Abstract
Purpose: The study was designed to evaluate clinical and laboratory determinants pulse wave velocity (PWV) ratio in women at the age of 50–65 years without overt cardiovascular disease but having elevated cardiovascular risk, such as hypertension, obesity, diabetes and hypercholesterolemia.
Materials and methods: We analyzed data from 1170 women enrolled in the national-wide primary prevention program. Univariate and multivariate linear regression analysis was used to establish independent risk factors in groups based on clinical data, laboratory values, and comorbidities. Arterial stiffness was evaluated using applanation tonometry technique (SphygmoCor). The PWV ratio was calculated by dividing cfPWV to crPWV.
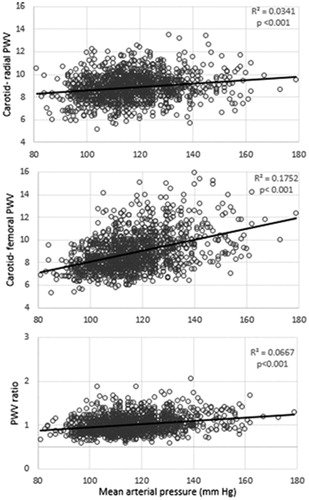
Results: In multivariate logistic regression analysis, age (OR = 1.109, p < .001), waist circumference (OR = 1.021, p = .001) and mean arterial pressure (OR = 1.031, p < .001) were found as independent clinical determinants of PWV ratio, while independent laboratory determinants were urine albumin to creatinine ratio (OR = 1.189, p = .010), triglycerides (OR = 1.161, p = .034), glucose (OR = 1.28, p = .001) and eGFR (OR = 0.998, p = .007). Diabetes (OR = 1.811, p = .029), hypertension (OR = 2.784, p = .042) and menopause (OR = 1.054, p = .018) were established as independent factors in comorbidities group. The analysis confirmed that PWV ratio (R2 = 0.0667, p < .001), as well as carotid radial (R2 = 0.0341, p < .001) and carotid femoral PWV (R2 = 0.1752, p < .001) is affected by mean arterial blood pressure.
Conclusions: Age, abdominal obesity, blood pressure, triglycerides, glucose, kidney function parameters and menopause all are associated with PWV ratio. More importance to women with high cardiovascular risk should be given whilst screening and stratifying further progression of the disease.
Introduction
Cardiovascular diseases (CVD) related mortality is the most common cause of death in Europe and reaches up to 45% of all-cause mortality. The rate is even higher among women, reaching up to 49% [Citation1–3]. Sex-specific characteristics in CVD biomarkers, phenotype and CVD outcomes have been observed [Citation4–6]. Furthermore, the CV risk increases dramatically in women after menopause due to a hormonal changes [Citation7], especially in those with obesity [Citation8]. It is important to emphasize gender related cardiovascular determinants, assuming that these changes may be more pronounced in middle-aged high-risk population.
Arterial stiffness plays a role as independent risk factor for CV morbidity and mortality in general population, as well as in women [Citation9]. In elderly patients large, elastic arteries become stiffer compared to peripheral muscular arteries, which are less effected [Citation10–13]. Even though, some patients have a tendency to have a decreased peripheral arterial stiffness [Citation12,Citation14–16]. These controversies are reported in patients with diabetes, patients on chronic dialysis and, most importantly, female patients. Therefore, 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk strongly recommends continued research to fill gaps in knowledge regarding short- and long-term atherosclerotic CVD risk assessment.
Given that with aging central arterial stiffness increases and peripheral arterial stiffness decreases in women, aortic-brachial stiffness gradient which reflects both elastic and muscular arterial stiffness may be an important tool in improving CVD risk stratification [Citation17,Citation18]. There are at least two studies analyzing the PWV ratio in the context of CV risk [Citation18,Citation19]. One of them [Citation18] included a healthy cohort (n = 2114) from the Framingham Heart Study without any prevalent cardiovascular disease and could not confirm the superiority of the PWV ratio over cfPWV in this population. Others [Citation19] have studied non-diabetic ESRD patients and showed that the PWV ratio is important in predicting CV mortality, but the main focus of the study was on arterial geometry and not the PWV ratio. We aimed to establish the determinants of pulse wave velocity (PWV) ratio comparing to cfPWV and crPWV in a large cohort of women with high cardiovascular risk and to look whether PWV ratio gives additional weight in evaluating the overall risk profile in these women.
