Abstract
Circulating cardiac natriuretic peptide levels are being used increasingly in a range of clinical circumstances. Since it is evident that drugs used in the treatment of cardiovascular disorders can modulate natriuretic peptide levels, we here review the literature documenting these effects. Diuretics, blockers of the renin‐angiotensin system, vasodilator agents, dopamine‐like agonists, amiodarone, and perhaps allopurinol and statins suppress natriuretic peptide levels, most obviously in heart failure. Beta‐blockers stimulate natriuretic peptide concentrations in hypertensive subjects, whereas in heart failure they have little effect or are stimulatory in the short term and inhibitory with sustained therapy. Digitalis compounds and aspirin tend to increase natriuretic peptide levels, and calcium channel blocking agents have varying effects depending on the individual drug and duration of administration. The effects of other drugs are less clear. Additional information is needed regarding the effects of medications along with dissection of the role of altered cardiac secretion versus changes in plasma clearance as explanation for drug‐induced perturbations in natriuretic peptide concentrations. In the meantime, clinicians need to consider the known effects of medications when interpreting plasma levels of the cardiac natriuretic peptides.
Introduction
The heart secretes the 28 amino acid atrial natriuretic peptide (ANP) and the 32 amino acid B‐type natriuretic peptide (BNP), both of which are biologically active and circulate normally in low picomolar concentrations. They are physiologically important protectors against volume overload and excessive vasoconstriction, acting in concert with other vasodilator/natriuretic/growth‐inhibitory systems to counterbalance contrary systems, most obviously, but not exclusively, the renin‐angiotensin system Citation1,2. These two bioactive cardiac peptides are cleaved from the carboxy‐terminal end of pro‐hormones (proANP and proBNP) yielding also N‐terminal fragments (98‐amino acid NT‐proANP and 76‐amino acid NT‐proBNP) which have no identified biological activity. Bioactive ANP and BNP are presumed to be secreted in a 1:1 ratio with their respective NT‐pro fragments, but the latter circulate at generally higher concentrations because, presumably again, their elimination rates from the circulation are slower.
As with all circulating hormones, their concentrations in blood reflect rates of secretion and clearance. In regard to secretion rates, stretch of the cardiac chambers (or strictly, transmural pressure) is the dominant stimulus Citation3,4 although additional factors are capable of modulating secretion. These include the renin‐angiotensin and sympathetic systems (alpha‐1‐agonists are generally seen to be stimulatory and beta‐agonists inhibitory), endothelin‐1, cytokines and growth factors, thyroid hormone, arginine vasopressin, thrombin, glucocorticoids, prostaglandins, and hypoxia/ischaemia Citation1.
The effect of medications on the secretory rate of natriuretic peptides will depend on change in the sum of the numerous input signals listed above, but dominated by alterations in stretch of the cardiac chambers. For example, angiotensin‐converting enzyme (ACE) inhibitors, when used in the treatment of severe grades of heart failure, reduce stretch of the cardiac chambers while also lowering plasma and cardiac angiotensin II levels and probably reducing sympathetic ‘traffic’ to the heart—all of which should contribute to a decline in the cardiac secretion rate of the natriuretic peptides. The situation is more complex for the beta‐blockers when used in heart failure. In the short term, the beta‐blockers may augment cardiac secretion of the natriuretic peptides concomitant with probable increased stretch of cardiac chambers together with blockade of the inhibitory effect of beta‐agonism, notwithstanding a fall in stimulatory angiotensin II levels Citation5. Clearance from plasma of biologically active ANP and BNP may be reduced by beta‐blockers Citation6 thereby contributing to the rise in their circulating levels. By contrast, with chronic beta‐blocker therapy and remodelling of the cardiac chambers (including reduced stretch and a decline in left ventricular mass), cardiac secretion of the cardiac peptides is likely to decline despite the above‐mentioned inhibitory action of these drugs on the plasma clearance rates of ANP and BNP Citation6. These issues are discussed more fully below.
Clearance of bioactive ANP and BNP from the circulation is accomplished through two pathways, the enzyme neutral endopeptidase 24.11 (neprilysin) and clearance receptors, although binding to their bioactive type A receptors presumably contributes to a small extent. Little is known regarding the clearance mechanisms for the NT‐propeptides and, accordingly, there is a dearth of information on the possibility that medications alter their clearance characteristics.
Circulating levels of the cardiac natriuretic peptides are of scientific interest and of actual, or potential, clinical use in a number of circumstances. First, their levels are of proven benefit in the diagnosis of heart failure for patients presenting with acute dyspnoea. Elevated values are consistent with a diagnosis of heart failure under these circumstances, and normal levels are powerful negative predictors of that diagnosis. Second, their levels in the circulation provide a robust prognostic index, either alone or when combined with additional prognostic factors in patients with established heart failure or post‐myocardial infarction, and also in patients with end‐stage chronic renal failure. Third, their levels hold the potential to function as relatively simple and cheap screening tests for cardiovascular disease, particularly left ventricular dysfunction, in predisposed subjects (diabetics, hypertensives, and the frail elderly, for example) and possibly in the general population. Fourth, guiding the intensity of drug treatment for patients with chronic heart failure according to circulating levels of these peptides has intuitive appeal, the results of the two completed studies are encouraging, and additional, larger studies are underway. Fifth, measurement of cardiac peptide levels might prove clinically useful in a variety of other clinical circumstances including the assessment of severity and progression of valvular heart disease and amyloid heart disease, the early detection of cardiotoxic doses of antimitotic drugs, as one determinant of myocardial status post‐cardiac transplantation, and in guiding the management of patients with end‐stage chronic renal failure.
Whereas elevated circulating levels of the cardiac natriuretic peptides are best known to be associated with heart failure based on systolic and/or diastolic left ventricular dysfunction, interpretation requires realization that their levels increase with age and as renal function deteriorates, are higher on average in females than males, are elevated by tachyarrhythmias, hypertension (especially with concomitant left ventricular hypertrophy and diastolic or systolic dysfunction) and hyperthyroidism, and tend to be lower in the obese and in hypothyroidism.
Apart from these factors, numerous articles attest to the fact that medications, especially those used to treat cardiovascular disorders, can alter levels of the cardiac peptides. Apart from exceptional circumstances, mitral stenosis for example Citation7,8, levels of the four peptides (ANP, NT‐proANP, BNP and NT‐proBNP) are closely correlated and almost invariably rise or fall in parallel even if their rates of change can differ Citation9. Accordingly, we will assume in this review that studies reporting drug‐induced perturbations in levels of one peptide are likely to reflect changes in the other three peptides. Nevertheless, it has been observed by a number of workers that the B‐type natriuretic peptides more closely relate to haemodynamic indices and prognosis in cardiac disorders than the two A‐type peptides. Furthermore, in contrast to assays for ANP and NT‐proANP, well validated commercial BNP and NT‐proBNP assays are in wide‐spread, and increasing Citation10, clinical use. We will tend, therefore, to emphasize B‐type natriuretic peptide responses to medications used to treat cardiovascular disorders whilst acknowledging studies reporting ANP and NT‐proANP levels—which reflect, by and large, the discovery of ANP before BNP and hence the earlier development of assays to measure the A‐type peptides. In this review paper we will restrict our attention largely to studies carried out in man and ignore the abundant studies performed in experimental animals, isolated tissues, cells, and organs. Since drugs which inhibit the enzyme neutral endopeptidase 24.11 along with ACE, the so‐called vasopeptidase inhibitors, are not in clinical use, we will not discuss their reported effects on circulating natriuretic peptide levels.
Key messages
Interpretation of circulating levels of cardiac natriuretic peptides requires awareness that many medications can alter their concentration through changes in secretion and/or clearance.
Diuretics, blockers of the renin‐angiotensin system, vasodilator drugs, dopamine‐like agonists, amiodarone, and perhaps allopurinol and statins reduce natriuretic peptide levels, whereas digitalis compounds, aspirin, oestrogens, and some immunosuppressive drugs tend to be stimulatory, and calcium channel blockers have varying effects.
The possibility that medications other than those noted above also modify plasma natriuretic peptide levels demands further study.
Diuretics
Healthy subjects, essential hypertension
Lijnen et al. reported a substantial (59%) decline in plasma ANP levels in healthy men receiving the chlorthalidone‐like diuretic, xipamide, for 16 weeks, most obvious after 1 week Citation11. By contrast, patients with essential hypertension were reported by Chalmers et al. to exhibit a rise in plasma ANP levels (from a mean of 92 to 145 pg/mL) over a 4‐week period of monotherapy with hydrochlorothiazide which reduced blood pressure significantly Citation12. In a study of hypertensive patients on chronic diuretic therapy alone (n = 8) or in combination with other antihypertensive drugs (n = 54), plasma ANP levels were not different from those in untreated hypertensive patients Citation13.
Heart failure
BNP levels in patients with severe, refractory heart failure fell by more than 50% with intravenous frusemide treatment (with or without concomitant hypertonic saline) over 6 days in association with a natriuresis, and a decline in arterial pressure and heart rate Citation14. Diuretics combined with nitroprusside reduced ANP, NT‐proANP and BNP levels significantly (by 26%–52%) and promptly (over 1.4 days) in patients with advanced, decompensated heart failure who were removed temporarily from their maintenance ACE inhibitors Citation15. The effects of diuretics versus nitroprusside in this study are impossible to separate. An apparently contradictory observation by Starklint and colleagues that a single 80‐mg dose of frusemide in patients with apparently stable chronic heart failure was without effect on plasma ANP and BNP levels Citation16 is not unexpected if, as is likely, daily maintenance diuretic administration elicits little immediate haemodynamic effect under these circumstances.
Addition of aldosterone receptor blockade to conventional medications (including beta‐blockers) for moderate to severe grades of heart failure, results in a fall in circulating natriuretic peptide levels. For example, in patients with New York Heart Association (NYHA) functional class III–IV heart failure associated with a low left ventricular ejection fraction, spironolactone 12.5–50 mg/day reduced plasma BNP levels 23% at 3 and 6 months in the Randomized ALdactone Evaluation Study (RALES) Citation17. Spironolactone compared to placebo, when added to conventional drug therapy (including beta‐blockers in one‐third of patients), reduced plasma ANP and BNP levels by 39%–55% along with left ventricular mass and volumes over 4 months in 20 patients with mild‐to‐moderate non‐ischaemic heart failure and a reduced left ventricular ejection fraction Citation18. A similar outcome for patients with moderate degrees of heart failure was reported with 6 months of spironolactone therapy versus placebo by Feola et al. in which BNP values declined by more than 50% Citation19. For patients with mild heart failure on optimal medical therapy, a statistically significant, if small, fall in BNP was documented over 3 months of spironolactone (12.5–50 mg/day) treatment versus placebo Citation20. Two studies report no significant effect. The first, by Roonsritong et al., showed that plasma BNP levels were not altered by the addition of spironolactone (25 mg daily) versus placebo for 4 months in elderly patients with mild isolated diastolic left ventricular dysfunction, most of whom were hypertensive Citation21. In the second study, Kinugawa et al. reported that spironolactone (25 mg/day) had a statistically insignificant suppressive effect on post‐exercise ANP levels in a small cohort (n = 9) of patients with heart failure (NYHA functional class II and III, left ventricular ejection fraction (LVEF) 10%–59%) receiving frusemide and low‐dose maintenance enalapril Citation22.
