Abstract
Background and aim
Centella asiatica (L.) Urb. (Apiaceae) is a renowned medicinal plant being used in the Ayurvedic system for its pharmacological effects on the central nervous system such as rejuvenating, sedative, anxiolytic and memory-enhancing properties. The present study was designed to investigate the effect of Centella asiatica (CA) extract on inflammatory responses induced by lipopolysaccharide (LPS) and resulting changes in cognitive behavior.
Materials and methods
Adult male Sprague-Dawley rats were divided into 4 groups as control, LPS, CA and LPS + CA. The treatments with LPS (5 mg/kg) were intraperitoneally (i.p) injected on day 4 and CA ethanol extract (200 mg/kg) were given orally for 14 days. Morris Water Maze (MWM) test was performed to assess spatial learning and memory performance. Acute oral toxicity of the extract at the highest dose of 5000 mg/kg was also conducted.
Results
Single administration of LPS was able to significantly elicit learning and memory impairment (p < .05) when compared to the control groups. Treatment with CA significantly improved the impaired learning ability in which the LPS + CA rats took the shortest time and route to find the hidden platform (15.85 ± 2.68 s (p < .001); 352.43 ± 88.10 cm (p < .001) on day 5) and induced differential cytokine responses in the blood. No mortality and no significant variation in the body and organ weights between the control and the treated group was observed after 14 days of acute toxicity study. Hematological analysis and biochemical parameters revealed no toxic effects of the extract. Pathologically, neither gross abnormalities nor histopathological changes were observed.
Discussion and conclusion
Centella asiatica extract exhibited significant learning and memory enhancement potential in animal model. Hence, indicating its putative preventive therapeutic effects in neuroinflammation related diseases.
A single dose of lipopolysaccharide (LPS) (5 mg/kg) administered systemically to mimic the consequences of LPS-induced inflammatory responses was able to affect some behavioral modification of spatial memory at the time point of study.
The study showed that the learning capability during the training trial was restored or ameliorated with the pre-emptive treatment of Centella asiatica extract (200 mg/kg).
Centella asiatica extract improves spatial memory, learning deficits and regulates proinflammatory responses in systemic LPS-treated rats.
KEY MESSAGE
Introduction
The mechanisms underlying the etiology of Alzheimer’s disease (AD) remain complex and unresolved. Alzheimer’s disease, the leading cause of dementia in the elderly, is characterized by amyloid-plaques and neurofibrillary tangles. The accumulation of the plaques in the central nervous system (CNS) leads to neuroinflammation due to the activation of microglia, the CNS’s resident immune cells. The significance of neuroinflammation in the pathophysiology of AD has recently come into focus [Citation1,Citation2]. Growing data reveals that AD pathogenesis is not limited to the neuronal compartment, but also involves strong interactions with immune processes in the brain [Citation3]. It is conceivable that external variables, such as systemic inflammation and obesity, interfere with the brain’s immunological processes and exacerbate disease progression. Potential future therapeutic or preventative methods for AD could involve regulating risk factors and targeting these immune pathways. Some forms of AD are typified by a neurodegenerative process that involves neuroinflammation and its degree and persistence, differ with its etiology. Therefore, to analyze the impact of inflammation in neurodegenerative pathologies like Parkinson’s and Alzheimer’s, animal studies of neuroinflammatory processes are essential [Citation4]. Different immune cells, referred as microglial cells, respond differently to this bacterial endotoxin, rendering it a potent inflammatory inducer. In order to look into the behavioral changes related with inflammation in rodents, it is customary to treat them with bacterial LPS and examine their responses [Citation5]. Animals given LPS intravenously or subcutaneously develop a behavioral condition known as ‘sickness,’ which includes symptoms like lethargy, decreased locomotor activity, and sleeping problem, as reported in a number of studies. Consequently, LPS-induced neuroinflammation is considered the standard method for examining neuroinflammation for both in vitro and in vivo.
Centella asiatica (L.) Urb. (Apiaceae), also known as gotu kola, pennywart and pegaga in Malaysia, is a perennial with kidney shaped-leaves commonly found and cultivated in Asian countries [Citation6]. Ayurveda and traditional Chinese medicines have relied on it for thousands of years to cure everything from skin diseases to memory loss. The plant’s antipyretic, antiseizure, antidepressant, wound-healing, anti-inflammatory, antioxidant, and neuroprotective properties are just a few examples of the various ways it has been studied for its therapeutic potential [Citation7,Citation8]. Its active ingredients are associated for many of its pharmacological effects, with pentacyclic triterpenes (among which are asiaticoside, asiatic acid, madecassic acid, and madecassoside) having a notably substantial role [Citation9,Citation10]. The versatility of its therapeutic functions, especially for the treatment of mental disorders, has sparked an interest in identifying agents that target various mechanism and pathways for the improvement of cognitive functions and performance [Citation11,Citation12]. Based on previous findings and accumulating evidences suggesting that involvement of peripheral inflammation in the development of neurodegenerative diseases, the present study was performed to investigate the effect of CA on neuroinflammation induced by LPS and resulting changes in cognitive behavior and inflammatory cytokines in LPS-induced inflammation rat model.
Several factors, including plant age, food availability, and the surrounding environment, affect the relative abundance of CA’s main bioactive components [Citation9,Citation13]. Differences in the chemical contents have been known between samples of CA originating from different countries; thus contributing to different pharmacological activity profiles. As the fresh leaves of CA have been consumed in salad preparation (also known as ulam), or dried leaves used to make tea as well as its supplements available in varying levels of purity, it is imperative to investigate the toxicity risk associated with the use of this medicinal plant. Previous work by Hafiz and colleague [Citation14] has demonstrated the method of preparation of the extract using non-toxic solvent, ethanol and its chemical characterization. Effect of the ethanol extract on the viability of neuroblastoma cells (SH-SY5Y) and murine macrophages cells (RAW 264.7) showed no significant degree of cytotoxicity at the highest concentration of 1000 µg/mL Nonetheless, in this context, our study was designed to evaluate the effect of ethanol CA extract on the impact of systemic inflammation on memory impairment and its safety profile using standard regulatory guideline for acute oral toxicity.
Material and methods
Plant material and preparation of the extract
Raw materials of CA were obtained from Herbagus Trading, Pulau Pinang, Malaysia in 2015. The plant samples were verified by a botanist of University Teknologi Mara (UiTM) Puncak Alam, Selangor, Malaysia. Voucher specimen (CA-K017) was prepared and deposited in the Atta-ur-Rahman Institute for Natural Product Discovery, UiTM Puncak Alam (Selangor, Malaysia) for future reference. The whole plant was washed, cleaned, and oven-dried at 40 °C. The powdered plant material was extracted using an optimized extraction protocol at the extraction facility of the Institute of Bioproducts Development (IBD), Universiti Teknologi Malaysia (UTM), Malaysia. The extraction was carried out by using 95% denaturated ethanol for 8 h at 60 °C as described in [Citation14].
