Abstract
Human embryonic stem (ES) cells serve as a potentially unlimited renewable source for cell transplantation targeted to treat several diseases. One advantage of embryonic stem (ES) cells over other stem cells under research is their apparently indefinite self‐renewal capacity if cultured appropriately, and their ready differentiation into various cell phenotypes of all three germ layers. To date, a number of studies have reported the derivation of specific functional derivatives from human ES cells in vitro. While there have been clinical trials of human embryonal carcinoma (EC) cell‐derived neurons in humans there has been no attempt as yet using human ES cell derivatives. However, the latter have been transplanted into recipient animals. In some cases ES‐derived cells were shown to undergo further maturation, displayed integration with host tissue and even ameliorated the disease condition in the animal model. Recently, it has been reported that human ES cells can be genetically manipulated. Such procedures could be used to direct differentiation to a specific cell type or to reduce graft rejections by the modification of immune responses. This review highlights some of the recent advances in the field and the challenges that lie ahead before clinical trials using ES‐derived cells can be contemplated.
Key messages
If differentiated human ES cells are to be used in therapies to treat degenerative diseases or as tools for drug discovery, then in vitro validation of their functionality is of paramount importance for establishing their true capabilities in vivo.
Discriminating between a direct effect of transplanted cells on tissue repair through their integration and proliferation and an indirect effect of providing beneficial factors for spontaneous recovery of organ recovery is often difficult to discern with the current animal models without carefully constructed controls.
Recent evidence interestingly suggests that human ES cells and their derivatives are much less susceptible to immune rejection than adult cells and they do not elicit direct allorejection in vivo
Culture and differentiation of human ES cells
Human embryonic stem (ES) cells are derived from inner cell mass of blastocyst‐stage human embryos from in vitro fertilization (IVF) treatment and cultured on mitotically‐inactivated feeders, most often mouse embryonic fibroblast cells Citation1, Citation2. Subsequently, these cells are maintained and passaged in culture by mechanically scraping human ES cell colonies after enzymatic treatment. Alternatively, good colonies judged by their morphological appearance (cells with a high nucleus to cytoplasm ratio and round borders; see Figure ) can be cut and subsequently reseeded Citation2. Human ES cells are maintained in media containing 20% foetal bovine serum (FBS) or Serum Replacement (SR) media supplemented with basic fibroblast growth factor (bFGF). KnockoutTM Serum Replacement (GIBCO Invitrogen) is a serum‐free formulation designed to directly replace FBS in human ES cell culture. Continuous culture of human ES cells in an undifferentiated state often requires the presence of feeder layers Citation3–7.
Human ES cells differentiate spontaneously upon culturing in small aggregates termed embryoid bodies (EBs) in non‐adherent culture (Figure ), or simply in an over‐confluent monolayer adherent culture. The formation of EBs recapitulates the three‐dimensional complexity of the embryo during gastrulation and usually results in the formation of progenitor cells representing the three initial germ layers of the embryo. EBs are often polarized and there are cell‐to‐cell interactions, asymmetric contacts and production of growth factors Citation8. All of these factors influence the initial events of cell differentiation Citation9. It should be emphasized, however, that EBs should not be considered reconstituted embryos as the correct order of developmental cues has been lost or are chaotic.
Schuldiner et al. (2000) demonstrated that the addition of specific growth factors can modify EB differentiation and promote directed differentiation and/or cell selection Citation10. ES cells differentiate in vitro as an adherent cell layer and this process has been utilized for derivation of neural Citation11 and haematopoietic progenitors Citation12. Such a protocol has the advantage of easy detection of differentiated cells by morphology assessment (in case of neurons) or fluorescent activated cell sorting (FACS) for appropriate cell surface markers (e. g. CD34 in the case of blood lineage) after an initial step of trypsinization into single cells. Selected cells can then be recovered for further expansion in culture.
Functional data from human ES cells
If differentiated human ES cells are to be used in therapies to treat degenerative diseases or as tools for drug discovery, then in vitro validation of their functionality is of paramount importance for establishing their true capabilities in vivo. Incumbent on this is a strong requirement for a comparative analysis of physiological mechanisms that regulate differentiated human ES cells and their native cell counterparts. The phenotypic properties of cells are largely determined by the proteins they express relevant to their cellular signalling and communication, including receptors and ion transporters (see Figure ). Hence, for example, characterization of ion channel expression and function in human ES cell‐derived cardiac, neuronal and endocrine cells is necessary at an early stage, for assessing their potential in strategies for transplantation. In this context, techniques such as electrophysiology and cell imaging for monitoring intracellular Ca2+, membrane potential and metabolism can be highly relevant since cells produce signature responses to physiological stimuli through electrical activity. Where possible, the parallel study of both foetal‐derived and mature human cell types is also desirable for comparison although this is often impossible due to limitations in the availability of human tissues.
Using human EBs, Kehat and colleagues were first to use the novel electrical mapping technique of MicroElectrode Arrays (MEA) to provide a high resolution two‐dimensional electrophysiological analysis of in vitro‐generated cardiomyocytes Citation13. Spontaneous contracting phenotypes within the EBs were plated on MEA plates, which allowed long‐term, high resolution electrophysiological profiling to be measured from 60 electrodes. Collectively these plots demonstrated the development of a functional syncytium with synchronized action potential propagation. Furthermore, the MEA system provided measurement of ion channel modulation and chronotropic effects upon pharmacological intervention Citation13, Citation14. Recently, Li and colleagues used comprehensive pharmacological intervention to establish the physiological mechanisms underlying human ES cell‐derived motor neurons. Whole cell patch clamp was used to determine the presence of characteristic Na+ currents which were sensitive to the sodium channel blocker, tetrodotoxin (TTX). Additionally, specific inhibitors were used to characterize the presence of postsynaptic currents detected under voltage clamp. Slow decaying inhibitory currents, which were completely blocked by a combination of bicuculline and strychnine respectively, confirmed the presence of gamma aminobutyric acid (GABA) neurotransmitters and glycine ligand‐gated chlorine channels. The presence of ionotropic glutamate receptors was substantiated via the inhibition of the rapidly decaying inward excitatory currents observed, by a combination of D‐2‐amino‐5‐phosphonovaleric acid (AP‐5) and 6‐cyano‐7‐nitroquinoxaline‐2,3‐dione (CNQX) which have been shown to block the excitatory currents in intact spinal cord Citation15, Citation16.
