Abstract
Atherosclerosis is characterized by chronic inflammation involving autoimmune components. The degree of inflammatory activity, as detectable both within the atherosclerotic plaque and in the circulation, is associated with plaque destabilization and atherothrombotic complications. Endogenous glucocorticoids are modulators of innate and acquired immune responses, and as such play a key role in the reciprocal interaction between neuroendocrine and immune systems. Abnormalities in hypothalamic-pituitary-adrenal axis (HPA) function have been described in several chronic inflammatory disorders, and evidence has emerged lately that HPA dysfunction may be implicated also in the pathogenesis of coronary artery disease. This review is an outline of knowledge gained so far by previous studies of glucocorticoids in coronary atherosclerosis and myocardial infarction. The results consistently point towards a dysregulated cortisol secretion that may involve a failure to contain inflammatory activity. A dysfunctional HPA axis and its possible implications for coronary artery disease progress, including the hypothetical link between stress and inflammation, are discussed.
Atherosclerosis—an inflammatory disease
Inflammation has a major influence on the development of atherosclerosis. In the atherosclerotic lesion, an excessive production of proinflammatory T helper (Th) 1 cytokines, like interferon (IFN)-γ and tumour necrosis factor (TNF)-α, is considered to drive the development towards plaque rupture and acute thrombotic complications Citation1, Citation2. Activated T cells, mainly of the Th1 type, and macrophages are frequently found in so-called high-risk lesions Citation3, Citation4. The Th1 polarization is associated with an overexpression of matrix-degrading enzymes, such as cathepsins and matrix metalloproteinase (MMP)-9 in the shoulder region of the plaque, thereby contributing to the weakening of the fibrous cap Citation5–7. Th1 cytokines also stimulate the secretion of prothrombotic and procoagulant factors Citation8. On the other hand, there is increasing evidence from experimental studies that anti-inflammatory cytokines, like interleukin (IL)-10, favour development towards plaque stability and a less prothrombotic and procoagulant state Citation8, Citation9. The enhanced proinflammatory activity is not only detectable within the arterial wall. A number of cross-sectional reports have shown an increased systemic inflammatory activity in patients with stable as well as unstable coronary artery disease (CAD), though most pronounced in unstable conditions. Such data include increased numbers of leukocytes, in particular activated T helper cells, and increased levels of serological markers, like C-reactive protein (CRP), proinflammatory cytokines, and MMP-9 Citation10–15. On the other hand, the low serum levels of IL-10 in patients with unstable CAD compared to patients with stable disease have been suggested to reflect a less efficient atheroprotection Citation16. The clinical importance of a proinflammatory state is further supported by a large number of epidemiological studies showing that elevations of inflammatory markers, like CRP and IL-6, are predictors of cardiovascular events Citation17–19. Conversely, high levels of IL-10 have been associated with an improved outcome in CAD patients Citation20. Altogether, data indicate that an imbalance between proinflammatory and anti-inflammatory systems has an important role in the clinical outcome of patients with atherosclerotic disease. A repeated or continuous exposure to potential self-antigens, like oxidized lipoproteins, may explain why inflammatory activity in atherosclerosis is not resolved. However, even if inflammation in atherosclerosis is initiated and driven by an antigen-specific response, it is likely that other mechanisms affecting the anti-inflammatory homeostasis are involved. During the last two decades, our understanding of the interactions between the hypothalamic-pituitary-adrenal (HPA) axis and immune-mediated inflammation has largely expanded. As will be reviewed herein, neuroendocrine dysregulation may contribute to both increased susceptibility to inflammatory/autoimmune disease and sustainment of the inflammatory process.
Key messages
Hypothalamic-pituitary-adrenal (HPA) axis dysfunction, mainly involving a blunted HPA response, is associated with inflammatory/autoimmune disease activity.
A flatter diurnal cortisol rhythm and a blunted HPA axis response is found in patients with preclinical as well as clinically established coronary artery disease (CAD).
The dysregulated cortisol secretion in CAD patients is associated with a systemic inflammatory activity, including an increased inflammatory response to stress. HPA axis dysfunction may, thereby, have implications for CAD progress.