In summary, what little information is available regarding the effects of diuretics on circulating natriuretic peptide levels in healthy subjects or patients with essential hypertension suggests there may be an initial decline followed by return to, or close to, baseline values. The isolated report that hydrochlorothiazide can stimulate levels of ANP in essential hypertensives Citation12 needs confirmation. The situation in heart failure is clearer. Diuretics, whether loop or aldosterone receptor blockers, reduce circulating natriuretic peptide levels, more obviously in patients with severe grades of heart failure and evidence of fluid retention than in those with mild cardiac failure. This presumably reflects, to a large extent, a decline in cardiac natriuretic peptide secretion in parallel with reduced pre‐load and stretch of the cardiac chambers. It is not known whether alterations in plasma clearance of the peptides contribute to (or perhaps limit) the fall in peptide levels. For patients who are stabilized on therapy, administration of their daily maintenance dose of diuretic may have little effect on natriuretic peptide levels on that day. Likewise, introduction of diuretics in those with mild degrees of left ventricular dysfunction may show little or no response in natriuretic peptide levels.
Beta‐adrenergic blockers
Of all the drug groups studied, beta‐blockers have received the most attention. The results have often varied between studies, but patterns have, nevertheless, emerged over time.
Normal subjects, hypertension, coronary artery disease
The accumulated evidence is that beta‐blockers, whether selective or non‐selective, with or without intrinsic sympathomimetic activity, with or without α1 blocking activity, whether given as a single dose or chronically, evoke a rise in circulating levels of the natriuretic peptides, more obvious with exercise than at rest ().
Table I. Studies reporting a stimulatory effect of beta‐blocker drugs on circulating levels of cardiac natriuretic peptides in healthy volunteers, or patients with coronary heart disease or hypertension.
A few studies, not shown in , have reported lesser stimulatory effects of beta‐blockade on natriuretic peptide levels. Dahlof and colleagues showed that levels of three peptides, ANP, BNP, and NT‐proBNP, rose slightly, though not statistically significantly, across 36 weeks of atenolol monotherapy in 99 patients with essential hypertension and left ventricular hypertrophy Citation40. Although there was no time‐matched placebo group in that study, there was a parallel group that received the angiotensin receptor blocker (ARB), losartan, in which levels of the three peptides declined significantly. The difference between atenolol and losartan groups regarding change in the circulating peptide levels was statistically significant for each of the three peptides. Similar results were reported by Davies and colleagues for 17 patients with essential hypertension randomized to atenolol and losartan each for 4 months in a randomized, cross‐over study in which BNP levels were significantly higher on atenolol than losartan Citation41. Differences between the groups in these two studies probably reflect a minor effect of atenolol to increase natriuretic peptide levels combined with a clearer effect of losartan to reduce them although, in the absence of a placebo‐control group, this remains speculative.
Of the far fewer studies reporting a suppressive action of beta‐blockers on natriuretic peptide levels, two were in subjects receiving beta‐blockers with β2 stimulating or α1 blocking activity. The first, by Omvik and colleagues, demonstrated a 27% decline in resting ANP levels (from 20.5 to 15 pmol/L) at 2 hours after the first dose of carvedilol but no change thereafter in supine, sitting or exercise‐related levels of ANP after 9 months of carvedilol monotherapy Citation42. In that there was no time‐matched placebo group, however, the initial 27% decline in ANP cannot be attributed confidently to carvedilol. The second study, by Bohlen et al., reported that celiprolol administered to healthy volunteers for 3 weeks reduced resting plasma levels of ANP and attenuated the rise in ANP associated with a glucose load Citation43. Again, however, interpretation is hindered by the absence of a time‐matched placebo‐control group. Of other studies reporting little or no effect of beta‐blockers, Kantola et al. showed, in 15 healthy volunteers, that a single dose of atenolol, propranolol or pindolol did not alter the response of plasma ANP to exercise compared to placebo, but pindolol enhanced slightly the post‐exercise decline in ANP Citation44. Legault et al. reported that administration of propranolol 80 mg had no effect on the ANP response to intravenous volume loading in healthy volunteers Citation45.
In summary, the vast majority of studies report that beta‐blockers increase circulating levels of the cardiac natriuretic peptides in healthy subjects and patients with essential hypertension or coronary artery disease. What might explain this stimulatory effect of beta‐blockers? Luchner and Schunkert, reviewing data from studies in animals and man, concluded that two mechanisms are likely be involved, one increasing cardiac production of the peptides, the other inhibiting their plasma clearance Citation6. First, withdrawal of beta‐receptor‐mediated inhibition of cardiac natriuretic peptide release from the heart would leave stimulatory, alpha‐receptor effects of cardiac sympathetic innervation, unopposed Citation6,Citation46. In addition, Olsen et al. Citation39 surmised, as have others Citation31, that beta‐blockers might augment cardiac production of the peptides through a bradycardia‐associated increase in diameter of the atria and ventricles which thereby heightens cardiac wall stress. Second, a beta‐blocker‐related decrease in extracardiac clearance of ANP and BNP through down‐regulation of natriuretic peptide clearance receptors Citation47,48 would, other factors being equal, increase circulating ANP and BNP levels and, in the presence of increased cardiac secretion, exaggerate the rise in their circulating concentrations. The latter mechanism cannot be invoked to explain beta‐blocker‐induced increases in NT‐proANP and NT‐proBNP, however, since these two peptides are almost certainly not cleared by natriuretic peptide clearance receptors.
Heart failure
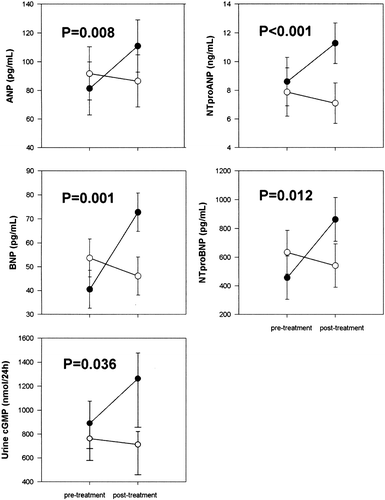
Early studies reported that beta‐blockers, when added to conventional maintenance therapy, stimulated an increase in circulating levels of the natriuretic peptides in the short term. For example, Sanderson et al. Citation49 showed that oral metoprolol 6.25 mg increased ANP and BNP levels at 5 hours and 24 hours (although the vasodilating beta‐blocker, celiprolol, suppressed both ANP and BNP, significantly so at some time points). The RESOLVD investigators demonstrated that controlled‐release metoprolol compared with placebo increased both NT‐proANP and BNP levels at 24 weeks in patients with NYHA functional class II–IV heart failure and a left ventricular ejection fraction (LVEF) <40% Citation50. Davis and colleagues Citation5 later reported that metoprolol increased significantly all four cardiac peptides (ANP, NT‐proANP, BNP and NT‐proBNP) at 6 weeks in a randomized, balanced, controlled, parallel‐group design (). This paper, which investigated also the effects of metoprolol on secretion and clearance rates of ANP and BNP, will be discussed further below.
Figure 1. Plasma cardiac natriuretic peptide levels(mean±SEM) in 16 patients with congestive heart failure before (pre‐treatment) and 6 weeks after the introduction of metoprolol (closed symbols) or unchanged treatment (open symbols). From reference Citation5 with permission.
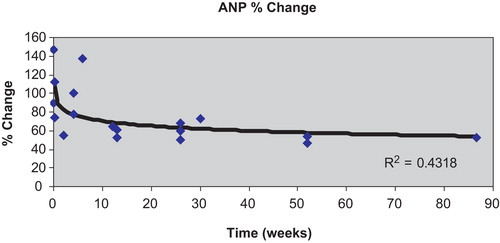
In contrast to the above observations, other reports, more numerous than those already quoted, are that natriuretic peptide levels decline when beta‐blockers are added to conventional pharmacotherapy for heart failure. It is noteworthy that in most, if not all Citation51, of these latter studies, the effects of beta‐blockers were assessed over a period of months Citation52–64, rather than days or weeks. The cumulative results suggest, therefore, that beta‐blockers often, though not invariably, have a biphasic effect on circulating natriuretic peptide concentrations, increasing their levels or evoking little change over days and weeks, but decreasing them in the longer term. The study by Fung et al. Citation60, in which peptide levels were measured before, then serially after introduction of beta‐blockade, illustrates well this biphasic effect. In that study, 49 patients with chronic heart failure, mean LVEF 26%, receiving maintenance diuretic, ACE inhibitor and digoxin therapy, were randomized to metoprolol or carvedilol therapy. Up to 4 weeks, ANP and NT‐proBNP levels were unaltered from baseline values, but by 12 weeks both peptides had fallen significantly and were lower again at 52 weeks (). Formal statistical analysis of results from all reported studies show a trend for a log‐linear relationship between duration of beta‐blocker treatment and percentage change in natriuretic peptide levels from baseline, most obvious for ANP () and NT‐proBNP. Statistical significance (P<0.05), however, was not achieved for any of the four cardiac natriuretic peptides.
Figure 2. Mean plasma levels of NT‐proBNP (N‐BNP) in 49 patients with chronic heart failure given either metoprolol (Meto) or carvedilol (Carv) for 52 weeks. From reference Citation60 with permission.
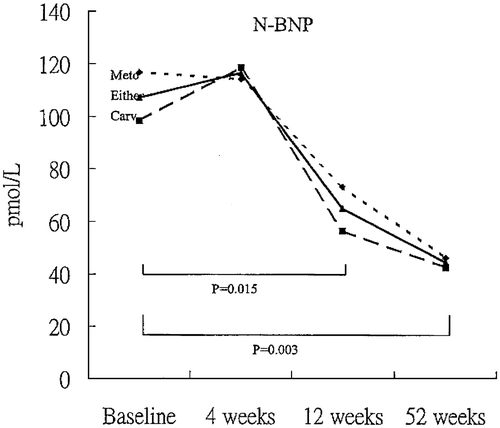
Figure 3. Percentage change from baseline(taken as 100%) in mean plasma levels of ANP with the introduction of beta‐blocker therapy in patients with heart failure according to duration of treatment with the beta‐blocker (range 5 hrs–20 months). Data were taken from all available studies reported in the literature. Although the percentage change in ANP appeared to relate to duration of therapy, the log‐linear association did not reach conventional statistical significance (P = 0.061).
It seems evident (and unsurprising) that changes in natriuretic peptide levels relate predominantly to the haemodynamic response to beta‐blocker therapy. Thus, for example, Fujimara and colleagues reported that plasma ANP and BNP levels fell in patients who ‘responded’ to 3–5 months of treatment with carvedilol or metoprolol and, furthermore, correlated with concomitant changes in LVEF Citation65. Kawai et al. similarly noted positive correlations between changes in haemodynamics and BNP levels across 6 months of carvedilol therapy Citation53 as did Hara and colleagues during metoprolol administration for 6 months Citation52. Numerous other authors have reported congruent haemodynamic and natriuretic hormone changes with beta‐blocker therapy in patients with chronic heart failure.
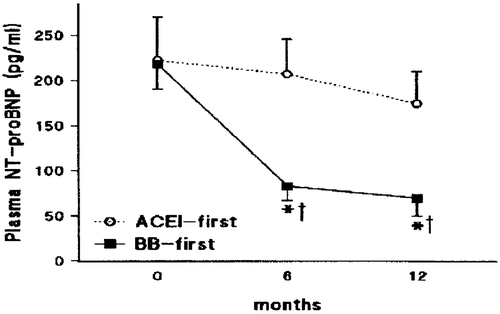
One factor which, seemingly, determines the magnitude of the natriuretic peptide response is whether beta‐blocker therapy is initiated before or, more conventionally, after ACE inhibition. Sliwa et al. Citation61 demonstrated that the effect of carvedilol to reduce plasma NT‐proBNP levels at 6 and 12 months in patients with newly diagnosed idiopathic cardiomyopathy (NYHA functional class II–IV and LVEF <40%) was much greater when the beta‐blocker was started before the ACE inhibitor perindopril, compared to when perindopril treatment was initiated first ().