In vivo studies: animal and treatments (LPS-induced neuroinflammation)
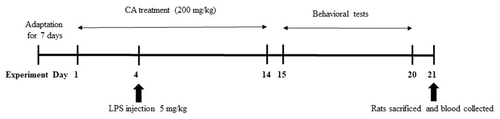
In this study, animals were maintained in accordance with the guidelines and approval from the Animal Ethics Committee of University Sains Malaysia (USM), Malaysia (USM/2016(98) (710). Twenty-four adult male Sprague-Dawley male rats, with body weight of 270 ± 30 g, were procured from Animal Research and Service Centre (ARASC), University Sains Malaysia, Malaysia. Prior to the beginning of the trial, the rats were given one week to adjust to their new surroundings. Every animal had access to a steady diet of food and water, and was housed in a clean, well-lit environment with a light/dark cycle of 12 h. The animals were randomly divided into 4 groups as described herein: (1) Control group (oral saline for 14 days); (2) LPS group (i.p 5 mg/kg on day 4); (3) CA group (oral 200 mg/kg for 14 days) and (4) LPS + CA (oral 200 mg/kg for 14 days). Lipopolysaccharide from E. coli 0111: B4 (Sigma-Aldrich, St Louis, MO) at the dose of 5 mg/kg was injected on Day 4. The present study was performed to investigate the effect of CA on inflammatory responses induced by LPS and resulting changes in cognitive behavior. Oral administration of CA extract (200 mg/kg/day) was administered for a duration of 14 days. On day 15 to day 19 until day 20, all of the rats were subjected through the Morris Water Maze (MWM) test, which assesses spatial learning and memory abilities. This was followed by the probe test on day 20. The animals were sacrificed on the following day and blood collected for further assessment as outlined in the timeline below ().
Behavioral test (Morris Water Maze, MWM)
In order to evaluate CA extract’s impact on spatial learning (from day 15 to day 19) and reference memory (day 20), the Morris water maze test was used. The rat was positioned in the maze so that it was faced the tank wall during the trials used for spatial learning. In principal, rats can primarily escape from swimming by climbing onto the platform, and throughout the acquisition phase, they can learn the platform’s spatial location from any point around the pool’s perimeter, demonstrating that the platform provides no local cues to assist the rats’ escape behavior. The animal’s sense of orientation is facilitated by the visual cues present outside the tank. The apparatus consists of circular water pool of 150 cm in diameter and 60 cm in height. It was filled with 23 ± 1 °C water with a depth of 40 cm. Skim milk powder was added to the pool water to create an opaque effect. A white Plexiglas platform, 12 cm in diameter, with holes drilled into the top for the animals’ grasp, was submerged 1 cm below the water’s surface. The pool’s rim was sectioned up into quadrants of equivalent size. As soon as the rat was placed in the tank, a timer or computer tracking software was activated. The rat was given one minute to find the submerging platform in the tank, and its escape latency was measured by the distance it swam in the pool. Throughout every trial, the same visual images were placed in the exact same spots around the pool where the rats could see them. The timer or computer program was halted as soon as the rat touched the platform. The rat was either placed on the platform or led to it if it did not locate it within the allotted time. The rat was allowed to be on the platform for 15 sec to memorize the distal cues around the tank. Over the course of 5 days (day 15 – day 19), each rat underwent the test four times per day with 10 min interval for each session. Each day, the beginning positions were rearranged in a different order.
On day 20, the probe test was administered to evaluate the reference memory. During the test, the tank’s platform was removed. The rat was placed in a new starting position and permitted to roam the tank for one minute. The number of targeted trips, overall distance covered, and total time spent in each quadrant were all evaluated. Video cameras were used to record all trials of the spatial learning and reference memory tests, and analysis was performed with video tracking software (SMART ver3.0 Panlab Harvard Apparatus, Barcelona, Spain) that enabled both real-time and post-hoc automatic rat path monitoring. All tests were performed during light cycle with consistent timing between groups (10 min interval for each session) and performed between 0900 and 1100 a.m in a soundproof laboratory.
Plasma cytokine measurement
Five cytokines, interleukin (IL)-1β, IL-6, interferon (IFN)-δ, tumor necrosis factor (TNF)-α and monocyte chemoattractant protein (MCP)-1 were measured using Procarta PrimePlex rat 5-plex proteomic assay kit (Panomics, CA, USA). On day 21 after the behavioral test, all rats were euthanized and blood was obtained through heart puncture into EDTA-coated tubes. Samples were centrifuged (6000 g for 15 min at 4 °C) and plasma was collected and stored frozen (–80 °C) until analysis. The plasma cytokines were measured using a Multi-Analyte ELISArray kit (cat. no. MER-004A; SABiosciences; Qiagen, Inc, Valencia, CA, USA) according to the manufacturer’s protocol. Samples were added to the array, which included the following specific cytokine capture antibodies from the above-mentioned kit: IL-1β, IL-6, TNF-α, IFN-γ and MCP-1. The reaction was measured by changes at 450 and 540 nm on a spectrophotometer.
Acute oral toxicity study
The evaluation of CA’s toxicity was performed in accordance with Organization for Economic Co-operation and Development (OECD) guideline 420 [Citation15]. For the investigation, Sprague Dawley female rats that were healthy, young, sterile, and not pregnant were employed. The animals were acclimated to their new environment for 7 days, during which duration they were housed in standard conditions with a 12 h dark/light cycle, given full access to water and a standard pellet meal, and allowed plenty of space to exercise. Except for water, all food was withheld 4 h before to the experiments. Following the recommendations of the OECD Guideline, the sighting dose of CA administered to an animal was 300 mg/kg body weight. The animals were given the single dose through oral gavage. Sighting dose results that exhibit some toxicity signs without generating severe toxic effects of mortality were used to establish the primary dosage. In the main dose trial of 2000 mg/kg, an additional four animals were examined in addition to the initial animal. For 48 h, the animals were observed for toxic symptoms, as well as behavioral changes, toxicity indicators, and death at the first, second, fourth, and sixth hours, and once a day for 14 days.
The five rats were observed on a daily basis for signs of mortality and abnormal clinical signs, such as piloerection, salivation, and lacrimation. The rats’ weights were recorded on days 1, 7, and 14. All animals were fasted overnight on the 14th, then euthanized on the 15th for necropsy analysis. Liver, spleen, and kidney specimens were removed, weighed, and examined macroscopically. Additional study on a higher dose at 5000 mg/kg was selected on the basis of the 2000 mg/kg dose results which did not produce any signs of toxicity and did not cause severe toxic effects of mortality. In the main dose study of dosage 5000 mg/kg, another 5 animals were tested in a similar acute toxicity evaluation. Blood samples were obtained after the 15th day of the experiment. The non-heparinized blood was allowed to coagulate before centrifuged and the serum separated. The serum was assayed for total protein, Urea, Alanine aminotransferase (ALT), Aspartate aminotransferase (AST) and Alkaline phosphatase (ALP). Immediately after the collection of blood samples, organs such as kidney, liver and spleen were removed and preserved in formalin solution. Tissues from the control group and treated with the high dose were subjected to dehydration process, embedded in paraffin, sectioned and followed by staining. Pathological observations of all tissues were performed. All procedures done in this study were performed according to the Institutional Animal Care and Use Committee (IACUC), Forest Research Institute Malaysia (FRIM) approval and review (IACUC-FRIM/1(2015)/02).
