Abstract
Purpose
To compare short-term anatomical outcomes observed in optical coherence tomography (OCT) between intravitreal injection (IVI) with anti-vascular endothelial growth factor (VEGF) agent aflibercept (IVA) and subthreshold micropulse laser (SML) therapy in chronic central serous chorioretinopathy (cCSC).
Methods
Thirty-nine eyes of 36 patients with symptomatic cCSC in this retrospective study received either IVA or SML between December 2020 and August 2022. Spectral-domain-OCT (SD-OCT) findings were compared between the two treatment groups in terms of central macular thickness (CMT), serous subretinal fluid (SRF) height, the presence of pigment epithelial detachment (PED) and subretinal hyperreflective foci (HF) at baseline and one-month follow-up visits.
Results
Both groups showed significant reductions in CMT and SRF at one-month follow-up visit. However, there were no statistically significant differences between the IVA and SML groups. Complete SRF resolution was observed in 10 out of 21 and 7 out of 18 eyes in the IVA and SML groups, respectively; however, retinal pigment epithelial (RPE) damage remained persistent in patients with PEDs at baseline.
Conclusions
Both IVA and SML were effective in treating cCSC. IVA and SML treatments had comparable effects in reducing CMT and SRF in eyes with cCSC. Further prospective studies with larger sample sizes and long-term follow-up visits are warranted to identify the long-term efficacy.
KEY MESSAGES
cCSC lead to subsequent irreversible photoreceptor damage and visual complaints.
IVA and SML treatments have comparable effects in reducing CMT and SRF in cCSC eyes.
Introduction
Central serous chorioretinopathy (CSC), the fourth most common retinopathy, can lead to visual complaints such as decreased visual acuity, metamorphopsia, dyschromatopsia, micropsia, macropsia, relative central scotoma, and disturbed contrast vision [Citation1–3]. CSC is characterized by the presence of serous subretinal fluid (SRF, defined as the accumulation of fluid beneath the neurosensory retina), retinal pigment epithelial (RPE) damage, as well as choroidal dysfunctions such as increased choroidal thickness, hyperpermeability, and congestion of choroidal vessels [Citation4]. CSC can be further categorized as acute CSC, if the SRF resolves spontaneously within 4 months and chronic CSC (cCSC) if the SRF persists longer than 4 months [Citation1]. A prolonged presence of SRF with persistent leakage can lead to RPE impairment with pigmentary changes, indicating a more aggressive form of this condition [Citation5,Citation6]. Patients with cCSC are more prone to developing choroidal neovascularization (CNV), with an estimated incidence of 4% to 8% [Citation7,Citation8]. The time of detection of CNV after the initial diagnosis of CSC varies from 1.65 ± 2.30 years to 17.0 ± 10.4 years [Citation9,Citation10]. Treatment is often advised for cases with persistent SRF [Citation1,Citation11]. Various forms of treatment have been reported for CSC, including conventional suprathreshold argon laser photocoagulation, subthreshold micropulse laser (SML), oral administration with mineralocorticoid antagonists eplerenone, intravitreal injection (IVI) with anti-vascular endothelial growth factor (VEGF) agents and photodynamic therapy (PDT).
PDT is a well-established treatment strategy for CSC. By activating photosensitive drugs via laser, PDT creates free oxygen radicals that lead to subsequent choroidal vascular remodelling [Citation12,Citation13], thus reducing choroidal hyperpermeability, which can then mitigate SRF leakage [Citation14]. Mounting evidence suggest that the effect of PDT, even half-dose PDT, is superior to other treatment regimens for CSC, such as 689-nm laser treatment, SML and orally administration with eplerenone [Citation1,Citation15–19]. PDT is commonly used off-label for conditions such as CSC [Citation20]. In line with most countries surveyed by Sirks et al. [Citation21], PDT was also our primary treatment choice for cCSC in China. However, a worldwide shortage of verteporfin (Visudyne®), a photosensitizer used to perform ocular PDT, has occurred since July 2021, rendering the clinical application of PDT in treating CSC difficult or impossible [Citation21]. Therefore, alternative treatment regimens are warranted [Citation22].
Because CSC is related to abnormally dilated choroidal vasculature, IVI with anti-VEGF agents, viz., bevacizumab, ranibizumab, and aflibercept, has been suggested as a possible treatment for CSC by modifying choroidal vascular permeability [Citation1]. IVI with anti-VEGF agents has effects comparable to PDT in CSC eyes with CNV. A comparative study concerning the prognostic outcomes between PDT and IVI administration with different anti-VEGF agents found no statistically significant difference between the two groups [Citation5]. Among the anti-VEGF agents, aflibercept has a stronger effect on choroidal hyperpermeability because of its additional molecular targets (i.e. VEGF-B, placental growth factor) other than VEGF-A [Citation23,Citation24]. Therefore, aflibercept was more effective in reducing the central retinal thickness and SRF than ranibizumab [Citation25].
SML is another treatment option [Citation26,Citation27]. In contrast to conventional suprathreshold argon laser photocoagulation, which is used for extrafoveal leakage, the SML energy is delivered in short pulses and lower total energy allowing its heat dissipation, thus preventing harmful thermal effects and minimizing structural tissue damage. SML is safer for treating subfoveal or juxtafoveal leakage [Citation26].
To date, it has remained unclear whether one treatment is more effective than another between IVI with anti-VEGF agent aflibercept (IVA) and SML. In this retrospective comparative study, we compared the efficacy of IVA and 577-nm SML treatment for cCSC. Using spectral domain-optical coherence tomography (SD-OCT), we compared short-term anatomical outcomes including central macular thickness (CMT), serous subretinal fluid (SRF) height, presence of pigment epithelial detachment (PED), and subretinal hyperreflective foci (HF), between patients treated with IVA versus SML, hoping to shed light on treatment options for cCSC.