Small changes in ion channel expression and function can have a major impact on the physiological competence of the organ and may prevent the tissue being therapeutically useful. For example in pancreatic β‐cells, altered channel densities of adenosine triphosphate (ATP)‐sensitive potassium ion channels underlie congenital hyperinsulinism (CHI) and defects in protein function lead to both CHI and permanent neonatal diabetes mellitus (PNDM) Citation17, Citation18. The reason for this is that altered channel numbers lead to mismatching in the decoding of information related to glucose sensing: either the membrane is permanently depolarized at resting glucose levels (CHI) or inactive at raised glucose levels (PNDM). Similarly, several ion channel defects have been associated with the spectrum of cardiac channelopathies giving rise to long‐QT and Brugada syndromes Citation19, Citation20 and neurological defects including migraine and epilepsy Citation21. Clearly it is essential to show that cells and tissues derived from human ES cells replicate their mature native counterparts in every major detail before they can be considered useful for potential replacement therapies.
Electrophysiological studies often require specialized equipment. However, simple studies of gene expression may give some indication of physiological competence of human ES‐derived material as long as a range of marker genes is used. If insulin‐secreting cells are taken as an example, then the expression of the insulin gene per se does not indicate that the cell can produce mature insulin as it also requires the enzymes necessary to convert pre‐proinsulin into insulin and mechanisms to secrete hormone. Moreover, gene expression profiles should always be coupled with studies of protein expression as RNA and protein expression can often be uncoupled. Thus, a positive result for insulin using reverse transcription‐polymerase chain reaction (RT‐PCR) does not necessary mean insulin is expressed within the sample at a protein level. Immunocytochemistry provides both quantitative results and information on intracellular localization and has been used by many research groups in an attempt to support any insulin expression detected using RT‐PCR Citation22–30. Furthermore, co‐localization studies can be carried out to determine the expression of more than one protein in the target cells. However, recent critical evidence has emerged which suggests insulin is not the most reliable marker to study in such experiments. Insulin detected in differentiating ES cells can be absorbed from the growth medium (which is often rich in insulin) and stored in the cells for up to 3 weeks Citation28. Therefore, any study published prior to the findings of Rajagopal and co‐workers, must clearly be interpreted with caution. Fortunately, in a normal β‐cell the insulin precursor proinsulin is cleaved to produce equal amounts of insulin and its metabolite, C‐peptide. Therefore current studies have avoided any confusion by the use of anti‐C‐peptide antibodies (in co‐localization studies with insulin).
The physiological function of neuronal cells and insulin‐secreting cells can also be monitored using assays to measure released protein, i.e. neurotransmitters or insulin/C‐peptide respectively. Examples of methods to quantify release include high‐performance liquid chromatography (HPLC) analysis Citation31, Citation32, radioimmunoassay (RIA) and enzyme‐linked immunosorbent assay (ELISA).
Examples of human ES cell applications in regenerative medicine
Stem cells and diabetes mellitus: Implications from the Edmonton protocol
Type 1 diabetes mellitus results from autoimmune destruction of the ß‐cells, leading to severe permanent insulin deficiency Citation33–35. It is a chronic disease that affects 5%–10% of all diabetic individuals and requires replacement therapy with insulin for life. Transplantation of islets of Langerhans serves as an effective form of treatment and each year approximately 1,300 diabetes patients receive whole organ pancreas transplants. After one year, 83% of these patients, on average, have no symptoms of diabetes or need to take insulin to maintain normal glucose homeostasis (figures released by National Institutes of Health, NIH). Despite this success, whole pancreas transplantation is of limited application since it entails major surgery and long‐term immunosuppression with its possible diabetogenic and oncogenic effects. In 2000, Shapiro and co‐workers from Edmonton, Canada reported on an improved technique of isolated islet transplantation which led to Type 1 diabetes patients being independent of exogenous insulin with minimal host versus graft and autoimmune reactions Citation36. The success of the Edmonton protocol relies on the use of a glucocorticoid‐free immunosuppressive regimen combined with the transplantation (portal vein transfusion) of purified islets from two freshly isolated donor pancreases using human albumin (rather than foetal calf serum) to reduce the loss of islet function and the risk of islet immunogenicity. However, this important breakthrough has led to the problem that under current donation procedures the amount of transplantable islets isolated from cadavers will never be sufficient to meet the demand of patients and therefore intensive research is being conducted to search for alternative sources of ß‐cells.
Some groups favour the derivation of insulin‐producing β‐cells from restricted lineages of various adult stem cells in human pancreas ductal cells Citation37, Citation38, human pancreatic islets of Langerhans Citation39 and human foetal pancreas Citation40. But currently these approaches have the disadvantage that human adult pancreatic stem cells display limited propagation in culture. In contrast, human ES cells can be propagated indefinitely in culture to theoretically provide an unlimited resource of a fully characterized cell line. It has been shown that spontaneously differentiated human ES cells express transcription factors characteristic of the establishment of the embryonic pancreas, including Insulin‐promoting factor‐1 (IPF‐1) and Neurogenin3 (NGN3), as well as pancreas‐specific transporters Glucokinase (GCK) and Glucose‐transporter isotype‐2 (GLUT‐2) Citation41. They are therefore an obvious cell source to search for β‐cells provided their pluripotent differentiation can be controlled or cell phenotypes can be specifically selected.
To date, many of the pancreatic β‐cell differentiation protocols conducted in human ES cells have been modified from studies in their mouse counterpart. Taking into account the close evolutionary and developmental relationship between endocrine and neural cell, recent studies have demonstrated the feasibility of inducing mouse ES cells to differentiate into insulin‐producing cells through the pathway used to generate neural cell types Citation22, Citation25, Citation27, Citation42. These mouse ES cell‐derived cells increased the circulating insulin levels, reduced weight loss, improved glycaemic control and were able to rescue survival in streptozocin (STZ)‐treated SCID (severe combined immunodeficient) diabetic mice Citation22, Citation25. Intriguingly, when a phosphoinositide 3‐kinase (P3K) inhibitor was used, transplanted cells completely rescued the survival of STZ‐treated SCID mice without detectable tumour formation Citation25. However, it is suggested that insulin‐producing cells produced from mouse ES cells differed significantly from pancreatic β‐cells Citation24, Citation30 and display an artefactual insulin release mechanism Citation24, Citation28. Whether this finding has a bearing on the use of human ES cells is hard to tell at present. There are numerous differences in the embryogenesis of the mouse and the human Citation43–45 and also differences in mouse and human ES cells and therefore caution is required when extrapolating findings from one species to another.
