Abstract
AURKA is a threonine or serine kinase that needs to be activated by TPX2, Bora and other factors. AURKA is located on chromosome 20 and is amplified or overexpressed in many human cancers, such as breast cancer. AURKA regulates some basic cellular processes, and this regulation is realized via the phosphorylation of downstream substrates. AURKA can function in either the cytoplasm or the nucleus. It can promote the transcription and expression of oncogenes together with other transcription factors in the nucleus, including FoxM1, C-Myc, and NF-κB. In addition, it also sustains carcinogenic signaling, such as N-Myc and Wnt signaling. This article will focus on the role of AURKA in the nucleus and its carcinogenic characteristics that are independent of its kinase activity to provide a theoretical explanation for mechanisms of resistance to kinase inhibitors and a reference for future research on targeted inhibitors.
KEY MESSAGES
AURKA plays an important role in the control of the proliferation, invasion, cell cycle regulation and self-renewal of cancer stem cells.
Small molecule kinase inhibitors targeting AURKA have been developed, but the overall response rate of patients in clinical trials is not ideal, prompting us to pay attention to the non-kinase activity of AURKA.
This review focuses on the nuclear function of AURKA and its oncogenic properties independent of kinase activity, demonstrating that the nuclear substrate of AURKA and the remote allosteric site of the kinase may be targets of anticancer therapy.
1. Introduction
AURKA is a threonine or serine kinase. Researchers first found a lineal homolog of AURKA, a protein called Ipl1, in germinating yeast [Citation1]. Since then, Ipl1 has been identified in Xenopus laevis, Drosophila melanogaster and Caenorhabditis elegans, and has been identified to regulate centrosome stability and maintain mitotic spindle formation [Citation2–4]. Studies further found that three orthologous proteins of Ipl1, namely, the Aurora kinases AURKA, AURKB and AURKC, exist in mammals. Overexpression of these proteins has been identified in several cancers; this phenotype is closely related to amplification of the AURKA gene on chromosome 20q13 [Citation5], and AURKA overexpression is found in skin, breast, ovarian, colon and other tumors [Citation6–11].
Given that AURKA has been found to be aberrantly expressed in a variety of tumors since its discovery, it was identified as an oncogene [Citation12]. High levels of AURKA are associated with malignant tumor proliferation [Citation13], invasion and metastasis [Citation14], splicing disorders [Citation15], epithelial-mesenchymal transformation [Citation16], radioresistance [Citation17], chemotherapy resistance [Citation18] and immune escape [Citation19]. Therefore, small molecule kinase inhibitors targeting AURKA have been successively developed. Although they have shown certain effects in preclinical studies [Citation20, Citation21], their efficacy in clinical trials is poor [Citation22]. The reason for this is that the understanding of AURKA itself is not comprehensive, and simply blocking AURKA kinase activity is usually not enough to achieve maximum therapeutic efficacy [Citation23, Citation24]. Many studies have focused on the way in which nuclear AURKA, rather than cytoplasmic AURKA, enhances the carcinogenic characteristics of tumors by activating Myc [Citation19, Citation25], HIF1 [Citation26], Ras [Citation27], and so on.
Here, we mainly focus on research related to the nuclear function of AURKA, starting with resistance to targeted inhibitors of AURKA kinase, review the research on the carcinogenic effects of AURKA that are independent of its kinase activity, and explain the relationship between AURKA spatial distribution and its carcinogenic properties, with the aim of improving the understanding of the unique characteristics of AURKA in relation to cancer. In particular, we hope to provide a deeper understanding of the unique dynamics, which will be a valuable reference for future patient classification for targeted therapy.
2. High nuclear AURKA expression is associated with a poor prognosis in cancer patients
One study revealed that AURKA staining in malignant tumor tissue is mainly located in the nucleus, and this staining pattern could predict adverse clinical prognosis of tumor patients [Citation28–30]. The expression of AURKA messenger ribonucleic acid (mRNA) can predict clinical prognosis and is highly positively correlated with advanced tumor stage in hepatocellular carcinoma patients [Citation31]. Immunohistochemistry can be used to label specific antigens by assessing the distribution of bound antibodies in tissues. Immunohistochemistry analysis showed that AURKA is expressed in the nucleus and cytoplasm, and this expression pattern was associated with high tumor grade, poor prognosis of patients and abnormal p53. The cytoplasm of ovarian cancer cells is the main location of phosphorylated AURKA. Ovarian cancer cells show no nuclear overexpression of AURKA protein, and instead, the protein is mostly found in the cytoplasm. Phosphorylated AURKA does not carry a nuclear localization signal at Thr288. The regulation of nuclear AURKA requires other mechanisms. When the protein acts in the cytoplasm, Aurora-A phosphorylation at Thr288 has a functional role [Citation32]. This phenomenon explains the correlation between aggressive clinical features and the presence of Aurora-A in the cytoplasm and phosphorylated Aurora-A. In Masaaki Tatsuka’s study, head and neck cancer cell nuclei showed overexpression of AURKA, suggesting that nuclear proteins can be targeted by AURKA; this finding is significant for understanding the relationship between the localization of AURKA in the nucleus and carcinogenic transformation [Citation27]. Lee et al. [Citation33] analyzed relative phosphorylated AURKA and total nuclear and cytoplasmic AURKA levels in HPV-negative head and neck squamous cell carcinoma (HNSCC) patients and found that a decreased survival rate was related to the phosphorylation and nuclear overexpression of AURKA in p16-negative HNSCC patients, but there was no correlation of OS with phosphorylated AURKA or cytoplasmic AURKA levels. Nuclear AURKA also plays a carcinogenic role in oral squamous cell carcinoma, and the survival of patients is not affected by the high nuclear expression of AURKA in tumor tissue [Citation34].
3. The AURKA core: functional diversification
Typical kinase domains of human Aurora-A contain residues 133 to 383 [Citation35]. Aurora-A is a protein with 403 amino acids. Researchers assessed the 1–333 and 1–383 amino acid sequences of AURKA and found that the nuclear localization signal (NLS) of AURKA determines the nuclear localization of AURKA. The NLS exists within amino acids 333–383 of the sequence. Furthermore, the nuclear output signal (NES) exists within amino acids 1–333, and the presence of an NLS or NES signal determines the subcellular localization of AURKA (in the nucleus or cytoplasm) [Citation25]. This is consistent with Masaaki Tatsuka’s finding that Aurora-A fuses with the nuclear output signal, thereby disrupting its nuclear localization [Citation27]. Moreover, overexpression of K/H-Ras (G12V mutant) resulted in a significant increase in the AURKA nuclear/cytoplasmic ratio in mouse embryonic fibroblasts [Citation25], while the K-Ras G12V mutant was also shown to be able to increase Aurora-A expression in ovarian, pancreatic and lung cancer cells [Citation36–38]. Recent studies have shown that the AURKA nuclear/cytoplasmic ratio is increased in hTERT RPE-1 cells during the G2 phase of mitosis [Citation39]. However, AURKA overexpression is not sufficient to increase AURKA nuclear localization, while LMB, epoxomicin and APC/C inhibitor treatment lead to AURKA nuclear enrichment [Citation39]. TPX2 and AURKA have been shown to be coexpressed in pancreatic cancer and breast cancer [Citation37, Citation39]. Interestingly, compared with AURKA-overexpressing cells, AURKA/TPX2-overexpressing cells exhibited AURKA nuclear enrichment, which was more evident after proteasome inhibition, while inhibition of AURKA kinase activity with kinase inhibitors did not affect AURKA nuclear localization [Citation39]. These results indicate that AURKA nuclear localization is regulated by the cell cycle, nuclear export, proteasome and TPX2, but AURKA kinase activity has no effect on its nuclear localization [Citation39]. This largely explains the resistance to and off-target effects of kinase inhibitor monotherapy in AURKA-overexpressing cancers. Therefore, how does the nuclear localization signal mediate the oncogenic properties of AURKA?