Materials and methods
Patients
This cohort study was conducted during the period 2010–2014 at Vilnius University Hospital Santaros Klinikos. Permission No. 158200-05-490-144 was issued by the Vilnius Regional Biomedical Research Ethics Committee. All study subjects signed special subject's information and informed consent form. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Participants with established high cardiovascular risk were enrolled in the study. The high cardiovascular risk category definition was based on at least one of the characteristics described in .
Table 1. Characteristics of the high cardiovascular risk.
They were referred by the primary health care institutions under Lithuanian High Cardiovascular Risk (LitHiR) primary prevention program. LitHiR program recruited men at the age of 40–55 years and women at the age of 50–65 years without overt cardiovascular disease. The exclusion criteria were as follows: clinically evident cardiovascular disease; end-stage oncological disease or any other end-stage somatic disease. We selected all the women of the cohort in order to establish the determinants of PWV ratio in this population.
Evaluation of cardiovascular risk
Participants underwent a physical examination, risk profile analysis, such as lifestyle (smoking, physical activity), personal and family patterns of cardiovascular disease and anthropometry (blood pressure, heart rate, weight, height, waist circumference and body mass index) assessment. Furthermore, the arterial stiffness was evaluated for all the participants.
Pulse wave velocity ratio assessment
Arterial stiffness was evaluated using applanation tonometry technique when pulse wave is registered using tonometer SphygmoCor (SphygmoCor version 8.0 with AtCor Medical Pty. Ltd. Software). Carotid-radial (cr-PWV) and carotid - femoral (cf- PWV) PWV were obtained, following the calculation of the aortic- brachial PWV ratio: cf-PWV/cr-PWV.
Statistical analysis
Study sample was retrieved retrospectively from the registry according to the definite period of time – from year 2010 to year 2014. Descriptive statistics were used to describe baseline characteristics of the patients. The normality of the distribution across the variables was assessed using Kolmogorov–Smirnov test. The data was further analyzed and presented accordingly.
Univariate logistic regression analysis was used to assess the risk factors associated with the PWV ratio. In further multivariate regression analysis data was divided into three uniform groups: (1) clinical data, (2) laboratory values, (3) comorbidities. Multivariate logistic regression analysis with forward selection process was used to establish independent risk factors in each group. A 2-tailed p value of less than .05 was considered to be significant. Statistical analysis was performed using statistical tools package IBM SPSS Statistics V22 (IBM Corporation, New York).
Results
Baseline characteristics of the participants
Total of 1170 women with mean age of 57.21 years referred by the primary health care institutions under Lithuanian High Cardiovascular Risk (LitHiR) primary prevention program and who had all data available were analyzed in this study. The most common comorbidities in the study group were hypertension, obesity, diabetes and hypercholesterolemia. These baseline characteristics of the patients are presented in .
Table 2. Baseline characteristics of the patients.
Regression analysis of pulse wave velocity ratio determinants
The comparison of factors that determine cfPWV, crPWV and pulse wave velocity ratio is available in Supplemental material (Supplement 1). Age, BMI, eGFR were associated with cfPWV and PWV ratio. High density lipoproteins were correlated with crPWV and PWV ratio. Urine albumin to creatinine ratio had relationship only with PWV ratio alone.
Univariate and multivariate logistic regression analysis revealed predictors of PWV ratio in each variable group (). Age, waist circumference and MAP were found as independent clinical determinants of PWV ratio, while independent laboratory determinants were urine albumin to creatinine ratio, triglycerides, glucose and eGFR. Diabetes, hypertension and menopause were established as independent factors in comorbidities group. Women in menopause had greater waist circumference, lower eGFR, greater sodium concentration in blood, higher cfPWV and higher PWV ratio (Supplement 2).
Table 3. Logistic regression analysis of pulse wave velocity ratio determinants.
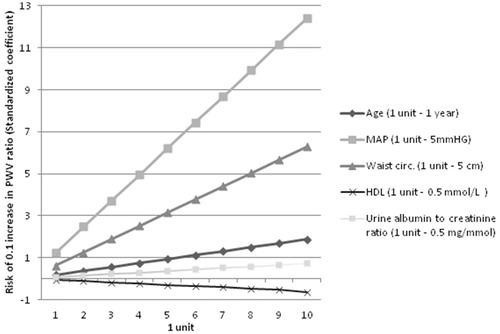
To get more insight into the PWV ratio determinants, linear regression analysis was performed and the effect of each determinant is presented in .