Neuroendocrine regulation of inflammation
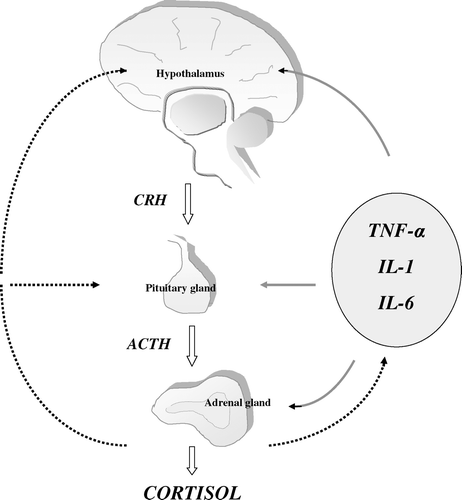
One major mechanism by which the central nervous system regulates the immune system is through the HPA axis and its end-product, glucocorticoids Citation21–23. Three cytokines—TNF-α, IL-1 and IL-6—account for most of the HPA axis-stimulating activity either directly through the systemic circulation or indirectly by, for example, activating neural mechanisms. The release of cytokines is not only triggered by immune stimuli but also by non-immune stress, presumably stimulated by catecholamines acting through β2-adrenergic receptors. The first step in HPA axis activation is the release of corticotropin-releasing hormone (CRH) from intrahypothalamic neurons. CRH travel from the hypothalamus via the hypophyseal–portal blood vessels to the anterior pituitary gland where it acts via specific receptors to trigger the release of the adrenocorticotrophic hormone (corticotrophin, ACTH) from specific ACTH-producing cells into the systemic circulation. ACTH in turn acts on the adrenal cortex to initiate the synthesis of cortisol, which is released immediately into the systemic circulation by diffusion. The magnitude of the HPA response to incoming stimuli is tempered by the glucocorticoids which act at the levels of the pituitary gland and hypothalamus to suppress the synthesis and release of ACTH and CRH, thus forming a negative feedback loop (). Cortisol is the predominant glucocorticoid in man. It also constitutes the active form while cortisone is its inactive precursor. The glucocorticoids exert wide-spread actions in the body, which are essential for the maintenance of homeostasis and enable the organism to prepare for, respond to, and cope with physical and emotional stress Citation24. They are important regulators of immune and inflammatory processes and are required for numerous processes associated with host defence. These properties underlie many of the stress-protective actions of the steroids as they quench the pathophysiological responses to tissue injury and inflammation and, thereby, prevent them from proceeding to a point where they threaten the survival of the host.
Abbreviations
Figure 1. Schematic diagram illustrating the bidirectional communication between the neuroendocrine and immune systems. Tumour necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6 stimulate the secretion of corticotropin-releasing hormone (CRH) from hypothalamic neurons; at high concentrations or over a prolonged period they may also stimulate the secretion of adrenocorticotrophic hormone (ACTH) from the pituitary gland and cortisol from adrenal cortex. The hypothalamic-pituitary-adrenal axis activity is modulated by a negative feedback system through which the release of CRH and ACTH is suppressed by cortisol itself. Cortisol inhibits the production of all three inflammatory cytokines and also inhibits most of their effects on target tissues. The solid lines indicate stimulation, the dotted lines indicate inhibition.
Initially, glucocorticoids were thought to have mainly immunosuppressive effects. In 1948 it was shown for the first time that a synthesized version of cortisone was capable of reversing the inflammation of rheumatoid arthritis Citation25. However, it is important to recognize that glucocorticoids in pharmacological doses exert different effects than they do under physiological conditions Citation26. Pharmacological doses (higher concentrations than physiological) are anti-inflammatory or immunosuppressive at virtually every level of immune and inflammatory responses, whereas physiological levels of glucocorticoids should rather be considered immunomodulatory. In vitro, their role at doses slightly higher than physiological is mainly exerted through genomic effects, among which are the induction of anti-inflammatory proteins annexin-1 (also called lipocortin 1) and mitogen-activated protein kinase (MAPK) phosphatase 1, and the repression of transcription of cyclo-oxygenase 2 Citation27. Glucocorticoids also block the transcriptional activity of nuclear factor κB, which is a major factor involved in the regulation of cytokines and other immune responses Citation28. As a consequence of the inhibition of nuclear factor κB, the expression of cytokines like IL-1, IL-6, IFN-γ, and TNF-α is downregulated. The net effect of glucocorticoids is a shift of cytokine production from a primarily proinflammatory to anti-inflammatory pattern, roughly corresponding to Th1 and Th2, respectively. The shift of Th1 to Th2 is considered to be due mainly to the downregulation of Th1 cytokines, thus allowing a dominant expression of Th2 cytokines Citation29, Citation30.
The transcriptional actions of glucocorticoids are mediated by supposed diffusion of the steroid hormone across the cell membrane and its binding to intracellular glucocorticoid receptors (GRs) Citation26, Citation31. Two human isoforms of the GR have been identified, termed GR-α and GR-β, which originate from the same gene by alternative splicing of the GR primary transcript Citation27, Citation32. GR-β is the predominant isoform of the receptor and the one that shows steroid-binding activity. The possible physiological role of GR-β is currently a matter for debate but it regulates GR-α activity in vivo and has been shown to act as a dominant negative inhibitor of GR-α activity.
Methodological considerations in the study of HPA axis activity
Cortisol shows a robust diurnal pattern in healthy adults with the strongest secretory activity of the adrenal cortex during the early morning hours. Peak cortisol levels are observed shortly after awakening, with steadily decreasing values thereafter, except for sizable, short-term increases in response to stimuli like lunch meal, exercise, or threat-provoking stressors. The nadir of cortisol secretion is reached around 02.00 or 03.00 with only minimal levels of the steroid detectable. The diurnal rhythm in the rate of cortisol secretion may thus explain the diurnal changes in disease-associated immune responses. For example, the delayed hypersensitivity reaction, which is particularly responsive to glucocorticoids, is most pronounced in the evening when cortisol secretion is low and least pronounced in the morning when secretion is high Citation33. Others have shown a circadian rhythm in the disease activity in rheumatoid arthritis with maximal activity between 02.00 and 04.00 Citation34.