3.1. AURKA is involved in regulating gene transcription
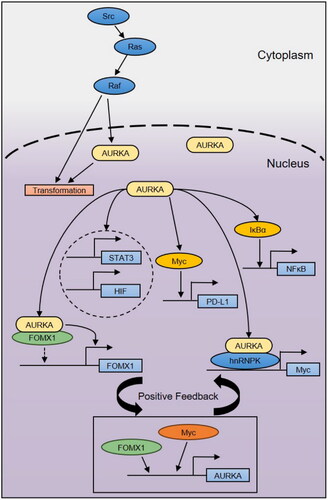
In addition to the physiological role of AURKA in cell division, its nonmitogenic role in cancer cells has received much attention in recent years [Citation35, Citation40]. AURKA exerts key functions in the nucleus as a transcription factor and transcription activator, roles by which it regulates gene transcription (). AURKA localization in the nucleus was variable in the study of Zhang et al. Fluorescence resonance energy transfer (FRET) technology exists between AURKA molecules and can enhance the proliferation of breast cancer stem cells and activate Myc transcription, which is supported by the direct action of the nuclear ribonucleoprotein hnRNPK and AURKA. However, this new function of AURKA is only applicable in the nucleus and cannot be performed by inactive AURKA [Citation21]. Moreover, the spatiotemporal location of AURKA outside mitosis is closely related to its carcinogenicity, and there is no correlation between the mitotic action of kinase and the transcriptional activation of AURKA on Myc, which has also been proven in the study [Citation25]. Myc can bind to the AURKA promoter and activate its transcription, indicating the existence of a positive feedback loop [Citation41]. Masaaki Tatsuka et al. proved that AURKA accumulated in the nucleus, as the downstream signal of Src-Ras-Raf signaling, transformed the Ras carcinogenic signal of cancer cells in the nucleus in head and neck cancer, supporting a functional role of AURKA in the processing of catalytic nuclear substrates [Citation27]. Transcription coactivators can also serve as oncogenes related to AURKA. Forkhead box subclass M1 (FOXM1) recruits nuclear AURKA. As FOXM1 is a cofactor, the activation of FOXM1 target genes mainly depends on a kinase-independent mechanism. FOXM1 and AURKA participate in the positive feedback circuit to enhance the BCSC phenotype of breast cancer [Citation42]. In invasive breast cancer patients, high expression of nuclear NF-κB mediated by amplification of AURKA can be detected and induces radiation resistance [Citation43, Citation44]. Through phosphorylation, IκBα activates NF-κB in the nucleus, which decreases the apoptosis-inducing effects of radiotherapy on cancer cells. In upper gastrointestinal adenocarcinoma, overexpression of AURKA can induce its nuclear translocation through phosphorylation of STAT3, directly activate STAT3 via transcription, and activate the expression of its target genes after the two interact [Citation45]. Nuclear AURKA has recently been found to bind to HIF1A/B and promote the transactivation of hypoxia response genes, thereby driving early breast cancer spread and metastasis, independent of kinase activity [Citation26]. In addition, nuclear/noncytoplasmic AURKA promotes PD-L1 expression in breast cancer cells through a Myc-dependent pathway, contributing to the immune escape of TNBC, suggesting that nuclear AURKA can regulate the immune response of tumors [Citation19]. Transcription factors are located in the nucleus and need a specific nuclear AURKA to activate them. STAT3 and NF-κB are the same as the transcription factor Myc. Thus, it is easy to infer that nuclear AURKA can activate multiple transcription factors at the same time. Transcription factors such as GSK3B and CTNNB1 should be studied to help clarify their activation sequence [Citation40].
AURKA functions as a transcription factor and transcriptional coactivator in the nucleus, participates in the regulatory mechanisms of cancer cells, and generates carcinogenic signals. AURKA in the nucleus affects biological processes of breast cancer stem cells through a kinase-independent mechanism. This review has focused on clarifying the relationship between AURKA in the nucleus and its downstream transcriptional network, as well as the function of AURKA in the nucleus from a new perspective, hoping to provide a theoretical foundation for the identification of chemotherapeutic drugs targeting AURKA.
3.2. AURKA promotes and sustains oncogenic signaling
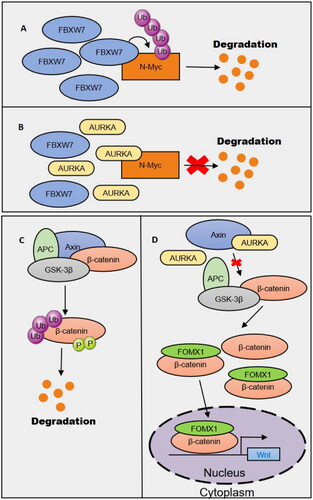
Abnormal Wnt signaling may underlie many diseases in humans. AURKA is a key protein affecting the stability of Wnt pathway signaling (). FoxM1 is a key molecule related to cellular accumulation of beta-catenin. In the Wnt signaling pathway, FoxM1 and beta-catenin combine in the nucleus with TCF4 in the promoter region of the Wnt gene to jointly regulate the stemness of GICs. However, FOXM1 decreases the overall expression of beta-catenin [Citation46]. Other studies have shown that overactivated AURKA in glioma-initiating cells (GICs) can bind AXIN (a multidomain scaffold protein that aids the assembly of the β-catenin destructive complex [Citation47]) to suppress β-catenin complex formation, which reduces the phosphorylation of β-catenin and its stability in the nucleus. Subsequently, the destructive complex activates Wnt signaling in the nucleus and promotes GIC self-renewal. This effect of AURKA is due to its protein expression, not its kinase activity [Citation48]. The mechanism by which AURKA affects oncogenic complexes through protein binding to inhibit oncoprotein degradation is not limited to Wnt signaling. Fbxw7 is a ubiquitin ligase that induces N-Myc degradation, and AURKA can fully bind to Fbxw7, thereby inhibiting the degradation of the oncogenic signaling protein N-Myc. Interestingly, AURKA kinase activity is not essential for N-Myc stabilization, indicating that kinase activity is not the only important carcinogenic characteristic of AURKA [Citation49,Citation50]. MYCN and AURKA are expressed at high levels in neurocytomas, medulloblastomas, acute myeloid leukemia, glioblastomas, astrocytoma, and prostate cancers, suggesting that the stabilization effect of Aurora-A on N-Myc may be present across solid tumors.