Statistical analysis
Data were presented as mean ± standard errors (SEM). The results of behavioral assessments for MWM were analyzed using two-way ANOVA, while spatial probe test was analyzed using one-way ANOVA, with Dunnet’s post hoc comparison was used where applicable. For cytokine analysis, data were presented as the mean ± standard error and assessed using one-way ANOVA followed by a Bonferroni Multiple Comparison Test (95% confidence). Significant differences were indicated by a probability value of less than 0.05. GraphPad Prism version 8.0.2(263) of GraphPad Software, Inc. was used for the data analysis.
Results
Escape latency and traveled distance
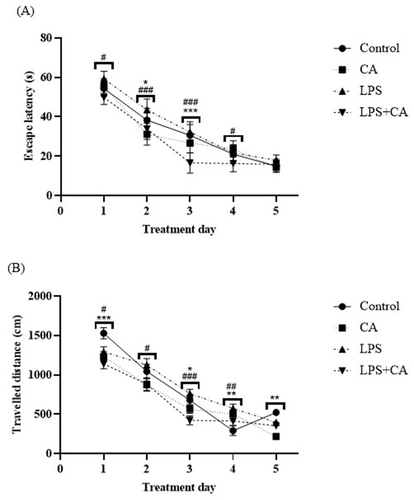
It was shown that the time for escape latency in all experimental groups reduced gradually during the acquisition phase for 5 days (), indicating normal learning abilities. The results showed that single injection of LPS was able to elicit behavioral alterations as shown by traveled path to reach the platform in LPS group was significantly more than in control group on day one (p < .05) and day four (p < .01) (). For the escape latency of the rats, the two-way ANOVA between the effect of treatment and days of treatment displayed progressive latency reduction [F4,100 = 289.9, p < .0001], with statistically significant treatment effect [F3,100 = 18.83, p < .0001] and interaction [F12,100 = 3.366, p = .0004]. Dunnet’s post hoc comparison revealed a statistically significant reduction (p < .05) in time to locate the hidden platform starting on day one to day three by the LPS + CA group when compared to the LPS group. LPS rats treated with CA were able to locate the immersed platform less than 20 sec starting on day three of the trials. The distance traveled by each group was correspondently associated with the escape latency. The two-way ANOVA demonstrated a significant treatment effect [F3,100 = 11.68, p < .0001], trial days [F4,100 = 158.6, p < .0001] and interaction between the effect of treatment and days of treatment on the average distance wandered by the rat groups in search of the hidden platform [F12,100 = 3.292, p = .0005]. It was interesting to note that LPS rats treated with CA exhibited a gradual reduction in searching distance during the trial period especially on the second and third day when compared to rats in LPS group with subsequent post-hoc comparison of p < .05 and p < .001, respectively (). Distance traveled to find the platform was also significantly greater on day 4 and 5 for CA rats compared to control (p = .004 and p = .002). Treatment with CA could alleviate spatial learning and memory deficit induced by LPS.
Figure 2. Behavioral assessments following treatment with CA extract on LPS-induced animals. (A) Comparison of escape latency (s) in reaching the hidden platform; (B) traveled distance (cm) by rats during the five days of training between four groups, data are presented as mean ± SEM (n = 6 in each group). Centella asiatica group (CA), lipopolysaccharide group (LPS) and lipopolysaccharide + Centella asiatica group (LPS + CA). *p < .05, **p < .01, ***p < .001 vs. control group. #p < .05, ##p < .01, ###p < .001 vs. LPS group.
Reference memory indices in the target quadrant (probe test)
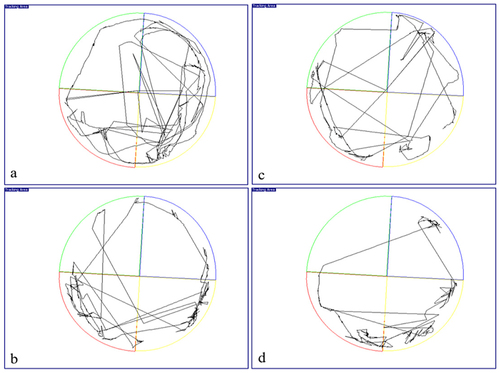
During the probing test (conducted on day 20), the experimental rats will attempt to recall the platform’s hidden location by swimming in a circle around the test area. Due to the location of the hidden platform in this quadrant, rats likely to swarm in the target quadrant. We found that rats in LPS group spent almost similar time in target quadrant (28.64 ± 3.93%) when compared to LPS + CA groups (28.69 ± 6.18%) during probe test (). However, a different pattern could be seen in target/platform crossover count, in which the LPS group crossover the target platform less (1.90 ± 0.52) compared to the LPS + CA group (2.33 ± 0.42) which cross the target platform more frequently (). Representative trajectory map of the rats showed that the LPS + CA group was found mostly swim at the edges of the pool compared to the other groups () as a mean of minimizing the stressful condition. Therefore, the LPS + CA group has low percentage activity in target quadrant (25.34 ± 2.25%) as compared to LPS group (28.66 ± 4.34%) (). However, no relevant differences were evident between experimental groups as revealed by one-way ANOVA on the percentage of time spent in the previously rewarded quadrant [F3,20 = 0.3046, p = n.s].
Figure 3. Reference memory indices in the target quadrant for all groups of rats. (A) Time spent (%) by rat in target quadrant during probe test of MWM test; (B) Activity (%) of rat in target quadrant during probe test of MWM test; (C) Target/platform crossover (count) in target quadrant during probe test by MWM test. Values are the mean ± SEM. n = 6 for each group.
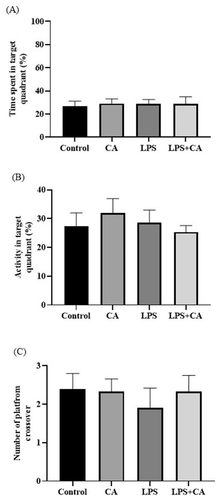
Figure 4. Representative trajectory map of rat for (a) control group; (b) Centella asiatica (CA) group; (c) lipopolysaccharide (LPS) group and (d) lipopolysaccharide + Centella asiatica (LPS + CA) group. Note that the pathways of Centella asiatica treated rats were more obvious in the target quadrant (red).