The measurement of free cortisol in 24-hour urine samples serves as an integrated measure of free cortisol concentrations during the entire day. To determine the circadian patterns of cortisol secretion or random cortisol concentrations, measurements of free cortisol can be performed in blood or saliva. However, measurements over 2–6 days are considered necessary to achieve reliable trait measures, since state factors may bias data from a single day Citation35. Free cortisol in saliva has been shown to have the same diurnal rhythm as blood cortisol. Furthermore, it has been shown that the transfer of cortisol from blood to saliva is rapid with a reflection in saliva of a cortisol increase in blood within 60 seconds and a state of equilibrium within 5 minutes Citation36, Citation37. Due to several advantages over blood cortisol analyses, such as stress-free sampling and laboratory independence, the determination of salivary cortisol has become the method of choice in basic research and clinical environments. It was first introduced to psychobiological stress research almost three decades ago, and since then a number of studies have investigated the association between psychological factors and cortisol in saliva Citation38. The activity of the HPA axis is often evaluated by measuring the levels of blood or salivary cortisol in response to different stimulation procedures such as CRH test, physical exertion or psychological stress. However, since the responses may show a large heterogeneity, standardized laboratory stress protocols that combine different stressors are preferable Citation39.
Cortisol in coronary artery disease
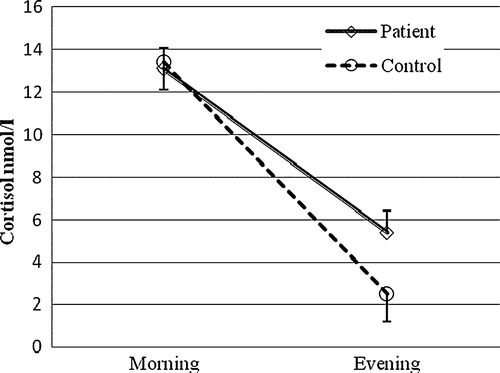
A growing body of evidence suggests an association between HPA axis activity and coronary atherosclerosis. A population-based prospective study showed that an elevated cortisol:testosterone ratio, based on one early morning blood sample, increased the risk of CAD mortality and incidence Citation40. However, data from earlier studies in CAD patients are both sparse and inconsistent. Positive correlations between morning plasma cortisol and the degree of CAD, as assessed by angiography, have been demonstrated Citation41–43, while others have failed to find associations between morning cortisol and number of diseased coronary vessels Citation44 or between 24-hour urinary cortisol and ischaemia, as assessed by stress echocardiography Citation45. On the other hand, we recently determined the total cortisol output in 24-hour urine collections and found significantly increased levels in patients with stable conditions of CAD when compared to age- and gender-matched healthy subjects () Citation46. One limitation is that neither single measurements of morning cortisol, nor 24-hour urine collections, provide any information on cortisol reactivity or daily cortisol profile. A dysregulation of the HPA axis can take the form of a smaller decline in cortisol throughout the day, i.e. a flatter diurnal slope. Although the determinants or consequences of having a flat cortisol rhythm are not clear, it is generally considered a result of long-term HPA overstimulation. A flat rhythm has also been proposed to constitute a marker of disease progression. Abnormal circadian rhythms have been observed in patients with cancer and, as an example, patients with metastatic breast cancer whose diurnal cortisol rhythms are flattened have earlier mortality Citation47. In a population-based study of middle-aged men, Rosmond and Björntorp showed that clusters of established risk factors for cardiovascular disease were tightly associated with a pathological HPA axis, characterized by low diurnal cortisol variability and a poor lunch-induced cortisol response Citation48. A number of studies have confirmed the link between subtle alterations in cortisol secretion and separate cardiovascular risk factors, including smoking, abdominal obesity, and hypertension Citation49–53. However, the most consistent findings have been reported in smokers and involve an increased cortisol release throughout the day as well as an attenuated HPA axis response to stress Citation51, Citation52. In a population-based study using a cross-sectional design, six salivary cortisol samples were collected from middle-aged adults on a single day, from awakening to bedtime. Results showed that the flatter the cortisol slopes throughout the day, the greater the likelihood of any coronary calcification Citation54. In addition, Rosmond and co-workers showed that the 5-year incidence of cardiovascular-related events was significantly higher in men with an abnormal cortisol slope along with low testosterone levels, compared to men with a normal hormone pattern Citation55. In order to evaluate the diurnal variation in cortisol secretion in patients with established CAD, we measured cortisol in saliva twice daily for 3 consecutive working days, the first sample taken 30 min after awakening and the second in the evening before going to bed. The morning cortisol levels did not differ between patients and controls, while the evening cortisol levels were significantly higher in patients, thus clearly indicating a flatter cortisol profile over the day in CAD patients () Citation46.
Figure 2. The total daily cortisol production, as assessed by free cortisol in 24-hour urine collection, in apparently healthy individuals versus patients with coronary artery disease (CAD) Citation46, P<0.05. Box plots summarize the median, interquartile range, and minimum and maximum values.
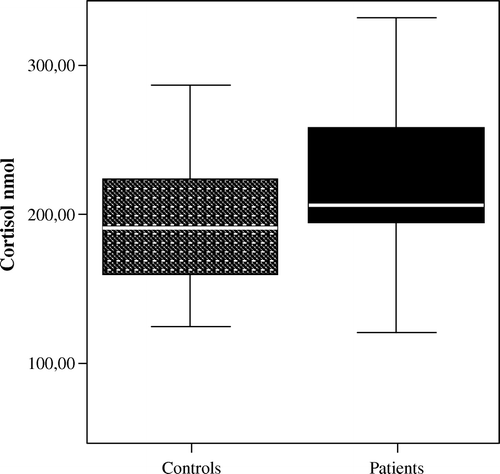
Figure 3. The salivary cortisol levels 30 minutes after awakening (morning) and at bedtime (evening) in apparently healthy individuals versus patients with coronary artery disease (CAD) Citation46, P<0.05. Values are given as mean (SD).