3.3. AURKA promotes the aberrant splicing of RNAs associated with cancer
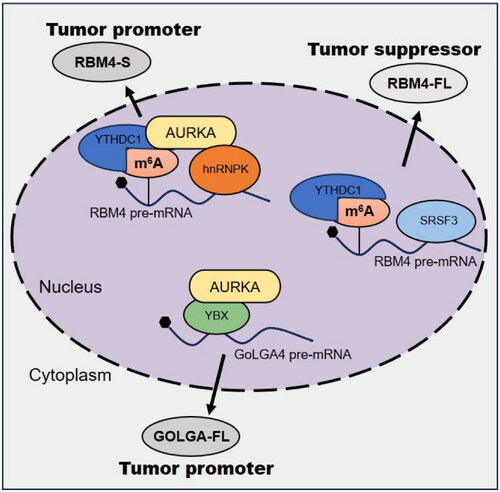
Aberrant RNA splicing can generate different gene subtypes, which can induce tumor invasion and metastasis [Citation51], epithelial-mesenchymal transition [Citation52], angiogenesis [Citation53], and anticancer resistance [Citation54]. Nuclear AURKA may promote tumor progression by regulating RNA splicing [Citation15,Citation55]. RNA splicing requires splicing factors such as heterogeneous nuclear ribonucleoprotein (HNRNP) proteins [Citation56], serin -and arginine-rich (SR) proteins [Citation57], and RNA binding motif (RBM) proteins [Citation58], which bind to regulatory elements in pre-mRNA to regulate its maturation. Previous studies have shown that RBM4 acts as a tumor suppressor by specifically controlling cancer-associated splicing [Citation59]. Recent studies have found that nuclear translocation of AURKA is a prerequisite for aberrant RNA splicing. Nuclear AURKA triggers the splicing of RBM4 from the complete isoform (RBM4-FL) to the short isoform (RBM4-S) in a kinase-independent manner, reversing the inhibition of SRSF1-mTORC1 activity by RBM4-FL and promoting tumor progression [Citation15]. The regulation of RBM4 splicing by nuclear AURKA is mainly dependent on the m6A reader YTHDC1, which inhibits the binding of YTHDC1 to SRSF3, thereby blocking the production of RBM4-FL induced by the m6A-YTHDC1-SRSF3 complex. In turn, it promotes the binding of hnRNPK and YTHDC1, thereby mediating m6A-YTHDC1-hnRNPK-dependent exon skipping leading to RBM4-S production [Citation15]. Further studies found that targeting AURKA kinase activity did not affect RBM4 splicing from RBM4-FL to RBM4-S, while the AURKA nuclear translocation inhibitor PHA-680632 and the E3 ligase inhibitor NJ-26854165 reversed RBM4-FL splicing to inhibit lung cancer growth [Citation15]. In breast cancer, the AURKA-YBX1/hnRNPK complex has been shown to be associated with a poor prognosis. AURKA binds to the splicing factor hnRNPK to promote RBM4 exon skipping, leading to RBM4-S production. Interaction with YBX1 promotes GOLGA4 exon inclusion to induce GOLGA4-FL production, and its aberrant splicing of RBM4 and GOLGA4 is blocked by nuclear translocation inhibitors [Citation55] ().
These data suggest that AURKA forms complexes with cofactors or oncogenic transcription factors to directly regulate RNA splicing but do not explain whether AURKA has a regulatory effect in RNA splicing. Therefore, targeting the AURKA complex can be used as a potential therapeutic means to inhibit aberrant RNA splicing and improve the prognosis of patients.
4. AURKA polymorphisms
The identification of single nucleotide polymorphisms related to key functions and the expression of genes will improve the understanding of the pathogenesis of tumors [Citation60]. Two single nucleotide polymorphisms of human Aurora-A (A < 91T and A < 169G) produce four isoforms: F31/V57, I31/V57, F31/I57 and I31/I57 [Citation61, Citation62]. Makoto T et al. found that different subtypes of AURKA have functional differences in esophageal epithelial cells. F31/I57 and I31/I57 exist not only in the nucleus but also in the cytoplasm but are only found in the cell. The kinase activity of the Aurora-A subtypes F31/V57 and I31/V57 was notably higher than that of F31/I57, but the activity of I31/I57 was the lowest, and I31/I57 phosphorylation was higher than F31/I57 phosphorylation, while I31/I57 lacked activity. These results show that the 57th amino acid residue is necessary for kinase activity [Citation63]. Therefore, at the subcellular level, there are obvious differences in the kinase activity of the four types of proteins. On the one hand, the activity of protein kinases in tumor patients affects the curative effects in tumor patients. In addition, subcellular localization impacts the effect of inhibitors. Aurora-A gene polymorphisms should be considered in the treatment of this type of tumor and in subsequent clinical trials. Aurora-A protein kinase (Aurora-A) plays key functions in the expansion of tumors. However, one study revealed that the lower the Aurora-A kinase activity was, the greater the risk of cancer. To determine how other Aurora-A subtypes cause tumors, further research is needed. Genetic testing for Aurora-A polymorphisms and Aurora-A kinase activity will be helpful for identifying high-risk patients. Relevant results will enable regular examination of and early detection of cancer in patients at high risk of tumors [Citation63].
5. Targeting AURKA
Due to the multiple functions that AURKA exhibits as a kinase during cancer cell mitosis, researchers have made great efforts to develop AURKA inhibitors for cancer therapy. Many inhibitors have been developed, some of which have entered clinical trial [Citation23, Citation64–93] (Supplementary Information). However, AURKA inhibitors are essentially small ATP analogs that target a highly homologous ATP-binding site (active site) on the kinase, which is highly conserved among kinase families, and targeting the ATP-binding site of the kinase can lead to poor selectivity and possible side effects in patients. In addition, kinase active site mutations are prone to lead to the emergence of drug resistance [Citation94]. To overcome inhibitor drug resistance, researchers have studied and designed type IV inhibitors that have an altered binding site to avoid activating the kinase and instead interact with its distal allosteric site [Citation95, Citation96]. The data thus far show that interaction with the distant allosteric point of the kinase can effectively inhibit cell mitosis and reduce drug resistance (). In addition, another way to circumvent drug resistance is to study inhibitors that can effectively stabilize the inactive conformation of AURKA. Burgess et al. identified the shark heavy chain antitrope monoclonal antibody vNAR. The antibody was used to develop a new allosteric inhibitor that can compete with TPX2. All these drugs bind to the corresponding site on SUPKA, which can change the α subunit. The C-helix is twisted, and inhibitor binding can destroy the lysine-glutamate bridge () [Citation97]. However, AURKA allosteric inhibitors () need to be further improved and have not yet entered clinical trials. Recently, researchers have explored potential AURKA kinase inhibitors based on computer-aided drug design. The MK8745 analog, lead compound 85 (NCI14040), SPB01812, JFD02217, SP01027 and KM00965 were identified as potential effective AURKA kinase inhibitors by 3D QSAR, MD simulation, molecular docking and virtual screening [Citation98–100]. The search for AURKA kinase inhibitors continues, 6-(2-amino-1H-benzo[d]imidazole-6-yl)-quinazolin-4(3H)-one derivatives [Citation101] and novel pyrazole analogs [Citation102] were found to bind AURKA. These compounds have the potential to inhibit AURKA kinase activity. In addition, a salicylic aldehyde-modified analog of PF-06447475 binds to AURKA in a covalent and noncovalent manner and inhibits AURKA kinase activity [Citation103].
Table 1. Allosteric inhibition of AURKA.