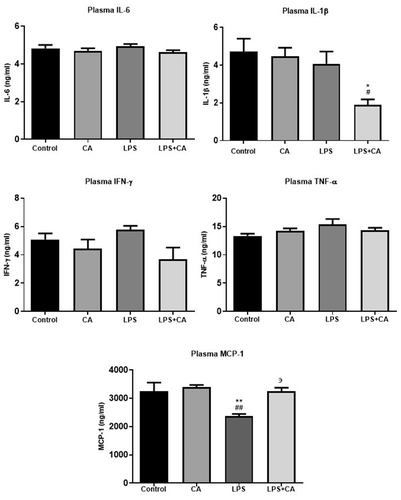
Effect of LPS-induced inflammatory cytokine levels
To investigate the systemic inflammatory responses after LPS exposure (17 days following injection), the expression levels at day 21 of plasma proinflammatory cytokines, such as IL-6, IL-1β, TNF-α, IFN-γ and MCP-1 were measured. As shown in , the results demonstrated that plasma cytokine levels were detectable in all the samples although these concentrations for several samples were very low particularly analytes IL-1β and IFN-γ. Plasma levels of IL-6, TNF-α and IFN-γ following peripheral administration showed no significant differences between all groups. Post hoc analysis demonstrated that plasma cytokine levels of IL-1β significantly decreased after 14 days treatment with LPS + CA (p < .001). The results also showed that the plasma MCP-1 levels also found to be significantly reduced in LPS treated animals as compared with control and CA groups (p < .01). However, the plasma levels of MCP-1 had returned to baseline values following treatment with CA extract at 200 mg/kg.
Figure 5. Effect of CA extract on LPS-induced inflammatory cytokines in plasma. The levels of cytokine were assessed by using specific ELISA, as described in the methodology section. Results are reported as histograms representing the mean concentrations with SEM error bars for each group. The animals (n = 6/group) were orally administered with C. asiatica at 200 mg/kg/day for 14 days. Saline was used as a negative control while LPS was used as a vehicle control. All data in the figures are presented as the mean ± standard error and assessed by using one way ANOVA analysis followed with Bonferroni Multiple comparison test (95% confidence), *p < .05 compared to control group, **p < .01 compared to control group, #p < .05 compared to CA group, ##p < .01 compared to CA group, ϶϶p < .05 compared to LPS group.
Acute toxicity effects of CA on SD rats
The main aim of toxicity study was to determine the adverse effects of CA extract [on the vital organs (liver, kidney and spleen) and the biochemical parameters (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, total protein and urea). There were no animal deaths of rats receiving 2000 mg/kg of CA. No sign of toxicity was observed in the wellness parameters during the 14-day observation period. There were no adverse effects on the behavioral responses of the treated animals. Second set of female rats that were treated with 5000 mg/kg CA also showed no mortality as well as no sign of toxicity. Physical observations indicated no changes in the skin, fur, eyes mucous membranes, behavior patterns, tremors, salivation and diarrhea in the animals. Therefore, the approximate acute lethal dose (LD50) of CA in female rats was estimated to be higher than 5000 mg/kg.
Effect of oral administration of CA on body and organ weights
The effects of CA on the changes of bodyweight of rats over dosing (2000 and 5000 mg/kg) were illustrated in . Overall, all groups showed increment of the body weight over time, which grew at rates of 1.83 ± 0.05, 3.37 ± 0.01, 1.92 ± 0.09 and 3.69 ± 0.04 for control 1, control 2, CA 2000 mg/kg and CA 5000 mg/kg, respectively. However, there were no significant difference between the control and CA treated rats.
Table 1. Effect of oral administration of CA extract on the changes in body weight of rats over the dosing period.
The mean organ-to-body weight ratio of rats that received the tested doses of CA showed no significant difference from the control group (). Although the relative organ weight of rats treated with 5000 mg/kg CA showed slightly higher for liver and spleen compared to rats treated with 2000 mg/kg, but the changes were not significantly different in comparison with the control groups. Meanwhile, there were no changes in relative organ weight of kidney for all groups.
Table 2. Relative organ weight of rats administered with various doses of CA extracts.
Effects of oral administration of CA on serum biochemical parameters
Biochemical analysis was conducted on serum of control and CA-treated (5000 mg/kg) rats. A reduction of serum levels for all parameters tested (AST, ALP, ALT, urea and total protein) was observed in treated rats after 14 days (). However, the reduction was not significantly different when compared to the control group. These results indicated that the administration of high dose (5000 mg/kg) of CA did not cause harm or liver damage to the exposed animals.
Table 3. Results of biochemical tests performed on the serum of control and treated (5000 mg/kg B.W.) rats in the acute toxicity study for 14 days.
Macroscopic and histopathological examination
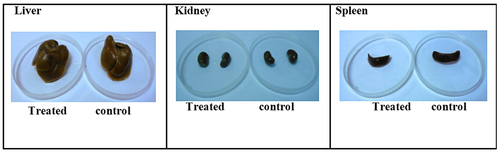
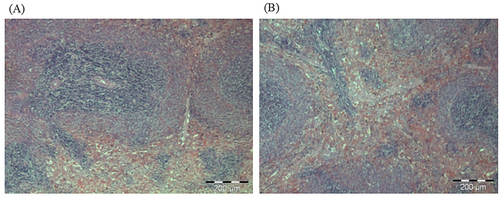
The macroscopic assessment of the vital organs of the treated animals showed no color or texture irregularities compared to the organs of the control group (). Sections of liver, kidney, and spleen were inspected histopathologically to assess the severity of any organ or tissue damage. The results of the liver, kidneys and spleen sections in rats treated with normal saline (control) and 5000 mg/kg CA are shown in respectively. The liver, kidneys and spleen of both the control and treatment groups showed no signs of abnormal histopathology. The liver presented with clear lumen of central vein and no evidence of lesion; the kidneys showed adequate glomeruli and normal tubes with no trace of edema and lesion: the spleen appeared normal with preserved internal architecture normal.
Figure 7. Photomicrographs from the liver of (A) control and (B) treated rats at dose 5000 mg/kg. There were no adverse histopathological conditions observed in the liver of the control and treatment groups. The liver for the treatment group appeared normal with preserved hepatic architecture. The was no sign of inflammation in the treated group.
Figure 8. Photomicrographs from the kidney of (A) control and (B) treated rats at dose 5000 mg/kg. There were no adverse histopathological conditions observed in the kidney of the control and treatment groups. The kidney for the treatment group appeared normal with preserved internal architecture.
Figure 9. Photomicrographs from the spleen of (A) control and (B) treated rats at dose 5000 mg/kg. There were no adverse histopathological conditions observed in the spleen of the control and treatment groups. The treated group showed normal cell observation with no sign of inflammation or congestion.
Discussion
It has been postulated that neuroinflammation plays a role in cognitive impairment and degenerative illnesses of the nervous system. Preceding studies have reported that chronic neuroinflammation elicited by systemic inflammation of LPS has been implicated in the persistent discriminative deficits of hippocampal-dependent tasks of location memory which is known to be impaired with aging process [Citation16–19]. The present study showed that LPS group of animal displayed learning and memory impairment which correlate with a deficit in learning ability and reference memory which are attributed to Alzheimer’s-like cognitive dysfunction. This finding is in line with a study by Bossu and colleague [Citation19] that demonstrated a single dose of LPS was able to evoke persistent cognitive impairment characterized by noticeable deficiencies in reacting to environment modifications which could be due to reduced motivational or attentional deficits. Hence, this model may represent a viable option for the investigation of Alzheimer’s-related diseases and the screening of therapeutic agents. By using Morris Water Maze (MWM) test, this study demonstrates the cognitive performance improvements of CA extract following systemic inflammation induced by LPS.