The association between HPA dysfunction and inflammation in CAD
Cytokines, like IL-1 and IL-6, can increase the glucocorticoid secretion by providing the synthesis and release of CRH and ACTH in hypothalamus and pituitary gland but also by enhancing the secretion of cortisol in adrenal cortex. Cortisol, in its turn, inhibits the production of TNF-α, IL-1, and IL-6. The interaction between the HPA axis and the immune system may result in reciprocally protective adaptations. For instance, immune suppression in Cushing's syndrome is mild, suggesting the development of tolerance to glucocorticoids. In animals with chronic inflammatory disease the hypercortisolism is mild rather than severe Citation56. However, disturbances at any level of the HPA axis or glucocorticoid action may lead to an imbalance of the system and an enhanced susceptibility to infection and inflammatory/autoimmune diseases. The association between a blunted HPA axis and susceptibility to autoimmune/inflammatory disease has been clearly shown in many animal models, e.g. when comparing two highly inbred rat strains, Fischer rats and Lewis rats Citation57–59. The Lewis rats are highly susceptible to a wide variety of autoimmune/inflammatory diseases, while Fischer rats are resistant to these diseases. The Lewis rats exhibit a blunted HPA axis response, compared to Fischer rats with an excessive HPA response compared to outbred rats. In Lewis rats treated with low-dose dexamethasone or transplanted intracerebroventricularly with fetal hypothalamic tissue from Fischer rats, the autoimmune disease was markedly attenuated Citation60. The abnormalities in Lewis rats may also have parallels in humans. In patients with CAD, we found that serum levels of IL-6 and CRP were strongly correlated to evening salivary cortisol but not to morning cortisol or 24-hour urinary cortisol Citation46. In addition, the circulating levels of MMP-9 were significantly correlated to evening cortisol levels and remained so after adjustment for CRP and IL-6 (unpublished observations, Nijm J, Jonasson L, 2007). These findings suggest that the low-grade systemic inflammation in CAD patients is associated with a flatter diurnal cortisol slope. In a previous clinical study, serum cortisol and IL-6 were measured in blood samples taken between 09.00 and 12.00 in patients with both stable and unstable conditions of CAD. In two-thirds of patients, the cortisol levels were found to be ‘inappropriately’ normal with concomitant high IL-6 levels thus giving rise to the interesting speculation that endogenous cortisol production was insufficient to limit inflammation in these patients Citation61. However, the correlations between cortisol and inflammatory markers do not prove a cause-and-effect relationship, and there are inherent difficulties in evaluating the appropriateness of a given level of cortisol for a particular level of on-going inflammation in individuals. In addition, the possible effects of medication on cortisol levels should be considered. However, data from previous studies investigating the effects of beta-blockers and statins have been consistent, giving no evidence for alterations in basal cortisol levels or ACTH-induced cortisol response Citation62–66.
The measurement of HPA axis response to stress is considered to be an important piece of information to include for a more precise study of HPA function. In patients with rheumatoid arthritis, the basal morning cortisol levels did not differ compared with healthy subjects but, still, the patients with rheumatoid arthritis showed a lower cortisol response after insulin-induced hypoglycaemia Citation67. Similar results have been demonstrated in patients with atopic dermatitis and systemic lupus erythematosus. Although the basal morning cortisol levels in these patient groups did not differ from healthy subjects, their ACTH and cortisol responses to acute psychological stress or insulin-induced hypoglycaemia were significantly lower Citation68, Citation69. When CAD patients were exposed to acute physical stress (a maximal bicycle exercise test), we found that their cortisol response was significantly attenuated compared to age- and gender-matched control subjects, thus indicating a hyporeactive HPA axis in the patients. Similarly, the cortisol response to a standardized laboratory stress test (‘anger recall’ followed by an arithmetic test) was markedly attenuated in CAD patients compared to controls (A) Citation46. The differences between patients and controls remained significant even after adjustment for possible confounding factors such as smoking and treatment with beta-blocker or statin. The study of stress response in CAD patients is the first to demonstrate a blunted cortisol response in patients with atherosclerotic disease, but findings are in line with previous results from the population-based LiVicordia study (Linkoping-Vilnius Coronary Risk Assessment Study) Citation70. In the 1990s, Lithuanian middle-aged men had a 4-fold risk for CAD mortality compared with Swedish men. The aim of the LiVicordia study was, therefore, to compare both traditional and new possible risk factors for CAD in 50-year-old men from each of the cities Vilnius, Lithuania and Linköping, Sweden. Small differences were found in traditional risk factors between Swedish and Lithuanian men but, interestingly, Lithuanian men exhibited a significantly lower cortisol response to a standardized laboratory stress test. In addition, the LiVicordia study showed a higher prevalence of subclinical atherosclerosis in Lithuanian men, as assessed by ultrasound measurements of intima-media thickness Citation71.
Figure 4. A: Salivary cortisol response, given as percentage increase, to physical stress (grey boxes) and psychological stress (white boxes) in apparently healthy individuals versus patients with coronary artery disease (CAD), P<0.01 for both tests. B: Inflammatory response, given as percentage increase in C-reactive protein (CRP), to physical stress (grey boxes) and psychological stress (white boxes) in apparently healthy individuals versus patients with CAD, P<0.01 for both tests Citation46.