Distribution of variables across pulse wave velocity ratio tertiles
PWV ratio was divided into tertiles in order to compare means of several risk factors. One- way ANOVA was used for parametric data and Kruskal- Wallis test for non-parametric data. Results are presented in .
Table 4. Comparison of risk factors of arterial stiffness gradient.
Discussion
Our study focused on women at the age of 50–65 years without overt cardiovascular disease but having elevated cardiovascular risk, such as hypertension, obesity, diabetes and hypercholesterolemia. We identified that abdominal obesity, blood pressure, triglycerides, glucose menopause and albuminuria, an important biomarker of kidney damage, all are associated with PWV ratio in this population.
Aortic-brachial gradient was firstly mentioned in a report of Fortier C et al. [Citation17]. They analyzed data of dialysis patients and the importance of PWV ratio calculation in the context of overall mortality. Based on latter studies, Covic et al. [Citation20] suggested that PWV ratio might serve as a “new gold standard “and improve CV risk assessment, especially in populations (women, diabetes mellitus, ESRD on dialysis) in which decreased stiffness in peripheral muscular arteries [Citation9,Citation14,Citation16,Citation17,Citation21] has been observed. However, the real value of PWV ratio remains unclear. The other important point is the difference between PWV and pulse pressure amplification (PPA). It has been shown [Citation22] that these two variables have diverse meaning in the context of target organ damage assessment. Due to elastin degradation in vascular wall in middle-age the large arteries become stiffer. In healthy population the aging is less evident in muscular arteries. This phenomenon is not common in above mentioned specific populations where due to the loss of VSMC’s and their contractility changes the muscular arterial stiffness decreases, and this mismatch between aortic and peripheral arterial stiffness in the long term might result in microvascular damage (kidney, brain, peripheral artery disease). PPA is more related to cardiac damage and CV outcomes [Citation23]. The different properties of arterial stiffness and PPA might be the key factor, why Fortier et al. [Citation17] did not succeed in showing better cfPWV and PWV ratio mortality representing ROC curves. Unfortunately, we did not collected data about PPA in our population.
Aortic-stiffness gradient calculation depends on the author and is calculated either cfPWV/crPWV [Citation17] or crPWV/cfPWV [Citation19]. The interpretation of this measure depends on the equation used. We suggest that PWV ratio gives additional weight in microvascular damage evaluation comparing to aortic PWV alone, because it represents both muscular and elastic arteries by using standardized applanation tonometry technique. For example in our study population neither cfPWV neither crPWV had any relationship to urine albumin to creatinine ratio. Albuminuria was significantly related only to PWV ration. But this hypothesis should be tested in further research projects.
We confirmed eGFR and urine albumin to creatinine ratio as independent arterial-stiffness gradient predictors in our study population. By comparing PWV ratio tertiles the decrease in eGFR and increase in urine albumin to creatinine ratio with increasing PWV ratio was observed. Although the mean albuminuria range was quite low, it might represent the early subclinical kidney damage [Citation24,Citation25]. Large cohort data from Sweden [Citation26] found no relationship between eGFR and cfPWV in community-based women population. In contrast, Rotterdam study revealed that arterial stiffness is associated with decline in renal function [Citation27]. Unfortunately, in both reports the data of albuminuria were absent.
There is evidence that PWV ratio might be blood pressure-independent variable [Citation16], but it is still the matter of debates. We found the relationship between MAP and arterial stiffness gradient; for example, if MAP reaches 90–100 mmHg there is 3 times higher risk of 0.1 increase in PWV ratio. In fact, MAP indirectly reflects systemic vascular resistance, cardiac output and central venous pressure [Citation28] and is closely related to the autonomic nervous system function [Citation29]. In women, there is a steep increase in sympathetic nerve activity after menopause resulting in augmented vascular resistance [Citation29,Citation30]. Furthermore, vascular dysfunction is also altered in presence of diabetes mellitus and its related autonomic neuropathy [Citation31]. It should be noted that part of our study women were in menopause and had diabetes mellitus, thus, we suggest that their sympathetic nerve activity was already altered. These mentioned changes eventually led to increased elastic arteries stiffness. In concordance with decreased pulse wave velocity in muscular arteries, it might create perfect conditions for target organ damage.
