.134; p among subgroups = 0.157. A cumulative analysis based on publication year in Supplementary Figure 3 showed that the pooled SMD did not substantially alter over time.
3.4. Relationship between circulating adiponectin levels and GDM risks
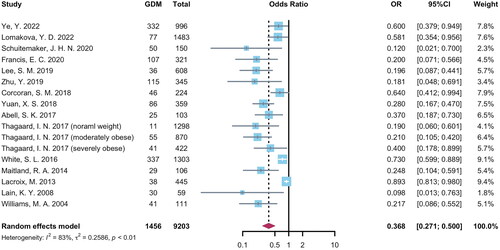
The OR and 95% CI of increased circulating adiponectin levels for GDM risks in individual studies ranged from 0.098 to 0.893 and summarized as 0.368 (95% CI = 0.271 to 0.500, p < .001, I2 = 83%) (). Sensitivity analysis was shown in Supplementary Figure 4: after deleting each study, the pooled OR remained stable between 0.344 and 0.384. The publication bias was observed by Egger’s test (p < .001) but not Begg’s test (p = .138) (Supplementary Figure 5).
Subgroup analyses of were shown in . The relationship between increased circulating adiponectin and decreased GDM risks were similar in different continents (Asia: OR = 0.339, 95% CI = 0.177–0.647; Europe: OR = 0.366, 95% CI = 0.233–0.600; North America: OR = 0.348, 95% CI = 0.177–0.687; Oceania: OR = 0.370, 95% CI = 0.187–0.730; p among subgroups = 0.997), GDM risk of study population (high-risk women: OR = 0.428, 95% CI = 0.279–0.658; low-risk women: OR = 0.323, 95% CI = 0.208–0.501; p among subgroups = 0.368), study design (cohort study: OR = 0.416, 95% CI = 0.293–0.589; matched nested case-control study: OR = 0.251, 95% CI = 0.116–0.544; non-matched nested case-control study: OR = 0.217, 95% CI = 0.086–0.552; p among subgroups = 0.270), GW of adiponectin detection (1st trimester: OR = 0.277, 95% CI = 0.157–0.489; 1st to 2nd trimester: OR = 0.445, 95% CI = 0.324–0.611; p among subgroups = 0.154), GDM diagnostic approach (1-step approach: OR = 0.428, 95% CI = 0.301–0.607; 2-step approach: OR = 0.259, 95% CI = 0.150–0.449; p among subgroups = 0.132), and methodological quality (high quality studies: OR = 0.379, 95% CI = 0.279–0.515; low quality studies: OR = 0.120, 95% CI = 0.021–0.700; p among subgroups = 0.208). A cumulative analysis based on publication year in Supplementary Figure 6 showed that the pooled OR did not substantially change over time after 2013.
4. Discussion
To the best of our knowledge, the current systematic review and meta-analysis, including 28 observational studies, is the first to identify a prospective association between circulating adiponectin levels and GDM risk. This study revealed that circulating adiponectin levels in GDM were significantly lower than those in NGT pregnant women. In addition, higher adiponectin levels are inversely associated with the incidence of GDM. These results were robust after sensitivity, subgroup, and cumulative analyses, suggesting that circulating adiponectin may be a potential early biomarker for screening or predicting GDM in pregnant women.