Materials and methods
Study population
A total of 36 patients with cCSC were evaluated in this retrospective study. All patients were recruited from the Department of Ophthalmology, Second Xiangya Hospital of Central South University, China. Demographic data, diagnosis, medical history, and baseline fundus photos were obtained for each participant. The retrospective data collection protocol was with the approval of the institutional review board from the Second Xiangya Hospital of Central South University and performed in agreement with the Declaration of Helsinki for research involving human subjects. The Second Xiangya Hospital of Central South University institutional review board waived the requirement for informed consent owing to the retrospective nature of the study. Each patient underwent a complete fundus examination, which included slit lamp ophthalmoscopy with a 90-diopter lens, ultra-widefield fundus photography (Optos Daytona Ultra-Widefield Fundus System, Optos Inc., Marlborough, MA, USA), FA and/or ICGA (Spectralis, Heidelberg Engineering, Germany), and spectral domain-OCT (SD-OCT, Optovue, USA, or Spectralis, Heidelberg, Germany) examinations at baseline and during the treatment follow-up. Angio OCT (OCT-A, Optovue, USA) was performed only in patients with suspicious CNV observed on fundus photography or FA. cCSC was diagnosed based on the presence of typical clinical features and symptoms as well as the presence of SRF with or without pigment epithelial detachment (PED) lasting more than 4 months. Three masked, experienced ophthalmologists (T Zhao, X Guo, and Y Sun) provided the diagnosis based on the examination results.
Inclusion criteria were: 1) age ≥18 years, 2) BCVA of at least 20/200 (Snellen equivalent), 3) presence of persistent SRF (> 4 month) and/or serous PED involving the macular fovea on OCT at the baseline examination, 4) presence of a single or multiple fluorescein leakages from the sites of RPE damage on FA and/or ICGA, 5) RPE window defects with areas of corresponding hyperfluorescence visible on ICGA, and 6) abnormally dilated choroidal vasculature and other features on ICGA. Exclusion criteria comprised: 1) evidence of CNV due to age-related macular degeneration, polypoidal choroidal vasculopathy, pathological myopia or other maculopathy on fundus examination, 2) refractive error ≥ 6.0 dioptres spherical equivalent, 3) the presence of retinopathies other than CSC, 4) prior cataract or retinal surgery (including IVI with anti-VEGF agents) within three months, 5) systemic or topical administration with corticosteroids within the past three months. Patients who received prior SML, conventional suprathreshold argon laser photocoagulation, or eplerenone were also excluded from this study.
Treatment regimens
Among the 39 eyes of 36 patients with cCSC enrolled in our study, 21 underwent IVA and 18 underwent SML. In the IVA group, the operations were performed by two experienced surgeons (Y Sun and X Guo). IVA was performed using a single injection regimen. The IVA procedure was performed as previously described [Citation28]. Briefly, after topical anaesthesia with an anaesthetic drop containing 0.4% oxybuprocaine (Benoxil, Santen Pharmaceutical, Osaka, Japan), 5% povidone-iodine was used to scrub eyelids and lashes, and aflibercept (2.0 mg, Eylea®, Regeneron, Tarrytown, NY, USA,) was injected with a 30-gauge needle through the pars plana into the vitreous cavity.
SML treatments were performed using a 577-nm yellow laser (MicroPulse® IQ577, IRIDEX, Mountain View, CA, USA) by an experienced ophthalmologist (T Zhao). Briefly, before the start of SML, the pupil was dilated with a topical 0.5% tropicamide and 5% phenylephrine hydrochloride mixture (Mydrin-P, Santen Pharmaceutical, Osaka, Japan). Then, an anaesthetic drop containing 0.4% oxybuprocaine (Benoxil, Santen Pharmaceutical, Osaka, Japan) was given. An area centralis lens (Volk Optical, Mentor, OH, USA) was used to visualize the fundus images during SML process. An energy titration process was used to prevent possible damage to the fovea, and SML treatment was performed by applying several adjacent non-overlapping spots. Energy titration was performed in the monospot mode and started at 60 mW in a distance of 2 DD outside the macular fovea. The power was then increased gradually until a light gray retinal spot was visible, and 4-fold of that energy level in micropulse mode was applied. The area to be treated in the macular region was determined based on hyperfluorescent areas on FA/ICGA compatible with SRF on OCT. The whole hyperfluorescent area was covered with a spot diameter of 200 μm, a duty cycle of 5%, an exposure time of 0.2 s and a laser spot spacing with 0.
Information regarding the settings for the IVA and SML was noted, and any adverse events were documented. The postoperative follow-up period was maintained for at least one month in both groups.
Assessment of retinal microstructures observed with optical coherence tomography
All patients underwent macular SD-OCT at baseline and follow-up. Morphological patterns observed with SD-OCT, such as CMT (defined as the distance between the internal limiting membrane and the ellipsoid zone on OCT), the height of the SRF (defined as the distance between the outer border of the sensory retina and the inner border of the RPE at the central fovea), the presence of PED and HFs, and the formation of CNV were carefully evaluated and recorded, as previously described [Citation29–31]. HFs were defined as discrete and punctiform hyperreflective focal lesions with reflectivity identical to that of the RPE signal [Citation32,Citation33]. RPE defect observed within the subretinal detachment area on OCT was classified into three types, as previously described [Citation32,Citation33]: regular PED (dome-shaped elevation of the RPE with hyporeflective content), RPE bump (shallow irregular elevation of the RPE without a hyporeflective area inside), and irregular PED (irregular elevation of the RPE with hyporeflective content).
Statistical analysis
All considered variables were checked for normal assumption using the Shapiro–Wilk test before statistical analysis. Means and standard deviations (SD) were computed for continuous variables, whereas percentages were computed for categorical variables. For comparison within one group, viz., CMT/SRF before and after IVA/SML, a paired-samples t-test was used to analyze differences in case of normal distribution, otherwise, a non-parametric Wilcoxon signed-ranks test was chosen for the comparison. For comparison between the two groups, an independent sample t-test was used to analyze differences in the case of the normal distribution; otherwise, a non-parametric Mann-Whitney test was chosen for the comparison. A p-value<0.05 was considered significant. Data were analyzed using SPSS 21.0 for Windows (IBM, Armonk, NY, USA).