Last year, Segev et al. (2004) modified protocols established in the mouse to generate cell clusters that mimic immature islets from human ES cells by reducing the glucose concentration at the later stage of differentiation and by introducing a subsequent aggregation step in suspension culture Citation29. A substantial number of cluster cells were co‐stained for insulin and glucagon and expressed several immature pancreatic endocrine genes such as Ngn3 but low expression of Islet‐1, IPF‐1 and GCK, suggesting that these islet‐clusters were not fully developed and resembled immature pancreatic embryonic cells. In this regard, although they showed a response to the different pancreatic signal pathway agonists and antagonists, their responsiveness to the glucose was much lower than expected and in keeping with an immature status. Therefore, it might be a matter of whether mature pancreatic β‐cells that can regulate insulin secretion can be generated from human ES cells rather than just precursor or immature cell types. Physiological functional properties of these clusters should also be further characterized to confirm that these differentiated insulin‐producing cells are true β‐cells. In summary, although human ES cells show considerable promise in generating β‐cells to treat diabetes, the derivation of a bone fide β‐cell remains elusive at the present time. Therefore it may be necessary to use genetic manipulation, such as over‐expression with pancreas‐specific transcription factor (i.e. Pax4 and IPF‐1) in conjunction with specific culture regimes to enrich insulin‐producing cells from human ES cell progeny.
Neuronal tissue repair
Human ES cell‐derived neural precursors transplanted into the neonatal mouse brain are incorporated into a variety of brain regions, where they differentiate into both neurons and astrocytes Citation46. Recently, Tabar et al. (2005) showed that neural precursors from human ES cells travelled along the rostral migratory stream to the olfactory bulb, where they contributed to neurogenesis, and were capable of responding appropriately to host cues in the subventricular zone Citation47. This success is similar to the outcome of transplantation involving human central nervous system (CNS)‐derived precursors into the adult rodent brain Citation48, Citation49, suggesting that differentiated derivatives from human ES cells might function like adult stem cells to generate renewable cells and integrate into diseased tissue, at least in a mouse model.
Human ES cell‐derived neuroepithelial cells are capable of generating motor neurons Citation50 at levels that may be therapeutically useful for treating human motor neuron diseases, including spinal muscular atrophy and amyotrophic lateral sclerosis. Although functional integration of human ES‐cell derived motor neurons has not been demonstrated, cells differentiated from pluripotent embryonic germ cells (the counterparts of ES cells derived from primordial germ cells) accounted for partial functional recovery in the rats Citation51. These cells migrated into the spinal cord parenchyma in paralysed rats, further differentiated into astrocytes and mature neurons. The transplantation procedure protected host neurons from death and facilitated reafferentiation of motor neuron cell bodies, but the efficiency of the process could not account for the functional recovery. It was assumed therefore that the differentiated human ES cells secreted growth factors that protected existing neurons and thereby aided recovery. Indeed, discriminating between a direct effect of transplanted cells on tissue repair through their integration and proliferation and an indirect effect of providing beneficial factors for spontaneous recovery of organ recovery is often difficult to discern with the current animal models without carefully constructed controls.
Parkinson's disease, with a relatively high prevalence in the USA and Europe (for example, affecting over 1.5 million Americans and 120,000 Britons) is another major target for regenerative medicine. Schulz et al. (2004) showed that human ES cells differentiate into dopaminergic (DA) neurons in serum‐free suspension culture, whereby neurons were detected in culture after 2–4 weeks. These neurons responded appropriately to electrophysiological cues, and released dopamine and other catecholamines in response to K+ depolarization. Surviving tyrosine hydroxylase‐positive (TH+) neurons were detected 8 weeks after transplantation to mouse model of Parkinson's disease Citation31. Ben‐Hur et al. (2004) transplanted human ES‐derived neural cells into the striatum of Parkinsonian rats, where the neural precursors differentiated in vivo into DA neurons, and resulted in functional improvement in behavioural deficit without any teratoma formation Citation52.
Cell transplantation pioneered in Parkinson's disease Citation53 has also been applied to other neurological diseases, including stroke. Stroke remains the leading cause of serious adult disability Citation54 and it is estimated that 200 new stroke cases are identified in a population of 100,000 each year. Phase I and phase II clinical trials of NTERA‐2 cell‐derived neuro‐transplantation have now been completed in stroke patients at the University of Pittsburgh, USA. NTERA‐2 cells are pluripotent stem cells of teratocarcinoma origin Citation55 that can differentiate to neuronal phenotype in vitro after retinoic acid treatment Citation56, Citation57. Upon transplantation, NTERA‐2‐derived neurons survived more than one year in host rodent brains and improved motor and cognitive capacity in animal models of ischaemic stroke Citation58–60. Phase I clinical study using NTERA‐2 neurons included 12 patients with stroke primarily involving the basal ganglia and producing significant motor deficits Citation60. Though the initial objective of this trial was to assess safety in these patients, neurological improvement was also measured using the European Stroke Scale (ESS) at the same time. Six of the 12 stroke patients showed motor improvement, with improved local cellular function and regional metabolism Citation61. Post‐mortem brain findings of one of the patients, who later died of an unrelated cause, revealed that transplanted NTERA‐2 neurons survived at the injection site within the area of infarction 27 months after implantation and did not revert to neoplasm Citation60. A recently completed Phase II dose‐response trial revealed that there was no serious complication from the NTERA‐2 neuro‐transplantation procedure Citation62.
Taken together, early successes in animal models have generated more interest for the use of human ES cells, and perhaps human EC cell‐derived neurons to restore or maintain neurological function in humans.
Cardiac tissue restoration
Ischaemic heart disease is the primary cause of death in elderly patients, and for individuals of 65 years of age and older, 16% have ischaemic disease (Information released by Pfizer). Cardiac ischaemia is caused by oxygen deprivation to heart tissue and subsequent oxygen perfusion initiates irreversible cell damage, leading to widespread cell death and loss of function Citation63. Repairing an infarcted heart would be of enormous medical benefit. However, evidence suggesting the regeneration of the adult mammal heart is scarce, although it has been observed that human cardiac myocytes divide after myocardial infarction Citation64, and that a high level of chimerism can happen as a result from migration of primitive cells from the recipient to the graft cells Citation65.
Interest in using human ES cells to generate functional cardiac muscle is high. Beating cardiac‐like structure can readily be seen in spontaneously differentiated EBs Citation66–69, suggesting that human ES cells undergo cardiogenesis during in vitro differentiation. Visceral endoderm has also been identified as a cellular source of signals that can induce the differentiation of human ES cells into cardiomyocytes that exhibit sarcomeric marker proteins, chronotropic responses and ion channel expression typical of foetal and adult heart Citation63. Real‐time determination of intracellular Ca2+ concentration showed that these cells were electrically coupled Citation13, Citation70. Human ES cell‐derived cardiomyocytes formed structural and electromechanical connections with cultured rat cardiomyocytes and when transplanted to the hearts of swine with complete atrioventricular block, were able to rescue pacemaker function as assessed by detailed three‐dimensional electrophysiological mapping and histopathological examination Citation68. These encouraging results indicate the potential of human ES‐cell cardiomyocytes to act as a rate‐responsive biological pacemaker and for myocardial regeneration strategies.