Findings in the current meta-analysis reported that the mean level of adiponectin in GDM patients was significantly lower than that in pregnant women with NGT (SMD = −1.514, 95% CI = −2.400 to −0.628). This was similar to several previous studies reporting decreased circulating adiponectin levels in patients with abnormal glucose metabolism. Bao et al. performed a systematic review and meta-analysis, including 12 prospective studies. They showed that adiponectin levels in the first to the second trimester significantly differed between GDM and non-GDM pregnant women [Citation56]. The pooled SMD was −1.20 (95% CI: −1.63 to −0.78], which was close to the current meta-analysis. Furthermore, another meta-analysis by Xu et al. pooled results from 15 studies and reported that circulating adiponectin levels in GDM were significantly lower than controls (WMD = −2.85 μg/mL, 95% CI = −3.64 to −2.06) [Citation57]. Although it was similar to the results of our meta-analysis, studies with a cross-sectional design were not excluded in the research by Xu et al. so it was hard to examine the prospective effect of adiponectin on new-onset GDM. Interestingly, lower pre-pregnant adiponectin levels were also demonstrated to be associated with a markedly higher risk of GDM in a subsequent pregnancy. Therefore adiponectin could be interpreted as playing a role as a bio-marker to identify women at high risk for GDM, even before pregnancy [Citation58]. A previous study by Fuglsang et al. showed that serum adiponectin concentrations would change during pregnancy. Before the second trimester of pregnancy, there is an increase in circulating levels of adiponectin which progressively decrease with the progress of gestation. Notably, after delivery, the decrease in adiponectin levels did not return to normal, which implies the long-term effect of adiponectin in pregnancy on maternal glucose metabolism may still exist after delivery [Citation59]. Retnakaran et al.’s research has proved this hypothesis. This research demonstrated that maternal adiponectin levels in women with postpartum IR or β-cell dysfunction were significantly reduced, indicating that adiponectin may participate in pathways linking GDM with T2DM [Citation60]. Similar results were found in the study by Thomann et al. which found adiponectin levels significantly decreased in subjects with previous GDM compared with healthy controls [Citation61]. Moreover, a recent study demonstrated a potential mediating role for adiponectin between vitamin D and GDM, given the increased expression of placental neoangiogenesis, inflammation markers, and parathyroid hormone-related protein [Citation62–64]. Outside pregnancy, similar decreases were showed in circulating adiponectin levels among patients with abnormal glucose metabolism. The study by Mir et al. showed that compared to the control group, the levels of adiponectin in T2DM patients was significantly reduced, especially in those diagnosed as obese and severely obese [Citation65]. Cnop et al. also found that adiponectin levels were significantly decreased in non-pregnant women with hyperglycemia, IR and T2DM, or those with higher BMI, intra-abdominal fat and atherogenic lipid profile participants [Citation66].
In the current meta-analysis, we found that increasing circulating adiponectin levels in the first to the second trimester could significantly reduce the risk of GDM: the pooled OR of the higher circulating adiponectin levels for GDM was 0.368 (95% CI = 0.271–0.500). A previous meta-analysis including 11 prospective studies showed that pregnant women with lower circulating adiponectin levels carrying the 45 T/G SNP in the ADIPOQ gene were 1.5–2 times more likely to develop GDM, especially in Asian populations [Citation67]. Another meta-analysis reported that the measurement of circulating adiponectin before pregnancy and early pregnancy might improve the ability to predict women at high risk of developing GDM (diagnostic OR = 6.4, 95% CI = 4.1–9.9) [Citation13]. Interestingly, they also found that adiponectin performs better in the ‘low risk of GDM’ compared with the ‘high risk of GDM’ population. This was consistent with findings in our meta-analysis: the protective effect of increasing adiponectin in ‘low-risk’ pregnant women was stronger than in ‘high-risk’ pregnant women (pooled OR 0.368 v.s. 0.428). In addition, studies conducted in non-pregnant populations have revealed that adiponectin levels are significantly associated with impaired glucose metabolism. The study by Hedderson et al. revealed that compared with the highest quartile of adiponectin levels, lower adiponectin levels during the non-pregnancy period could increase GDM risk by 1.5–5.2 times in the following pregnancy [Citation58]. A meta-analysis by Li et al. reported that an increase in adiponectin levels of 1 log μg/mL could reduce the risk of T2DM by nearly 30% [Citation12]. A similar inverse association was also found between adiponectin levels and pre-diabetes risk. Compared with the lowest quartile of adiponectin, the risk of pre-diabetes in the highest quartile was decreased by 61% in young-healthy subjects, and this reduction in the risk of pre-diabetes could be as high as 85% in obese subjects [Citation68]. A cross-sectional study performed among 202 subjects also revealed that adiponectin was inversely associated with metabolic syndrome (OR = 0.634, 95% CI = 0.519–0.775), which is characterized by dysfunction of glucose and lipid metabolism [Citation69]. This evidence indicates that the effect of adiponectin on abnormal glucose metabolism is relatively consistent, regardless of pregnancy status.