Results
A total of 39 eyes of 36 patients met the study criteria and were enrolled in this retrospective study, of which 21 eyes of 19 patients received IVA and 18 eyes of 17 patients received SML treatment. Six of 21 eyes in the IVA group, and 6 of 18 eyes in the SML group combined with PED. One eye with CNV in the IVA group was evidenced by FA, ICGA and OCT-A. The demographics and clinical characteristics of the two groups are summarized in and Citation2. There were no significant age-based differences between groups (47.37 ± 8.88 vs. 47.00 ± 7.30 years old, p = 0.893 by independent sample t-test). No sex-based differences were present (7 and 3 females in the IVA and SML groups, respectively, p = 0.274 by Fisher’s exact test) at baseline. depicts a representative case of a patient with bilateral cCSC.
Figure 1. Example of a 53-year-old male patient with bilateral chronic central serous chorioretinopathy (cCSC). (A–E) Multimodal imaging at baseline from the affected right eye. (A) Fundus photography showed serous detachment at macular area with diffuse atrophic RPE in the right eye. (B) Spectral domain optical coherence tomography (SD-OCT) scan at baseline from the right eye showing presence of subretinal fluid (SRF) with serous pigment epithelial detachment (PED, arrows). Presence of posterior cystoid retinal degeneration (arrowhead), pachychoroid with dilated vessels in haller’s layer (asterisks) can also be visible. (C–E) Early-, intermediate- and late-phase images from fluorescein angiography (FA, left panel) and indocyanine green angiography (ICGA, right panel). (C,D) Diffuse hyperfluorescent areas due to increased transmission of the choroidal fluorescence as well as hyperfluorescent areas due to window defects, staining and accumulation were visible in the early and intermediate phase of FA. (E) Maintenance of hyperfluorescence in areas of dye staining and pooling was visible in the late phase of FA. In the early phase of ICGA, dilated choroidal vessels can be observed (arrow). In the mid-phase of ICGA, geographic areas of hyperfluorescence with blurred contours due to choroidal vascular hyperpermeability was visible, areas of RPE atrophy appeared hypofluorescent (D). Such mid-phase hyperfluorescent areas was washed-out, forming a hyporfluorescent with a blurred hyperfluorescent rings (E). (F–J) Multimodal imaging at baseline from the left eye affected by secondary choroidal neovascularization (CNV). (F) Fundus photography showed serous sub-macular detachment with pigmentary abnormalities in the left eye. (G) SD-OCT B-scan centred to the fovea showing the flat irregular PED with sub-RPE hyperreflectivity (double layer sign). (H–J) Early-, intermediate- and late-phase images from FA (left panel) and ICGA (right panel) showed hyperfuorescence at macula.
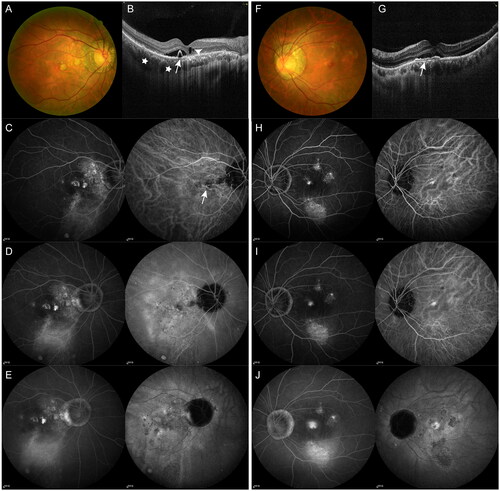
Table 1. Baseline demographics characteristics of patients that underwent intravitreal injection of aflibercept or subthreshold micropulse laser for chronic central serous chorioretinopathy.
Table 2. OCT characteristics of eyes that underwent intravitreal injection of aflibercept or subthreshold micropulse laser for chronic central serous chorioretinopathy at baseline and one-month follow-up visit.
Changes of central macular thickness and serous subretinal fluid in optical coherence tomography
There were no significant differences in CMT and SRF values between the two groups at baseline (p = 0.494 and p = 0.282, respectively, by Mann-Whitney test). At one-month visit, CMT reduced significantly to 221.62 ± 80.85 μm in the IVA group, and 257.42 ± 73.57 μm in the SML group. Both groups showed significant improvements in the reduction of CMT (p<0.001 by Wilcoxon signed ranks test). Both groups also showed significant improvements in the reduction of SRF (p<0.001 by Wilcoxon signed-ranks test). SRF reduced significantly to 56.74 ± 62.00 μm and 98.28 ± 74.55 μm in IVA and SML groups, respectively. However, the reduction of CMT and SRF between the two groups showed no significant differences (p = 0.813 and 0.945, respectively, Mann-Whitney test). Nine eyes of eight patients in the IVA group showed complete SRF resolution, whereas five eyes of five patients in the SML group achieved complete SRF resolution at a one-month visit.
In those patients combined with PED at baseline, small PED were discernible, and persistent RPE abnormalities were visible, even in cases in which complete reabsorption of the SRF had been achieved. and depict representative cases of the changes in SRF and PED after IVA. and depict representative cases of the changes in SRF and PED after SML.
Figure 2. Examples of the change in serous subretinal fluid (SRF) with spectral domain optical coherence tomography (SD-OCT) in patients with chronic central serous chorioretinopathy (cCSC) after intravitreal injections with aflibercept (IVA). The left panel represents SD-OCT B-scan images at baseline visit, the right panel represents SD-OCT B-scan images at one-month follow-up visit after IVA. (A) SD-OCT B-scan at baseline showed sub-macular SRF and irregularly appeared RPE from the right eye of a 65-year-old male patient (left). Hyperreflective foci (HFs) are visible between the ellipsoid zone and RPE (arrow). At the one-month visit after IVA treatment, SRF were resolved, however, disruptions in the ellipsoid zone remained (right). (B) SD-OCT B-scan at baseline showed sub-macular SRF from the right eye of a 49-year-old male patient (left). At the one-month visit after IVA treatment, SRF were completely resolved, the ellipsoid zone and RPE are intact (right). (C) SD-OCT B-scan at baseline showed presence of SRF and irregularly appeared RPE from the right eye of a 56-year-old male patient (left). The HFs are visible between the ellipsoid zone and RPE (arrow). At the one-month visit after IVA treatment, SRF were partially resolved. The presence of HFs remained, with disruptions in the ellipsoid zone and irregularly thickened RPE (right).