However, AURKA kinase inhibitors do not address any kinase activity-independent effects of nuclear AURKA, which may explain the poor clinical outcomes of kinase inhibitor therapy. In view of the regulation of oncogenes by nuclear AURKA at the transcriptional and protein levels, AURKA combined with other targeted therapies may have synergistic effects. AKI6038, an AURKA kinase inhibitor, combined with thiostrepton, a FOXM1 inhibitor, can effectively inhibit AURKA activity and the AURKA/FOXM1 positive feedback loop, and synergistically inhibit the tumorigenesis of breast cancer stem cells [Citation42]. Through screening the drug library, researchers found that the Aurora kinase inhibitor PHA-680632 and the E3 ligase inhibitor JNJ-26854165 could promote AURKA accumulation in the cytoplasm of A549 and NCI-H460 cells and prevent AURKA nuclear translocation [Citation15]. In addition, proteolytic targeting chimeras (PROTACs) are bifunctional molecules consisting of ligands that bind to E3 ubiquitin ligases, appropriate adaptors and the parent ligand of the target protein (POI). PROTACs induce binding of the POI to E3 ubiquitin ligases and stimulate POI ubiquitination for proteasomal degradation [Citation104]. The PROTAC utilizing the AURKA kinase inhibitor alisertib was recently found to rapidly and specifically degrade the AURKA protein in cells and avoid mitotic arrest, instead arresting cells in the S phase of the cell cycle, which is different from the mitotic arrest observed with alisertib-mediated inhibition [Citation105]. Mk-5108-based PROTACs degrades AURKA in a proteasome - and E3 ligase-dependent manner [Citation106]. PROTAs induces sequential degradation of AURKA in AML stem cells, CRBN-based dAurA383 preferentially degrades mitotic AURKA, and CIAP-based dAurA450 degrades interphase AURKA, indicating that it is a promising spatiotemporal drug delivery strategy [Citation107]. This strategy has the advantage of inhibiting both the catalytic and noncatalytic functions of AURKA [Citation107, Citation108]. Therefore, targeting nuclear AURKA and inhibiting its nuclear translocation may help to solve kinase inhibitor resistance and make better use of AURKA as a target for antitumor therapy.
6. Discussion and outlook
AURKA is a signaling protein closely related to cell proliferation, invasion, survival and cell stage. AURKA is related to drug resistance [Citation114, Citation115], radiotherapy resistance [Citation43, Citation116–118] and other functions. High AURKA expression in cells is a common phenomenon in cancer, and protein kinase inhibitors targeting AURKA have been developed. However, increasing evidence shows that the clinical applications of AURKA kinase inhibitors are limited. Many recently developed inhibitors have not been successfully transitioned into clinical use due to drug resistance or off-target effects when they are applied as monotherapy and the toxic side effects of combination drugs. Here, we reviewed recent studies on the oncogenic functions of AURKA independent of its kinase activity and revealed key factors related to AURKA kinase inhibitor resistance. We believe that identifying the unique functions of specific kinases will enable new strategies to inactivate kinases through gene knockdown, gene knock-in and other means, which can then be compared with inhibitors. Such studies can be used to determine whether inhibitors or proteins can better target the kinase. The above problems must be overcome before AURKA can be employed as a target of tumor therapy. At present, there are still certain risks in the clinical application of AURKA inhibitors [Citation119].
AURKA non-kinase functions are often overlooked. On the one hand, AURKA can cause changes in cell function by interacting with oncoproteins such as MYCN to form protein complexes. In this case, small molecule inhibitors cannot directly target the protein because they do not have suitable binding sites. The development of allosteric inhibitors can overcome this problem; such inhibitors can be redirected to bind with the remote allosteric site of the kinase and affect stability [Citation120]. On the other hand, the non-kinase functions of AURKA are in part related to its nuclear localization, which is essential for its regulation of oncogene (C-Myc, FOXM1, NF-κB, STAT3) transcription and stabilization of oncogenic signals (Wnt, N-Myc). Therefore, it is necessary to focus on the subcellular localization of AURKA in future studies. Most previous studies have focused on the series of reactions caused by AURKA substrate phosphorylation, but this phosphorylation process is mostly carried out in the cytoplasm. Studies have shown that extremely high levels of nuclear AURKA are related to its biological function in cancer, and introducing relevant substrates into the nucleus may be a novel anticancer therapeutic strategy.
Authors’ contributions
The authors’ contributions to this review are as follows: Study conception: WL and JW; manuscript draft preparation: MC and JW; data collection, critical review, commentary, revision, and figure design: MC, HZ, JL, DL and JZ; and supervision of the prepared manuscript: JW and WL. All the authors verified the conclusion and approved the final version of the manuscript.
Supplemental Material
Download MS Excel (14.9 KB)Acknowledgements
We thank all participants.
Disclosure statement
The authors report that there are no relevant conflicts of rights or interests.
Data availability statement
All information obtained by the authors in this manuscript can be found in the literature.
Additional information
Funding
References
- Chan CS, Botstein D. Isolation and characterization of chromosome-gain and increase-in-ploidy mutants in yeast. Genetics. 1993;135(3):677–691. doi: 10.1093/genetics/135.3.677.
- Glover DM, Leibowitz MH, McLean DA, et al. Mutations in Aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81(1):95–105. doi: 10.1016/0092-8674(95)90374-7.
- Giet R, Uzbekov R, Kireev I, et al. The Xenopus laevis centrosome Aurora/Ipl1-related kinase. Biol Cell. 1999;91(6):461–470.
- Schumacher JM, Ashcroft N, Donovan PJ, et al. A highly conserved centrosomal kinase, AIR-1, is required for accurate cell cycle progression and segregation of developmental factors in Caenorhabditis elegans embryos. Development. 1998;125(22):4391–4402. doi: 10.1242/dev.125.22.4391.
- Isola JJ, Kallioniemi OP, Chu LW, et al. Genetic aberrations detected by comparative genomic hybridization predict outcome in node-negative breast cancer. Am J Pathol. 1995;147(4):905–911.
- Kahl I, Mense J, Finke C, et al. The cell cycle-related genes RHAMM, AURKA, TPX2, PLK1, and PLK4 are associated with the poor prognosis of breast cancer patients. J Cell Biochem. 2022;123(3):581–600. doi: 10.1002/jcb.30205.
- Puig-Butille JA, Vinyals A, Ferreres JR, et al. AURKA overexpression is driven by FOXM1 and MAPK/ERK activation in melanoma cells harboring BRAF or NRAS mutations: impact on melanoma prognosis and therapy. J Invest Dermatol. 2017;137(6):1297–1310. doi: 10.1016/j.jid.2017.01.021.
- Takahashi Y, Sheridan P, Niida A, et al. The AURKA/TPX2 axis drives Colon tumorigenesis cooperatively with MYC. Ann Oncol. 2015;26(5):935–942. doi: 10.1093/annonc/mdv034.
- Lassmann S, Shen Y, Jütting U, et al. Predictive value of Aurora-a/STK15 expression for late stage epithelial ovarian cancer patients treated by adjuvant chemotherapy. Clin Cancer Res. 2007;13(14):4083–4091. doi: 10.1158/1078-0432.Ccr-06-2775.
- de Paula Careta F, Gobessi S, Panepucci RA, et al. The Aurora a and B kinases are up-regulated in bone marrow-derived chronic lymphocytic leukemia cells and represent potential therapeutic targets. Haematologica. 2012;97(8):1246–1254. doi: 10.3324/haematol.2011.054668.
- Goldenson B, Crispino JD. The Aurora kinases in cell cycle and leukemia. Oncogene. 2015;34(5):537–545. doi: 10.1038/onc.2014.14.