The effect of adiponectin on GDM has been shown to be biologically reasonable. Briefly, the reduction of adiponectin levels may inhibit glucose consumption, stimulate lipolysis, and increase hepatic glucose production, leading to a decrease in insulin sensitivity [Citation70,Citation71]. In addition, decreased adiponectin levels may stimulate the hepatic production of TNF-α and increase plasma concentrations of this proinflammatory cytokine to promote an inflammatory status, which could decrease insulin sensitivity and enhance gluconeogenesis [Citation72]. Compared to non-pregnant women, the sensitivity of tissues to insulin during pregnancy failed significantly. Hence, a decrease in adiponectin levels may aggravate this process and result in GDM [Citation9]. Studies performed on pregnant women have shown that adiponectin levels are inversely associated with fasting glucose, insulin, and insulin resistance [Citation73,Citation74]. As hyperinsulinemia caused by GDM may further reduce adiponectin levels, increasing adiponectin levels in pregnant women will help improve insulin sensitivity and perinatal outcomes [Citation75].
To the best of our knowledge, this study is the first to pool circulating adiponectin levels in the first or second trimester between GDM and NGT pregnant women and to estimate the effect of increasing circulating adiponectin on the incidence of GDM. These findings provide comprehensive new evidence for adiponectin, which may be a potential screening and prediction biomarker or therapeutic target in GDM. Our study has some limitations. First, all the original studies included in the meta-analysis were observational studies, which might have resulted in additional biases resulting from the study design. However, we only included nested case-control or cohort studies that measured circulating adiponectin levels before GDM diagnosis. Therefore, our findings regarding the difference in circulating adiponectin levels between GDM and NTG and the effect of adiponectin on GDM are reliable. Second, as the current study is a literature-based meta-analysis, the lack of access to individual patient data might have led to heterogeneities across the original studies. However, to reduce the effect of heterogeneity in the meta-analyses, random-effect models were adopted to estimate the pooled effect sizes. Moreover, subgroup analyses based on the six characteristics of the original study showed that the associations between circulating adiponectin levels and GDM were consistent. Furthermore, cumulative and sensitivity analyses were performed to examine the stability of the results. Third, significant publication bias existed in the current meta-analysis. This may be due to the exclusion of original studies that were difficult to extract data from or insufficient to calculate the effect sizes. Additional prospective studies are needed to confirm the effect of circulating adiponectin levels on the risk of GDM.
5. Conclusions
In conclusion, our systematic review and meta-analysis of 28 studies 12,256 pregnant women indicated that the mean levels of circulating adiponectin in the first to second trimesters among GDM women were significantly lower than those in NGT pregnant women. More importantly, the incidence of GDM was negatively associated with circulating adiponectin levels in the first to the second trimester. These findings suggest that circulating adiponectin may be a potential early biomarker for screening or predicting GDM in pregnant women. Further well-designed large prospective cohort or intervention studies are needed to confirm our findings.
Author contributions
Chenghong Yin, Ruixia Liu and Wentao Yue initiated the research. Shen Gao, Shaofei Su and Enjie Zhang searched and identified literatures. Enjie Zhang, and Yue Zhang extracted data. Jianhui Liu and Shuanghua Xie assessed the methodology quality of the studies. Shen Gao and Shaofei Su performed meta-analyses. Shen Gao and Shaofei Su wrote the draft of the manuscription. Chenghong Yin, Ruixia Liu and Wentao Yue contributed to manuscript revision and supervision. All authors approved the final version of our manuscript.
Supplemental Material
Download JPEG Image (4.8 MB)Supplemental Material
Download JPEG Image (24.8 KB)Supplemental Material
Download JPEG Image (5.6 MB)Supplemental Material
Download JPEG Image (6.4 MB)Supplemental Material
Download JPEG Image (414.8 KB)Supplemental Material
Download JPEG Image (6.3 MB)Data availability statement
All data in the article and its supplementary materials are available.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Magliano DJ, Boyko EJ; IDF Diabetes Atlas 10th edition scientific committee. IDF DIABETES ATLAS [Internet]. 10th edition. Brussels: International Diabetes Federation; 2021.