The ancient use of CA in Ayurveda and various Asian traditional medicine systems as a memory-enhancing medication and for the treatment of mental diseases has been acknowledged for centuries [Citation7]. Neuronal morphology, learning ability, and memory retention have all been found to be able improve with CA. Several mechanisms of action underlying the cognitive enhancing effect of CA have been well documented in various experimental designs both in vitro and in vivo, such as the inhibition of oxidative stress and acetylcholinesterase activity [Citation14], restoration of neural network [Citation20], increased synaptic density [Citation12], activation of antioxidant and mitochondrial response pathways and enhanced synthesis of brain-derived neurotrophic factor and other related molecules [Citation21–23]. The protective effect on the central nervous is generally attributed to its most prominent group of active compounds identified as pentacyclic triterpene saponins including asiaticoside, madecassoside, asiatic acid and madecassic acid [Citation10,Citation24].
The cognitive enhancing effects of aqueous CA extract within the range of concentration of 100 − 300 mg/kg/day have been supported by quite a number of rodent studies using various standard behavioral tests including elevated plus maze, passive avoidance test and step through paradigm [Citation6,Citation21]. The study by Kumar and Gupta [Citation21] indicated that aqueous extract at the dose of 200 mg/kg in both shuttle box and step through paradigms demonstrated much better improvement in learning and memory than that of 100 mg/kg and not significantly different than 300 mg/kg, thus justified the selection of our dosage treatment of CA extract. The selected extract used in this study was prepared and quantified previously by Hafiz and colleague [Citation14] according to an optimized protocol and contained a high amount of saponin glycosides than the aglycones, with madecosside is the highest component, followed by their sapogenins, asiatic acid. The same study also has elucidated the neuroprotective of CA extract against inflammation and oxidative stress via inhibition of acetylcholinesterase and nitrite production as well as suppression of reactive oxygen species production in a concentration dependent manner (treatment extracts ranging from 3.90 up to 1000 µg/mL) with highest concentration of 1000 µg/mL Having observed the encouraging anti-inflammatory and antioxidant effects of CA extract in vivo and in vitro, we further explore its potential in cognitive enhancing effects using LPS rat model. A Morris Water Maze was conducted to elucidate the LPS-induced cognitive impairment such as learning and memory in animal [Citation25]. Using the maze test, we discovered that rats from all groups significantly reduced their overall escape latency time to locate the submerged platform and traveled shorter distances during the course of the training trial days (days 1 through 5). However, the LPS + CA group took the least amount of time to locate the concealed platform compared to the LPS group beginning on day three and continuing through day five. In a similar trend, the LPS + CA group swam the shortest distance before arriving at the target platform, as opposed to the LPS group, which had to swim the farthest to get there. In general, therapy with CA demonstrates a beneficial effect that mitigated the learning and memory deficits in the LPS group. Treatment with CA extract improved the impaired learning ability in which the animals exhibit a learning curve requiring less time and less distance to find the hidden platform. This finding implies that the presence of a well-known triterpene components in the extract: asiatic acid and madecosside which has been previously associated with neuroprotective and neutropic effects supports the cognition functions relevance to the treatment of AD [Citation26].
Inflammation has been highlighted as being one of the most contributory factors to the pathophysiology of AD [Citation3]. In addition to neuronal cells, microglia and astrocytes are implicated in inflammatory processes in the central nervous system, and stimulation of these cells results in a cascade of inflammatory features. A number of cellular neuroscience studies have shown evidence that activation of astrocytes and microglia is associated with plaques and parallel tangles formations in AD [Citation27,Citation28]. Activated astrocytes and microglia are closely associated with increased levels of inflammatory cytokines and some other neurotoxic substances. It is critical to induce inflammation in neurodegenerative models in order to explore its multifaceted repercussions [Citation29]. Among the pathogenic mechanisms underlying AD is chronic neuroinflammation mediated by cytokines released by activated microglia and astrocytes [Citation30]. Cytokines could play a role in the emergence and progression of neurodegenerative conditions as important mediators of chronic neuroinflammation that drives progressive tissue destruction in the brain. Pro-inflammatory cytokines are key players in inflammation and may contribute to changes in the brain during both normal and pathological processes due to their impact on the immune and neurological systems [Citation16,Citation28]. Thus, establishment of an appropriate animal model is important in characterizing neuroinflammation-associated cognitive impairment and neurodegenerative diseases. This work shows that a single injection of LPS mimicking a systemic infection can produce persisting neuroinflammatory responses, and that intraperitoneal administration produces a more physiologically relevant inflammatory stimulation [Citation31]. In the event of neuroinflammation, these cytokines mediate many of the physiological and behavioral changes after infection or trauma and become dysregulated in multiple disease states.
Since LPS causes the early brain production of inflammatory cytokines notably IL-1, IL-6, and TNF-α, which resemble systemic illness, peripheral injection of the LPS has been routinely utilized to cause neuroinflammation [Citation32]. These inflammatory mediators, in turn, appear central in driving behavioral modifications, as in the case of the behavioral responses to LPS, known as sickness behavior, which is the acute consequence of cytokine elevation [Citation33]. Due to the diversity of the LPS injection model in terms of dosage, frequency, and timing, as well as tissue or serum samples, the outcomes of these tests may be influenced by these variables [Citation34]. These variabilities make it quite laborious to compare results between research groups, thus making it improbable to determine the exact time and dose-dependent changes in neuroinflammation and behavior. A study by Somman and colleague [Citation31] shows that following different mode of LPS administrations in the animal induce different timing in the cytokine cascade, thus further defining the inflammatory responses. The current research shows that peripheral injection of bacterial endotoxin (LPS) that models systemic infection is capable of eliciting inflammatory responses, but displays some variances, probably as a result of different regulatory influences on various cytokine levels. Here, we show that a single peripheral inflammatory insult with LPS causes long-lasting abnormalities in spatial navigation and cognitive function, including learning and memory impairment. Drug discovery and development studies can benefit greatly from the behavioral approach taken in this study, as it can be employed to assess compounds for possible cognitive improving effects and to delineate adverse effects of neurotoxicants on cognition. Here, we demonstrate that learning and memory deficits were lessened with CA treatment. A single episode of systemic inflammation can lead to long-lasting behavioral changes and the production of pro-inflammatory cytokines. Despite the fact that the cytokines generated in the peripheral tissues do not diffuse across the blood–brain barrier, they may nevertheless transmit the signal to the brain. Peripheral injection of LPS has been found to activate astrocytes and microglia as well as other pro-inflammatory responses in the brain. Pro-inflammatory signaling pathways are activated by lipopolysaccharide (LPS) through toll like receptor 4 (TLR4) [Citation35]. This activation in microglia up-regulates the expression of inflammatory mediators and cytokines such as IL-1β, IL-6, TNF-α and chemokines among many others as reviewed by [Citation36,Citation37]. By using animal models, scientists have looked into the molecules and cells that are involved in relaying signals from the periphery to the brain during times of inflammation [Citation19,Citation38–40].