- Bischoff JR, Anderson L, Zhu Y, et al. A homologue of drosophila Aurora kinase is oncogenic and amplified in human colorectal cancers. Embo J. 1998;17(11):3052–3065. doi: 10.1093/emboj/17.11.3052.
- Wang LH, Xiang J, Yan M, et al. The mitotic kinase Aurora-a induces mammary cell migration and breast cancer metastasis by activating the Cofilin-F-actin pathway. Cancer Res. 2010;70(22):9118–9128. doi: 10.1158/0008-5472.Can-10-1246.
- Miligy IM, Toss MS, Gorringe KL, et al. Aurora kinase a is an independent predictor of invasive recurrence in breast ductal carcinoma in situ. Pathobiology. 2022;89(6):382–392. doi: 10.1159/000522244.
- Li S, Qi Y, Yu J, et al. Nuclear Aurora kinase a switches m(6)a reader YTHDC1 to enhance an oncogenic RNA splicing of tumor suppressor RBM4. Signal Transduct Target Ther. 2022;7(1):97. doi: 10.1038/s41392-022-00905-3.
- Li M, Sun C, Bu X, et al. ISL1 promoted tumorigenesis and EMT via Aurora kinase A-induced activation of PI3K/AKT signaling pathway in neuroblastoma. Cell Death Dis. 2021;12(6):620. doi: 10.1038/s41419-021-03894-3.
- Shen ZT, Chen Y, Huang GC, et al. Aurora-a confers radioresistance in human hepatocellular carcinoma by activating NF-κB signaling pathway. BMC Cancer. 2019;19(1):1075. doi: 10.1186/s12885-019-6312-y.
- Miralaei N, Majd A, Ghaedi K, et al. Integrated pan-cancer of AURKA expression and drug sensitivity analysis reveals increased expression of AURKA is responsible for drug resistance. Cancer Med. 2021;10(18):6428–6441. doi: 10.1002/cam4.4161.
- Sun S, Zhou W, Li X, et al. Nuclear Aurora kinase a triggers programmed death-ligand 1-mediated immune suppression by activating MYC transcription in triple-negative breast cancer. Cancer Commun (Lond). 2021;41(9):851–866. doi: 10.1002/cac2.12190.
- Xie Y, Zhu S, Zhong M, et al. Inhibition of Aurora kinase a induces necroptosis in pancreatic carcinoma. Gastroenterology. 2017;153(5):1429–1443.e5. e1425. doi: 10.1053/j.gastro.2017.07.036.
- Du J, Yan L, Torres R, et al. Aurora A-Selective inhibitor LY3295668 leads to dominant mitotic arrest, apoptosis in cancer cells, and shows potent preclinical antitumor efficacy. Mol Cancer Ther. 2019;18(12):2207–2219. doi: 10.1158/1535-7163.Mct-18-0529.
- Bavetsias V, Linardopoulos S. Aurora kinase inhibitors: current status and outlook. Front Oncol. 2015;5:278. doi: 10.3389/fonc.2015.00278.
- Melichar B, Adenis A, Lockhart AC, et al. Safety and activity of alisertib, an investigational Aurora kinase a inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: a five-arm phase 2 study. Lancet Oncol. 2015;16(4):395–405. doi: 10.1016/s1470-2045(15)70051-3.
- Chen A, Wen S, Liu F, et al. CRISPR/Cas9 screening identifies a kinetochore-microtubule dependent mechanism for Aurora-a inhibitor resistance in breast cancer. Cancer Commun (Lond). 2021;41(2):121–139. doi: 10.1002/cac2.12125.
- Zheng F, Yue C, Li G, et al. Nuclear AURKA acquires kinase-independent transactivating function to enhance breast cancer stem cell phenotype. Nat Commun. 2016;7(1):10180. doi: 10.1038/ncomms10180.
- Whately KM, Voronkova MA, Maskey A, et al. Nuclear Aurora-a kinase-induced hypoxia signaling drives early dissemination and metastasis in breast cancer: implications for detection of metastatic tumors. Oncogene. 2021;40(37):5651–5664. doi: 10.1038/s41388-021-01969-1.
- Tatsuka M, Sato S, Kanda A, et al. Oncogenic role of nuclear accumulated Aurora-A. Mol Carcinog. 2009;48(9):810–820. doi: 10.1002/mc.20525.
- Burum-Auensen E, De Angelis PM, Schjølberg AR, et al. Subcellular localization of the spindle proteins Aurora A, Mad2, and BUBR1 assessed by immunohistochemistry. J Histochem Cytochem. 2007;55(5):477–486. doi: 10.1369/jhc.6A7077.2007.
- Tamotsu K, Okumura H, Uchikado Y, et al. Correlation of Aurora-a expression with the effect of chemoradiation therapy on esophageal squamous cell carcinoma. BMC Cancer. 2015;15(1):323. doi: 10.1186/s12885-015-1329-3.
- Tanaka E, Hashimoto Y, Ito T, et al. The clinical significance of Aurora-a/STK15/BTAK expression in human esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11(5):1827–1834. doi: 10.1158/1078-0432.Ccr-04-1627.
- Wang R, Wang JH, Chu XY, et al. Expression of STK15 mRNA in hepatocellular carcinoma and its prognostic significance. Clin Biochem. 2009;42(7-8):641–647. doi: 10.1016/j.clinbiochem.2009.01.023.
- Lassus H, Staff S, Leminen A, et al. Aurora-a overexpression and aneuploidy predict poor outcome in serous ovarian carcinoma. Gynecol Oncol. 2011;120(1):11–17. doi: 10.1016/j.ygyno.2010.09.003.
- Lee JW, Parameswaran J, Sandoval-Schaefer T, et al. Combined Aurora kinase A (AURKA) and WEE1 inhibition demonstrates synergistic antitumor effect in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2019;25(11):3430–3442. doi: 10.1158/1078-0432.Ccr-18-0440.
- Kao SY, Chen YP, Tu HF, et al. Nuclear STK15 expression is associated with aggressive behaviour of oral carcinoma cells in vivo and in vitro. J Pathol. 2010;222(1):99–109. doi: 10.1002/path.2737.
- Nikonova AS, Astsaturov I, Serebriiskii IG, et al. Aurora a kinase (AURKA) in normal and pathological cell division. Cell Mol Life Sci. 2013;70(4):661–687. doi: 10.1007/s00018-012-1073-7.
- Yang G, Mercado-Uribe I, Multani AS, et al. RAS promotes tumorigenesis through genomic instability induced by imbalanced expression of Aurora-a and BRCA2 in midbody during cytokinesis. Int J Cancer. 2013;133(2):275–285. doi: 10.1002/ijc.28032.
- Gomes-Filho SM, Dos Santos EO, Bertoldi ERM, et al. Aurora a kinase and its activator TPX2 are potential therapeutic targets in KRAS-induced pancreatic cancer. Cell Oncol (Dordr). 2020;43(3):445–460. doi: 10.1007/s13402-020-00498-5.
- Dos Santos EO, Carneiro-Lobo TC, Aoki MN, et al. Aurora kinase targeting in lung cancer reduces KRAS-induced transformation. Mol Cancer. 2016;15(1):12. doi: 10.1186/s12943-016-0494-6.
- Asteriti IA, Polverino F, Stagni V, et al. AurkA nuclear localization is promoted by TPX2 and counteracted by protein degradation. Life Sci Alliance. 2023;6(5):e202201726. doi: 10.26508/lsa.202201726.