- Ye W, Luo C, Huang J, et al. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2022;377:1. doi: 10.1136/bmj-2021-067946.
- Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62(6):905–915. doi: 10.1007/s00125-019-4840-2.
- Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361.
- Chodick G, Tenne Y, Barer Y, et al. Gestational diabetes and long-term risk for dyslipidemia: a population-based historical cohort study. BMJ Open Diabetes Res Care. 2020;8(1):e000870. doi: 10.1136/bmjdrc-2019-000870.
- Pathirana MM, Lassi ZS, Ali A, et al. Association between metabolic syndrome and gestational diabetes mellitus in women and their children: a systematic review and meta-analysis. Endocrine. 2021;71(2):310–320. doi: 10.1007/s12020-020-02492-1.
- Lowe WLJr, Scholtens DM, Kuang A, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42(3):372–380. doi: 10.2337/dc18-1646.
- Rowland J, Wilson CA. The association between gestational diabetes and ASD and ADHD: a systematic review and meta-analysis. Sci Rep. 2021;11(1):5136. doi: 10.1038/s41598-021-84573-3.
- Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005;115(3):485–491. doi: 10.1172/JCI24531.
- Fasshauer M, Blüher M, Stumvoll M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol. 2014;2(6):488–499. doi: 10.1016/S2213-8587(13)70176-1.
- Zavalza-Gómez AB, Anaya-Prado R, Rincón-Sánchez AR, et al. Adipokines and insulin resistance during pregnancy. Diabetes Res Clin Pract. 2008;80(1):8–15. doi: 10.1016/j.diabres.2007.12.012.
- Li S, Shin HJ, Ding EL, et al. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302(2):179–188. doi: 10.1001/jama.2009.976.
- Iliodromiti S, Sassarini J, Kelsey TW, et al. Accuracy of circulating adiponectin for predicting gestational diabetes: a systematic review and meta-analysis. Diabetologia. 2016;59(4):692–699. doi: 10.1007/s00125-015-3855-6.
- Schuitemaker JHN, Beernink RHJ, Franx A, et al. First trimester secreted Frizzled-related protein 4 and other adipokine serum concentrations in women developing gestational diabetes mellitus. PLOS One. 2020;15(11):e0242423. doi: 10.1371/journal.pone.0242423.
- Ye Y, Wu P, Wang Y, et al. Adiponectin, leptin, and leptin/adiponectin ratio with risk of gestational diabetes mellitus: a prospective nested case-control study among Chinese women. Diabetes Res Clin Pract. 2022;191:110039. doi: 10.1016/j.diabres.2022.110039.
- Florian AR, Cruciat G, Pop RM, et al. Predictive role of altered leptin, adiponectin and 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid secretion in gestational diabetes mellitus. Exp Ther Med. 2021;21(5):520. doi: 10.3892/etm.2021.9951.
- Al-Musharaf S, Sabico S, Hussain SD, et al. Inflammatory and adipokine status from early to midpregnancy in Arab women and its associations with gestational diabetes mellitus. Dis Markers. 2021;2021:8862494. doi: 10.1155/2021/8862494.
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008.
- Wells G, Shea B, O’connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [cited 2022 Nov]. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- Roshanzamir F, Miraghajani M, Rouhani MH, et al. The association between circulating fetuin-A levels and type 2 diabetes mellitus risk: systematic review and meta-analysis of observational studies. J Endocrinol Invest. 2018;41(1):33–47. doi: 10.1007/s40618-017-0697-8.
- Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183.
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557.
- Blettner M, Sauerbrei W, Schlehofer B, et al. Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int J Epidemiol. 1999;28(1):1–9. doi: 10.1093/ije/28.1.1.
- Abell SK, Shorakae S, Boyle JA, et al. Role of serum biomarkers to optimise a validated clinical risk prediction tool for gestational diabetes. Aust N Z J Obstet Gynaecol. 2019;59(2):251–257. doi: 10.1111/ajo.12833.