Haematopoietic lineage
The haematopoietic differentiation of human ES includes the derivation of erythrocytes and platelets for transfusions, and the derivation of haematopoietic stem cells (HSCs) for haematopoietic cell transplantation Citation12. Human ES cells differentiated into haematopoietic precursor cells when co‐cultured with the murine bone marrow or yolk sac cell line, which can form myeloid, erythryoid and megakaryocyte colonies, identical to those produced from adult bone marrow cells. A ventral mesoderm patterning factor, bone morphogenic protein‐4 (BMP‐4) and haematopoietic growth and differentiation cytokines strongly promoted haematopoietic commitment during EB differentiation Citation71. Alternatively, different types of haematopoietic forming colonies can be isolated with the addition of specific growth factors. For example, development of erythroid cells in human ES cells can be enhanced by the addition of BMP‐4, cytokines and an angiogenic factor (vascular endothelial growth factor, VEGF‐A165) Citation72. Therefore, with further characterization of human ES cell‐derived haematopoietic progenitor cells, these cells would supply an alternative source of cells for transplantation.
Osteoblast derivation for the treatment of orthopaedic disease
Clinical application for regenerating bone tissue from human ES cells would be for the treatment of traumas and skeletal defects arising from developmental abnormalities, bone inflammation, tumours, and degenerative diseases, such as osteoporosis, and osteoarthritis. Sottile et al. (2003) were the first to describe osteogenic differentiation from human ES cells, which included nodule formation and mineral deposition in the differentiated cultures. When cells harvested from EBs were replated in a medium containing osteogenic supplements, mineralizing cells that are immunostained positive for osteocalcin could be identified Citation73. Transplanting mineralizing cells into SCID mice, regions of mineralized tissue could be detected 35 days after implantation. This observation further suggests that human ES cell‐bone derivatives underwent further maturation in vivoCitation74. Future work would be required to elucidate the functional contribution of human ES cell‐derived osteoblasts and whether they can promote repair to a fracture site or aid recovery after orthopaedic disease.
Requirements and possible complications for using human ES cells in transplantation therapy
Despite the considerable progress since human ES cells were first generated in 1998, we are still some time away from making tissue restoration a reality using these cells. There remain a number of significant problems including: 1) human ES cells demonstrate high genomic instability and non‐predictable differentiation after long‐term growth in culture; 2) the outcomes of some ES cell differentiation protocols can be inconsistent Citation75 and cells with mixed phenotypes are often present in the differentiation culture, therefore the quality of these cells are suboptimal for therapeutic uses. For example, the differentiated culture may consist of neurons and cells that appear to secrete insulin, in other words, a purified population that contains only one differentiated cell type is hard to achieve; 3) differentiated human ES cells express histocompatability antigens which cause immune rejection.
Genetic concern of human ES cells
Although original reports of human ES cell derivation and maintenance indicated a stable diploid karyotype in these cells Citation1, karyotypic changes, involving the gain of chromosome 17q, and occasionally, one or more isochromosomes 12p have been noted in human ES cells maintained for long period of time Citation76. Currently, it is not known whether this extra‐chromosomal acquisition will affect the differentiation pattern of human ES cells, and whether they will demonstrate enhanced tumorigenic capacity. It is possible that acquisition of karyotypic changes in human ES cells resulted from the adaptation of cells to the culture condition but these cells might still maintain their ability to differentiate in culture. As terminally differentiated cells are known to have none or very limited proliferative capacity, cells derived from karyotypically abnormal human ES cells may not necessarily evoke any greater tumorigenic reaction in transplanted patients than normal cells. None the less, before clinical trials are begun in humans, the issue of clinical complications that might arise from potential karyotypic changes needs to be very carefully evaluated.
Possible risk of uncontrolled cell proliferation following transplantation
Even if cells remain genetically normal during in vitro differentiation, cells from residual undifferentiated pluripotent stem cells may persist and might contribute to the formation of teratomas due to the uncontrollable cellular proliferation that might occur after transplantation Citation31. In order to selectively reduce tumour growth upon transplantation, Schuldiner et al. (2003) engineered several human ES cell lines stably transfected with the herpes simplex thymidine kinase (HSV‐tk) gene. Expression of HSV‐tk gene causes sensitivity to the US Food and Drug Administration‐approved prodrug ganciclovir, hence, any tumour proliferated from stem cell residue could be killed. Tumour mass and vascularization was reduced when the experimental mice were subjected to ganciclovir treatment Citation77 with one tumour disappearing completely, and with no signs of recurrence. This success is encouraging but more studies are required over longer time spans. However, with the appropriate drug administration, the activation of cell suicide genes may provide the insurance required to implement the use of phenotypes derived from ES cells.
A major emphasis must now be to selectively derive and purify tissue‐specific cell types during in vitro differentiation. There are several ways to achieve this. Differentiation of human ES cells should be carried out in a defined serum‐free condition so that the outcome of differentiated progenitors would be consistent and it can easily be scaled for the production of a large number of differentiated lineage‐specific cell types. Genetic manipulation could facilitate the use of human ES cells for pre‐clinical applications.
Genetic manipulation of human ES cells
While there are only few descriptions of genetic manipulation of human ES cells, in the mouse, over‐expressions of various genes lead to improved differentiation into specific cell lineages, such as midbrain dopamine neurons Citation78, insulin producing islet‐like clusters Citation22, erythroid/myeloid cells Citation79, myoblasts Citation80, osteoblasts Citation81 and myocardials Citation82. In many cases, not only did transgene expression result in an increased number of target cell types, but also improvement of functionality and physiological responses. The development of homologous recombination technology in human ES cells Citation83 will have important implications for elucidating gene function in vitro and for modifying specific ES cell‐derived tissues for therapeutic applications in transplantation medicine.
One important aspect of genetically engineered human ES cells has been the use of reporter for the purification and tracing of specific cell types Citation84. For example, using FACS, Lavon et al. (2004) isolated hepatic‐like cells from differentiated human EBs tagged by enhanced green fluorescence protein (eGFP) reporter gene encoded by albumin minimal promoter region Citation85. These cells express various liver markers, such as albumin and α‐fetoprotein, and could further be expanded without any loss of proliferation capacity. Secondly, evidence of functional integration of human ES‐cell derivatives into the target tissue could also be identified using a marker gene. In our laboratory we have derived several eGFP‐expressing transgenic clones from human ES cells following their stable transfection. When expressed from a strong, ubiquitous and constitutive promoter, eGFP could be detected in all undifferentiated stem cells and differentiated derivatives (Figure ). eGFP‐expressing differentiated derivatives and neurons have also been derived and these terminally‐differentiated progeny will be a useful tool for in vitro and in vivo tracing of grafted cells in pre‐clinical transplantation studies (Fig ).