Based on the finding in our study, plasma levels of mediators, IL-1β, IL-6, IFN-γ and TNF-α in the LPS group, albeit measurable in plasma after 17 days of LPS administration but did not show a significant change compared to control group. Work from various laboratory has systematically examined the temporal effect of LPS administration in various animal settings. Injection with LPS induced a transient release of cytokines in the circulation and also CNS, for example IL-6 was observed to increase 1 h and reached its peak in 1.5 h after LPS administration [Citation41]. While the plasma TNF-α upregulated as early as 30 min after LPS and reached its peak 1 h post-injection. The previous study also indicated that there were differences in the magnitude of elevation (expressed as fold from the basal value) after stimulation with LPS. It was noted that serum levels of most pro-inflammatory cytokines in LPS-treated group restored to its basal value within 24 h post administration. However, the serum level of IL-6 and MCP-1 remained elevated even after 24 h [Citation38]. Long after LPS administration as measured on day 17, the plasma levels of these cytokines had presumably returned to baseline values, while level of MCP-1 was significantly affected in LPS-treated animals, demonstrating that the immune system was still mildly prevail at this time point. We report here that LPS treatment alone show a reduced level of chemokine MCP-1 although previous work has demonstrated an upregulation of this chemokine in the brain and periphery after a peripheral injection of LPS as observed at different time points [Citation38]. Treatment with CA extract for the indicated period was able to counteract the effect of LPS. While no significant changes in levels of other analytes were observed in the plasma at later time point after peripheral LPS injection. This could be attributed to the transient increase in MCP-1 which explains that circulating levels of some cytokines increase soon after the endotoxin insult, and after acute response they subside in the blood. Although the temporal changes in plasma levels of these circulating inflammatory markers was not determined in this work, and did not account for regional differences of cytokines profiles in the brain, a number or works have shown that synthesis and secretion of various pro-inflammatory cytokines in the periphery elicited centrally or systematically are determined by its specific spatio-temporal patterns. Temporal profiles of peripheral cytokines after stimulation with LPS may differ on its route of administration, i.e. intraperitoneal vs. intravenous [Citation41,Citation42]. In a different animal setting, it was shown that repeated dose of LPS intraperitoneally caused neuroinflammation via activation of pro-inflammatory cytokines TNF-α and PGE2 in LPS-induced rats and that treatment with ethanol CA extract at doses of 300 and 350 mg/kg were able to reduce the levels of both mediators [Citation14]. Based on the findings in this study, we interpret our present data as suggesting that differential cytokines responses in the blood after single insult of LPS may play an important role in mediating long-lasting inflammatory responses which gradually leads to neurodegeneration. These results support the view that a systemic inflammatory response evoked by a single LPS injection is able to affect some cognitive impairments which in part appear central in driving some behavioral modifications implicated in AD.
There is an emerging trend in the public’s use of herbal preparations due to the general perception that these remedies are derived from nature and thus devoid of harmful or undesirable side effects. Nonetheless, the toxicity of traditional herbal medications in any form of preparation must be thoroughly explored and documented to provide information on the risk and toxicity associated with the use of these botanicals to treat illnesses [Citation43]. In this context, toxicological studies could address some of the inherent risks that may be associated to the use of herbs, hence preventing negative consequences when used as medications. For the evaluation of safety profiles of herbal remedies or pharmaceuticals intended for oral consumption, acute and sub-acute toxicity tests on rodents are commonly carried out [Citation44]. The acute toxicity test is the initial step in determining the harmful effects of a chemical within 14 days of a single dosage delivery. It is usually administered via oral routes to determine the median lethal dose (LD50) for a particular toxic substance in testing animals (usually rats or mice). In a toxicity test, internal animal organs such as the liver, kidneys, and spleen are used to assess the safety or toxicity of plant materials and extracts [Citation45]. The weight of the animal’s organs is a useful indicator of its physiological and pharmacological conditions. Significant differences in relative organ weight between normal and treated animals served as a sign of toxic effects. It provides a preliminary assessment of any edema or damage caused by noxious substances [Citation45,Citation46]. The present study focused on the acute toxicity evaluation of the CA extract. The results showed that both 2000 mg/kg and 5000 mg/kg CA extracts did not affect the relative organ weight in the treated animals. Thus, indicating that both CA extracts did not interfere with the ordinary growth and normal development of the internal organs in the treated animals. Gross macroscopic examination of the organs of the treated animals revealed no abnormalities in color or texture when compared with the organs of the animals in control group.
The initial physiological and metabolic status of the animals is also reflected by changes in animal body weight, which is another crucial indicator or signal for the evaluation of toxicity [Citation47]. An abnormal rise or fall in the treated animal’s body weight may be an indicator of toxicity. The body weight of the animals was obtained on the first day of the study and weekly during the 14-day period toxicity test. Both the animals in the treatment group and those in the control group exhibited an increase in their overall body weight. No difference in body weight gain occurred between rats given a control diet and those treated with either 2000 or 5000 milligrams per kilogram (mg/kg) of CA extracts. Both CA extracts were shown to have no adverse effects on the well-being or growth of the rats altogether, and neither extract was observed to have any impact on metabolism. The primary organs i.e. kidneys and liver are susceptible to the noxious effects of medicines and involved in the detoxification and excretions of the ingested harmful substances, which are more likely to manifest negative effects in the cells of the organs [Citation46]. In the serum biochemical analysis, the levels of total protein, urea, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) are important indicators for good liver and kidney functions [Citation48]. Damage on kidney and liver released higher levels of ALT, AST and ALP. In this study, the levels of these parameters in the treated group were not significantly different when compared to the control group. These results indicated that the administration of high dose (5000 mg/kg) of CA did not cause harm or damage to the liver and kidney of the treated animals.
Histopathological analysis is an important technique to detect any possible damage in the internal organs caused by any exposure to treatments in the animal system [Citation49]. Gross examination of the kidneys, liver and spleen did not show any signs of morphological abnormalities changes. Histopathological slides were prepared from the liver, kidneys and spleen of the animal treated with the 5000 mg/kg CA extracts. The microscopic examination on the organ slides of the treated animals also recorded insignificant changes of tissue morphology. The findings further strengthen the claim that treatment with the higher dose of 5000 mg/kg did not cause tissue damage in the internal organs, thus LD50 value was classified as lowest toxicity class (LD50 > 5000 mg/kg). This study is congruent with a study by Chivapat and colleague [Citation50] that revealed standardized extract of CA up to 10 g/kg did not cause any fatality in animal in acute toxicity study and no severe pathological abnormalities were seen in any organs. In a similar experimental setting, oral administration of juice of whole plant of CA at the dose up to 7 g/kg was shown to be non-toxic to the tested animal [Citation51]. Meanwhile, a study done by Chauhan and Singh [Citation52] using acetone leaf extract of CA revealed that the mean lethal dose (LD50) for the animals is greater than 4000 mg/kg and subacute treatment did not show any change in corporal weight and hematological parameters. In the toxicology evaluation of a test substance, mortality is a crucial and important criterion [Citation53]. Findings from this study showed both test doses at 2000 mg/kg and at a higher dose of 5000 mg/kg body weight did not cause death to the treated animal in the duration of 14 days’ treatment test. The results of biochemical analysis corroborated with histological examination of the organs that indicated the extract did not cause toxicity towards the organs. The no-observed-adverse-effect level was determined at the dosage of 5000 mg/kg body weight/day, henceforth suggesting the safety margin of CA for further development of therapeutic agent. However, acutely non-toxic components may become toxic on long term exposure due to its accumulation which leads to the disruption of physiological and biochemical homeostasis, indicating the need for further evaluation of this plant extract.