- Correa PJ, Venegas P, Palmeiro Y, et al. First trimester prediction of gestational diabetes mellitus using plasma biomarkers: a case-control study. J Perinat Med. 2019;47(2):161–168. doi: 10.1515/jpm-2018-0120.
- Coussa A, Hasan HA, Barber TM. Early predictors of gestational diabetes mellitus in IVF-Conceived pregnancies. Endocr Pract. 2021;27(6):579–585. doi: 10.1016/j.eprac.2020.10.020.
- Sir-Petermann T, Echiburú B, Maliqueo MM, et al. Serum adiponectin and lipid concentrations in pregnant women with polycystic ovary syndrome. Hum Reprod. 2007;22(7):1830–1836. doi: 10.1093/humrep/dem090.
- Xiao L, Zhao JP, Nuyt AM, et al. Female fetus is associated with greater maternal insulin resistance in pregnancy. Diabet Med. 2014;31(12):1696–1701. doi: 10.1111/dme.12562.
- Yang X, Ye Y, Wang Y, et al. Association between early-pregnancy serum C-peptide and risk of gestational diabetes mellitus: a nested case-control study among Chinese women. Nutr Metab. 2022;19(1):56. doi: 10.1186/s12986-022-00691-3.
- Abell SK, Shorakae S, Harrison CL, et al. The association between dysregulated adipocytokines in early pregnancy and development of gestational diabetes. Diabetes Metab Res Rev. 2017;33(8). doi: 10.1002/dmrr.2926.
- Corcoran SM, Achamallah N, Loughlin JO, et al. First trimester serum biomarkers to predict gestational diabetes in a high-risk cohort: striving for clinically useful thresholds. Eur J Obstet Gynecol Reprod Biol. 2018;222:7–12. doi: 10.1016/j.ejogrb.2017.12.051.
- Ferreira AF, Rezende JC, Vaikousi E, et al. Maternal serum visfatin at 11–13 weeks of gestation in gestational diabetes mellitus. Clin Chem. 2011;57(4):609–613. doi: 10.1373/clinchem.2010.159806.
- Francis EC, Li M, Hinkle SN, et al. Adipokines in early and mid-pregnancy and subsequent risk of gestational diabetes: a longitudinal study in a multiracial cohort. BMJ Open Diab Res Care. 2020;8(1):e001333. doi: 10.1136/bmjdrc-2020-001333.
- Gao XL, Yang HX, Zhao Y. Variations of tumor necrosis factor-alpha, leptin and adiponectin in mid-trimester of gestational diabetes mellitus. Chin Med J (Engl). 2008;121(8):701–705.
- Georgiou HM, Lappas M, Georgiou GM, et al. Screening for biomarkers predictive of gestational diabetes mellitus. Acta Diabetol. 2008;45(3):157–165. doi: 10.1007/s00592-008-0037-8.
- Ianniello F, Quagliozzi L, Caruso A, et al. Low adiponectin in overweight/obese women: association with diabetes during pregnancy. Eur Rev Med Pharmacol Sci. 2013;17(23):3197–3205.
- Lacroix M, Battista MC, Doyon M, et al. Lower adiponectin levels at first trimester of pregnancy are associated with increased insulin resistance and higher risk of developing gestational diabetes mellitus. Diabetes Care. 2013;36(6):1577–1583. doi: 10.2337/dc12-1731.
- Lain KY, Daftary AR, Ness RB, et al. First trimester adipocytokine concentrations and risk of developing gestational diabetes later in pregnancy. Clin Endocrinol (Oxf). 2008;69(3):407–411. doi: 10.1111/j.1365-2265.2008.03198.x.
- Lee SM, Kwak SH, Koo JN, et al. Non-alcoholic fatty liver disease in the first trimester and subsequent development of gestational diabetes mellitus. Diabetologia. 2019;62(2):238–248. doi: 10.1007/s00125-018-4779-8.
- Lomakova YD, Chen X, Stein TP, et al. Decreased adiponectin levels in early pregnancy are associated with high risk of prematurity for African American women. J Clin Med. 2022;11(11):3213. doi: 10.3390/jcm11113213.