Immunorejection and potential cloning strategy
Survival of transplanted cells correlates with the number of differences in major histocompatibility (MHC) antigens between donor and recipient that triggers T‐cell responses and rejection of cells with disparate MHC profiles Citation86. Since human ES cells and their derivatives express MHC Citation87, an important concern is that human ES‐derived cells would avoid rejection by patients following transplantation. While conventional immunosuppression treatment remains an option, the development of long‐term human ES cell therapy strategies offers the possibility of eliminating entirely the problem of cell rejection. One major route is through the use of somatic cell nuclear transfer (SCNT) where the genome of the patient replaces the genome of a donated egg from which ES cell lines are subsequently generated (a form of cloning termed therapeutic cloning). This method ensures complete genetic congruence and therefore histocompatability between human ES cell‐derived phenotypes and the patient. Hwang and co‐workers Citation88, Citation89 have demonstrated that pluripotent human embryonic stem cells can efficiently be generated by nuclear transfer and have used donors who had conditions that are potentially amenable to stem‐cell therapy such as congenital hypogammaglobulinaemia, spinal cord injury, and juvenile diabetes. It might be argued however that SCNT may not be economically viable for large scale transplantation therapies with ES (or in many countries ethically acceptable) and therefore other techniques are also being pursued.
Interestingly, recent evidence suggests that human ES cells and their derivatives are much less susceptible to immune rejection than adult cells and they did not elicit direct allorejection in vivoCitation90. There is also demonstration that human ES cells failed to stimulate proliferation of alloreactive primary human T cells, thereby providing protection to human ES cell‐derived allografts Citation86. Alternatively, human ES cells might generate both the cell phenotype necessary for the treatment of disease and haematopoietic stem cells (HSCs) or terminally differentiated dendritic cells (DCs) that might facilitate the induction of transplantation tolerance Citation91. Therefore, the use of immunosuppressive regimes for human ES cell‐based transplantation therapy could be much reduced in comparison to conventional organ transplantation.
Derivation of new human ES cell lines to GMP standard
The availability of more human ES cell lines in highly defined and controlled culture conditions would allow the generation of master cell banks to support cell transplants. However, it is a major hurdle to produce human ES cell lines that meet the criteria required for clinical utility. None of the many lines derived to date (probably many more than 100) meet the good manufacturing protocols (GMP) required by the regulatory authorities such as the Food and Drug Administration (FDA) in the USA or the Medical and Healthcare Regulatory Authority (MHRA) in the UK and therefore can only be used for research purposes and not clinical treatment. There are likely to be distinct differences in the characteristics of ES cell lines as they originate from genetically different individuals and therefore it is imperative to work on deriving specific cell phenotypes from cell lines which have been approved for future transplantation purposes. To meet GMP, human ES cells must be derived under stringent conditions of cleanliness (clean room requirements) and screened for the absence of a range of infectious agents; all equipment and processes must be validated and audited and the provenance of source tissue (pre‐implantation embryos) needs to be confirmed in terms of ethical procedures (e.g. fully‐informed patient consent) and health‐status of the donors Citation92. Apart from the fact that these requirements are usually quite different from those employed in research facilities, a number of specific hurdles must be faced. The use of animal cells and products must be eliminated or minimized to reduce the risk of the transfer of animal infectious agents to patients. This means that embryos should be cultured and manipulated preferably without using any animal products and that human ES cell lines should be derived using animal‐free culture conditions with human feeder cells (that have also met GMP) or preferably in the absence of feeder cells entirely. While many of these conditions have been achieved experimentally Citation93, Citation94, the amalgamation of the various procedures into a seamless GMP protocol represents a considerable challenge to overcome in the next few years. Moreover, contingent on generating GMP cell lines is the need for procedures to ensure quality control and assurance of any cell phenotypes that might be produced. There is therefore the need to have defined cell markers of ES cells that are to an international standard Citation95.
Summary
Although in vitro studies are essential for understanding the detailed physiological function of human ES‐derived tissues, in vivo studies are equally important to establish the usefulness of the generated material for future replacement therapy. In vivo studies widely differ according to the target organ of interest but it is essential to demonstrate both efficacy in treating an animal model of disease over a prolonged period of time, and safety of the transplanted material including tumorigenicity. Therefore, it is necessary to characterize human ES cell‐derived tissues at a functional level both in vitro and in vivo in order to discover their full potential for use in cell transplantation therapies.
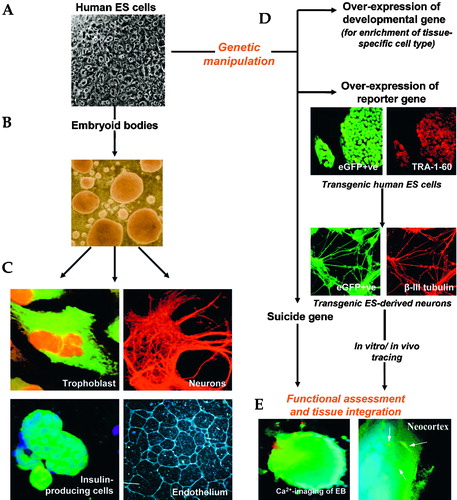
Figure 1 From undifferentiated human embryonic stem(ES) cells to regenerative medicine. A shows an undifferentiated human ES cell colony and the typical morphology of undifferentiated stem cells with high nuclear to cytoplasm ratio and prominent nuclei in the cells. One of the most commonly used methods for studying human ES cell differentiation relies on the differentiation within cellular aggregates known as embryoid bodies (B), resulting in the formation of cells containing elements of ectoderm, endoderm and mesoderm. C. Subsequently, these differentiated progenitors can be selectively induced to differentiate into tissue‐specific cell derivatives, such as neurons, endothelium and trophoblast. D. Additionally, human ES cells can be genetically manipulated in vitro and used as vectors in cell‐based therapies. Over‐expression of key developmental gene would enhance the differentiation of human ES cells into lineage‐specific cell derivatives, and with the reporter gene‐tagging technology, these differentiated cells can also be used to trace the trafficking of the transplanted cells in pre‐clinical studies. Shown here, are the eGFP‐expressing human ES cell clones which express TRA‐1‐60 stem cell marker, and eGFP‐expressing neurons stained with TUJ‐1 antibody which detects neuronal β‐III tubulin. On the other hand, it has been proven that possible tumorigenesis evoked by the persistent stem cells amongst the differentiated cultures can be selectively eliminated by the over‐expression of a ‘suicide gene’ and appropriate drug administration. E. It is also important to ensure that differentiated cells acquire cell surface signals and ascertain whether integration occurs upon transplantation. Left: changes in the intracellular Ca2+ concentration, measured using fluorescent probe Fura‐2AM can be used to record the transient Ca2+ changes in putative β‐cells derived from an EB, showing that differentiated cells express voltage‐dependent Ca2+‐channels. Right: eGFP‐expressing human EC‐derived neurons that are integrated into rat neocortex upon transplantation. The outcome is therefore pertinent to potential clinical use of human ES cells in transplantation therapy.