Figure 4. Impact on plasma levels of NT‐proBNP of initiating treatment with carvedilol (BB) before perindopril (ACEI) versus perindopril before carvedilol in patients with newly diagnosed heart failure (n = 38 and 40, respectively). In each case, the alternative drug was added after 6 months of treatment with the initial drug. *P<0.0005 versus baseline data; †P<0.01 versus change from baseline in the ACEI‐first group. From reference Citation61 with permission.
Finally, the magnitude of haemodynamic dysfunction and level of natriuretic peptides at baseline may be, again unsurprisingly, another determinant of natriuretic peptide response. In this regard, Yoshizawa and colleagues showed that BNP levels declined with 16 or more weeks of carvedilol or metoprolol therapy in patients with heart failure (NYHA functional class II–IV, LVEF <40%, and taking diuretics, ACE inhibitors or ARBs, and digoxin in most cases) who had the highest baseline BNP levels but increased in those who were in the lowest quartile for BNP at baseline Citation63.
What factors determine these beta‐blocker‐induced biphasic effects on circulating natriuretic peptide levels in heart failure? Davis et al. showed that the introduction of metoprolol increased the secretion rate of ANP, NT‐proANP, BNP, and NT‐proBNP at 6 weeks (when intracardiac pressures were likely to have risen) while also reducing the clearance rate of bioactive ANP and BNP from the circulation Citation5. As discussed already, longer‐term beta‐blocker therapy, whilst possibly still reducing the clearance rate of ANP and BNP, would be expected to inhibit cardiac secretion of the four peptides as cardiac remodelling and improved myocardial functioning develops—notwithstanding withdrawal of any direct beta‐adrenergic inhibitory action on natriuretic peptide secretion from the heart.
End‐stage renal failure
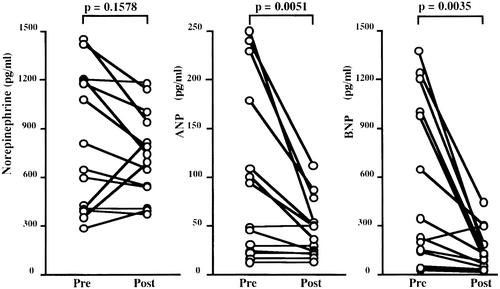
Hara and colleagues Citation66 showed a marked fall in plasma ANP and BNP levels across 4 months of treatment with metoprolol in 14 patients with dilated left ventricles on chronic haemodialysis (). End‐systolic and end‐diastolic left ventricular dimensions decreased over the same time interval and, presumably, accounted for the fall in ANP and BNP levels. If, as in heart failure, beta‐blockade reduced the clearance rate of the two bioactive peptides from the circulation, the decline in cardiac secretion of ANP and BNP may be greater than was reflected by the fall in their plasma levels.
Figure 5. Plasma levels of norepinephrine and natriuretic peptides before and after 4 months of treatment with metoprolol in 14 patients on chronic haemodialysis. Changes in norepinephrine were not statistically significant. From referenceCitation66 with permission.
Angiotensin‐converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs)
Essential hypertension
Of the reports in the literature, most document a fall in circulating natriuretic peptide levels during chronic ACE inhibitor or ARB therapy in patients with essential hypertension Citation39,40,Citation67–75. In almost all studies, blood sampling was carried out with the patients supine or sitting, but Januszewicz et al. documented also that the exercise‐induced rise in ANP was attenuated by 6 months of captopril therapy Citation69. Similarly, Dessi‐Fulgheri and colleagues showed that 4 weeks of benazapril therapy not only reduced ANP to 60% of baseline values but also blunted the ANP response to a 1‐litre saline infusion Citation67. That both A‐type and B‐type natriuretic peptides respond similarly to these drugs is shown in the study of Dahlof where ANP, BNP, and NT‐proBNP levels all declined across 36 weeks of losartan treatment Citation40 and in the report by Olsen et al. of a decline in both NT‐proANP and NT‐proBNP during losartan administration Citation39. The combination of an ACE inhibitor and ARB was shown by Anan and colleagues to have a greater BNP‐lowering effect than either drug alone Citation75. That study, along with many of the others quoted above, involved patients with left ventricular hypertrophy amongst whom natriuretic peptide levels are commonly elevated. Some workers have recorded a statistically significant positive association between the treatment‐related declines in left ventricular mass and plasma natriuretic peptide levels Citation72,Citation74.
In contrast to the above observations, a small number of studies have documented no change in circulating natriuretic peptide levels in association with ACE inhibitor or ARB therapy Citation12,13,Citation33,Citation37,Citation41. Of these, only the study of Davies et al. was in patients with left ventricular hypertrophy, and in this small cohort (n = 17) plasma levels of BNP did fall (from 17.9±9 (SD) to 10.9±7.4 pg/mL), albeit not statistically significantly, across 4 months of treatment with losartan Citation41.
Chronic heart failure
These drugs, which inhibit the formation or actions of angiotensin II, reduce circulating levels of the natriuretic peptides in patients with heart failure secondary to reduced systolic function of the left ventricle Citation61,Citation76–91. This is so after the first dose of drug Citation76,Citation78, at least for patients with severe grades of heart failure and disturbed central haemodynamics, and also in the longer term with maintenance therapy Citation61,Citation79,Citation81–83,Citation85,Citation87. The fall in the A‐type natriuretic peptides with the first dose of ACE inhibitor is faster than for the B‐type peptides Citation78, a phenomenon seen also with acute vasodilator administration in heart failure (vidae infra). Since the suppressive effect of these drugs on natriuretic peptide levels is dose‐dependent Citation85,86,Citation92, it is no particular surprise that two studies by Kasama et al., in which a low dose of enalapril (5 mg daily), given for 6 months to patients with NYHA functional class II or III heart failure, altered neither haemodynamic indices nor plasma BNP levels whereas comparator drugs (perindopril 2 mg daily and valsartan 80 mg/day) improved haemodynamics and reduced BNP levels Citation90,91. This 5‐mg daily dose of enalapril is well below that used commonly in clinical practice and is considerably lower than the mean dose (18.4 mg/day) shown in the CONSENSUS study to increase longevity in patients with NYHA functional class IV heart failure Citation93.
Does addition of an ARB to maintenance ACE inhibitor therapy for heart failure alter natriuretic peptide levels? Latini and colleagues showed that the ARB, valsartan, reduced plasma BNP levels in patients in the Valsartan Heart Failure Trial (Val‐HeFT) whether or not they were on maintenance ACE inhibitor and/or beta‐blocker therapy Citation82. Of course it is probable that the outcome depends upon the maintenance dose of ACE inhibitor since, as mentioned already, there is a dose‐response relationship between ACE inhibitors and natriuretic peptide levels.
With regard to possible mechanisms to explain the above observations, Crozier et al. demonstrated, in the earliest of these studies, that the first dose of an ACE inhibitor, ramipril, induced parallel falls in plasma ANP, plasma angiotensin II, arterial pressure, pulmonary artery diastolic pressure and right atrial pressure Citation76. Longer‐term therapy with an ACE inhibitor or ARB likewise induced concordant changes in haemodynamic indices (including left ventricular volumes) and both ANP and BNP levels in the RESOLVD study Citation94. These and subsequent data point to the probability that treatment‐induced changes in transmural pressure in the cardiac chambers, perhaps contributed to also by the fall in levels (or actions of) angiotensin II Citation95,96, a known stimulator of natriuretic peptide synthesis, accounted for the decline in circulating natriuretic peptide levels. A prompt decline in plasma angiotensin II concentrations and perhaps withdrawal of a high level of sympathetic ‘traffic’ to the heart with initiation of ACE inhibitor therapy might account for the temporal dissociation between an early fall in plasma ANP and BNP levels and a later reduction in left ventricular diastolic diameter reported by Yoshimura et al. Citation85.
Whether ACE inhibition slows the rate of clearance of ANP and BNP from the circulation is open to dispute. If so, the decline in cardiac secretion of the biologically active natriuretic peptides might be greater than is reflected by the fall in their plasma levels during ACE inhibitor therapy.
Calcium channel blockers
The effect of calcium channel blockers on natriuretic peptide levels seems to depend on the individual drug, its duration of administration and possibly, the disorder being treated.
Verapamil
Verapamil given orally or intravenously as a single dose has little or no effect on resting ANP levels in normal volunteers or in patients with essential hypertension or coronary heart disease Citation97–99 although it reportedly impairs the rise in ANP induced by rapid right ventricular pacing Citation97 or by an intravenous saline load Citation100. With longer‐term administration, verapamil has generally been found to stimulate plasma ANP levels in healthy volunteers and patients with essential hypertension Citation101–104.
Amlodipine
Amlodipine, in contrast to verapamil, has been reported, from well designed studies, to suppress circulating levels of ANP, NT‐proANP, and BNP over 2–6 weeks in essential hypertensives Citation105,106 and over 2 and 26 weeks in patients with heart failure due to non‐ischaemic cardiomyopathy Citation107. Additional studies have shown either no effect of amlodipine when added to ACE inhibitor therapy, or a statistically insignificant suppressive action on plasma ANP levels in essential hypertensives Citation108,109.
Nifedipine
Nifedipine given acutely stimulates a rise in ANP levels in normal subjects and essential hypertensives Citation98,99,Citation110. No effect on ANP levels was seen by Colantonio et al. in ten patients with essential hypertension receiving nifedipine over 7 days Citation29. When given for 14 days to healthy volunteers or patients with Raynaud's phenomenon, nifedipine suppressed the rise in ANP induced by a cold‐pressor test Citation111. Monotherapy for essential hypertension with nifedipine for 4–6 months reduced ANP concentrations by approximately 40% Citation70,Citation112. It appears, therefore, that nifedipine stimulates natriuretic peptide levels in the short term (possibly through activation of the sympathetic nervous system) but with chronic administration, at least in essential hypertension, it has a suppressive action.
Diltiazem
Diltiazem 60 mg, given orally, increased plasma ANP levels by approximately 80% at 90 and 120 minutes in seven healthy volunteers Citation98. Four weeks of diltiazem therapy, 180 mg daily, altered neither resting nor exercise‐induced increments in plasma ANP in 16 patients with angina pectoris Citation32. In patients with myocardial infarction and reperfusion therapy, diltiazem compared to placebo reduced plasma BNP levels over 4 weeks Citation113.
Others
Felodipine, like nifedipine, increased ANP levels over 2 and 24 hours in patients with essential hypertension Citation99. Again, as with nifedipine, felodipine tended to suppress ANP levels with longer‐term administration in patients with essential hypertension Citation114. It also attenuated the rise in ANP levels over 3 and 12 months in patients with heart failure Citation115.
Nitrendipine 20 mg daily reduced ANP levels in plasma by 16% over 6 weeks in patients with hypertension and type 1 diabetes mellitus Citation116.
Chronic intake of calcium channel antagonists, alone (n = 17) or in combination (n = 28) for hypertension was associated with higher ANP levels than in untreated patients Citation13. Interpretation of these data is hampered by pooling of data from patients taking different agents within the group of calcium channel blockers.
In summary, it is difficult to perceive a uniform pattern of response of the natriuretic peptides to calcium channel antagonists. Interpretation of much available data is compromised by the absence of time‐matched placebo administration. It appears, nevertheless, that chronic administration of verapamil stimulates natriuretic peptide levels whereas amlodipine, nifedipine, felodipine, nitrendipine, and perhaps diltiazem, have a suppressive action.