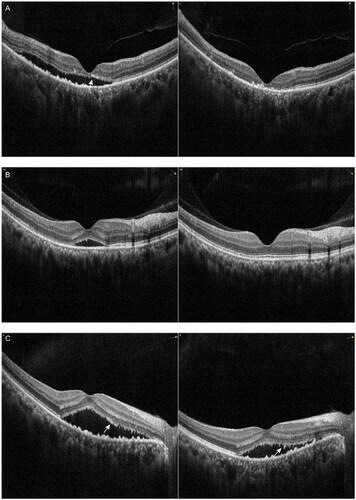
Figure 3. Examples of the change in pigment epithelial detachment (PED) with spectral domain optical coherence tomography (SD-OCT) in patients with chronic central serous chorioretinopathy (cCSC) after intravitreal injections with aflibercept (IVA). The left panel represents SD-OCT B-scan images at baseline visit, the right panel represents SD-OCT B-scan images at one-month follow-up visit after IVA. (A) SD-OCT scan at baseline from the right eye of the patient depicted in (left). At the one-month visit after IVA treatment, subretinal fluid (SRF) was completely absorbed, small PED in the temporal macula was resolved. The large dome-shaped PED height remained relatively stable and small posterior cystoid retinal degeneration and dilated choroidal vessels were visible (right). (B) SD-OCT B-scan at baseline showed sub-macular SRF and a small PED from the right eye of a 31-year-old female patient (left). Presence of RPE micro-rip at the peak of PED was visible (arrow). At the one-month visit, SRF as well as PED were resolved, undulating RPE was visible subfoveally (right). (C) SD-OCT B-scan at baseline showed sub-macular SRF and a small PED from the right eye of a 44-year-old male patient (left). At the one-month visit (right), SRF was partially absorbed, small PED was resolved, a discontinuous RPE was visible, likely representing the leakage spot in RPE (arrow).
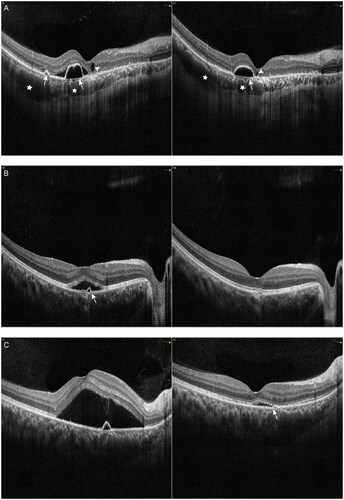
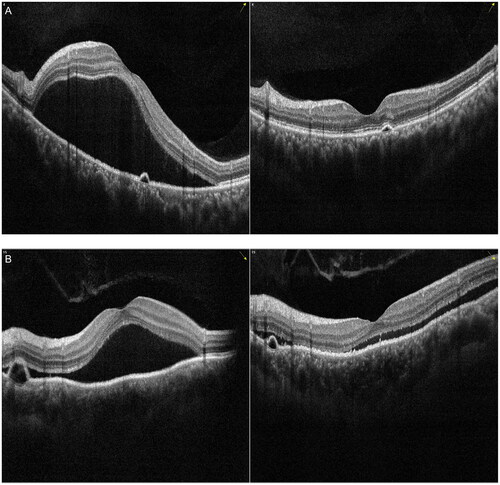
Figure 4. Examples of the change in serous subretinal fluid (SRF) with spectral domain optical coherence tomography (SD-OCT) in patients with chronic central serous chorioretinopathy (cCSC) after subthreshold micropulse laser (SML). The left panel represents SD-OCT B-scan images at baseline visit, the right panel represents SD-OCT B-scan images at one-month follow-up visit after SML. (A) SD-OCT B-scan at baseline showed sub-macular SRF from the left eye of a 39-year-old male patient (left). At the one-month visit after SML treatment, SRF were completely resolved, the ellipsoid zone and RPE were intact (right). (B) SD-OCT B-scan at baseline showed sub-macular SRF from the left eye of a 43-year-old male patient (left). At the one-month visit, SRF were completely resolved, however, disruptions in the ellipsoid zone can be visible (arrow) (right). (C) SD-OCT B-scan at baseline showed presence of SRF and irregularly appeared RPE from the left eye of a 52-year-old male patient (left). The HFs were visible between the ellipsoid zone and RPE. Small irregular PED was visible (arrowhead). At the one-month visit, SRF were resolved, however, discontinuous ellipsoid zone was visible (arrow), the disruptions in RPE remained visible (arrowhead) (right).
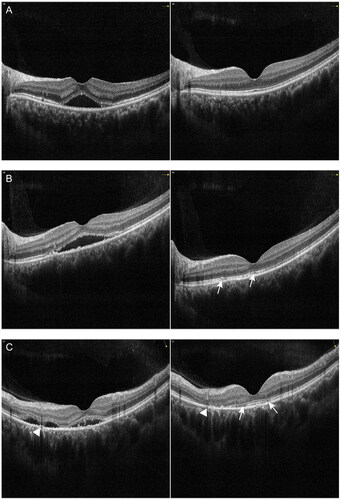
Figure 5. Examples of the change in pigment epithelial detachment (PED) with spectral domain optical coherence tomography (SD-OCT) in patients with chronic central serous chorioretinopathy (cCSC) after subthreshold micropulse laser (SML). The left panel represents SD-OCT B-scan images at baseline visit, the right panel represents SD-OCT B-scan images at one-month follow-up visit after SML. (A) SD-OCT B-scan at baseline showed sub-macular SRF and a small PED from the left eye of a 41-year-old male patient (left). SRF was completely absorbed, small PED remained visible with a discontinuous ellipsoid zone at one-month visit (right). (B) SD-OCT B-scan at baseline showed sub-macular SRF and a dome-shaped PED from the left eye of a 48-year-old female patient (left). At the one-month visit, SRF as well as PED were partially resolved (right).