- Bertolin G, Tramier M. Insights into the non-mitotic functions of Aurora kinase A: more than just cell division. Cell Mol Life Sci. 2020;77(6):1031–1047. doi: 10.1007/s00018-019-03310-2.
- den Hollander J, Rimpi S, Doherty JR, et al. Aurora kinases a and B are up-regulated by myc and are essential for maintenance of the malignant state. Blood. 2010;116(9):1498–1505. doi: 10.1182/blood-2009-11-251074.
- Yang N, Wang C, Wang Z, et al. FOXM1 recruits nuclear Aurora kinase a to participate in a positive feedback loop essential for the self-renewal of breast cancer stem cells. Oncogene. 2017;36(24):3428–3440. doi: 10.1038/onc.2016.490.
- Baba Y, Nosho K, Shima K, et al. Aurora-a expression is independently associated with chromosomal instability in colorectal cancer. Neoplasia. 2009;11(5):418–425. doi: 10.1593/neo.09154.
- Briassouli P, Chan F, Savage K, et al. Aurora-a regulation of nuclear factor-kappaB signaling by phosphorylation of IkappaBalpha. Cancer Res. 2007;67(4):1689–1695. doi: 10.1158/0008-5472.Can-06-2272.
- Katsha A, Arras J, Soutto M, et al. AURKA regulates JAK2-STAT3 activity in human gastric and esophageal cancers. Mol Oncol. 2014;8(8):1419–1428. doi: 10.1016/j.molonc.2014.05.012.
- Zhang N, Wei P, Gong A, et al. FoxM1 promotes β-catenin nuclear localization and controls wnt target-gene expression and glioma tumorigenesis. Cancer Cell. 2011;20(4):427–442. doi: 10.1016/j.ccr.2011.08.016.
- Zeng L, Fagotto F, Zhang T, et al. The mouse fused locus encodes axin, an inhibitor of the wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90(1):181–192. doi: 10.1016/s0092-8674(00)80324-4.
- Xia Z, Wei P, Zhang H, et al. AURKA governs self-renewal capacity in glioma-initiating cells via stabilization/activation of β-catenin/wnt signaling. Mol Cancer Res. 2013;11(9):1101–1111. doi: 10.1158/1541-7786.Mcr-13-0044.
- Otto T, Horn S, Brockmann M, et al. Stabilization of N-myc is a critical function of Aurora a in human neuroblastoma. Cancer Cell. 2009;15(1):67–78. doi: 10.1016/j.ccr.2008.12.005.
- Meraldi P, Honda R, Nigg EA. Aurora-a overexpression reveals tetraploidization as a major route to centrosome amplification in p53-/- cells. Embo J. 2002;21(4):483–492. doi: 10.1093/emboj/21.4.483.
- Zhou HZ, Li F, Cheng ST, et al. DDX17-regulated alternative splicing that produced an oncogenic isoform of PXN-AS1 to promote HCC metastasis. Hepatology. 2022;75(4):847–865. doi: 10.1002/hep.32195.
- Hu X, Harvey SE, Zheng R, et al. The RNA-binding protein AKAP8 suppresses tumor metastasis by antagonizing EMT-associated alternative splicing. Nat Commun. 2020;11(1):486. doi: 10.1038/s41467-020-14304-1.
- Huang G, Zhou Z, Wang H, et al. CAPER-α alternative splicing regulates the expression of vascular endothelial growth factor165 in ewing sarcoma cells. Cancer. 2012;118(8):2106–2116. doi: 10.1002/cncr.26488.
- Tanaka I, Chakraborty A, Saulnier O, et al. ZRANB2 and SYF2-mediated splicing programs converging on ECT2 are involved in breast cancer cell resistance to doxorubicin. Nucleic Acids Res. 2020;48(5):2676–2693. doi: 10.1093/nar/gkz1213.
- Li S, Qi Y, Yu J, et al. Aurora kinase a regulates cancer-associated RNA aberrant splicing in breast cancer. Heliyon. 2023;9(7):e17386. doi: 10.1016/j.heliyon.2023.e17386.
- Geuens T, Bouhy D, Timmerman V. The hnRNP family: insights into their role in health and disease. Hum Genet. 2016;135(8):851–867. doi: 10.1007/s00439-016-1683-5.
- Howard JM, Sanford JR. The RNAissance family: SR proteins as multifaceted regulators of gene expression. Wiley Interdiscip Rev RNA. 2015;6(1):93–110. doi: 10.1002/wrna.1260.
- Li Z, Guo Q, Zhang J, et al. The RNA-Binding motif protein family in cancer: friend or foe? Front Oncol. 2021;11:757135. doi: 10.3389/fonc.2021.757135.
- Wang Y, Chen D, Qian H, et al. The splicing factor RBM4 controls apoptosis, proliferation, and migration to suppress tumor progression. Cancer Cell. 2014;26(3):374–389. doi: 10.1016/j.ccr.2014.07.010.
- Imyanitov EN, Togo AV, Hanson KP. Searching for cancer-associated gene polymorphisms: promises and obstacles. Cancer Lett. 2004;204(1):3–14. doi: 10.1016/j.canlet.2003.09.026.
- Haga H, Yamada R, Ohnishi Y, et al. Gene-based SNP discovery as part of the japanese millennium genome project: identification of 190,562 genetic variations in the human genome. Single-nucleotide polymorphism. J Hum Genet. 2002;47(11):605–610. doi: 10.1007/s100380200092.
- Venter JC, Adams MD, Myers EW, et al. The sequence of the human genome. Science. 2001;291(5507):1304–1351. doi: 10.1126/science.1058040.
- Kimura MT, Mori T, Conroy J, et al. Two functional coding single nucleotide polymorphisms in STK15 (Aurora-A) coordinately increase esophageal cancer risk. Cancer Res. 2005;65(9):3548–3554. doi: 10.1158/0008-5472.Can-04-2149.
- Robbrecht DGJ, Lopez J, Calvo E, et al. A first-in-human phase 1 and pharmacological study of TAS-119, a novel selective Aurora a kinase inhibitor in patients with advanced solid tumours. Br J Cancer. 2021;124(2):391–398. doi: 10.1038/s41416-020-01100-3.
- Chu QS, Bouganim N, Fortier C, et al. Aurora kinase a inhibitor, LY3295668 erbumine: a phase 1 monotherapy safety study in patients with locally advanced or metastatic solid tumors. Invest New Drugs. 2021;39(4):1001–1010. doi: 10.1007/s10637-020-01049-3.
- Diamond JR, Bastos BR, Hansen RJ, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of ENMD-2076, a novel angiogenic and Aurora kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2011;17(4):849–860. doi: 10.1158/1078-0432.Ccr-10-2144.
- Yee KW, Chen HW, Hedley DW, et al. A phase I trial of the Aurora kinase inhibitor, ENMD-2076, in patients with relapsed or refractory acute myeloid leukemia or chronic myelomonocytic leukemia. Invest New Drugs. 2016;34(5):614–624. doi: 10.1007/s10637-016-0375-2.
- Matulonis UA, Lee J, Lasonde B, et al. ENMD-2076, an oral inhibitor of angiogenic and proliferation kinases, has activity in recurrent, platinum resistant ovarian cancer. Eur J Cancer. 2013;49(1):121–131. doi: 10.1016/j.ejca.2012.07.020.