Conclusions
The present study demonstrates that peripheral injection of bacterial endotoxin that models systemic infection was able to induce differential cytokines responses in the blood which appear central in driving behavioral modifications. The results show that in a behavioral testing of single injection of LPS and treatment with CA extract (200 mg/kg), per oral) for a period of 14 days was able to attenuate the LPS-induced spatial and memory impairment, indicating its important role in inhibition of neuroinflammation and its putative preventive therapeutic effects. The CA extract was found to be relatively non-toxic even at high dose of 5000 mg/kg body weight. Thus, the plant, which is generally consumed as fresh ulam is presumed safe to be used as an oral health supplement when used for a short duration. However, further toxicity assessment including subacute, chronic and genotoxic studies using repeated dose are warranted before this extract can be further developed as therapeutic agents.
Author contributions
All authors contributed to the paper in the following ways. Conceptualization, Mazura, MP; methodology, Mazura, MP, Chee BJ, Asiah O and Idris L; validation and formal analysis, Mazura, MP, Chee BJ and Idris L; resources and project administration, Asiah O; writing-original draft preparation, Mazura MP; writing-review and editing, Mazura MP, Chee BJ and Idris L.
Acknowledgements
The author would like to thank the technical staff of the animal facility at University Sains Malaysia, Kelantan, Malaysia and Biology Laboratory of Forest Research Institute Malaysia for their technical assistance during the whole period of the experimental study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data generated during this study are included in this article. Further enquiries can be directed to the corresponding author.
Additional information
Funding
References
- Hensley K. Neuroinflammation in Alzheimer’s disease: mechanisms, pathologic consequences and potential for therapeutic manipulation. J Alzheimers Dis. 2010;21(1):1–16. doi: 10.3233/JAD-2010-1414.
- Zhang F, Jiang L. Neuroinflammation in Alzheimer’s disease. Neuropsychiatr Dis Treat. 2015;11:243–256. doi: 10.2147/NDT.S75546.
- Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer’s disease. J Neuroimmunol. 2007;184(1–2):69–91. doi: 10.1016/j.jneuroim.2006.11.017.
- Nazem A, Sankowski R, Bacher M, et al. Rodent models of neuroinflammation for Alzheimer’s disease. J Neuroinflammation. 2015;12:74. doi: 10.1186/s12974-015-0291-y.
- Lee JW, Lee YK, Yuk DY, et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflamm. 2008;5:37–50.
- Gupta YK, Veerendra Kumar MH, Srivastava AK. Effects of Centella asiatica on pentylenetetrzole-induced kindling, cognition and oxidative stress in rats. Pharmacol Biochem Behav. 2003;74(3):579–585. doi: 10.1016/s0091-3057(02)01044-4.
- Gohil KJ, Patel JA, Gajjar AK. Pharmacological review on Centella asiatica: a potential herbal cure-all. Indian J Pharm Sci. 2010;72(5):546–556. doi: 10.4103/0250-474X.78519.
- Visweswari G, Prasad KS, Chetan PS, et al. Evaluation of anticonvulsants effects of Centella asiatica (gotu kala) in pentelynetetrazole-induced seizures with respect to cholinergic neurotransmission. Epilepsy Behav. 2010;17(3):332–335. doi: 10.1016/j.yebeh.2010.01.002.
- James JT, Dubery IA. Pentacyclic triterpenoids from the medicinal herb, centella asiatica (L.) urban. Molecules. 2009;14(10):3922–3941. doi: 10.3390/molecules14103922.
- Jantwal A, Durgapal S, Upadhyay J, et al. Centella asiatica. In: Belwal T, Nabavi SM, Nabavi SF, Dehpour AR, editors. Naturally occurring chemicals against Alzheimer’s Disease. London: academic Press. 2021; p. 257–269.
- Lokanathan Y, Omar N, Puzi A, et al. R. Recent updates in neuroprotective and neurodegenerative potential of Centella asiatica. Malaysia J Med Sci. 2015;23(1):4–14.
- Gray NE, Zweig JA, Caruso M, et al. Centella asiatica increases hippocampal synaptic density and improves memory and executive function in aged mice. Brain Behav. 2018;8(7):e01024. doi: 10.1002/brb3.1024.
- Randriamampionona D, Diallo B, Rakotoniriana F, et al. Comparative analysis of active constituents in centella asiatica samples from Madagascar: application for ex situ conservation and clonal propagation. Fitoterapia. 2007;78(7–8):482–489. doi: 10.1016/j.fitote.2007.03.016.
- Hafiz ZZ, Amin MM, Johari James RM, et al. Inhibitory effects of raw extract Centella asiatica (RECA) on acetylcholinesterase, inflammations, and oxidative stress activities via in vitro and in vivo. Molecules. 2020;25(4):892–912. doi: 10.3390/molecules25040892.
- OECD Guideline for Testing of Chemicals. Acute oral Toxicity-Fixed dose procedure. Paris: Organisation for Economic Co-operation and Development; 2001. p. 1–14.
- Magalingam KB, Radhakrishnan A, Ping NS, et al. Current concepts of neurodegenerative mechanisms in Alzheimer’s disease. Biomed Res Int. 2018;2018:3740461. doi: 10.1155/2018/3740461.
- Bluthe RM, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992;573(2):318–320. doi: 10.1016/0006-8993(92)90779-9.
- Jacewicz M, Czapski GA, Katkowska I, et al. Systemic administration of lipopolysaccharide impairs glutathione redox state and object recognition in male mice. The effect of PARP-1 inhibitor. Folia Neuropathol. 2009;47(4):321–328.
- Bossu P, Cutuli D, Palladino I, et al. A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain levels of TNF-α and IL-18. J Inflamm. 2012;9:101–113.
- Abdul Hannan M, Nazmul Haque M, Munni YA, et al. Centella asiatica promotes early differentiation, axodendritic maturation and synaptic formation in primary hippocampal neurons. Neurochem Int. 2021;144:104957. doi: 10.1016/j.neuint.2021.104957.
- Kumar MHV, Gupta YK. Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. J Ethnopharmacol. 2002;79(2):253–260. doi: 10.1016/s0378-8741(01)00394-4.