Changes of subretinal hyperreflective foci in optical coherence tomography
Subretinal HFs at the leakage site were found to be positively associated with recurrences and can be used as a predictive factor for the course of anatomical and functional recovery in cCSC [Citation32,Citation33]. We also evaluated changes in subretinal HFs using OCT. At the baseline visit, HFs in the subretinal space were present in 18 of 21 eyes in the IVA group and 16 of 18 eyes in the SML group. At the one-month follow-up visit, subretinal HFs in six and three eyes had completely disappeared in the IVA and SML groups, respectively, along with complete SRF resolution.
Safety of the treatments
No systemic or ocular adverse events associated with treatment were observed. No new-onset RPE atrophy or CNV was observed during follow-up visits.
Discussion
Although the underlying pathogenesis remained unclear, CSC is considered a pachychoroid (choroidal thickening) spectrum disease. Choroidal abnormalities are believed to be the primary underlying pathophysiology [Citation1] Choroidal abnormalities, including choroidal thickening and hyperpermeability, venous congestion and leakage, together with increased hydrostatic pressure, result in impaired RPE function [Citation34]. In cCSC, RPE damage results from persistent SRF, leading to irreversible photoreceptor damage [Citation1–3,Citation35].
Inhibiting VEGF exerts an anti-proliferative and anti-hyperpermeability effect on choroidal endothelial cells [Citation36]. In addition, inhibiting VEGF has an inhibitory effect on leakage and fibrovascular proliferation, decreasing choroidal blood flow, and reducing central choroidal thickness [Citation37–39]. Intriguingly, previous studies indicated a reduced plasma VEGF level in both acute and chronic CSC, suggesting that VEGF-independent arteriogenesis rather than angiogenesis may underlie vascular abnormalities in CSC [Citation22,Citation40,Citation41]. In contrast, elevated levels of pro-inflammatory cytokines were observed in CSC and were correlated with mean choroidal thickness and choroidal vessel abnormalities [Citation22,Citation42]. These results suggest that anti-VEGF agents may partly exert anti-inflammatory effects in the treatment of CSC.
The effect of SML treatment has been suggested to modulate both the production and release of vasoactive cytokines and to increase the expression of heat shock proteins by targeting the RPE without causing visible damage to the retina [Citation1,Citation43]. In this case, SML may exert an anti-inflammatory effect, to some extent, in a similar manner as IVI-VEGF agents in treating CSC. The energy delivered by the 577-nm SML, which is absorbed by both melanin and oxyhaemoglobin, increases its absorption in the RPE and choriocapillaris rather than in the inner and outer cluster layers of the macula [Citation44]. Moreover, the SML energy delivered in short pulses allows heat dissipation to prevent thermal damage; therefore, the 577-nm SML is safer for treating subfoveal leakage [Citation26,Citation45]. Previous studies have revealed a favourable efficiency of 577-nm SML in treating either acute and chronic CSC [Citation27,Citation46]. However, SML was suggested to be less efficient than PDT [Citation15,Citation16,Citation47], and SML treatment often did not provide the desired treatment effect in reducing SRF volume [Citation48] and PED resolution [Citation49].
In our study, all patients who received either IVA or SML showed a favourable aresponse. Overall, we observed significantly reduced CMT and SRF values. The mean reduction in CMT was 174.52 ± 161.67 μm and 193.81 ± 218.31 μm for IVA and SML groups at one month visit, respectively. 30% and 16.7% of eyes had complete reabsorption of SRF in IVA and SML groups, respectively. In contrast to our study, previous studies reported complete resolution of SRF in 33–75% after the SML treatment [Citation50–53]. This less favourable outcome in our study is potentially attributed to the short-term post-treatment follow-up time, as well as the severity of the disease in our patients with long-term persistent chronic retinal and choroidal changes.
Damage to the outer blood-retina barrier, which is formed by RPE, causes SRF accumulation under the retina, resulting in serous retinal detachment [Citation34]; therefore, it is important to evaluate the effect of treatment on PEDs in cCSC [Citation1,Citation15]. In our study, none of the patients with PED at baseline achieved complete resolution, even in cases with complete SRF reabsorption. This less favourable outcome in our study may be attributed to chronic choroidal damage. PEDs observed on OCT were reported present in 70% to 100% of the affected eyes of CSC patients [Citation54]. PEDs in CSC result from underlying choroidal dysfunction [Citation34,Citation55,Citation56]. A previous study reported that half-dose PDT was superior to SML treatment in decreasing the height and inducing complete resolution of macular PEDs in cCSC [Citation49]. The superiority of half-dose PDT over SML is because PDT primarily targets the choroidal vasculature, which is thought to play a primary role in cCSC [Citation1]. Significant increase in deep capillary plexus and choriocapillaris perfusion, as well as the decrease of the luminal areas after half-dose and half-fluence PDT in patients with cCSC, were evidenced by OCT-A [Citation57–59]. In contrast, the SML only targets the RPE without addressing choroidal dysfunction [Citation60].