Vasodilators
In patients with heart failure, especially those in whom central haemodynamics are severely perturbed, oral or intravenous vasodilators (nitroglycerine, nitroprusside, and nicorandil) reduce plasma natriuretic peptide levels Citation9,Citation15,Citation117–121. Plasma ANP and NT‐proANP concentrations fall promptly and in parallel with pulmonary artery wedge and right atrial pressures but, according to Larsen and colleagues, the B‐type peptides respond less briskly, paralleling the slower decline in systemic vascular resistance Citation9. Nitroprusside, which reduced arterial pressure levels to baseline in healthy volunteers receiving noradrenaline infusions, reduced also ANP to baseline values Citation122.
Alpha‐adrenergic blockers
Of the few studies addressing the effects of alpha‐adrenergic blocking drugs on natriuretic peptide levels in healthy volunteers or hypertensive patients, most report little or no change in ANP and BNP either at rest or with exercise following single‐dose or sustained (4–6 weeks) therapy Citation106,Citation123–125. Kohno et al, however, demonstrated a decline in plasma ANP levels at rest and with exercise in 26 elderly hypertensive patients after acute prazosin administration Citation30. A similar outcome was observed in a study of eight healthy volunteers who, after a single dose of prazosin 1 mg, exhibited a lesser response in plasma ANP to exercise than with placebo although the difference was not statistically significant Citation31. One contrary report, that terazosin therapy over 30 days stimulated plasma ANP levels slightly (by 16.4%) in patients with hypertension and prostatic hypertrophy, is not readily interpreted in the absence of a time‐matched control group Citation126. This might be especially pertinent since maintenance medications were withdrawn 2 weeks prior to the study and this alone may have resulted in a ‘moving baseline’ for ANP levels.
In summary, the sparse literature available suggests that alpha‐adrenergic blockade has little effect or an inhibitory action on natriuretic peptide levels in man. An inhibitory effect might be anticipated from in vitro and animal studies that demonstrate that alpha‐adrenergic agonists stimulate ANP and BNP secretion whereas blockade of alpha‐adrenergic receptors is inhibitory Citation6.
Positive inotropic agents
Digitalis
Of the three papers reporting effects of digitalis compounds on ANP levels in patients with heart failure, all demonstrated a stimulatory effect over 40 minutes–6 days Citation127–129 and this was so also for BNP in the report by Tsutamoto Citation127. Interestingly, two of these reports showed a clear dissociation between digitalis‐induced changes in pulmonary capillary wedge pressure and in ANP and BNP levels Citation127,Citation129: whereas wedge pressures fell, natriuretic peptide levels rose. These data suggest that the natriuretic peptide‐stimulatory action of digitalis compounds, demonstrated ex vivoCitation130,131, must be powerful in order to overcome a decline in cardiac chamber stretch. The possibility that digitalis compounds might act to reduce the plasma clearance rate of ANP and BNP, thereby contributing to the rise in their circulating levels, however, cannot be discounted. Whether the observed increase in ANP and BNP levels contributes to the beneficial therapeutic effects of digitalis compounds, as has been suggested Citation129, remains to be determined.
Dopamine‐like agents
Dobutamine and dopamine agonists, administered over minutes to 2 days, reportedly reduce circulating ANP and BNP levels in patients with severe grades of heart failure Citation132–135. Treatment with ibopamine, an orally active dopamine agonist, for 24 weeks likewise suppressed plasma ANP levels, this time in patients with mild to moderate heart failure Citation79. An exception to this general rule was reported by Avgeropoulou et al. who showed no change in plasma BNP following a 24‐hour infusion of dobutamine in patients with advanced, decompensated heart failure Citation136. These workers, however, measured BNP levels only at baseline (pre‐infusion) and 1 and 4 days post‐infusion, not during the infusion itself.
For patients not in heart failure, usually undergoing investigations for coronary artery disease, intravenous dobutamine had little or a minor stimulatory effect on natriuretic peptide levels Citation137–139. Dopamine infusion in premature infants had no effect on ANP levels Citation140.
Phosphodiesterase III inhibitors
Milrinone reduced ANP levels along with cardiac filling pressures over 2 hours in seven patients with congestive heart failure Citation141. The same drug, given intermittently by intravenous infusion over 2 years to a patient with chronic pulmonary thromboembolism, was associated with a substantial (40%–50%) decline in both ANP and BNP levels and improved clinical status, although no decline in mean pulmonary artery pressure was observed Citation142.
Levosimendan
This drug, which has both positive inotropic (calcium sensitization of cardiac myofilaments) and vasodilator actions, was shown to suppress plasma BNP levels significantly and substantially for up to 5 days following 24 hours of intravenous infusion in patients with decompensated heart failure Citation136,Citation143. Serial 24‐hour infusions every 3 weeks likewise reduced plasma NT‐proBNP levels while simultaneously reducing left ventricular volume indices in 17 patients with decompensated chronic heart failure Citation144.
Xamoterol
Compared with placebo, this beta‐1‐partial agonist drug, given over 10 days, was without effect on plasma levels of ANP in patients with or without heart failure who had suffered acute myocardial infarction Citation145.
Miscellaneous
Amiodarone
Two studies of patients with heart failure demonstrated a substantial decline in plasma levels of natriuretic peptides during chronic amiodarone therapy in a dose of 100–200 mg daily. The first Citation146 was in 13 patients intolerant of beta‐blockers but otherwise receiving diuretics (all), ACE inhibitors (n = 10) and digitalis (n = 10). Compared with controls (n = 9), those receiving amiodarone showed a steady fall in BNP levels to less than 50% baseline values at 3 months. The second study, by Shiga and colleagues, was in patients with chronic heart failure (NYHA functional class II–IV) and arrhythmias who were receiving diuretics, ACE inhibitors or ARBs and beta‐blockers with or without digoxin Citation147. Here again, those receiving amiodarone (n = 46) compared to placebo (n = 21 patients who, instead of amiodarone, received an implantable cardioverter defibrillator) reduced plasma BNP levels steadily over 6 months (from a mean of 287 to 180 pg/mL).
Central alpha‐2 agonists
Clonidine injection increased ANP levels in healthy control women and normotensive obese women Citation148. This fits well with an abundant literature from animal studies which also, in general, describes a stimulatory action of clonidine on natriuretic peptide levels. In contrast, Spinetti et al. reported a 50% reduction in plasma ANP levels and suppression of the ANP response to saline infusion after 3 months of clonidine administration in five post‐menopausal women Citation149. Interpretation of these directionally opposite observations is difficult given the different patient cohorts and time frames involved.
Aspirin
In a study of 42 survivors of acute myocardial infarction who required an ACE inhibitor because of heart failure or a reduced LVEF (<40%) during their hospital stay, 27 were randomized to aspirin (150 mg daily) and 15 to warfarin. There were no changes in plasma ANP levels across 3 months in either group Citation150. In contrast, two other studies reported aspirin to have a stimulatory effect on natriuretic peptide levels. The first, by Jug et al., showed that aspirin 100 mg/day increased plasma NT‐proBNP slightly but significantly over 8 weeks in 18 patients with stable chronic heart failure (NYHA functional class II or III and LVEF <40%) and ischaemic heart disease who were taking ACE inhibitors Citation151. The second study, by Meune and colleagues, also in patients (n = 19) with stable chronic heart failure, reduced LVEF, and taking an ACE inhibitor, demonstrated a rise in plasma BNP levels (from a mean of 107 to 144 pg/mL) during treatment with aspirin 325 mg/day for 14 days Citation152. A parallel group of 17 patients receiving clopidogrel exhibited no change in BNP.
Heparin
Of 102 patients with chronic heart failure due to dilated cardiomyopathy and receiving conventional ACE inhibitor, beta‐blocker, and diuretic therapy, those randomized to low‐molecular‐weight heparin daily for 12 months (n = 52) exhibited a significant decline in plasma NT‐proBNP levels (and left ventricular diameters) at 6 and 12 months compared with those (n = 50) who did not receive heparin Citation153. Confirmation of this observation is needed since the study was neither blinded nor placebo‐controlled.
Histamine H2 receptor blockade
Kim and colleagues reported from a retrospective study and from a randomized, open‐label protocol, that the histamine H2 receptor blocker famotidine, given to patients with congestive heart failure and gastro‐oesophageal reflux disease, induced a decline in plasma BNP levels in parallel, apparently, with a fall in left ventricular diameters and arterial pressure Citation154. Most likely the fall in BNP was secondary to improved cardiac function as manifest by the reduction in left ventricular diameters rather than to any direct effect of histamine blockade on BNP secretion since, at least in rabbits, histamine has been shown to inhibit (rather than stimulate) secretion of natriuretic peptides by atrial myocytes via H2 receptors Citation155.
Non‐steroidal anti‐inflammatory drugs
Indomethacin given acutely, by mouth or intravenously, failed to alter circulating ANP levels in healthy subjects, patients with chronic glomerulonephritis or patients on chronic intermittent haemodialysis Citation156–158. In ten patients with essential hypertension, however, indomethacin administration for 1 week increased mean plasma ANP levels from 16 to 21.5 pg/mL Citation159.
Allopurinol
Gavin and Struthers showed, in a randomized, parallel trial of 50 patients with heart failure (NYHA functional class II or III) associated with reduced systolic function and receiving conventional medications, that allopurinol, 300 mg/day, reduced plasma BNP levels slightly but significantly compared with placebo over the 3‐month study Citation160. The authors suggested that the fall in BNP levels might have reflected allopurinol‐induced improvements in endothelial and cardiac function.
Statins
Strey and colleagues reported that treatment with atorvastatin 40 mg daily for 6 weeks resulted in slightly, though statistically significantly, lower levels of plasma ANP levels compared to placebo (42 versus 51 pmol/L) in a double‐blind, placebo‐controlled, cross‐over study of 23 patients with chronic heart failure of non‐ischaemic aetiology and normocholesterolaemia Citation161.
Oestrogens
Oestrogen replacement therapy activates the cardiac natriuretic peptide system Citation162–164, perhaps via renal sodium retention Citation164 or stimulation of the renin‐angiotensin system Citation165.
Immunosuppressive drugs
Notwithstanding the fact that the immunosuppressive drug, doxorubicin, is capable of suppressing ANP and BNP gene expression in cultured myocytes in vitroCitation166, this and similar cardiotoxic agents stimulate circulating levels of the cardiac natriuretic peptides in those who have, or are developing, cardiac failure Citation167–171.
Overview
As noted in the introduction, natriuretic peptide levels and particularly the B‐type peptides, are being used increasingly in clinical practice under a variety of circumstances. The effects of drug therapy on circulating levels of natriuretic peptides need to be taken into account when interpreting such levels. Of the drugs used to treat cardiovascular disorders, the diuretics reduce elevated levels of natriuretic peptides in heart failure, but their effects in patients with hypertension and in healthy volunteers are less clear. Beta‐blocking drugs stimulate natriuretic peptide levels in normal subjects and in patients with hypertension or coronary artery disease. For patients with cardiac failure, the effect of beta‐blockers is more complex. In the short term they tend to increase peptide levels whereas in the longer term they are inhibitory, at least when clinical and haemodynamic improvement results. Drugs which block the renin‐angiotensin system reduce natriuretic peptide levels, most obviously when pre‐treatment levels are elevated as in severe grades of heart failure but also in essential hypertension, especially when there is left ventricular hypertrophy. Different calcium channel blockers appear to have different effects. Thus, verapamil has little or no effect in the short term but often stimulates natriuretic peptide levels with chronic administration. Amlodipine tends to suppress, or alter little, natriuretic peptide levels whereas nifedipine and felodipine are stimulatory in the short term and inhibitory with chronic administration. Vasodilator drugs, when used in the management of heart failure, appear to lower natriuretic peptides in parallel with haemodynamic responses. Alpha‐adrenergic receptor blocking drugs suppress, or have little effect. Whereas digoxin stimulates natriuretic peptide levels even, apparently, when haemodynamic indices improve, dopamine‐like agents reduce their circulating concentration congruent with improvements in central haemodynamics. Amiodarone, allopurinol, and atorvastatin have been shown to reduce natriuretic peptide levels in patients with heart failure whilst aspirin has a minimal or stimulatory effect. Histamine H2 receptor blockade and low‐molecular‐weight heparin have each been reported to reduce B‐type natriuretic peptides in heart failure. In the few studies completed, opposite effects of clonidine on ANP have been reported, and indomethacin increased slightly, or was without effect on, ANP.