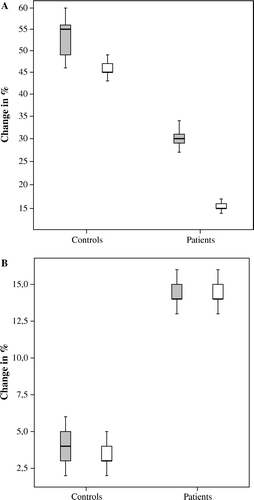
In the CAD patients, serum levels of CRP and IL-6 were determined before and 24 hours after the stress tests Citation46. None of the inflammatory markers differed between patients and controls at base-line. Neither were there any differences in IL-6 levels between patients and controls after 24 hours, probably reflecting that IL-6 levels had returned to base-line levels. The hepatic synthesis of CRP is largely under the regulation of IL-6. In humans, CRP is increased following IL-6 infusion, reaching a peak level 21 hours after the cessation of IL-6 infusion Citation72. In the CAD patients, acute physical stress induced a significant increase in CRP in the patients compared to the controls after 24 hours. Similarly, the psychological stress test induced a significant increase in CRP in the patients but not in the controls (B). It also deserves to be emphasized that all CAD patients participating in the stress tests were on long-term treatment with statin, a drug with well known CRP-lowering effects Citation73. Altogether, the findings support the hypothesis that a hypofunctional HPA axis in CAD patients fails to suppress stress-induced inflammatory activity. In an earlier study, patients with rheumatoid arthritis showed a failure to increase cortisol secretion following surgery, despite high levels of IL-1β and IL-6, compared to subjects with chronic osteomyelitis, the latter a joint disease not associated with systemic inflammation Citation74. The authors thus proposed that the activity of HPA axis in patients with rheumatoid arthritis was insufficient to inhibit on-going inflammation. In agreement with this, it was recently shown that an acute psychological stress test induced an increase in CRP in patients with rheumatoid arthritis, but not in patients with chronic osteomyelitis Citation75. Interestingly, these authors speculated that the inflammatory reaction to stress could underlie the increased risk for myocardial infarction in patients with rheumatoid arthritis.
There is a strong association between external triggers and onset of myocardial infarction and sudden cardiac death beyond that expected by chance alone. Several studies have shown that both physical exertion and acute emotional stress such as a burst of anger are potent triggers of myocardial infarction Citation76, Citation77. In one multicentre study, possible triggers were identified by 49% of the population; the most common were emotional upset (18%) and moderate physical activity (14%) Citation78. Lately, a case-control study with 11,119 patients with a first myocardial infarction and 13,648 age- and sex-matched controls from 52 countries (the INTERHEART study) investigated the relation of psycho-social factors to the risk of myocardial infarction. People with myocardial infarction reported a significantly higher prevalence of stress factors including stress at work and at home, financial stress, and major life events in the past year Citation79. The mechanism by which stress increases the risk of myocardial infarction is not clarified, but the interplay between stress and inflammation could play an important role. Thus, one intriguing possibility is that individuals with a blunted cortisol response will be more susceptible to atherosclerosis progression and plaque instability due to an ‘abnormally’ high inflammatory response to stressful stimuli.
A suppressed release of cortisol in response to stressful stimuli may be one mechanism linking HPA dysfunction to inflammatory disease, and glucocorticoid resistance in the target tissue may be another Citation80, Citation81. Glucocorticoid resistance is characterized by increased cortisol secretion without clinical evidence of hypercortisolism. The circadian rhythm of cortisol is intact albeit at higher concentrations, and there is resistance of the HPA axis to dexamethasone treatment. Up to 30% of patients with autoimmune or chronic inflammatory diseases have a form of glucocorticoid resistance, yet the mechanisms are not fully established. In experimental studies, the treatment with TNF-α or IL-1 leads to the development of glucocorticoid resistance by inducing a relative overexpression of GR-β Citation82. GR gene variants may also be related to changes in cortisol sensitivity. The haplotype 3, characterized by the GR-9β polymorphism, leads to an increased GR-β expression by generating a more stable GR-β messenger RNA transcript Citation83. This polymorphism has been found to be associated with rheumatoid arthritis in a small observational study Citation84. Moreover, in a large population-based prospective cohort, haplotype 3 was recently shown to be associated with a more active proinflammatory state, as assessed by elevations in CRP and IL-6, and greater carotid intima-media thickness Citation85. Interestingly, in this study the carriers of haplotype 3 also showed a more than 2-fold risk of myocardial infarction during a 9-year follow-up.