- Madhu SV, Bhardwaj S, Jhamb R, et al. Prediction of gestational diabetes from first trimester serum adiponectin levels in Indian women. Indian J Endocrinol Metab. 2019;23(5):536–539. doi: 10.4103/ijem.IJEM_319_19.
- Maitland RA, Seed PT, Briley AL, et al. Prediction of gestational diabetes in obese pregnant women from the UK pregnancies better eating and activity (UPBEAT) pilot trial. Diabet Med. 2014;31(8):963–970. doi: 10.1111/dme.12482.
- Nanda S, Savvidou M, Syngelaki A, et al. Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks. Prenat Diagn. 2011;31(2):135–141. doi: 10.1002/pd.2636.
- Paradisi G, Ianniello F, Tomei C, et al. Longitudinal changes of adiponectin, carbohydrate and lipid metabolism in pregnant women at high risk for gestational diabetes. Gynecol Endocrinol. 2010;26(7):539–545. doi: 10.3109/09513591003632084.
- Ramachandrayya SA, D’Cunha P, Rebeiro C. Maternal circulating levels of adipocytokines and insulin resistance as predictors of gestational diabetes mellitus: preliminary findings of a longitudinal descriptive study. J Diabetes Metab Disord. 2020;19(2):1447–1452. doi: 10.1007/s40200-020-00672-4.
- Ravnsborg T, Andersen LL, Trabjerg ND, et al. First-trimester multimarker prediction of gestational diabetes mellitus using targeted mass spectrometry. Diabetologia. 2016;59(5):970–979. doi: 10.1007/s00125-016-3869-8.
- Savvidou M, Nelson SM, Makgoba M, et al. First-trimester prediction of gestational diabetes mellitus: examining the potential of combining maternal characteristics and laboratory measures. Diabetes. 2010;59(12):3017–3022. doi: 10.2337/db10-0688.
- Sweeting AN, Wong J, Appelblom H, et al. A novel early pregnancy risk prediction model for gestational diabetes mellitus. Fetal Diagn Ther. 2019;45(2):76–84. doi: 10.1159/000486853.
- Thagaard IN, Krebs L, Holm JC, et al. Adiponectin and leptin as first trimester markers for gestational diabetes mellitus: a cohort study. Clin Chem Lab Med. 2017;55(11):1805–1812. doi: 10.1515/cclm-2017-0427.
- White SL, Lawlor DA, Briley AL, et al. Early antenatal prediction of gestational diabetes in obese women: development of prediction tools for targeted intervention. PLOS One. 2016;11(12):e0167846. doi: 10.1371/journal.pone.0167846.
- Williams MA, Qiu C, Muy-Rivera M, et al. Plasma adiponectin concentrations in early pregnancy and subsequent risk of gestational diabetes mellitus. J Clin Endocrinol Metab. 2004;89(5):2306–2311. doi: 10.1210/jc.2003-031201.
- Yuan XS, Shi H, Wang HY, et al. Ficolin-3/adiponectin ratio for the prediction of gestational diabetes mellitus in pregnant women. J Diabetes Investig. 2018;9(2):403–410. doi: 10.1111/jdi.12688.
- Zhu Y, Hedderson MM, Quesenberry CP, et al. Central obesity increases the risk of gestational diabetes partially through increasing insulin resistance. Obesity. 2019;27(1):152–160. doi: 10.1002/oby.22339.
- Bao W, Baecker A, Song Y, et al. Adipokine levels during the first or early second trimester of pregnancy and subsequent risk of gestational diabetes mellitus: a systematic review. Metabolism. 2015;64(6):756–764. doi: 10.1016/j.metabol.2015.01.013.
- Xu J, Zhao YH, Chen YP, et al. Maternal circulating concentrations of tumor necrosis factor-alpha, leptin, and adiponectin in gestational diabetes mellitus: a systematic review and meta-analysis. ScientificWorldJournal. 2014;2014:926932. doi: 10.1155/2014/926932.
- Hedderson MM, Darbinian J, Havel PJ, et al. Low prepregnancy adiponectin concentrations are associated with a marked increase in risk for development of gestational diabetes mellitus. Diabetes Care. 2013;36(12):3930–3937. doi: 10.2337/dc13-0389.