Acknowledgements
We are grateful to Barbara Griffin for secretarial assistance.
References
- Clerico A., Recchia F. A., Passino C., Emdin M. Cardiac endocrine function is an essential component of the homeostatic regulation network: physiological and clinical implications. Am J Physiol 2006; 290: H17–29
- Potter L. R., Abbey‐Hosch S., Dickey D. M. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate‐dependent signaling functions. Endocr Rev 2006; 27: 47–72
- Edwards B. S., Zimmerman R. S., Schwab T. R., Heublein D. M., Burnett J. C., Jr. Atrial stretch, not pressure, is the principal determinant controlling the acute release of atrial natriuretic factor. Circ Res 1988; 62: 191–5
- Kinnunen P., Vuolteenaho O., Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology 1993; 132: 1961–70
- Davis M. E., Richards A. M., Nicholls M. G., Yandle T. G., Frampton C. M., Troughton R. W. Introduction of metoprolol increases plasma B‐type cardiac natriuretic peptides in mild, stable heart failure. Circulation 2006; 113: 977–85
- Luchner A., Schunkert H. Interactions between the sympathetic nervous system and the cardiac natriuretic peptide system. Cardiovasc Res 2004; 63: 443–9
- Nakamura M., Kawata Y., Yoshida H., Arakawa N., Koeda T., Ichikawa T., et al. Relationship between plasma atrial and brain natriuretic peptide concentrations and hemodynamic parameters during percutaneous transvenous mitral valvulotomy in patients with mitral stenosis. Am Heart J 1992; 124: 1283–8
- Yoshimura M., Yasue H., Okumura K., Ogawa H., Jougasaki M., Mukoyama M., et al. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation 1993; 87: 464–9
- Larsen A. I., Dickstein K., Ahmadi N. S., Aarsland T., Kvaløy J. T., Hall C. The effect of altering haemodynamics on the plasma concentrations of natriuretic peptides in heart failure. Eur J Heart Fail 2006; 8: 628–33
- Yandle T., Fisher S., Livesey J., Espiner E., Richards M., Nicholls G. Exponential increase in clinical use of plasma brain natriuretic peptide (BNP) assays. N Z Med J 2004; 117: U956
- Lijnen P., Hespel P., Fagard R., Staessen J., Goossens W., Lissens W., et al. Plasma atrial natriuretic peptide and the renin‐aldosterone system during long‐term administration of the diuretic xipamide in man. Eur J Clin Pharmacol 1989; 36: 111–17
- Chalmers J. P., Wing L. M. H., West M. J., Bune A. J. C., Elliott J. M., Morris M. J., et al. Effects of enalapril and hydrochlorothiazide on blood pressure, renin‐angiotensin system, and atrial natriuretic factor in essential hypertension: a double blind factorial cross‐over study. Aust NZ J Med 1986; 16: 475–80
- Schunkert H., Hense H. W., Brockel U., Luchner A., Muscholl M., Holmer S. R., et al. Differential effects of antihypertensive drugs on neurohormonal activation: insights from a population‐based sample. J Intern Med 1998; 244: 109–19
- Paterna S., Di Pasquale P., Parrinello G., Fornaciari E., Di Gaudio F., Fasullo S., et al. Changes in brain natriuretic peptide levels and bioelectrical impedance measurements after treatment with high‐dose furosemide and hypertonic saline solution versus high‐dose furosemide alone in refractory congestive heart failure. J Am Coll Cardiol 2005; 45: 1997–2003
- Johnson W., Omland T., Hall C., Lucas C., Myking O. L., Collins C., et al. Neurohormonal activation rapidly decreases after intravenous therapy with diuretics and vasodilators for class IV heart failure. J Am Coll Cardiol 2002; 39: 1623–9
- Starklint J., Bech J. N., Nyvad O., Jensen P., Pedersen E. B. Increased urinary aquaporin‐2 excretion in response to furosemide in patients with chronic heart failure. Scand J Clin Lab Invest 2006; 66: 55–66
- Rousseau M. F., Gurné O., Duprez D., Van Mieghem W., Robert A., Ahn S., et al. Beneficial neurohormonal profile of spironolactone in severe congestive heart failure. J Am Coll Cardiol 2002; 40: 1596–601
- Tsutamoto T., Wada A., Maeda K., Mabuchi N., Hayashi M., Tsutsui T., et al. Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol 2001; 37: 1228–33
- Feola M., Menardi E., Ribichini F., Vado A., Deorsola A., Ferrero V., et al. Effects of the addition of a low dose of spironolactone on brain natriuretic peptide plasma level and cardiopulmonary function in patients with moderate congestive heart failure. Med Sci Monit 2003; 9: CR341–5
- Macdonald J. E., Kennedy N., Struthers A. D. Effects of spironolactone on endothelial function, vascular angiotensin converting enzyme activity, and other prognostic markers in patients with mild heart failure already taking optimal treatment. Heart 2004; 90: 765–70
- Roongsritong C., Sutthiwan P., Bradley J., Simoni J., Power S., Meyerrose G. E. Spironolactone improves diastolic function in the elderly. Clin Cardiol 2005; 28: 484–7
- Kinugawa T., Ogino K., Kato M., Furuse Y., Shimoyama M., Mori M., et al. Effects of spironolactone on exercise capacity and neurohormonal factors in patients with heart failure treated with loop diuretics and angiotensin‐converting enzyme inhibitor. Gen Pharmacol 1998; 31: 93–9
- Nakaoka H., Kitahara Y., Amano M., Imataka K., Fujii J., Ishibashi M., et al. Effect of β‐adrenergic receptor blockade on atrial natriuretic peptide in essential hypertension. Hypertension 1987; 10: 221–5
- Thamsborg G., Sykulski R., Larsen J., Storm T., Keller N. Effect of β1‐adrenoceptor blockade on plasma levels of atrial natriuretic peptide during exercise in normal man. Clin Physiol 1987; 7: 313–8
- Bouissou P., Galen F‐X., Richalet J. P., Lartigue M., Devaux F., Dubray C., et al. Effects of propranolol and pindolol on plasma ANP levels in humans at rest and during exercise. Am J Physiol 1989; 257: R259–64
- Donckier J. E., De Coster P. M., Buysschaert M., Van Hoof M., Cauwe F. M., Robert A., et al. Effect of beta‐adrenergic blockade on plasma atrial natriuretic factor and cardiac volumes during exercise in normal men. Am J Cardiol 1989; 63: 1000–2
- Deray G., Berlin I., Maistre G., Martinez F., Legrand S., Carayon A., et al. Beta‐adrenoceptor blockade potentiates exercise‐induced release of atrial natriuretic peptide. Eur J Clin Pharmacol 1990; 38: 363–6
- Tsai R‐C., Yamaji T., Ishibashi M., Takaku F., Hsu S‐T., Yeh S‐J., et al. Role of atrial natriuretic peptide in hemoconcentration during exercise. Am J Hypertens 1990; 3: 833–7
- Colantonio D., Casale R., Desiati P., Giandomenico G., Bucci V., Pasqualetti P. Short‐term effects of atenolol and nifedipine on atrial natriuretic peptide, plasma renin activity, and plasma aldosterone in patients with essential hypertension. J Clin Pharmacol 1991; 31: 238–42
- Kohno M., Yokokawa K., Yasunari K., Murakawa K., Kurihara N., Takeda T. Acute effects of α‐ and β‐adrenoceptor blockade on plasma atrial natriuretic peptides during exercise in elderly patients with mild hypertension. Chest 1991; 99: 847–54
- Berlin I., Lechat P., Deray G., Landault C., Maistre G., Chermat V., et al. Beta‐adrenoceptor blockade potentiates acute exercise‐induced release of atrial natriuretic peptide by increasing atrial diameter in normotensive healthy subjects. Eur J Clin Pharmacol 1993; 44: 127–33
- Riley M., Elborn J. S., Onuoha G., Erwin C., Shaw C., Khan M. M., et al. Effect of beta‐adrenoceptor blockade on atrial natriuretic peptide levels during exercise in angina pectoris. Br J Clin Pharmacol 1993; 35: 209–12
- Elijovich F., Laffer C. L., Schiffrin E. L. The effects of atenolol and zofenopril on plasma atrial natriuretic peptide are due to their interactions with target organ damage of essential hypertensive patients. J Hum Hypertens 1997; 11: 313–9
- Luchner A., Burnett J. C., Jr., Jougasaki M., Hense H‐W., Riegger G. A. J., Schunkert H. Augmentation of the cardiac natriuretic peptides by beta‐receptor antagonism: evidence from a population‐based study. J Am Coll Cardiol 1998; 32: 1839–44
- Papadopoulos C. L., Kokkas B., Kotridis P., Karamouzis M., Haldoupi M., Platis A., et al. Effect of the β1‐blocker/β2‐agonist celiprolol on atrial natriuretic peptide plasma levels in hypertensive patients. Cardiovasc Drugs Ther 1998; 12: 345–6
- Deary A. J., Schumann A. L., Murfet H., Haydock S., Foo R. S., Brown M. J. Influence of drugs and gender on the arterial pulse wave and natriuretic peptide secretion in untreated patients with essential hypertension. Clin Sci 2002; 103: 493–9
- van den Meiracker A. H., Lameris T. W., van de Ven L. L. M., Boomsma F. Increased plasma concentration of natriuretic peptides by selective β1‐blocker bisoprolol. J Cardiovasc Pharmacol 2003; 42: 462–8
- Marie P‐Y., Mertes P. M., Hassan‐Sebbag N., de Talence N., Djaballah K., Djabballah W., et al. Exercise release of cardiac natriuretic peptides is markedly enhanced when patients with coronary artery disease are treated medically by beta‐blockers. J Am Coll Cardiol 2004; 43: 353–9
- Olsen M. H., Wachtell K., Tuxen C., Fossum E., Bang L. E., Hall C., et al. Opposite effects of losartan and atenolol on natriuretic peptides in patients with hypertension and left ventricular hypertrophy: a LIFE substudy. J Hypertens 2005; 23: 1083–90
- Dahlof B., Zanchetti A., Diez J., Nicholls M. G., Yu C‐M., Barrios V., et al. Effects of losartan and atenolol on left ventricular mass and neurohormonal profile in patients with essential hypertension and left ventricular hypertrophy. J Hypertens 2002; 20: 1855–64
- Davies J., Carr E., Band M., Morris A., Struthers A. Do losartan and atenolol have differential effects on BNP and central haemodynamic parameters?. J Renin Angiotensin Aldosterone Syst 2005; 6: 151–3
- Omvik P., Lund‐Johansen P., Myking O. Effects of carvedilol on atrial natriuretic peptide, catecholamines, and hemodynamics in hypertension at rest and during exercise. J Cardiovasc Pharmacol 1992; 19(Suppl 1)S90–6
- Böhlen L. M., de Courten M., Hafezi F., Shaw S., Riesen W., Weidmann P. Insulin sensitivity and atrial natriuretic factor during β‐receptor modulation with celiprolol in normal subjects. J Cardiovasc Pharmacol 1994; 23: 877–83
- Kantola I., Tarssanen L., Scheinin M., Ruskoaho H., Vinamäki O., Kaila T. β‐blockade, atrial natriuretic peptide and exercise. Int J Clin Pharmacol Ther 1996; 34: 12–16