- Diamond JR, Eckhardt SG, Pitts TM, et al. A phase II clinical trial of the Aurora and angiogenic kinase inhibitor ENMD-2076 for previously treated, advanced, or metastatic triple-negative breast cancer. Breast Cancer Res. 2018;20(1):82. doi: 10.1186/s13058-018-1014-y.
- Lheureux S, Tinker A, Clarke B, et al. A clinical and molecular phase II trial of oral ENMD-2076 in ovarian clear cell carcinoma (OCCC): a study of the princess margaret phase II consortium. Clin Cancer Res. 2018;24(24):6168–6174. doi: 10.1158/1078-0432.Ccr-18-1244.
- Veitch Z, Zer A, Loong H, et al. A phase II study of ENMD-2076 in advanced soft tissue sarcoma (STS). Sci Rep. 2019;9(1):7390. doi: 10.1038/s41598-019-43222-6.
- Abou-Alfa GK, Mayer R, Venook AP, et al. Phase II multicenter, Open-Label study of oral ENMD-2076 for the treatment of patients with advanced fibrolamellar carcinoma. Oncologist. 2020;25(12):e1837–e1845. doi: 10.1634/theoncologist.2020-0093.
- Amin M, Minton SE, LoRusso PM, et al. A phase I study of MK-5108, an oral Aurora a kinase inhibitor, administered both as monotherapy and in combination with docetaxel, in patients with advanced or refractory solid tumors. Invest New Drugs. 2016;34(1):84–95. doi: 10.1007/s10637-015-0306-7.
- Dees EC, Infante JR, Cohen RB, et al. Phase 1 study of MLN8054, a selective inhibitor of Aurora a kinase in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;67(4):945–954. doi: 10.1007/s00280-010-1377-y.
- Macarulla T, Cervantes A, Elez E, et al. Phase I study of the selective Aurora a kinase inhibitor MLN8054 in patients with advanced solid tumors: safety, pharmacokinetics, and pharmacodynamics. Mol Cancer Ther. 2010;9(10):2844–2852. doi: 10.1158/1535-7163.Mct-10-0299.
- Beltran H, Oromendia C, Danila DC, et al. A phase II trial of the Aurora kinase a inhibitor alisertib for patients with castration-resistant and neuroendocrine prostate cancer: efficacy and biomarkers. Clin Cancer Res. 2019;25(1):43–51. doi: 10.1158/1078-0432.Ccr-18-1912.
- Falchook G, Kurzrock R, Gouw L, et al. Investigational Aurora a kinase inhibitor alisertib (MLN8237) as an enteric-coated tablet formulation in non-hematologic malignancies: phase 1 dose-escalation study. Invest New Drugs. 2014;32(6):1181–1187. doi: 10.1007/s10637-014-0121-6.
- Falchook G, Coleman RL, Roszak A, et al. Alisertib in combination with weekly paclitaxel in patients with advanced breast cancer or recurrent ovarian cancer: a randomized clinical trial. JAMA Oncol. 2019;5(1):e183773. doi: 10.1001/jamaoncol.2018.3773.
- Barr PM, Li H, Spier C, et al. Phase II intergroup trial of alisertib in relapsed and refractory peripheral T-cell lymphoma and transformed mycosis fungoides: SWOG 1108. J Clin Oncol. 2015;33(21):2399–2404. doi: 10.1200/jco.2014.60.6327.
- Mossé YP, Fox E, Teachey DT, et al. A phase II study of alisertib in children with recurrent/refractory solid tumors or leukemia: children’s oncology group phase I and pilot consortium (ADVL0921). Clin Cancer Res. 2019;25(11):3229–3238. doi: 10.1158/1078-0432.Ccr-18-2675.
- Mossé YP, Lipsitz E, Fox E, et al. Pediatric phase I trial and pharmacokinetic study of MLN8237, an investigational oral selective small-molecule inhibitor of Aurora kinase A: a children’s oncology group phase I consortium study. Clin Cancer Res. 2012;18(21):6058–6064. doi: 10.1158/1078-0432.Ccr-11-3251.
- Zhou X, Mould DR, Yuan Y, et al. Population pharmacokinetics and Exposure-Safety relationships of alisertib in children and adolescents with advanced malignancies. J Clin Pharmacol. 2022;62(2):206–219. doi: 10.1002/jcph.1958.
- Siddiqi T, Frankel P, Beumer JH, et al. Phase 1 study of the Aurora kinase a inhibitor alisertib (MLN8237) combined with the histone deacetylase inhibitor vorinostat in lymphoid malignancies. Leuk Lymphoma. 2020;61(2):309–317. doi: 10.1080/10428194.2019.1672052.
- Venkatakrishnan K, Kim TM, Lin CC, et al. Phase 1 study of the investigational Aurora a kinase inhibitor alisertib (MLN8237) in east asian cancer patients: pharmacokinetics and recommended phase 2 dose. Invest New Drugs. 2015;33(4):942–953. doi: 10.1007/s10637-015-0258-y.
- Brunner AM, Blonquist TM, DeAngelo DJ, et al. Alisertib plus induction chemotherapy in previously untreated patients with high-risk, acute myeloid leukaemia: a single-arm, phase 2 trial. Lancet Haematol. 2020;7(2):e122–e133. doi: 10.1016/s2352-3026(19)30203-0.
- Fathi AT, Wander SA, Blonquist TM, et al. Phase I study of the Aurora a kinase inhibitor alisertib with induction chemotherapy in patients with acute myeloid leukemia. Haematologica. 2017;102(4):719–727. doi: 10.3324/haematol.2016.158394.
- Semrad TJ, Kim EJ, Gong IY, et al. Phase 1 study of alisertib (MLN8237) and weekly irinotecan in adults with advanced solid tumors. Cancer Chemother Pharmacol. 2021;88(2):335–341. doi: 10.1007/s00280-021-04293-3.
- Kelly KR, Shea TC, Goy A, et al. Phase I study of MLN8237–investigational Aurora a kinase inhibitor–in relapsed/refractory multiple myeloma, non-Hodgkin lymphoma and chronic lymphocytic leukemia. Invest New Drugs. 2014;32(3):489–499. doi: 10.1007/s10637-013-0050-9.
- Necchi A, Lo Vullo S, Mariani L, et al. An open-label, single-arm, phase 2 study of the Aurora kinase a inhibitor alisertib in patients with advanced urothelial cancer. Invest New Drugs. 2016;34(2):236–242. doi: 10.1007/s10637-016-0328-9.
- Necchi A, Pintarelli G, Raggi D, et al. Association of an Aurora kinase a (AURKA) gene polymorphism with progression-free survival in patients with advanced urothelial carcinoma treated with the selective Aurora kinase a inhibitor alisertib. Invest New Drugs. 2017;35(4):524–528. doi: 10.1007/s10637-017-0440-5.
- Kelly KR, Friedberg JW, Park SI, et al. Phase I study of the investigational Aurora a kinase inhibitor alisertib plus rituximab or rituximab/vincristine in relapsed/refractory aggressive B-cell lymphoma. Clin Cancer Res. 2018;24(24):6150–6159. doi: 10.1158/1078-0432.Ccr-18-0286.
- Shah HA, Fischer JH, Venepalli NK, et al. Phase I study of Aurora a kinase inhibitor alisertib (MLN8237) in combination with selective VEGFR inhibitor pazopanib for therapy of advanced solid tumors. Am J Clin Oncol. 2019;42(5):413–420. doi: 10.1097/coc.0000000000000543.