- Kumar A, Samrita D, Prakash A. Neuroprotective effects of centella asiatica against intracerebroventricular colchicine-induced cognitive impairment and oxidative stress. Int J Alzheimer’s Dis. 2009;2009:1–8. doi: 10.4061/2009/972178.
- Chiroma SM, Hidayat Baharuldin MT, Mat Taib CN, et al. Protective effect of Centella asiatica against D-galactose and aluminium chloride induced rats: behavioral and ultrastructural approaches. Biomed Pharmacother. 2019;109:853–864. doi: 10.1016/j.biopha.2018.10.111.
- Wu ZW, Li WB, Zhou J, et al. Oleanane and ursane-type triterpene saponins from centella asiatica exhibit neuroprotective effects. J Agric Food Chem. 2020;68(26):6977–6986. doi: 10.1021/acs.jafc.0c01476.
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116.
- Soumyanath A, Zhong YP, Gold SA, et al. Centella asiatica accelerates nerve regeneration upon oral administration and contains multiple active fractions increasing neurite elongation in vitro. J Pharm Pharmacol. 2005;57(9):1221–1229. doi: 10.1211/jpp.57.9.0018.
- Serrano-Pozo A, Mielke ML, Gómez-Isla T, et al. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am J Pathol. 2011;179(3):1373–1384. doi: 10.1016/j.ajpath.2011.05.047.
- Meraz-Ríos MA, Toral-Rios D, Franco-Bocanegra D, et al. Inflammatory process in Alzheimer’s disease. Front Integr Neurosci. 2013;7:59. doi: 10.3389/fnint.2013.00059.
- McLarnon JG. Correlated inflammatory responses and neurodegeneration in peptide-injected animal models of Alzheimer’s disease. Biomed Res Int. 2014;2014:923670. doi: 10.1155/2014/923670.
- Steinman L. Inflammatory cytokines at the summits of pathological signal Cascades in brain diseases. Sci Signal. 2013;6(258):pe3. doi: 10.1126/scisignal.2003898.
- Somann JP, Wasilczuk KM, Neihouser KV, et al. Characterization of plasma cytokine response to intraperitoneally administered LPS & subdiaphragmatic branch Vagus nerve stimulation in rat model. PLoS One. 2019;14(3):e0214317. doi: 10.1371/journal.pone.0214317.
- Barrientos RM, Higgins EA, Biedenkapp JC, et al. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27(5):723–732. doi: 10.1016/j.neurobiolaging.2005.03.010.
- Dantzer R. Cytokine-induced sickness behavior: Where do we stand? Brain Behav Immun. 2001;15(1):7–24. doi: 10.1006/brbi.2000.0613.
- Laye S, Parnet P, Goujon E, et al. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res. 1994;27(1):157–162. doi: 10.1016/0169-328x(94)90197-x.
- Chen Z, Jalabi W, Shpargel KB, et al. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci. 2012;32(34):11706–11715. doi: 10.1523/JNEUROSCI.0730-12.2012.
- Zhao Y, Lukiw WJ. Bacteroidetes neurotoxins and inflammatory neurodegeneration. Mol Neurobiol. 2018;55(12):9100–9107. doi: 10.1007/s12035-018-1015-y.
- Batista CRA, Gomes GF, Candelario-Jalil E, et al. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int J Mol Sci. 2019;20(9):2293–2324. doi: 10.3390/ijms20092293.
- Biesmans S, Meert TF, Bouwknecht JA, et al. Systemic immune activation leads to neuroinflammation and sickness behavior in mice. Mediators Inflamm. 2013; 2013:271359. doi: 10.1155/2013/271359.
- Anaeigoudari A, Shafei MN, Soukhtanloo M, et al. Lipopolysaccharide-induced memory impairment in rats is preventable using 7-nitroindazole. Arq Neuropsiquiatr. 2015;73(9):784–790. doi: 10.1590/0004-282X20150121.
- Czerniawski J, Miyashita T, Lewandowski G, et al. Systemic lipopolysaccharide administration impairs retrieval of context-object discrimination, but not spatial memory: evidence for selective disruption of specific hippocampus-dependent memory functions during acute neuroinflammation. Brain Behav Immun. 2015;44:159–166. doi: 10.1016/j.bbi.2014.09.014.
- Kakizaki Y, Watanobe H, Kohsaka A, et al. Temporal profiles of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in the plasma and hypothalamic paraventricular nucleus after intravenous or intraperitoneal administration of lipopolysaccharide in the rat: estimation by push-pull perfusion. Endocr J. 1999;46(4):487–496. doi: 10.1507/endocrj.46.487.
- Vitkovic L, Konsman JP, Bockaert J, et al. Cytokine signals propagate through the brain. Mol Psychiatry. 2000;5(6):604–615. doi: 10.1038/sj.mp.4000813.
- Ifeoma O, Oluwakanyinsola S. Screening of herbal medicines for potential toxicities. In: Gowler S, editor. New insights into toxicity and drug testing. London: Intech Open; 2013. p. 63–88.
- Bhardwaj S, Gupta D. Study of acute, subacute and chronic toxicity test. Int J Adv Res Pharmaceu BioSci. 2012;2(2):103–129.
- S. Satyapa U, J. Kadam V, Ghosh R. Hepatoprotective activity of livobond a polyherbal formulation against CCl-4 induced hepatotoxicity in rats. Int J Pharmacol. 2008;4(6):472–476. doi: 10.3923/ijp.2008.472.476.
- Burcham PC. An introduction to toxicology. In: Target-organ toxicity: liver and kidney. London: Springer; 2014. p. 151–186.
- Vahalia MK, Thakur KS, Nadkarni S, et al. Chronic toxicity study for Tamra Bhasma (a generic ayurvedic mineral formulation) in laboratory animals. Recent Res Sci Technol. 2011;3(11):76–79.
- Pallavi OD, Vishwaraman M, Prasad T. Preclinical safety assessment of standardized extract of centella asiatica (L.) urban leaves. Toxicol Int. 2015;22(1):10–20.
- Yadav MK, Santosh KS, Manish S, et al. In vivo toxicity study of ethanolic extracts of Evolvulus alsinoides & Centella asiatica in Swiss albino mice. Open Access Maced J Med Sci. 2019;7(7):1071–1076. doi: 10.3889/oamjms.2019.209.
- Chivapat S, Chavalittumrong P, Tantisira MH. Acute and Sub chronic toxicity studies of a standardized extract of Centella asiatica. Thai J Pharm Sci. 2011;35:55–64.
- Pingale SS. Acute toxicity study for Centella asiatica whole plant powder. Newsletter Pharmacologyonline. 2008;3:80–84.
- Chauhan PK, Singh V. Acute and Sub-acute toxicity study of the acetone leaf extract of Centella asiatica in experimental animal models. Asian Pacific J Tropic Biomed. 2012;2(2):S511–S513. doi: 10.1016/S2221-1691(12)60263-9.
- Asare GA, Gyan B, Bugyei K, et al. Toxicity potentials of the nutraceutical Moringa oleifera at super-supplementation levels. J Ethnopharmacol. 2012;139(1):265–272. doi: 10.1016/j.jep.2011.11.009.