- Legault L., van Nguyen P., Holliwell D. L., Leenen F. H. H. Hemodynamic and plasma atrial natriuretic factor responses to cardiac volume loading in young versus older normotensive humans. Can J Physiol Pharmacol 1992; 70: 1549–54
- Shields P. P., Glembotski C. C. Regulation of atrial natriuretic factor‐(99‐126) secretion from neonatal rat primary atrial cultures by activators of protein kinases A and C. J Biol Chem 1989; 264: 9322–8
- Yoshimoto T., Naruse M., Tanabe A., Naruse K., Seki T., Imaki T., et al. Potentiation of natriuretic peptide action by the β‐adrenergic blocker carvedolol in hypertensive rats: a new antihypertensive mechanism. Endocrinology 1998; 139: 81–8
- Ohta Y., Watanabe K., Nakazawa M., Tamamoto T., Ma M., Fuse K., et al. Carvedolol enhances atrial and brain natriuretic peptide mRNA expression and release in rat heart. J Cardiovasc Pharmacol 2000; 36(Suppl 2)S19–23
- Sanderson J. E., Chan W. W. M., Hung Y. T., Chan S. K. W., Shum I. O. L., Raymond K., et al. Effect of low dose β blockers on atrial and ventricular (B type) natriuretic factor in heart failure: a double blind, randomised comparison of metoprolol and a third generation vasodilating β blocker. Br Heart J 1995; 74: 502–7
- The RESOLVD Investigators. Effects of metoprolol CR in patients with ischemic and dilated cardiomyopathy. The Randomized Evaluation of Strategies for Left Ventricular Dysfunction Pilot Study. Circulation 2000; 101: 378–84
- Yoshikawa T., Handa S., Anzai T., Nishimura H., Baba A., Akaishi M., et al. Early reduction of neurohumoral factors plays a key role in mediating the efficacy of β‐blocker therapy for congestive heart failure. Am Heart J 1996; 131: 329–36
- Hara Y., Hamada M., Shigematsu Y., Suzuki M., Kodama K., Kuwahara T., et al. Effect of beta‐blocker on left ventricular function and natriuretic peptides in patients with chronic heart failure treated with angiotensin‐converting enzyme inhibitor. Jpn Circ J 2000; 64: 365–9
- Kawai K., Hata K., Takaoka H., Kawai H., Yokoyama M. Plasma brain natriuretic peptide as a novel therapeutic indicator in idiopathic dilated cardiomyopathy during β‐blocker therapy: a potential of hormone‐guided treatment. Am Heart J 2001; 141: 925–32
- Gabrielli O., Puyó A. M., De Rosa A., Armando I., Barontini M., Levin G. Atenolol improves ventricular function without changing plasma noradrenaline but decreasing plasma atrial natriuretic factor in chronic heart failure. Auton Autacoid Pharmacol 2002; 22: 261–8
- Hara Y., Hamada M., Ohtsuka T., Ogimoto A., Saeki H., Matsunaka T., et al. Comparison of treatment effects of bevantolol and metoprolol on cardiac function and natriuretic peptides in patients with dilated cardiomyopathy. Heart Vessels 2002; 17: 53–6
- Rahman M. A., Hara K., Daly P. A., Wigle E. D., Floras J. S. Reductions in muscle sympathetic nerve activity after long term metoprolol for dilated cardiomyopathy: preliminary observations. Br Heart J 1995; 74: 431–6
- Stanek B., Frey B., Hülsmann M., Berger R., Sturm B., Strametz‐Juranek J., et al. Prognostic evaluation of neurohumoral plasma levels before and during beta‐blocker therapy in advanced left ventricular dysfunction. J Am Coll Cardiol 2001; 38: 436–42
- Ohtsuka T., Hamada M., Saeki H., Ogimoto A., Hiasa G., Hara Y., et al. Comparison of effects of carvedilol versus metoprolol on cytokine levels in patients with idiopathic dilated cardiomyopathy. Am J Cardiol 2002; 89: 996–9
- Persson H., Andréasson K., Kahan T., Eriksson S. V., Tidgren B., Hjemdahl P., et al. Neurohormonal activation in heart failure after acute myocardial infarction treated with beta‐receptor antagonists. Eur J Heart Fail 2002; 4: 73–82
- Fung J. W. H., Yu C. M., Yip G., Chan S., Yandle T. G., Richards A. M., et al. Effect of beta blockade (carvedilol or metoprolol) on activation of the renin‐angiotensin‐aldosterone system and natriuretic peptides in chronic heart failure. Am J Cardiol 2003; 92: 406–10
- Sliwa K., Norton G. R., Kone N., Candy G., Kachope J., Woodiwiss A. J., et al. Impact of initiating carvedilol before angiotensin converting enzyme inhibitor therapy on cardiac function in newly diagnosed heart failure. J Am Coll Cardiol 2004; 44: 1825–30
- Takeda Y., Fukutomi T., Suzuki S., Yamamoto K., Ogata M., Kondo H., et al. Effects of carvedilol on plasma B‐type natriuretic peptide concentration and symptoms in patients with heart failure and preserved ejection fraction. Am J Cardiol 2004; 94: 448–53
- Yoshizawa A., Yoshikawa T., Nakamura I., Satoh T., Moritani K., Suzuki M., et al. Brain natriuretic peptide response is heterogenous during β‐blocker therapy for congestive heart failure. J Card Fail 2004; 10: 310–5
- Frantz R. P., Olson L. J., Grill D., Moualla S. K., Nelson S. M., Nobrega T. P., et al. Carvedilol therapy is associated with a sustained decline in brain natriuretic peptide levels in patients with congestive heart failure. Am Heart J 2005; 149: 541–7
- Fujimura M., Yasumura Y., Ishida Y., Nakatani S., Komamura K., Yamagishi M., et al. Improvement in left ventricular function in response to carvedolol is accompanied by attenuation of neurohumoral activation in patients with dilated cardiomyopathy. J Card Fail 2000; 6: 3–10
- Hara Y., Hamada M., Shigematsu Y., Murakami B., Hiwada K. Beneficial effect of β‐adrenergic blockade on left ventricular function in haemodialysis patients. Clin Sci 2001; 101: 219–25
- Dessi‐Fulgheri P., Motolese M., Di Noto G., Delfino D., Giacchetti G., Boria C., et al. Blunting of atrial natriuretic factor response to volume expansion by benazepril in hypertensive patients. J Hypertens 1989; 7(suppl)S300–1
- Fioretto P., Muollo B., Ben G. P., Mollo F., Frigato F., Opocher G., et al. Resistance to the actions of atrial natriuretic factor in insulin‐dependent diabetic hypertensives and improvement with angiotensin converting enzyme inhibitor treatment. J Hypertens 1991; 9(suppl 6)S262–3
- Januszewicz A., Lapinski M., Stepniakowski K., Szczypaczewska M., Kowalik‐Borowka E., Chlebus M., et al. The exercise‐induced rise in atrial natriuretic factor is reduced by chronic angiotensin converting enzyme inhibition in patients with primary hypertension. J Hypertens 1991; 9(suppl 6)S386–7
- Kuriyama S., Tokutome G., Kimura Y., Shimada T., Nakamura K., Tomonari H., et al. Atrial natriuretic peptide lowering effect of antihypertensives in patients with essential hypertension. Am J Hypertens 1991; 4: 289–90
- Kohno M., Yokokawa K., Yasunari K., Kano H., Minami M., Hanehira T., et al. Changes in plasma cardiac natriuretic peptides concentrations during 1 year treatment with angiotensin‐converting enzyme inhibitor in elderly hypertensive patients with left ventricular hypertrophy. Int J Clin Pharmacol Ther 1997; 35: 38–42
- Kohno M., Minami M., Kano H., Yasunari K., Maeda K., Hanehira T., et al. Effect of angiotensin‐converting enzyme inhibitor on left ventricular parameters and circulating brain natriuretic peptide in elderly hypertensives with left ventricular hypertrophy. Metabolism 2000; 49: 1356–60
- Yalcin F., Aksoy F. G., Muderrisoglu H., Sabah I., Garcia M. J., Thomas J. D. Treatment of hypertension with perindopril reduces plasma atrial natriuretic peptide levels, left ventricular mass, and improves echocardiographic parameters of diastolic function. Clin Cardiol 2000; 23: 437–41
- Anan F., Takahashi N., Ooie T., Hara M., Yoshimatsu H., Saikawa T. Candesartan, an angiotensin II receptor blocker, improves left ventricular hypertrophy and insulin resistance. Metabolism 2004; 53: 777–81
- Anan F., Takahashi N., Ooie T., Yufu K., Hara M., Nakagawa M., et al. Effects of valsartan and perindopril combination therapy on left ventricular hypertrophy and aortic arterial stiffness in patients with essential hypertension. Eur J Clin Pharmacol 2005; 61: 353–9
- Crozier I. G., Nicholls M. G., Ikram H., Espiner E. A., Yandle T. G. Atrial natriuretic peptide levels in congestive heart failure in man before and during converting enzyme inhibition. Clin Exper Pharmacol Physiol 1989; 16: 417–24
- Kettunen R. V. J., Vuolteenaho O., Ukkola O., Lilja M., Jounela A., Kesäniemi A., et al. Effects of early administration of enalapril on radionuclide left ventricular ejection fraction and plasma N‐terminal atrial natriuretic peptide after acute myocardial infarction. Am J Cardiol 1994; 73: 865–7
- Yoshimura M., Yasue H., Tanaka H., Kikuta K., Sumida H., Kato H., et al. Responses of plasma concentrations of A type natriuretic peptide and B type natriuretic peptide to alacepril, an angiotensin‐converting enzyme inhibitor, in patients with congestive heart failure. Br Heart J 1994; 72: 528–33
- Dohmen H. J. M., Dunselman P. H. J. M., Poole‐Wilson P. A. Comparison of captopril and ibopamine in mild to moderate heart failure. Heart 1997; 78: 285–90
- Inoko M., Fujita M., Nakae I., Tamaki S., Watanuki M., Hashimoto T., et al. Effect of angiotensin‐converting enzyme inhibition on sympathetic tone in patients with mild to moderate heart failure. Jpn Circ J 2001; 65: 395–8
- Kinugawa T., Osaki S., Kato M., Ogino K., Shimoyama M., Tomikura Y., et al. Effects of the angiotensin‐converting enzyme inhibitor alacepril on exercise capacity and neurohormonal factors in patients with mild‐to‐moderate heart failure. Clin Exper Pharmacol Physiol 2002; 29: 1060–5
- Latini R., Masson S., Anand I., Judd D., Maggioni A. P., Chiang Y‐T., et al. Effects of valsartan on circulating brain natriuretic peptide and norepinephrine in symptomatic chronic heart failure. The Valsartan Heart Failure Trial (Val‐HeFT). Circulation 2002; 106: 2454–8
- Maggioni A. P., Anand I., Gottlieb S. O., Latini R., Tognoni G., Cohn J. N., et al. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin‐converting enzyme inhibitors. J Am Coll Cardiol 2002; 40: 1414–21
- Sheth T., Parker T., Block A., Hall C., Adam A., Pfeffer M. A., et al. Comparison of the effects of omapatrilat and lisinopril on circulating neurohormones and cytokines in patients with chronic heart failure. Am J Cardiol 2002; 90: 496–500
- Yoshimura M., Mizuno Y., Nakayama M., Sakamoto T., Sugiyama S., Kawano H., et al. B‐type natriuretic peptide as a marker of the effects of enalapril in patients with heart failure. Am J Med 2002; 112: 716–20