- Dickson MA, Mahoney MR, Tap WD, et al. Phase II study of MLN8237 (alisertib) in advanced/metastatic sarcoma. Ann Oncol. 2016;27(10):1855–1860. doi: 10.1093/annonc/mdw281.
- Panicker RC, Coyne AG, Srinivasan R. Allosteric targeting of Aurora a kinase using small molecules: a step forward towards next generation medicines? Curr Med Chem. 2019;26(13):2234–2242. doi: 10.2174/0929867324666170727120315.
- Zhang J, Adrián FJ, Jahnke W, et al. Targeting Bcr-Abl by combining allosteric with ATP-binding-site inhibitors. Nature. 2010;463(7280):501–506. doi: 10.1038/nature08675.
- Cox KJ, Shomin CD, Ghosh I. Tinkering outside the kinase ATP box: allosteric (type IV) and bivalent (type V) inhibitors of protein kinases. Future Med Chem. 2011;3(1):29–43. doi: 10.4155/fmc.10.272.
- Burgess SG, Oleksy A, Cavazza T, et al. Allosteric inhibition of Aurora-a kinase by a synthetic vNAR domain. Open Biol. 2016;6(7):160089. doi: 10.1098/rsob.160089.
- Almilaibary A. Targeting Aurora kinase a (AURKA) in cancer: molecular docking and dynamic simulations of potential AURKA inhibitors. Med Oncol. 2022;39(12):246. doi: 10.1007/s12032-022-01852-3.
- Hijjawi MS, Abutayeh RF, Taha MO. Structure-Based discovery and bioactivity evaluation of novel Aurora-a kinase inhibitors as anticancer agents via Docking-Based comparative intermolecular contacts analysis (dbCICA). Molecules. 2020;25(24):6003. doi: 10.3390/molecules25246003.
- Swamy PMG, Abbas N, Dhiwar PS, et al. Discovery of potential Aurora-a kinase inhibitors by 3D QSAR pharmacophore modeling, virtual screening, docking, and MD simulation studies. J Biomol Struct Dyn. 2023;41(1):125–146. doi: 10.1080/07391102.2021.2004236.
- Fan Y, Luo F, Su M, et al. Structure optimization, synthesis, and biological evaluation of 6-(2-amino-1H-benzo[d]imidazole-6-yl)-quinazolin-4(3H)-one derivatives as potential multi-targeted anticancer agents via Aurora a/PI3K/BRD4 inhibition. Bioorg Chem. 2023;132:106352. doi: 10.1016/j.bioorg.2023.106352.
- Yevale DB, Teraiya N, Lalwani TD, et al. A novel class of pyrazole analogues as Aurora kinase a inhibitor: design, synthesis, and anticancer evaluation. Bioorg Chem. 2023;141:106901. doi: 10.1016/j.bioorg.2023.106901.
- Wang W, Wang X, Tang G, et al. Multitarget inhibitors/probes that target LRRK2 and Aurora a kinases noncovalently and covalently. Chem Commun (Camb). 2023;59(72):10789–10792. doi: 10.1039/d3cc03530a.
- Nieto-Jiménez C, Morafraile EC, Alonso-Moreno C, et al. Clinical considerations for the design of PROTACs in cancer. Mol Cancer. 2022;21(1):67. doi: 10.1186/s12943-022-01535-7.
- Wang R, Ascanelli C, Abdelbaki A, et al. Selective targeting of non-centrosomal AURKA functions through use of a targeted protein degradation tool. Commun Biol. 2021;4(1):640. doi: 10.1038/s42003-021-02158-2.
- Rishfi M, Krols S, Martens F, et al. Targeted AURKA degradation: towards new therapeutic agents for neuroblastoma. Eur J Med Chem. 2023;247:115033. doi: 10.1016/j.ejmech.2022.115033.
- Liu F, Wang X, Duan J, et al. A temporal PROTAC Cocktail-Mediated sequential degradation of AURKA abrogates acute myeloid leukemia stem cells. Adv Sci (Weinh). 2022;9(22):e2104823. doi: 10.1002/advs.202104823.
- Adhikari B, Bozilovic J, Diebold M, et al. PROTAC-mediated degradation reveals a non-catalytic function of Aurora-a kinase. Nat Chem Biol. 2020;16(11):1179–1188. doi: 10.1038/s41589-020-00652-y.
- Janeček M, Rossmann M, Sharma P, et al. Allosteric modulation of AURKA kinase activity by a small-molecule inhibitor of its protein-protein interaction with TPX2. Sci Rep. 2016;6(1):28528. doi: 10.1038/srep28528.
- Karthigeyan D, Siddhanta S, Kishore AH, et al. SERS and MD simulation studies of a kinase inhibitor demonstrate the emergence of a potential drug discovery tool. Proc Natl Acad Sci U S A. 2014;111(29):10416–10421. doi: 10.1073/pnas.1402695111.
- Kesisova IA, Nakos KC, Tsolou A, et al. Tripolin A, a novel small-molecule inhibitor of Aurora a kinase, reveals new regulation of HURP's distribution on microtubules. PLoS One. 2013;8(3):e58485. doi: 10.1371/journal.pone.0058485.
- Lewis JOE. inventor; EUROPEAN MOLECULAR BIOLOGY LABORATORY (EMBL), assignee. AURORA KINASE INHIBITORS. WO2007115805. 2007 Apr 05.
- Conti E, Bayliss R, Schultz C, et al. inventor; EMBL, assignee. Crystals of an Aurora-a tpx2 complex, tpx2 binding site of Aurora-A, Aurora-a ligands and their use. WO2005040368. 2004 Nov 11.
- Sumi K, Tago K, Kasahara T, et al. Aurora kinase a critically contributes to the resistance to anti-cancer drug cisplatin in JAK2 V617F mutant-induced transformed cells. FEBS Lett. 2011;585(12):1884–1890. doi: 10.1016/j.febslet.2011.04.068.
- Shah KN, Bhatt R, Rotow J, et al. Aurora kinase a drives the evolution of resistance to third-generation EGFR inhibitors in lung cancer. Nat Med. 2019;25(1):111–118. doi: 10.1038/s41591-018-0264-7.
- Liu N, Wang YA, Sun Y, et al. Inhibition of Aurora a enhances radiosensitivity in selected lung cancer cell lines. Respir Res. 2019;20(1):230. doi: 10.1186/s12931-019-1194-8.
- Liu JB, Hu L, Yang Z, et al. Aurora-a/NF-ĸB signaling is associated with radio-resistance in human lung adenocarcinoma. Anticancer Res. 2019;39(11):5991–5998. doi: 10.21873/anticanres.13804.
- Ma Y, Yang J, Wang R, et al. Aurora-a affects radiosenstivity in cervical squamous cell carcinoma and predicts poor prognosis. Oncotarget. 2017;8(19):31509–31520. doi: 10.18632/oncotarget.15663.
- Damodaran AP, Vaufrey L, Gavard O, et al. Aurora a kinase is a priority pharmaceutical target for the treatment of cancers. Trends Pharmacol Sci. 2017;38(8):687–700. doi: 10.1016/j.tips.2017.05.003.
- Gustafson WC, Meyerowitz JG, Nekritz EA, et al. Drugging MYCN through an allosteric transition in Aurora kinase A. Cancer Cell. 2014;26(3):414–427. doi: 10.1016/j.ccr.2014.07.015.