Acknowledgements:
The authors are indebted to Ruth Shepherd, Hyeon‐Seon Park, Kyoji Ohyama and Kei Cho for functional study of human ES cells. This research was supported by grants from the Juvenile Diabetes Research Foundation (JDRF).
References
- Thomson J., Itskovitz‐Eldor J., Shapiro S., Waknitz M., Swiergiel J., Marshall V., et al. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282: 1145–7
- Reubinoff B. E., Pera M. F., Fong C. Y., Trounson A., Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol 2000; 18: 399–404
- Amit M., Carpenter M. K., Inokuma M. S., Chiu C. P., Harris C. P., Waknitz M. A., et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol 2000; 227: 271–8
- Hovatta O., Mikkola M., Gertow K., Stromberg A. ‐M., Inzunza J., Hreinsson J., et al. A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum Reprod 2003; 18: 1404–9
- Park J. H., Kim S. J., Oh E. J., Moon S. Y., Roh S. I., Kim C. G., et al. Establishment and maintenance of human embryonic stem cells on STO, a permanently growing cell line. Biol Reprod 2003; 69: 2007–14
- Richards M., Fong C. ‐Y., Chan W. ‐K., Wong P. ‐C., Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cell lines. Nat Biotechnol 2002; 20: 933–6
- Richards M., Tan S., Fong C. ‐Y., Biswas A., Chan W. ‐K., Bongso A. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells 2003; 21: 546–56
- Soria B. In‐vitro differentiation of pancreatic beta‐cells. Differentiation 2001; 4: 205–19
- Studer L., Csete M., Lee S. ‐H., Kabbani N., Walikonis J., Wold B., et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J. Neurosci 2000; 20: 7377–83
- Schuldiner M., Yanuka O., Itskovitz‐Eldor J., Melton D. A., Benvenisty N. From the Cover: Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A 2000; 97: 11307–12
- Reubinoff B. E., Itsykson P., Turetsky T., Pera M. F., Reinhartz E., Itzik A., et al. Neural progenitors from human embryonic stem cells. Nat Biotechnol 2001; 19: 1134–40
- Kaufman D. S., Hanson E. T., Lewis R. L., Auerbach R., Thomson J. A. Hematopoietic colony‐forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A 2001; 98: 10716–21
- Kehat I., Gepstein A., Spira A., Itskovitz‐Eldor J., Gepstein L. High‐resolution electrophysiological assessment of human embryonic stem cell‐derived cardiomyocytes: a novel in vitro model for the study of conduction. Circ Res 2002; 91: 659–61
- Reppel M., Boettinger C., Hescheler J. Beta‐adrenergic and muscarinic modulation of human embryonic stem cell‐derived cardiomyocytes. Cell Physiol Biochem 2004; 14: 187–96
- Gao B. ‐X., Cheng G., Ziskind‐Conhaim L. Development of spontaneous synaptic transmission in the rat spinal cord. J Neurophysiol 1998; 79: 2277–87
- Li X. J., Du Z. W., Zarnowska E. D., Pankratz M., Hansen L. O., Pearce R. A., et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol 2005; 23: 215–21
- Dunne M. J., Cosgrove K. E., Shepherd R. M., Aynsley‐Green A., Lindley K. J. Hyperinsulinism in infancy: from basic science to clinical disease. Physiol Rev 2004; 84: 239–75
- Slingerland A. S., Hattersley A. T. Mutations in the Kir6.2 subunit of the KATP channel and permanent neonatal diabetes: new insights and new treatment. Ann Med 2005; 37: 186–95
- Roberts R., Brugada R. Genetics and arrhythmias. Ann Rev Med 2003; 54: 257–67
- Tester D. J., Will M. L., Haglund C. M., Ackerman M. J. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm 2005; 2: 507–17
- Graves T. D., Hanna M. G. Neurological channelopathies. Postgrad Med J 2005; 81: 20–32
- Blyszczuk P., Czyz J., Kania G., Wagner M., Roll U., St‐Onge L., et al. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin‐positive progenitor and insulin‐producing cells. Proc Natl Acad Sci U S A 2003; 100: 998–1003
- Blyszczuk P., Asbrand C., Rozzo A., Kania G., St‐Onge L., Rupnik M., et al. Embryonic stem cells differentiate into insulin‐producing cells without selection of nestin‐expressing cells. Int J Dev Biol 2004; 48: 1095–104
- Hansson M., Tonning A., Frandsen U., Petri A., Rajagopal J., Englund M. C., et al. Artifactual insulin release from differentiated embryonic stem cells. Diabetes 2004; 53: 2603–9
- Hori Y., Rulifson I. C., Tsai B. C., Heit J. J., Cahoy J. D., Kim S. K. Growth inhibitors promote differentiation of insulin‐producing tissue from embryonic stem cells. Proc Natl Acad Sci U S A 2002; 99: 16105–10
- Ku H. T., Zhang N., Kubo A., O'Connor R., Mao M., Keller G., et al. Committing embryonic stem cells to early endocrine pancreas in vitro. Stem Cells 2004; 22: 1205–17
- Miyazaki S., Yamato E., Miyazaki J. Regulated expression of pdx‐1 promotes in vitro differentiation of insulin‐producing cells from embryonic stem cells. Diabetes 2004; 53: 1030–7
- Rajagopal J., Anderson W. J., Kume S., Martinez O. I., Melton D. A. Insulin staining of ES cell progeny from insulin uptake. Science 2003; 299: 363
- Segev H., Fishman B., Ziskind A., Shulman M., Itskovitz‐Eldor J. Differentiation of human embryonic stem cells into insulin‐producing clusters. Stem Cells 2004; 22: 265–74
- Sipione S., Eshpeter A., Lyon J. G., Korbutt G. S., Bleackley R. C. Insulin expressing cells from differentiated embryonic stem cells are not beta cells. Diabetologia 2004; 47: 499–508
- Schulz T. C., Noggle S. A., Palmarini G. M., Weiler D. A., Lyons I. G., Pensa K. A., et al. Differentiation of human embryonic stem cells to dopaminergic neurons in serum‐free suspension culture. Stem Cells 2004; 22: 1218–38
- Park C. H., Minn Y. K., Lee J. Y., Choi D. H., Chang M. Y., Shim J. W., et al. In vitro and in vivo analyses of human embryonic stem cell‐derived dopamine neurons. J Neurochem 2005; 92: 1265–76