To conclude, coronary atherosclerosis is associated with hypercortisolism, involving a flatter diurnal rhythm. In addition, patients with clinically established CAD show a blunted cortisol response to stress, a feature previously described in autoimmune diseases, like rheumatoid arthritis. The relationship between a flat diurnal cortisol pattern and an attenuated cortisol response to stress is not clarified but both phenomena have been associated with reduced activity of the HPA axis. In the CAD patients, the low diurnal cortisol decline correlates with systemic inflammatory activity and, upon stressful stimuli, their attenuated cortisol response is accompanied by a significant increase in CRP. It remains unclear if the HPA dysfunction is mainly a secondary phenomenon due to a long-lasting inflammatory process or if it is more actively involved in the pathogenesis of CAD. Yet, the role of the HPA axis in atherogenesis has to be demonstrated in animal models. However, chronic or repeated immune insults on the HPA axis function may eventually lead to a poorer regulation of the inflammatory response, possibly earlier and more pronounced in genetically predisposed individuals. The imbalance between anti-inflammatory and proinflammatory mechanisms may thus lead to an increased susceptibility to immune insults and a maintenance of a proinflammatory state. It is a well known fact that a proinflammatory state is closely related to atherosclerosis and risk of cardiovascular events. Although the clinical consequences of a hyporesponsive HPA axis are still unknown, it is intriguing to speculate that it may favour the development towards plaque instability, thereby providing a link between stress and inflammation in CAD.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005; 352: 1685–95
- Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006; 6: 508–19
- Frostegård J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999; 145: 33–43
- de Boer OJ, van der Wal AC, Verhagen CE, Becker AE. Cytokine secretion profiles of cloned T cells from human aortic atherosclerotic plaques. J Pathol. 1999; 188: 174–9
- Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994; 94: 2493–503
- Brown DL, Hibbs MS, Kearney M, Loushin C, Isner JM. Identification of 92-kD gelatinase in human coronary atherosclerotic lesions. Association of active enzyme synthesis with unstable angina. Circulation. 1995; 91: 2125–31
- Li W, Kornmark L, Jonasson L, Forssell C, Yuan X-M. Cathepsin L is significantly associated with apoptosis and plaque destabilization in human atherosclerosis. Atherosclerosis. 2008 Apr 18 (Epub ahead of print).
- Del Prete G, De Carli M, Lammel RM, D'Elios MM, Daniel KC, Giusti B, et al. Th1 and Th2 T-helper cells exert opposite regulatory effects on procoagulant activity and tissue factor production by human monocytes. Blood. 1995; 1: 250–7
- Caligiuri G, Rudling M, Olivier V, Jacob MP, Michel JB, Hansson GK, et al. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003; 9: 10–17
- Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, et al. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med. 1994; 331: 417–24
- Biasucci LM, Vitelli A, Liuzzo G, Altamura S, Caligiuri G, Monaco C, et al. Elevated levels of interleukin-6 in unstable angina. Circulation. 1996; 94: 874–7
- Neri Serneri GG, Prisco D, Martini F, Gori AM, Brunelli T, Poggesi L, et al. Acute T cell activation is detectable in unstable angina. Circulation. 1997; 95: 1806–12
- Jonasson L, Linderfalk C, Olsson J, Wikby A, Olsson AG. Systemic T-cell activation in stable angina pectoris. Am J Cardiol. 2002; 89: 754–6
- Kai H, Ikeda H, Yasukawa H, Kai M, Seki Y, Kuwahara F, et al. Peripheral blood levels of matrix metalloproteinases-2 and -9 are elevated in patients with acute coronary syndromes. J Am Coll Cardiol. 1998; 32: 368–72
- Nilsson L, Jonasson L, Nijm J, Hamsten A, Eriksson P. Increased plasma concentration of matrix metalloproteinase-7 in patients with coronary artery disease. Clin Chem. 2006; 52: 1522–7
- Smith DA, Irving SD, Sheldon J, Cole D, Kaski JC. Serum levels of the anti-inflammatory cytokine interleukin-10 are decreased in patients with unstable angina. Circulation. 2001; 104: 746–9
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997; 336: 973–9
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000; 342: 836–43
- Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000; 321: 199–204
- Heeschen C, Dimmeler S, Hamm CW, Fichtlscherer S, Boersma E, Simoons ML, et al. Serum level of the antiinflammatory cytokine interleukin-10 is an important prognostic determinant in patients with acute coronary syndromes. Circulation. 2003; 107: 2109–14
- Buckingham JC, Loxley HD, Taylor AD, Flower RJ. Cytokines, glucocorticoids and neuroendocrine function. Pharmacol Res. 1994; 30: 35–42
- Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995; 332: 1351–63
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation ofimmunity. Annu Rev Immunol. 2002; 20: 125–63
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000; 21: 55–89
- Hench PS, Kendall EC, Slocumb CH, Polley HF. Effects of cortisone acetate and pituitary ACTH on rheumatoid arthritis, rheumatic fever and certain other conditions. Arch Intern Med (Chic) 1950; 85: 545–666