- Mitrovic V., Willenbrock R., Miric M., Seferovic P., Spinar J., Dabrowski M., et al. Acute and 3‐month treatment effects of candesartan cilexetil on hemodynamics, neurohormones, and clinical symptoms in patients with congestive heart failure. Am Heart J 2003; 145: e14
- Krum H., Carson P., Farsang C., Maggioni A. P., Glazer R. D., Aknay N., et al. Effect of valsartan added to background ACE inhibitor therapy in patients with heart failure: results from Val‐HeFT. Eur J Heart Fail 2004; 6: 937–45
- Shinohara H., Fukuda N., Soeki T., Sakabe K., Onose Y., Tamura Y. Effects of angiotensin II receptor antagonists on [123] metaiodobenzylguanidine myocardial imaging findings and neurohumoral factors in chronic heart failure. Heart Vessels 2002; 17: 47–52
- Falcao L. M., Pinto F., Ravara L., van Zwieten P. A. BNP and ANP as diagnostic and predictive markers in heart failure with left ventricular systolic dysfunction. J Renin Angiotensin Aldosterone Syst 2004; 5: 121–9
- Kasama S., Toyama T., Kumakura H., Takayama Y., Ichikawa S., Suzuki T., et al. Effects of perindopril on cardiac sympathetic nerve activity in patients with congestive heart failure: comparison with enalapril. Eur J Nucl Med Mol Imaging 2005; 32: 964–71
- Kasama S., Toyama T., Hatori T., Sumino H., Kumakura H., Takayama Y., et al. Comparative effects of valsartan and enalapril on cardiac sympathetic nerve activity and plasma brain natriuretic peptide in patients with congestive heart failure. Heart 2006; 92: 625–30
- Brunner‐La Rocca H. P., Weilenmann D., Kiowski W., Maly F. E., Candinas R., Follath F. Within‐patient comparison of effects of different dosages of enalapril on functional capacity and neurohormone levels in patients with chronic heart failure. Am Heart J 1999; 138: 654–62
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316: 1429–35
- Yan R. T., White M., Yan A. T., Yusuf S., Rouleau J. L., Maggioni A. P., et al. Usefulness of temporal changes in neurohormones as markers of ventricular remodeling and prognosis in patients with left ventricular systolic dysfunction and heart failure receiving either candesartan or enalapril or both. Am J Cardiol 2005; 96: 698–704
- Leskinen H., Vuolteenaho O., Ruskoaho H. Combined inhibition of endothelin and angiotensin II receptors blocks volume load‐induced cardiac hormone release. Circ Res 1997; 80: 114–23
- Rademaker M. T., Charles C. J., Espiner E. A., Frampton C. M., Nicholls M. G., Richards A. M. Combined inhibition of angiotensin II and endothelin suppresses the brain natriuretic peptide response to developing heart failure. Clin Sci 2004; 106: 569–76
- Haufe M. C., Gerzer R., Weil J., Ernst J. E., Theisen K. Verapamil impairs secretion of stimulated atrial natriuretic factor in humans. J Am Coll Cardiol 1988; 11: 1199–203
- Shima J., Ogihara T., Hara H., Iinuma K., Kumahara Y. Effects of calcium antagonists on the secretion of atrial natriuretic peptide in normal volunteers. Curr Ther Res 1987; 42: 115–23
- Shamiss A., Peleg E., Rosenthal T., Ezra D. The role of atrial natriuretic peptide in the diuretic effect of Ca2+ entry blockers. Eur J Pharmacol 1993; 233: 113–7
- Fioretto P., Frigato F., Velussi M., Riva F., Muollo B., Carraro A., et al. Effects of angiotensin converting enzyme inhibitors and calcium antagonists on atrial natriuretic peptide release and action and on albumin excretion rate in hypertensive insulin‐dependent diabetic patients. Am J Hypertens 1992; 5: 837–46
- Antonicelli R., Tomassini P. F., Galletti P., Gambini C., Marini M., Amadio L., et al. Age‐related antihypertensive and haemodynamic effects of verapamil ST: clinical results and effects on atrial natriuretic peptide. Eur J Clin Pharmacol 1990; 39(suppl 1)S29–33
- Van Bortel L. M. A. B., Schiffers P. M. H., Böhm R. O. B., Mooij J. M. V., Rahn K. H., Struyker Boudier H. A. J. The influence of chronic treatment with verapamil on plasma atrial natriuretic peptide levels in young and elderly hypertensive patients. Eur J Clin Pharmacol 1990; 39(suppl 1)S39–40
- Buckley J. W., Hedner T., Masotto C., Posvar E., Negro‐Villar A., Cubeddu L. X. Comparative effects of verapamil and volume overload on atrial natriuretic factors and the renin‐angiotensin aldosterone‐vasopressin system. J Clin Pharmacol 1992; 32: 1120–7
- Kokkas B., Kotridis P., Karamouzis M., Kanonidis I., Sakadamis G., Dadous G., et al. Plasma atrial natriuretic peptide levels in essential hypertension after treatment with verapamil. Eur J Drug Metab Pharmacokinet 2002; 27: 45–8
- Cappuccio F. P., Markandu N. D., Sagnella G. A., Singer D. R., Buckley M. G., Miller M. A., et al. Effects of amlodipine on urinary sodium excretion, renin‐angiotensin‐aldosterone system, atrial natriuretic peptide and blood pressure in essential hypertension. J Hum Hypertens 1991; 5: 115–9
- Deary A. J., Schumann A. L., Murfet H., Haydock S. F., Foo R. S‐Y., Brown M. J. Double‐blind, placebo‐controlled crossover comparison of five classes of antihypertensive drugs. J Hypertens 2002; 20: 771–7
- Wijeysundera H. C., Hansen M. S., Stanton E., Cropp A. S., Hall C., Dhalla N. S., et al. Neurohormones and oxidative stress in nonischemic cardiomyopathy: relationship to survival and the effect of treatment with amlodipine. Am Heart J 2003; 146: 291–7
- Stokes G. S., Monaghan J. C., Berman K., Ryan M., Campbell D. J. Double‐blind crossover study of the interaction between perindopril and amlodipine on blood pressure and hormones related to fluid and electrolyte balance in patients with essential hypertension. J Hum Hypertens 1998; 12: 129–34
- Lennox S., Penney M., Woodhouse K. Plasma atrial natriuretic peptide levels in elderly hypertensives: effects of blood pressure reduction with amlodipine. Arch Gerontol Geriatr 1994; 19: 223–7
- Shigematsu S., Yamada T., Aizawa T., Takasu N., Shimizu Z. Differential effects of nifedipine on plasma atrial natriuretic peptide in normal subjects and hypertensive patients. Angiology 1992; 43: 40–6
- Ringqvist I., Hedner T., Leppert J., Niklasson U., Edvinsson L. Effects of cold pressor test on circulating atrial natriuretic peptide 99‐126 (ANP) in patients with Raynaud's phenomenon and influence of treatment with magnesium sulphate and nifedipine. Clin Physiol 1993; 13: 271–80
- Phillips R. A., Ardeljan M., Shimabukuro S., Goldman M. E., Garbowit D. L., Eison H. B., et al. Normalization of left ventricular mass and associated changes in neurohormones and atrial natriuretic peptide after 1 year of sustained nifedipine therapy for severe hypertension. J Am Coll Cardiol 1991; 17: 1595–602
- Maki N., Yoshiyama M., Omura T., Yoshimura T., Kawarabayashi T., Sakamoto K., et al. Effect of diltiazem on cardiac function assessed by echocardiography and neurohumoral factors after reperfused myocardial infarction without congestive heart failure. Cardiovasc Drugs Ther 2001; 15: 493–9
- Erbas B., Ozdemir O., Pasaoglu I., Ugur O., Varoglu E., Koray Z., et al. Short‐ and long‐term effects of felodipine on circulating endothelin and atrial natriuretic peptide levels in essential hypertension. Nephron 1993; 63: 363–4
- Smith R. F., Germanson T., Judd D., Wong M., Ziesche S., Anand I. S., et al. Plasma norepinephrine and atrial natriuretic peptide in heart failure: influence of felodipine in the third Vasodilator Heart Failure Trial. V‐HeFT III investigators. J Card Fail 2000; 6: 97–107
- Lehnert H., Schmitz H., Preuss K., Küstner E., Krause U., Beyer J. Effects of nitrendipine on blood pressure, renin‐angiotensin‐system and atrial natriuretic peptide in hypertensive type 1 diabetic patients. Horm Metab Res 1993; 25: 24–8
- Keller N., Sykulski R., Thamsborg G., Storm T., Larsen J. Changes in atrial natriuretic factor during preload reduction with nitroglycerin in patients with congestive heart failure. Clin Physiol 1988; 8: 57–64
- Webster M. W. I., Sharpe D. N., Coxon R., Murphy J., Hannan S., Nicholls M. G., et al. Effect of reducing atrial pressure on atrial natriuretic factor and vasoactive hormones in congestive heart failure secondary to ischemic and nonischemic dilated cardiomyopathy. Am J Cardiol 1989; 63: 217–21
- Lewis B. S., Makhoul N., Dakak N., Flugelman M. Y., Yechiely H., Halon D. A., et al. Atrial natriuretic peptide in severe heart failure: response to controlled changes in atrial pressures during intravenous nitroglycerin therapy. Am Heart J 1992; 124: 1009–16
- Tsutamoto T., Kinoshita M., Nakae I., Maeda Y., Wada A., Yabe T., et al. Absence of hemodynamic tolerance to nicorandil in patients with severe congestive heart failure. Am Heart J 1994; 127: 866–73
- Ferreira A., Bettencourt P., Dias P., Pestana M., Serrao P., Soares‐da‐Silva P., et al. Neurohormonal activation, the renal dopaminergic system and sodium handling in patients with severe heart failure under vasodilator therapy. Clin Sci 2001; 100: 557–66
- Uehlinger D. E., Zaman T., Weidmann P., Shaw S., Gnadinger M. P. Pressure dependence of atrial natriuretic peptide during norepinephrine infusion in humans. Hypertension 1987; 10: 249–53
- Tomiyama T., Baba T., Murabayashi S., Ishizaki T. Acute effect of an alpha 1‐adrenoceptor antagonist on urinary sodium excretion, plasma atrial natriuretic peptide, arginine vasopressin, and the renin‐aldosterone system in healthy subjects. Eur J Clin Pharmacol 1992; 43: 17–21
- Semplicini A., Valle R., Serena L., Fazari G., Fontebasso A., Gebbin A., et al. Long‐term alpha‐1 adrenoceptor blockade does not affect plasma atrial natriuretic peptide in hypertensive patients. Horm Metab Res 1994; 26: 207–8
- Valle R., Semplicini A., Serena L., Gebbin A., Fontebasso A., Gerardi G., et al. Pressure and metabolic effects of terazosin in essential hypertension. Cardiologia 1994; 39: 421–4
- Kotridis P., Kokkas B., Kyriakou P., Karamouzis M., Salpigidis G., Karantona C., et al. Plasma atrial natriuretic peptide in essential hypertension after treatment with terazocin. Eur J Drug Metab Pharmacokinet 2003; 28: 143–6
- Tsutamoto T., Wada A., Maeda K., Hisanaga T., Fukai D., Maeda Y., et al. Digitalis increases brain natriuretic peptide in patients with severe congestive heart failure. Am Heart J 1997; 134: 910–6