- Peltoniemi P., Yki‐Jarvinen H., Oikonen V., Oksanen A., Takala T. O., Ronnemaa T., et al. Resistance to exercise‐induced increase in glucose uptake during hyperinsulinemia in insulin‐resistant skeletal muscle of patients with type 1 Diabetes. Diabetes 2001; 50: 1371–7
- Vuorinen‐Markkola H., Sinisalo M., Koivisto V. Guar gum in insulin‐dependent diabetes: effects on glycemic control and serum lipoproteins. Am J Clin Nutr 1992; 56: 1056–60
- Yki‐Järvinen H. D., Mott A. A., Young K. S., Bogardus C. Regulation of glycogen synthase and phosphorylase activities by glucose, insulin and basal enzyme activity in human skeletal muscle. J Clin Invest 1987; 80: 95–100
- Shapiro A. M. J., Lakey J. R. T., Ryan E. A., Korbutt G. S., Toth E., Warnock G. L., et al. Islet transplantation in seven patients with type 1 Diabetes Mellitus using a glucocorticoid‐free immunosuppressive Regimen. N Engl J Med 2000; 343: 230–8
- Bonner‐Weir S., Taneja M., Weir G. C., Tatarkiewicz K., Song K. ‐H., Sharma A., et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A 2000; 97: 7999–8004
- Hui H. X., Wright C., Perfetti R. Glucagon‐like peptide 1 induces differentiation of islet duodenal homeobox‐1‐positive pancreatic ductal cells into insulin‐secreting cells. Diabetes 2001; 50: 785–96
- Zulewski H., Abraham E. J., Gerlach M. J., Daniel P. B., Moritz W., Muller B., et al. Multipotential nestin‐positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes 2001; 50: 521–33
- Beattie G. M., Otonkoski T., Lopez A. D., Hayek A. Functional beta‐cell mass after transplantation of human foetal pancreatic cells: differentiation or proliferation?. Diabetes 1997; 46: 244–8
- Assady S., Maor G., Amit M., Itskovitz‐Eldor J., Skorecki K. L., Tzukerman M. Insulin production by human embryonic stem cells. Diabetes 2001; 50: 1691–7
- Lumelsky N., Blondel O., Laeng P., Velasco I., Ravin R., McKay R. Differentiation of embryonic stem cells to insulin‐secreting structures similar to pancreatic islets. Science 2001; 292: 1389–94
- Fougerousse F., Bullen P., Herasse M., Lindsay S., Richard I., Wilson D., et al. Human–mouse differences in the embryonic expression patterns of developmental control genes and disease genes. Human Molecular Genetics 2000; 9: 165–73
- Piper K., Brickwood S., Turnpenny L., Cameron I., Ball S., Wilson D., et al. Beta cell differentiation during early human pancreas development. J Endocrinol 2004; 181: 11–23
- Richardson M. K., Hanken J., Gooneratne M. L., Pieau C., Raynaud A., Selwood L., et al. There is no highly conserved embryonic stage in the vertebrates: implications for current theories of evolution and development. Anat Embryol 1997; 196: 91–106
- Zhang S. C., Wernig M., Duncan I. D., Brustle O., Thomson J. A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 2001; 19: 1129–33
- Tabar V., Panagiotakos G., Greenberg E. D., Chan B. K., Sadelain M., Gutin P. H., et al. Migration and differentiation of neural precursors derived from human embryonic stem cells in the rat brain. Nat Biotechnol 2005; 23: 601–6
- Akiyama Y., Honmou O., Kato T., Uede T., Hashi K., Kocsis J. D. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Experimental Neurology 2001; 167: 27–39
- Fricker R. A., Carpenter M. K., Winkler C., Greco C., Gates M. A., Bjorklund A. Site‐specific migration and neuronal differentiation of human neural progenitor cells after transplantation in the adult rat brain. J Neurosci 1999; 19: 5990–6005
- Shin S., Dalton S., Stice S. Human motor neuron differentiation from human embryonic stem cells. Stem Cells Dev 2005; 14: 266–9
- Kerr D. A., Llado J., Shamblott M. J., Maragakis N. J., Irani D. N., Crawford T. O., et al. Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury. J Neurosci 2003; 23: 5131–40
- Ben‐Hur T., Idelson M., Khaner H., Pera M., Reinhartz E., Itzik A., et al. Transplantation of human embryonic stem cell‐derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells 2004; 22: 1246–55
- Freed C. R., Breeze R. E., Rosenberg N. L., Schneck S. A., Wells T. H., Barrett J. N., et al. Transplantation of human fetal dopamine cells for Parkinson's disease. Results at 1 year. Arch Neurol 1990; 47: 505–12
- Kojima S., Omura T., Wakamatsu W., Kishi M., Yamazaki T., Iida M., et al. Prognosis and disability of stroke patients after 4 years in Akita, Japan. Stroke 1990; 21: 72–7
- Andrews P. W., Damjanov I., Simon D., Banting G., Carlin C., Dracopoli N. C., et al. Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera‐2: differentiation in vivo and in vitro. Lab Invest 1984; 50: 147–62
- Andrews P. W. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol 1984; 103: 285–93
- Pleasure S. J., Page C., Lee V. M. ‐Y. Pure, postmitotic, polarized human neurons derived from Ntera2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons. J Neurosci 1992; 12: 1802–15
- Hartley R. S., Margulis M., Fishman P. S., Lee V. M. ‐Y., Tang C. ‐M. Functional synapses are formed between human NTera2 (NT2N, hNT) neurons grown on astrocytes. J Comp Neurol 1999; 407: 1–10
- Lee V. M. ‐Y., Hartley R. S., Trojanowski J. Q. Neurobiology of human neurons (NT2N) grafted into mouse spinal cord: implications for improved therapy of spinal cord injury. Prog Brain Res 2000; 128: 299–307
- Nelson P. T., Kondziolka D., Wechsler L., Goldstein S., Gebel J., Decesare S., et al. Clonal human (hNT) neuron grafts for stroke therapy: neuropathology in a patient 27 months after implantation. Am J Pathol 2002; 160: 1201–6
- Meltzer C. C., Kondziolka D., Villemagne V. L., Wechsler L., Goldstein S., Thulborn K. R., et al. Serial [18F]fluorodeoxyglucose positron emission tomography after human neuronal implantation for stroke. Neurosurgery 2001; 49: 586–92
- Wechsler L. R. Stem cell transplantation for stroke. Cleve Clin J Med 2004; 71: S40–1