- Buckingham JC. Glucocorticoids: exemplars of multi-tasking. Br J Pharmacol. 147 Suppl 2006; 1: S258–68
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005; 353: 1711–23
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003; 100: 1920–5
- Almawi WY, Beyhum HN, Rahme AA, Rieder MJ. Regulation of cytokine and cytokine receptor expression by glucocorticoids. J Leukoc Biol. 1996; 60: 563–72
- John CD, Buckingham JC. Cytokines: regulation of the hypothalamo-pituitary-adrenocortical axis. Curr Opin Pharmacol. 2003; 3: 78–84
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998; 19: 269–301
- Bamberger CM, Bamberger AM, de Castro M, Chrousos GP. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest. 1995; 95: 2435–41
- Cove-Smith JR, Kabler P, Pownall R, Knapp MS. Circadian variation in an immune response in man. Br Med J. 1978; 2: 253–4
- Harkness JA, Richter MB, Panayi GS, Van de Pette K, Unger A, Pownall R, et al. Circadian variation in disease activity in rheumatoid arthritis. BMJ. 1982; 284: 551–4
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology. 2007; 32: 80–6
- Aardal E, Holm AC. Cortisol in saliva—reference ranges and relation to cortisol in serum. Eur J Clin Chem Clin Biochem. 1995; 33: 927–32
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, et al. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997; 61: 2539–49
- Kirschbaum C, Hellhammer DH. Noise and stress—salivary cortisol as a non-invasive measure of allostatic load. Noise Health. 1999; 4: 57–65
- Kirschbaum C, Pirke K-M, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993; 28: 76–81
- Smith GD, Ben-Shlomo Y, Beswick A, Yarnell J, Lightman S, Elwood P. Cortisol, testosterone, and coronary heart disease: prospective evidence from the Caerphilly study. Circulation. 2005; 112: 332–40
- Troxler RG, Sprague EA, Albanese RA, Fuchs R, Thompson AJ. The association of elevated plasma cortisol and early atherosclerosis as demonstrated by coronary angiography. Atherosclerosis. 1977; 26: 151–62
- Koertge J, Al-Khalili F, Ahnve S, Janszky I, Svane B, Schenck-Gustafsson K. Cortisol and vital exhaustion in relation to significant coronary artery stenosis in middle-aged women with acute coronary syndrome. Psychoneuroendocrinology. 2002; 27: 893–906
- Alevizaki M, Cimponeriu A, Lekakis J, Papamichael C, Chrousos GP. High anticipatory stress plasma cortisol levels and sensitivity to glucocorticoids predict severity of coronary artery disease in subjects undergoing coronary angiography. Metabolism. 2007; 56: 222–6
- Whitehead DL, Perkins-Porras L, Strike PC, Magid K, Steptoe A. Cortisol awakening response is elevated in acute coronary syndrome patients with type-D personality. J Psychosom Res. 2007; 62: 419–25
- Otte C, Marmar CR, Pipkin SS, Moos R, Browner WS, Whooley MA. Depression and 24-hour urinary cortisol in medical outpatients with coronary heart disease: The Heart and Soul Study. Biol Psychiatry. 2004; 56: 241–7
- Nijm J, Kristenson M, Olsson AG, Jonasson L. Impaired cortisol response to acute stressors in patients with coronary disease. Implications for inflammatory activity. J Intern Med. 2007; 262: 375–84
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000; 92: 994–1000
- Rosmond R, Björntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med. 2000; 247: 188–97
- Gluck ME, Geliebter A, Lorence M. Cortisol stress response is positively correlated with central obesity in obese women with binge eating disorder (BED) before and after cognitive-behavioral treatment. Ann N Y Acad Sci. 2004; 1032: 202–7
- Duclos M, Marquez PP, Barat P, Gatta B, Roger P. Increased cortisol bioavailability, abdominal obesity, and the metabolic syndrome in obese women. Obes Res. 2005; 13: 1157–66
- Rohleder N, Kirschbaum C. The hypothalamic-pituitary-adrenal (HPA) axis in habitual smokers. Int J Psychophysiol. 2006; 59: 236–43
- Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J Clin Endocrinol Metab. 2007; 92: 819–24
- Wirtz PH, von Känel R, Emini L, Ruedisueli K, Groessbauer S, Maercker A, et al. Evidence for altered hypothalamus-pituitary-adrenal axis functioning in systemic hypertension: Blunted cortisol response to awakening and lower negative feedback sensitivity. Psychoneuroendocrinology. 2007; 32: 430–6
- Rosmond R, Wallerius S, Wanger P, Martin L, Holm G, Björntorp P. A 5-year follow-up study of disease incidence in men with an abnormal hormone pattern. J Intern Med. 2003; 254: 386–90
- Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med. 2006; 68: 657–66
- Harbuz MS, Lightman SL. Stress and the hypothalamo-pituitary-adrenal axis: acute, chronic and immunological activation. J Endocrinol. 1992; 134: 327–39
- Sternberg EM, Young WS, 3rd, Bernardini R, Calogero AE, Chrousos GP, Gold PW, et al. A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall-induced arthritis in Lewis rats. Proc Natl Acad Sci U S A. 1989; 86: 4771–5
- Wick G, Hu Y, Schwarz S, Kroemer G. Immunoendocrine communication via the hypothalamo-pituitary-adrenal axis in autoimmune diseases. Endocr Rev. 1993; 14: 539–63
- Brown DH, Sheridan J, Pearl D, Zwilling BS. Regulation of mycobacterial growth by the hypothalamus-pituitary-adrenal axis: differential responses of mycobacterium bovis BCG-resistant and -susceptible mice. Infect Immun. 1993; 61: 4793–800