The present study had several limitations. First, the sample size was relatively small, and a study with larger samples is needed with more detailed medical records to delineate the comparative results of efficiency and safety between IVA and SML treatments for cCSC. Another limitation is that it is impossible to compare the levels of systemic biochemical parameters with the multimodal imaging characteristics before and after these two treatment regimens because of the retrospective nature of our study. A previous study using plasma samples from patients with cCSC revealed elevated levels of pro-inflammatory cytokines [Citation22]. We still need to clarify whether the levels of plasma cytokines can provide a readout for the systemic inflammation status and be associated with the prognosis under the various treatment strategies, and help to choose better personalized management of cCSC in the future. Clinical trials with a valid measure of prognosis (such as vision-related outcomes and structural alterations in OCT) based on baseline significantly differential cytokines are needed to clarify this issue. Third, a case-matched control arm who did not receive any treatments was not included in this study due to the severity of the disease in our patients whose SRF persist with a minimum duration of 4 months. In chronic CSC, even a small amount of remaining SRF can lead to irreversible RPE changes, photoreceptor damage, and persistent vision loss [Citation1,Citation8,Citation61–63], thus, in the case of persistent SRF, treatment is often advised [Citation1,Citation11]. Further prospective studies with larger cohorts and control subject group are warranted.
In conclusion, we demonstrated a good short-term anatomic outcome for patients receiving either IVA therapy or SML treatment for cCSC. Further prospective studies with larger sample sizes and long-term follow-up visits are necessary to identify the efficiency of achieving complete resolution of PED and complete reabsorption of SRF in both treatment strategies.
Author contributions
Tantai Zhao: Conceptualization, Methodology, Investigation, Writing – original draft, Visualization, Funding acquisition. Jiani Li: Investigation, Writing – original draft, formal analysis. Yanbin Wang: Software, Formal analysis. Xiaojian Guo: Investigation, Validation, Formal analysis. Yun Sun: Conceptualization, Methodology, Writing – review and editing, project administration, Supervision, Funding acquisition.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting the findings of this study are available from the corresponding author, YS, upon reasonable request.
Additional information
Funding
References
- van Rijssen TJ, van Dijk EHC, Yzer S, et al. Central serous chorioretinopathy: towards an evidence-based treatment guideline. Prog Retin Eye Res. 2019;73:1. doi: 10.1016/j.preteyeres.2019.07.003.
- van Dijk EHC, Boon CJF. Serous business: delineating the broad spectrum of diseases with subretinal fluid in the macula. Prog Retin Eye Res. 2021;84:100955. doi: 10.1016/j.preteyeres.2021.100955.
- Spaide RF, Gemmy Cheung CM, Matsumoto H, et al. Venous overload choroidopathy: a hypothetical framework for central serous chorioretinopathy and allied disorders. Prog Retin Eye Res. 2022;86:100973. doi: 10.1016/j.preteyeres.2021.100973.
- Wang M, Munch IC, Hasler PW, et al. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86(2):126–13. doi: 10.1111/j.1600-0420.2007.00889.x.
- Peiretti E, Caminiti G, Serra R, et al. Anti-Vascular endothelial growth factor therapy versus photodynamic therapy in the treatment of choroidal neovascularization secondary to Central serous chorioretinopathy. Retina. 2018;38(8):1526–1532. doi: 10.1097/IAE.0000000000001750.
- Pitcher JD, 3rd, Witkin AJ, DeCroos FC, et al. A prospective pilot study of intravitreal aflibercept for the treatment of chronic Central serous chorioretinopathy: the contain study. Br J Ophthalmol. 2015;99(6):848–852. doi: 10.1136/bjophthalmol-2014-306018.
- Bandello F, Virgili G, Lanzetta P, et al. ICG angiography and retinal pigment epithelial decompensation (CRSC and epitheliopathy). J Fr Ophtalmol. 2001;24:448–451.
- Loo RH, Scott IU, Flynn HW, Jr., et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002;22(1):19–24. doi: 10.1097/00006982-200202000-00004.
- Kim RY, Ma GJ, Park WK, et al. Clinical course after the onset of choroidal neovascularization in eyes with central serous chorioretinopathy. Medicine. 2021;100(34):e26980. doi: 10.1097/MD.0000000000026980.
- Mrejen S, Balaratnasingam C, Kaden TR, et al. Long-term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology. 2019;126(4):576–588. doi: 10.1016/j.ophtha.2018.12.048.
- Mohabati D, van Dijk EH, van Rijssen TJ, et al. Clinical spectrum of severe chronic Central serous chorioretinopathy and outcome of photodynamic therapy. Clin Ophthalmol. 2018;12:2167–2176. doi: 10.2147/OPTH.S174573.
- Kwiatkowski S, Knap B, Przystupski D, et al. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098–1107. doi: 10.1016/j.biopha.2018.07.049.
- Chan WM, Lam DS, Lai TY, et al. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003;87(12):1453–1458. doi: 10.1136/bjo.87.12.1453.
- Schmidt-Erfurth U, Hasan T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv Ophthalmol. 2000;45(3):195–214. doi: 10.1016/s0039-6257(00)00158-2.
- van Dijk EHC, Fauser S, Breukink MB, et al. Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: the PLACE trial. Ophthalmology. 2018;125(10):1547–1555. doi: 10.1016/j.ophtha.2018.04.021.
- van Rijssen TJ, van Dijk EHC, Scholz P, et al. Crossover to photodynamic therapy or micropulse laser after failure of primary treatment of chronic central serous chorioretinopathy: the REPLACE trial. Am J Ophthalmol. 2020;216:80–89. doi: 10.1016/j.ajo.2020.04.007.
- Lotery A, Sivaprasad S, O’Connell A, et al. Eplerenone for chronic central serous chorioretinopathy in patients with active, previously untreated disease for more than 4 months (VICI): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395(10220):294–303. doi: 10.1016/S0140-6736(19)32981-2.
- van Rijssen TJ, van Dijk EHC, Tsonaka R, et al. Half-Dose photodynamic therapy versus eplerenone in chronic Central serous chorioretinopathy (SPECTRA): a randomized controlled trial. Am J Ophthalmol. 2022;233:101–110. doi: 10.1016/j.ajo.2021.06.020.
- Russo A, Turano R, Morescalchi F, et al. Comparison of half-dose photodynamic therapy and 689 nm laser treatment in eyes with chronic Central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255(6):1141–1148. doi: 10.1007/s00417-017-3626-9.