- Mummery C., Ward D., van den Brink C. E., Bird S. D., Doevendans P. A., Opthof T., et al. Cardiomyocyte differentiation of mouse and human embryonic stem cells. J Anat 2002; 200: 233–42
- Beltrami A. P., Urbanek K., Kajstura J., Yan S. M., Finato N., Bussani R., et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med 2001; 344: 1150–7
- Quaini F., Urbanek K., Beltrami A. P., Finato N., Beltrami C. A., Nadal‐Ginard B., et al. Chimerism of the transplanted heart. N Engl J Med 2002; 346: 5–15
- He J. Q., Ma Y., Lee Y., Thomson J. A., Kamp T. J. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res 2003; 93: 32–9
- Kehat I., Kenyagin‐Karsenti D., Snir M., Segev H., Amit M., Gepstein A., et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Invest 2001; 108: 407–14
- Kehat I., Khimovich L., Caspi O., Gepstein A., Shofti R., Arbel G., et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol 2004; 22: 1282–9
- Xu C., Inokuma M. S., Denham J., Golds K., Kundu P., Gold J. D., et al. Feeder‐free growth of undifferentiated human embryonic stem cells. Nat Biotechnol 2001; 19: 971–4
- Mummery C., Ward‐van Oostwaard D., Doevendans P., Spijker R., van den Brink S., Hassink R., et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm‐like cells. Circulation 2003; 107: 2733–40
- Chadwick K., Wang L., Li L., Menendez P., Murdoch B., Rouleau A., et al. Cytokines and BMP‐4 promote hematopoietic differentiation of human embryonic stem cells. Blood 2003; 102: 906–15
- Cerdan C., Rouleau A., Bhatia M. VEGF‐A165 augments erythropoietic development from human embryonic stem cells. Blood 2004; 103: 2504–12
- Sottile V., Thomson A., McWhir J. In vitro osteogenic differentiation of human ES cells. Cloning Stem Cells 2003; 5: 149–55
- Bielby R. C., Boccaccini A. R., Polak J. M., Buttery L. D. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng 2004; 10: 1518–25
- Leon‐Quinto T., Jones J., Skoudy A., Burcin M., Soria B. In vitro directed differentiation of mouse embryonic stem cells into insulin‐producing cells. Diabetologia 2004; 47: 1442–51
- Draper J. S., Smith K., Gokhale P., Moore H. D., Maltby E., Johnson J., et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol 2004; 22: 53–4
- Schuldiner M., Itskovitz‐Eldor J., Benvenisty N. Selective ablation of human embryonic stem cells expressing a “suicide” gene. Stem Cells 2003; 21: 257–65
- Chung S., Sonntag K. ‐C., Andersson T., Bjorklund L. M., Park J. ‐J., Kim D. ‐W., et al. Genetic engineering of mouse embryonic stem cells by Nurr1 enhances differentiation and maturation into dopaminergic neurons. Eur J Neurosci 2002; 16: 1829–38
- Helgason C., Sauvageau G., Lawrence H., Largman C., Humphries R. Overexpression of HOXB4 enhances the hematopoietic potential of embryonic stem cells differentiated in vitro. Blood 1996; 87: 2740–9
- Prelle K., Wobus A. M., Krebs O., Blum W. F., Wolf E. Overexpression of insulin‐like growth factor‐II in mouse embryonic stem cells promotes myogenic differentiation. Biochem Biophys Res Commun 2000; 277: 631–8
- Tai G., Polak J. M., Bishop A. E., Christodoulou I., Buttery L. D. Differentiation of osteoblasts from murine embryonic stem cells by overexpression of the transcriptional factor osterix. Tissue Eng 2004; 10: 1456–66
- Sudou A., Muramatsu H., Kaname T., Kadomatsu K., Muramatsu T. Le(X) structure enhances myocardial differentiation from embryonic stem cells. Cell Struct Funct 1997; 22: 247–51
- Zwaka T. P., Thomson J. A. Homologous recombination in human embryonic stem cells. Nat Biotechnol 2003; 21: 319–21
- Lavon N., Benvenisty N. Differentiation and genetic manipulation of human embryonic stem cells and the analysis of the cardiovascular system. Trends Cardiovasc Med 2003; 13: 47–52
- Lavon N., Yanuka O., Benvenisty N. Differentiation and isolation of hepatic‐like cells from human embryonic stem cells. Differentiation 2004; 72: 230–8
- Li L., Baroja M. L., Majumdar A., Chadwick K., Rouleau A., Gallacher L., et al. Human embryonic stem cells possess immune‐privileged properties. Stem Cells 2004; 22: 448–56
- Drukker M., Katz G., Urbach A., Schuldiner M., Markel G., Itskovitz‐Eldor J., et al. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci U S A 2002; 99: 9864–9
- Hwang W. S., Ryu Y. J., Park J. H., Park E. S., Lee E. G., Koo J. M., et al. Evidence of a pluripotent human embryonic stem cell line derived from a cloned blastocyst. Science 2004; 303: 1669–74
- Hwang W. S., Roh S. I., Lee B. C., Kang S. K., Kwon D. K., Kim S., et al. Patient‐specific embryonic stem cells derived from human SCNT blastocysts. Science 2005; 308: 777–83
- Drukker M., Katchman H., Katz G., Even‐Tov Friedman S., Shezen E., Horenstein E., et al. Human embryonic stem cells and their differentiated derivatives are less susceptible for immune rejection than adult cells. Stem Cells. 2005; Aug. (Epub ahead of print 2005‐0188). 0000
- Fairchild P. J., Cartland S., Nolan K. F., Waldmann H. Embryonic stem cells and the challenge of transplantation tolerance. Trends Immunol 2004; 25: 465–70
- Draper J. S., Moore H. D., Ruban L. N., Gokhale P. J., Andrews P. Culture and characterization of human embryonic stem cells. Stem Cells Dev 2004; 4: 325–36
- Hovatta O., Skottman H. Feeder‐free derivation of human embryonic stem‐cell lines. Lancet 2005; 365: 1601–3
- Klimanskaya I., Chung Y., Meisner L., Johnson J., West M. D., Lanza R. Human embryonic stem cells derived without feeder cells. Lancet 2005; 365: 1636–41
- Andrews P. W., Benvenisty N., McKay R., Pera M. F., Rossant J., Semb H., et al. The International Stem Cell Initiative: toward benchmarks for human embryonic stem cell research. Nat Biotechnol 2005; 23: 795–7