- Karalis K, Crofford L, Wilder RL, Chrousos GP. Glucocorticoid and/or glucocorticoid antagonist effects in inflammatory disease-susceptible Lewis rats and inflammatory disease-resistant Fischer rats. Endocrinology. 1995; 136: 3107–12
- Fantidis P, Perez De Prada T, Fernandez-Ortiz A, Carcia-Touchard A, Alfonso M, Sabaté M, et al. Morning cortisol production in coronary heart disease patients. Eur J Invest. 2002; 32: 304–8
- Nugent AM, Neely D, Young I, McDowell I, O'Kane M, Bell N, et al. Stress responses after treatment of hypercholesterolemia with simvastatin. Br J Clin Pharmacol. 1993; 36: 474–7
- Travia D, Tosi F, Negri C, Faccini G, Moghetti P, Muggeo M. Sustained therapy with 3-hydroxy-3-hethylglutaryl-coenzyme-A reductase inhibitors does not impair steroidogenesis by adrenals and gonads. J Clin Endocrinol Metab. 1995; 80: 836–40
- Oberbeck R, Schurmeyer T, Jacobs R, Benschop RJ, Sommer B, Schmidt RE, et al. Effects of beta-adrenoceptor-blockade on stress-induced adrenocorticotropin release in humans. Eur J Appl Physiol Occup Physiol. 1998; 77: 523–6
- Nonell A, Kerk S, Lederbogen F, Kopf D, Hamann B, Lewicka S, et al. No major effect of orciprenaline and propranolol upon ACTH-induced cortisol secretion. Exp Clin Endocrinol Diabetes. 2004; 112: 59–61
- Bohm M, Herrmann W, Wassmann S, Laufs U, Nickenig G. Does statin therapy influence steroid hormone synthesis?. Z Kardiol. 2004; 93: 43–8
- Gutiérrez MA, García ME, Rodriguez JA, Mardonez G, Jacobelli S, Rivero S. Hypothalamic-pituitary-adrenal axis function in patients with active rheumatoid arthritis: a controlled study using insulin hypoglycemia stress test and prolactin stimulation. J Rheumatol. 1999; 26: 277–81
- Gutiérrez MA, Garcia ME, Rodriguez JA, Rivero S, Jacobelli S. Hypothalamic-pituitary-adrenal axis function and prolactin secretion in systemic lupus erythematosus. Lupus. 1998; 7: 404–8
- Buske-Kirschbaum A, Geiben A, Höllig H, Morschhäuser E, Hellhammer D. Altered responsiveness of the hypothalamus-pituitary-adrenal axis and the sympathetic adrenomedullary system to stress in patients with atopic dermatitis. J Clin Endocrinol Metab. 2002; 87: 4245–51
- Kristenson M, Orth-Gomér K, Kucinskienë Z, Bergdahl B, Calkauskas H, Balinkyniene I, et al. Attenuated cortisol response to a standardized stress test in Lithuanian versus Swedish men: the LiVicordia study. Int J Behav Med. 1998; 5: 17–30
- Kristenson M, Lassvik C, Bergdahl B, Kucinskiène Z, Aizieniène L, Ziedén B, et al. Ultrasound determined carotid and femoral atherosclerosis in Lithuanian and Swedish men: the LiVicordia study. Atherosclerosis. 2000; 151: 501–8
- Steensberg A. The role of IL-6 in exercise-induced immune changes and metabolism. Exerc Immunol Rev. 2003; 9: 40–7
- Jialal I, Stein D, Balis D, Grundy SM, Adams-Huet B, Devaraj S. Effect of hydroxymethyl glutaryl coenzyme a reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001; 103: 1933–5
- Chikanza IC, Petrou P, Kingsley G, Chrousos G, Panayi GS. Defective hypothalamic response to immune and inflammatory stimuli in patients with rheumatoid arthritis. Arthritis Rheum. 1992; 35: 1281–8
- Veldhuijzen van Zanten JJ, Ring C, Carroll D, Kitas GD. Increased C reactive protein in response to acute stress in patients with rheumatoid arthritis. Ann Rheum Dis. 2005; 64: 1299–304
- Willich SN, Lewis M, Löwel H, Arntz HR, Schubert F, Schröder R. Physical exertion as a trigger of acute myocardial infarction. Triggers and Mechanisms of Myocardial Infarction Study Group. N Engl J Med. 1993; 329: 1684–90
- Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, et al. Triggering of acute myocardial infarction onset by episodes of anger. Determinants of Myocardial Infarction Onset Study Investigators. Circulation. 1995; 92: 1720–5
- Tofler GH, Stone PH, Maclure M, Edelman E, Davis VG, Robertson T, et al. Analysis of possible triggers of acute myocardial infarction (the MILIS study). Am J Cardiol. 1990; 66: 22–7
- Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004; 364: 953–62
- Leung DY, Hamid Q, Vottero A, Szefler SJ, Surs W, Minshall E, et al. Association of glucocorticoid insensitivity with increased expression of glucocorticoid receptor beta. J Exp Med. 1997; 186: 1567–74
- Lewis-Tuffin LJ, Cidlowski JA. The physiology of human glucocorticoid receptor beta (hGRbeta) and glucocorticoid resistance. Ann N Y Acad Sci. 2006; 1069: 1–9
- Webster JC, Oakley RH, Jewell CM, Cidlowski JA. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci U S A. 2001; 98: 6865–70
- Schaaf MJ, Cidlowski JA. AUUUA motifs in the 3′UTR of human glucocorticoid receptor alpha and beta mRNA destabilize mRNA and decrease receptor protein expression. Steroids. 2002; 67: 627–36
- Derijk RH, Schaaf MJ, Turner G, Datson NA, Vreugdenhil E, Cidlowski J, et al. A human glucocorticoid receptor gene variant that increases the stability of the glucocorticoid receptor beta-isoform mRNA is associated with rheumatoid arthritis. J Rheumatol. 2001; 28: 2383–8
- van den Akker ELT, Koper JW, van Rossum EFC, Dekker MJH, Russcher H, de Jong FH, et al. Glucocorticoid receptor gene and risk of cardiovascular disease. Arch Intern Med. 2008; 168: 33–9