- van Dijk EHC, van Rijssen TJ, Subhi Y, et al. Photodynamic therapy for chorioretinal diseases: a practical approach. Ophthalmol Ther. 2020;9(2):329–342. doi: 10.1007/s40123-020-00250-0.
- Sirks MJ, van Dijk EHC, Rosenberg N, et al. Clinical impact of the worldwide shortage of verteporfin (visudyne(R)) on ophthalmic care. Acta Ophthalmol. 2022;100(7):e1522–e1532.
- Karska-Basta I, Pociej-Marciak W, Chrzaszcz M, et al. Altered plasma cytokine levels in acute and chronic Central serous chorioretinopathy. Acta Ophthalmol. 2021;99(2):e222–e231. doi: 10.1111/aos.14547.
- Hata M, Oishi A, Tsujikawa A, et al. Efficacy of intravitreal injection of aflibercept in neovascular age-related macular degeneration with or without choroidal vascular hyperpermeability. Invest Ophthalmol Vis Sci. 2014;55(12):7874–7880. doi: 10.1167/iovs.14-14610.
- Julien S, Biesemeier A, Taubitz T, et al. Different effects of intravitreally injected ranibizumab and aflibercept on retinal and choroidal tissues of monkey eyes. Br J Ophthalmol. 2014;98(6):813–825. doi: 10.1136/bjophthalmol-2013-304019.
- Schworm B, Luft N, Keidel LF, et al. Response of neovascular central serous chorioretinopathy to an extended upload of anti-VEGF agents. Graefes Arch Clin Exp Ophthalmol. 2020;258(5):1013–1021. doi: 10.1007/s00417-020-04623-w.
- Scholz P, Altay L, Fauser S. Comparison of subthreshold micropulse laser (577 nm) treatment and half-dose photodynamic therapy in patients with chronic central serous chorioretinopathy. Eye (Lond). 2016;30(10):1371–1377. doi: 10.1038/eye.2016.142.
- Long H, Liu M, Hu Q, et al. 577 nm subthreshold micropulse laser treatment for acute central serous chorioretinopathy: a comparative study. BMC Ophthalmol. 2022;22(1):105. doi: 10.1186/s12886-022-02330-0.
- Smretschnig E, Hagen S, Glittenberg C, et al. Intravitreal anti-vascular endothelial growth factor combined with half-fluence photodynamic therapy for choroidal neovascularization in chronic central serous chorioretinopathy. Eye (Lond). 2016;30(6):805–811. doi: 10.1038/eye.2016.41.
- Sulzbacher F, Schutze C, Burgmuller M, et al. Clinical evaluation of neovascular and non-neovascular chronic Central serous chorioretinopathy (CSC) diagnosed by swept source optical coherence tomography angiography (SS OCTA). Graefes Arch Clin Exp Ophthalmol. 2019;257(8):1581–1590. doi: 10.1007/s00417-019-04297-z.
- Hata M, Oishi A, Shimozono M, et al. Early changes in foveal thickness in eyes with Central serous chorioretinopathy. Retina. 2013;33(2):296–301. doi: 10.1097/IAE.0b013e31826710a0.
- Davoudi S, Papavasileiou E, Roohipoor R, et al. Optical coherence tomography characteristics of macular edema and hard exudates and their association with lipid serum levels in type 2 diabetes. Retina. 2016;36(9):1622–1629. doi: 10.1097/IAE.0000000000001022.
- Lee H, Lee J, Chung H, et al. Baseline spectral domain optical coherence tomographic hyperreflective foci as a predictor of visual outcome and recurrence for central serous chorioretinopathy. Retina. 2016;36(7):1372–1380. doi: 10.1097/IAE.0000000000000929.
- Matet A, Daruich A, Zola M, et al. Risk factors for recurrences of central serous chorioretinopathy. Retina. 2018;38(7):1403–1414. doi: 10.1097/IAE.0000000000001729.
- Daruich A, Matet A, Dirani A, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118. doi: 10.1016/j.preteyeres.2015.05.003.
- Cheung CMG, Lee WK, Koizumi H, et al. Pachychoroid disease. Eye (Lond). 2019;33(1):14–33. doi: 10.1038/s41433-018-0158-4.
- Peters S, Julien S, Heiduschka P, et al. Antipermeability and antiproliferative effects of standard and frozen bevacizumab on choroidal endothelial cells. Br J Ophthalmol. 2007;91(6):827–831. doi: 10.1136/bjo.2006.109702.
- Koizumi H, Kano M, Yamamoto A, et al. Subfoveal choroidal thickness during aflibercept therapy for neovascular age-related macular degeneration: twelve-month results. Ophthalmology. 2016;123(3):617–624. doi: 10.1016/j.ophtha.2015.10.039.
- Nourinia R, Ahmadieh H, Nekoei E, et al. Changes in central choroidal thickness after treatment of diabetic macular edema with intravitreal bevacizumab correlation with central macular thickness and best-corrected visual acuity. Retina. 2018;38(5):970–975. doi: 10.1097/IAE.0000000000001645.
- Roohipoor R, Sharifian E, Ghassemi F, et al. Choroidal thickness changes in proliferative diabetic retinopathy treated with panretinal photocoagulation versus panretinal photocoagulation with intravitreal bevacizumab. Retina. 2016;36(10):1997–2005. doi: 10.1097/IAE.0000000000001027.
- Chrząszcz M, Pociej-Marciak W, Żuber-Łaskawiec K, et al. Changes in plasma VEGF and PEDF levels in patients with Central serous chorioretinopathy. Medicina. 2021;57(10):1063. doi: 10.3390/medicina57101063.
- Karska-Basta I, Pociej-Marciak W, Chrzaszcz M, et al. Imbalance in the levels of angiogenic factors in patients with acute and chronic Central serous chorioretinopathy. J Clin Med. 2021;10(5):1087.