- Kobusiak‐Prokopowicz M., Swidnicka‐Szuszkowska B., Mysiak A. Effect of digoxin on atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and cyclic 3', 5'‐guanosine monophosphate (cGMP) in patients with chronic congestive heart failure. Pol Arch Med Wewn 2001; 105: 475–82
- Miller W. L., Bailey K. R., Weston S. A., Burnett J. C., Jr., Rodeheffer R. J. Hemodynamic and plasma atrial natriuretic peptide responses to acute digitalis therapy in patients with normal and impaired left ventricular function. Eur J Heart Fail 2002; 4: 63–72
- Bloch K. D., Zamir N., Lichtstein D., Seidman C. E., Seidman J. G. Ouabain induces secretion of proatrial natriuretic factor by rat atrial myocytes. Am J Physiol 1988; 255: E383–7
- Morise T., Takeuchi Y., Okamoto S., Takeda R. Stimulation of atrial natriuretic peptide secretion and synthesis by Na‐K‐ATPase inhibitors. Biochem Biophys Res Commun 1991; 176: 875–81
- Stangl K., Baumann G., Gerzer R., Weil J. Acute effects of beta‐adrenergic stimulation with dobutamine on the plasma levels of atrial natriuretic peptide and cyclic guanosine monophosphate in patients with chronic heart failure. Eur Heart J 1991; 12: 917–23
- La Vecchia L., Bottero M., Centofante P., Bedogni F., Ometto R., Cera A., et al. Acute changes in the hemodynamic profile and circulating levels of atrial natriuretic peptide induced by dobutamine in severe heart failure. G Ital Cardiol 1996; 26: 863–74
- Van der Ent M., van den Heuvel A. F., Remme W. J. Neurohumoral response to carmoxirole, a selective dopamine (D2) receptor agonist, in patients with chronic moderate heart failure. Cardiovasc Drugs Ther 1998; 12: 387–94
- Hayabuchi Y., Matsuoka S., Kuroda Y. Plasma concentrations of atrial and brain natriuretic peptides and cyclic guanosine monophosphate in response to dobutamine infusion in patients with surgically repaired tetralogy of fallot. Pediatr Cardiol 1999; 20: 343–50
- Avgeropoulou C., Andreadou I., Markantonis‐Kyroudis S., Demopoulou M., Missovoulos P., Androulakis A., et al. The Ca2+‐sensitizer levosimendan improves oxidative damage, BNP and pro‐inflammatory cytokine levels in patients with advanced decompensated heart failure in comparison to dobutamine. Eur J Heart Fail 2005; 7: 882–7
- Cherng W. J., Wang C. H., Hung M. J. Changes of endothelin‐1 and atrial natriuretic peptide during dobutamine stress echocardiography. J Formos Med Assoc 1998; 97: 812–8
- Asada J., Tsuji H., Iwasaka T., Thomas J. D., Lauer M. S. Usefulness of plasma brain natriuretic peptide levels in predicting dobutamine‐induced myocardial ischemia. Am J Cardiol 2004; 93: 702–4
- Karabinos I., Karvouni E., Chiotinis N., Papadopoulos A., Simeonidis P., Tsolas O., et al. Acute changes in N‐terminal pro‐brain natriuretic peptide induced by dobutamine stress echocardiography. Eur J Echocardiogr 2006 Jul 15, [Epub ahead of print]
- Tulassay T., Rascher W., Hajdu J., Lang R. E., Toth M., Seri I. Influence of dopamine on atrial natriuretic peptide level in premature infants. Acta Paediatr Scand 1987; 76: 42–6
- Berglund H., Bevegard S., Carlens P., Hedner J., Hedner T. Atrial natriuretic peptide during acute treatment of congestive heart failure. Clin Physiol 1988; 8: 155–62
- Sonoda K., Ikeda S., Miyahara Y., Kohno S. Successful treatment of chronic pulmonary thromboembolism by long‐term intermittent administration of milrinone, a phosphodiesterase‐III inhibitor. Int Med 2002; 41: 961–6
- Moertl D., Berger R., Huelsmann M., Bojic A., Pacher R. Short‐term effects of levosimendan and prostaglandin E1 on hemodynamic parameters and B‐type natriuretic peptide levels in patients with decompensated chronic heart failure. Eur J Heart Fail 2005; 7: 1156–63
- Parissis J. T., Adamopoulos S., Farmakis D., Filippatos G., Paraskevaidis I., Panou F., et al. Effects of serial levosimendan infusions on left ventricular performance and plasma biomarkers of myocardial injury and neurohormonal and immune activation in patients with advanced heart failure. Heart 2006; 92: 1768–72
- McMurray J. J., Lang C. C., MacLean D., McDevitt D. G., Struthers A. D. Neuroendocrine changes post myocardial infarction: effects of xamoterol. Am Heart J 1990; 120: 56–62
- Takemura K., Yasumura Y., Hirooka K., Hanatani A., Nakatani S., Komamura K., et al. Low‐dose amiodarone for patients with advanced heart failure who are intolerant of beta‐blockers. Circ J 2002; 66: 441–4
- Shiga T., Hosaka F., Wakaumi M., Matsuda N., Tanizaki K., Kajimoto K., et al. Amiodarone decreases plasma brain natriuretic peptide level in patients with heart failure and ventricular tachyarrhythmia. Cardiovasc Drugs Ther 2003; 17: 325–33
- Baranowska B., Wasilewska‐Dziubinska E., Radzikowska M., Plonowski A., Roguski K. Impaired response of atrial natriuretic peptide to acute water load in obesity and in anorexia nervosa. Eur J Endocrinol 1995; 132: 147–51
- Spinetti A., Margutti A., Bertolini S., Bernardi F., BiFulco G., Degli Uberti E. C., et al. Hormonal replacement therapy affects calcitonin gene‐related peptide and atrial natriuretic peptide secretion in postmenopausal women. Eur J Endocrinol 1997; 137: 664–9
- Hurlen M., Hole T., Seljeflot I., Arnesen H. Aspirin does not influence the effect of angiotensin‐converting enzyme inhibition on left ventricular ejection fraction 3 months after acute myocardial infarction. Eur J Heart Fail 2001; 3: 203–7
- Jug B., Šebeštjen M., Šabovič M., Keber I. Clopidogrel is associated with a lesser increase in NT‐proBNP when compared to aspirin in patients with ischemic heart failure. J Card Fail 2006; 12: 446–51
- Meune C., Wahbi K., Fulla Y., Cohen‐Solal A., Duboc D., Mahé I., et al. Effects of aspirin and clopidogrel on plasma brain natriuretic peptide in patients with heart failure receiving ACE inhibitors. Eur J Heart Fail 2006 Aug 14, [Epub ahead of print]
- Wojnicz R., Nowak J., Szygula‐Jurkiewicz B., Wilczek K., Lekston A., Trzeciak P., et al. Adjunctive therapy with low‐molecular‐weight heparin in patients with chronic heart failure secondary to dilated cardiomyopathy: one year follow‐up results of the randomized trial. Am Heart J 2006; 152: 713e1–e7
- Kim J., Ogai A., Nakatani S., Hashimura K., Kanzaki H., Komamura K., et al. Impact of blockade of histamine H2 receptors on chronic heart failure revealed by retrospective and prospective randomized studies. J Am Coll Cardiol 2006; 48: 1378–84
- Li D., Wen J. F., Jin J. Y., Jin H., Ann H. S., Kim S. Z., et al. Histamine inhibits atrial myocytic ANP release via H2 receptor‐cAMP‐protein kinase signaling. Am J Physiol Regul Integr Comp Physiol 2003; 285: R380–93
- Manner T., Kanto J., Ruskoaho H., Karhuvaara S., Viinamaki O., Valimaki M., et al. Hormonal, haemodynamic, and subjective effects of intravenously infused indomethacin: no change in the physiological response to hypertonic saline challenge. Pharmacol Toxicol 1989; 65: 231–5
- Nielsen C. B., Sørensen S. S., Pedersen E. B. Effects of indomethacin on renal function in normotensive patients with chronic glomerulonephritis with preserved renal function. Scand J Clin Lab Invest 1994; 54: 523–9
- Pedersen E. B., Bacevicius E., Bech J. N., Solling K., Pedersen H. B. Abnormal rhythmic oscillations of atrial natriuretic peptide and brain natriuretic peptide in chronic renal failure. Clin Sci 2006; 110: 491–501
- Olsen M. E., Thomsen T., Hassager C., Ibsen H., Dige‐Petersen H. Hemodynamic and renal effects of indomethacin in losartan‐treated hypertensive individuals. Am J Hypertens 1999; 12: 209–16
- Gavin A. D., Struthers A. D. Allopurinol reduces B‐type natriuretic peptide concentrations and haemoglobin but does not alter exercise capacity in chronic heart failure. Heart 2005; 91: 749–53
- Strey C. H., Young J. M., Lainchbury J. H., Frampton C. M., Nicholls M. G., Richards A. M., et al. Short‐term statin therapy improves endothelial function and neurohormonal imbalance in normocholesterolaemic patients with non‐ischaemic heart failure. Heart 2006; 92: 1603–9
- Davidson B. J., Rea C. D., Valenzuela G. J. Atrial natriuretic peptide, plasma renin activity, and aldosterone in women on estrogen therapy and with premenstrual syndrome. Fertil Steril 1988; 50: 743–6
- Maffei S., Del Ry S., Prontera C., Clerico A. Increase in circulating levels of cardiac natriuretic peptides after hormone replacement therapy in postmenopausal women. Clin Sci (Lond) 2001; 101: 447–53
- Karjalainen A. H., Ruskoaho H., Vuolteenaho O., Heikkinen J. E., Bäckström A‐C., Savolainen M. J., et al. Effects of estrogen replacement therapy on natriuretic peptides and blood pressure. Maturitas 2004; 47: 201–8
- Kuroski de Bold M. L. Estrogen, natriuretic peptides and the renin‐angiotensin system. Cardiovasc Res 1999; 41: 524–31
- Chen S., Garami M., Gardner D. G. Doxorubicin selectively inhibits brain versus atrial natriuretic peptide gene expression in cultured neonatal rat myocytes. Hypertension 1999; 34: 1223–31
- Meinardi M. T., van Veldhuisen D. J., Gietema J. A., Dolsma W. V., Boomsma F., van den Berg M. P., et al. Prospective evaluation of early cardiac damage induced by epirubicin‐containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J Clin Oncol 2001; 19: 2746–53
- Hayakawa H., Komada Y., Hirayama M., Hori H., Ito M., Sakurai M. Plasma levels of natriuretic peptides in relation to doxorubicin‐induced cardiotoxicity and cardiac function in children with cancer. Med Pediatr Oncol 2001; 37: 4–9
- Nousiainen T., Vanninen E., Jantunen E., Puustinen J., Remes J., Rantala A., et al. Natriuretic peptides during the development of doxorubicin‐induced left ventricular diastolic dysfunction. J Intern Med 2002; 251: 228–34
- Perik P. J., De Vries E. G., Boomsma F., van der Graaf W. T., Sleijfer D. T., van Veldhuisen D. J., et al. Use of natriuretic peptides for detecting cardiac dysfunction in long‐term disease‐free breast cancer survivors. Anticancer Res 2005; 25: 3651–7
- Pichon M. F., Cvitkovic F., Hacene K., Delaunay J., Lokiec F., Collignon M. A., et al. Drug‐induced cardiotoxicity studied by longitudinal B‐type natriuretic peptide assays and radionuclide ventriculography. In Vivo 2005; 19: 567–76