- Fuglsang J, Skjaerbaek C, Frystyk J, et al. A longitudinal study of serum adiponectin during normal pregnancy. BJOG. 2006;113(1):110–113. doi: 10.1111/j.1471-0528.2005.00792.x.
- Retnakaran R, Qi Y, Connelly PW, et al. Low adiponectin concentration during pregnancy predicts postpartum insulin resistance, beta cell dysfunction and fasting glycaemia. Diabetologia. 2010;53(2):268–276. doi: 10.1007/s00125-009-1600-8.
- Thomann R, Rossinelli N, Keller U, et al. Differences in low-grade chronic inflammation and insulin resistance in women with previous gestational diabetes mellitus and women with polycystic ovary syndrome. Gynecol Endocrinol. 2008;24(4):199–206. doi: 10.1080/09513590801893398.
- Sirico A, Rossi ED, Degennaro VA, et al. Placental diabesity: placental VEGF and CD31 expression according to pregestational BMI and gestational weight gain in women with gestational diabetes. Arch Gynecol Obstet. 2023;307(6):1823–1831. doi: 10.1007/s00404-022-06673-3.
- Sirico A, Dell’Aquila M, Tartaglione L, et al. PTH-rP and PTH-R1 expression in placentas from pregnancies complicated by gestational diabetes: new insights into the pathophysiology of hyperglycemia in pregnancy. Diagnostics (Basel). 2021;11(8):1356. doi: 10.3390/diagnostics11081356.
- Mousa A, Abell SK, Shorakae S, et al. Relationship between vitamin D and gestational diabetes in overweight or obese pregnant women may be mediated by adiponectin.Mol Nutr Food Res. 2017;61(11). doi: 10.1002/mnfr.201700488.
- Mir MM, Mir R, Alghamdi MAA, et al. Differential association of selected adipocytokines, adiponectin, leptin, resistin, visfatin and chemerin, with the pathogenesis and progression of type 2 diabetes mellitus (T2DM) in the Asir region of Saudi Arabia: a case control study. J Pers Med. 2022;12(5):735. doi: 10.3390/jpm12050735.
- Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–469. doi: 10.1007/s00125-003-1074-z.
- Huang LT, Wu SL, Liao X, et al. Adiponectin gene polymorphisms and risk of gestational diabetes mellitus: a meta-analysis. World J Clin Cases. 2019;7(5):572–584. doi: 10.12998/wjcc.v7.i5.572.
- Gong X, You L, Li F, et al. The association of adiponectin with risk of pre-diabetes and diabetes in different subgroups: cluster analysis of a general population in South China. Endocr Connect. 2021;10(11):1410–1419. doi: 10.1530/EC-21-0235.
- Vecchiola A, García K, González-Gómez LM, et al. Plasminogen activator inhibitor-1 and adiponectin are associated with metabolic syndrome components. Am J Hypertens. 2022;35(4):311–318. doi: 10.1093/ajh/hpab138.
- Orrù S, Nigro E, Mandola A, et al. A functional interplay between IGF-1 and adiponectin. Int J Mol Sci. 2017;18(10):2145. doi: 10.3390/ijms18102145.
- Frankenberg ADV, Reis AF, Gerchman F. Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: a literature review. Arch Endocrinol Metab. 2017;61(6):614–622. doi: 10.1590/2359-3997000000316.
- Xu A, Wang Y, Keshaw H, et al. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112(1):91–100. doi: 10.1172/JCI17797.
- Wang Q, Du J, Liu F. Changes of serum adiponectin and glycated albumin levels in gestational diabetes mellitus patients and their relationship with insulin resistance. Iran J Public Health. 2020;49(7):1252–1261. doi: 10.18502/ijph.v49i7.3578.
- Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol. 2002;13(1):51–59. doi: 10.1097/00041433-200202000-00008.
- Mallardo M, Ferraro S, Daniele A, et al. GDM-complicated pregnancies: focus on adipokines. Mol Biol Rep. 2021;48(12):8171–8180. doi: 10.1007/s11033-021-06785-0.