- Terao N, Koizumi H, Kojima K, et al. Association of upregulated angiogenic cytokines with choroidal abnormalities in chronic Central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2018;59(15):5924–5931. doi: 10.1167/iovs.18-25517.
- Yu AK, Merrill KD, Truong SN, et al. The comparative histologic effects of subthreshold 532- and 810-nm diode micropulse laser on the retina. Invest Ophthalmol Vis Sci. 2013;54(3):2216–2224. doi: 10.1167/iovs.12-11382.
- Mainster MA. Wavelength selection in macular photocoagulation. Tissue optics, thermal effects, and laser systems. Ophthalmology. 1986;93(7):952–958. doi: 10.1016/s0161-6420(86)33637-6.
- Scholz P, Altay L, Fauser S. A review of subthreshold micropulse laser for treatment of macular disorders. Adv Ther. 2017;34(7):1528–1555. doi: 10.1007/s12325-017-0559-y.
- Kim JY, Park HS, Kim SY. Short-term efficacy of subthreshold micropulse yellow laser (577-nm) photocoagulation for chronic Central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253(12):2129–2135. doi: 10.1007/s00417-015-2965-7.
- van Rijssen TJ, Hahn LC, van Dijk EHC, et al. RESPONSE oF CHOROIDAL ABNORMALITIES tO PHOTODYNAMIC THERAPY vERSUS MICROPULSE LASER in CHRONIC Central SEROUS CHORIORETINOPATHY: place trial report no. 4. Retina. 2021;41(10):2122–2131. doi: 10.1097/IAE.0000000000003157.
- Subhi Y, Bjerager J, Boon CJF, et al. Subretinal fluid morphology in chronic Central serous chorioretinopathy and its relationship to treatment: a retrospective analysis on PLACE trial data. Acta Ophthalmol. 2022;100(1):89–95. doi: 10.1111/aos.14901.
- Feenstra HMA, Hahn LC, van Rijssen TJ, et al. Efficacy of Half-Dose photodynamic therapy versus High-Density subthreshold micropulse laser for treating pigment epithelial detachments in chronic Central serous chorioretinopathy. Retina. 2022;42(4):721–729. doi: 10.1097/IAE.0000000000003363.
- Gupta B, Elagouz M, McHugh D, et al. Micropulse diode laser photocoagulation for Central serous chorio-retinopathy. Clin Exp Ophthalmol. 2009;37(8):801–805. doi: 10.1111/j.1442-9071.2009.02157.x.
- Lavinsky D, Palanker D. Nondamaging photothermal therapy for the retina: initial clinical experience with chronic Central serous retinopathy. Retina. 2015;35(2):213–222. doi: 10.1097/IAE.0000000000000340.
- Koss MJ, Beger I, Koch FH. Subthreshold diode laser micropulse photocoagulation versus intravitreal injections of bevacizumab in the treatment of Central serous chorioretinopathy. Eye. 2012;26(2):307–314. doi: 10.1038/eye.2011.282.
- Yadav NK, Jayadev C, Mohan A, et al. Subthreshold micropulse yellow laser (577 nm) in chronic Central serous chorioretinopathy: safety profile and treatment outcome. Eye (Lond). 2015;29(2):258–264; quiz 265. doi: 10.1038/eye.2014.315.
- Song IS, Shin YU, Lee BR. Time-periodic characteristics in the morphology of idiopathic Central serous chorioretinopathy evaluated by volume scan using spectral-domain optical coherence tomography. Am J Ophthalmol. 2012;154(2):366–375.e4. e364. doi: 10.1016/j.ajo.2012.02.031.
- Yang L, Jonas JB, Wei W. Optical coherence tomography-assisted enhanced depth imaging of Central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2013;54(7):4659–4665. doi: 10.1167/iovs.12-10991.
- Mrejen S, Sarraf D, Mukkamala SK, et al. Multimodal imaging of pigment epithelial detachment: a guide to evaluation. Retina. 2013;33(9):1735–1762. doi: 10.1097/IAE.0b013e3182993f66.
- Demirel S, Ozcan G, Yanik O, et al. Vascular and structural alterations of the choroid evaluated by optical coherence tomography angiography and optical coherence tomography after half-fluence photodynamic therapy in chronic Central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2019;257(5):905–912. doi: 10.1007/s00417-018-04226-6.
- Karasu B, Akbas YB, Aykut A, et al. Comparison of retinochoroidal vascular and structural changes after Half-Dose photodynamic therapy versus Half-Fluence photodynamic therapy based on optical coherence tomography angiography in eyes with chronic Central serous chorioretinopathy. Ophthalmologica. 2022;245(4):323–334. doi: 10.1159/000523704.
- Christou EE, Stavrakas P, Kozobolis V, et al. Evaluation of the choriocapillaris after photodynamic therapy for chronic central serous chorioretinopathy. A review of optical coherence tomography angiography (OCT-A) studies. Graefes Arch Clin Exp Ophthalmol. 2022;260(6):1823–1835. doi: 10.1007/s00417-022-05563-3.
- van Rijssen TJ, van Dijk EHC, Scholz P, et al. Long-term follow-up of chronic Central serous chorioretinopathy after successful treatment with photodynamic therapy or micropulse laser. Acta Ophthalmol. 2021;99(7):805–811. doi: 10.1111/aos.14775.
- Wang MS, Sander B, Larsen M. Retinal atrophy in idiopathic central serous chorioretinopathy. Am J Ophthalmol. 2002;133(6):787–793. doi: 10.1016/s0002-9394(02)01438-1.
- Levine R, Brucker AJ, Robinson F. Long-term follow-up of idiopathic Central serous chorioretinopathy by fluorescein angiography. Ophthalmology. 1989;96(6):854–859. doi: 10.1016/s0161-6420(89)32810-7.
- Wong R, Chopdar A, Brown M. Five to 15 year follow-up of resolved idiopathic Central serous chorioretinopathy. Eye (Lond). 2004;18(3):262–268. doi: 10.1038/sj.eye.6700637.