Recently, paper on The Framingham Heart Study based trial [Citation18] evaluated the prognostic significance of PWV ratio in the community setting. They found no significant difference in age among high vs. low cfPWV and low crPWV. Similar to our results, the independent relationship of arterial stiffness gradient with age, female sex, diabetes mellitus and MAP in multivariate linear regression was mentioned. In contrast, they found no correlation between aging and crPWV. Initially our study couldn’t confirm this correlation as well, but after excluding the outliers there was a weak correlation between age and muscular arteries stiffness.
The other important finding of our study is that women in menopause had not only significantly higher aortic arterial stiffness and PWV ratio, but also higher sodium plasma concentration. It is known that both estrogens and progesterone interact with rennin-angiotensin-aldesterone system and impact pressure-natriuresis relationship resulting in change in salt sensivity in menopausal women [Citation32]. Long term estrogen supplementation in postmenopausal women influence sodium retention acting independently from aldosterone in kidney, and supplemental progesterone acts as aldosterone antagonist and can bind to mineralcorticoid receptors in the distal tubule [Citation33]. Moreover, sodium retention and consumption is strongly related to hypertension onset and arterial stiffness [Citation34,Citation35] leading to increased cardiovascular risk.
The relationship of arterial stiffness gradient and metabolic syndrome clusters such as waist circumference, glycaemia and blood pressure corresponded to Metabolic syndrome and Arteries REsearch (MARE) consortium [Citation36] in which cfPWV was measured. In concordance with some reports [Citation8] we also found nonlinear association of cfPWV with visceral, but not total adiposity.
The main strength of our study is the large cohort of high cardiovascular risk women in whom PWV ratio might improve further outcome stratification. A few limitations should be also mentioned. Firstly, the cross-sectional design could not evaluate the causal relationship between variables. Secondly, the specific age range could not reflect other age groups, i.e. younger or older women. Thirdly, we did not test for vascular calcification, which is a significant determinant of arterial stiffness. Additional limitation is quite low albuminuria levels with no follow-up and absence of information about drugs used at inclusion to the study. Therefore, we could not eliminate an impact of medication on arterial-stiffness gradient values.
Conclusion
Age, visceral adiposity, mean arterial blood pressure, urine albumin to creatinine ratio, triglycerides, glucose level, eGFR, diabetes mellitus, hypertension and menopause are the important determinants of arterial stiffness in both large and peripheral arteries in high cardiovascular risk women population. Additionally, albuminuria, a biomarker of kidney damage, is also assocciated to PWV ratio in studied population.
Author contributions
Research idea and study design: LRil, ALC, MM, LRim, JB; data acquisition: LRim, LRil, MM, ALC, JB; data analysis/interpretation: LRim, AV, ALC; statistical analysis: ALC, AV. All authors of the study have contributed significantly, based on all ICMJE recommended criteria, and are in agreement with content of the manuscript.
Supplement_2.docx
Download MS Word (12.1 KB)Supplement.docx
Download MS Word (12.2 KB)Disclosure statement
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
References
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322.
- CVD Statistics [Internet]. [cited 2017 Oct 23]. Available from: http://www.ehnheart.org/images/CVD-statistics-report-August-2017.pdf.
- Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722.
- Meyer S, van der Meer P, Massie BM, et al. Sex-specific acute heart failure phenotypes and outcomes from PROTECT. Eur J Heart Fail. 2013;15:1374–1381.
- Gori M, Lam CSP, Gupta DK, et al. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16:535–542.
- Daniels LB, Maisel AS. Cardiovascular biomarkers and sex: the case for women. Nat Rev Cardiol. 2015;12:588–596.
- Lloyd-Jones DM, Sutton-Tyrrell K, Patel AS, et al. Ethnic variation in hypertension among premenopausal and perimenopausal women: Study of Women’s Health Across the Nation. Hypertens Dallas Tex 1979. 2005;46:689–695.
- Scuteri A, Orru' M, Morrell CH, et al. Associations of large artery structure and function with adiposity: effects of age, gender, and hypertension. The SardiNIA Study. Atherosclerosis. 2012;221:189–197.
- Coutinho T. Arterial stiffness and its clinical implications in women. Can J Cardiol. 2014;30:756–764.
- Avolio AP, Chen SG, Wang RP, et al. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50–58.
- Bortolotto LA, Hanon O, Franconi G, et al. The aging process modifies the distensibility of elastic but not muscular arteries. Hypertension. 1999;34:889–892.
- Cameron JD, Bulpitt CJ, Pinto ES, et al. The aging of elastic and muscular arteries: a comparison of diabetic and nondiabetic subjects. Diabetes Care. 2003;26:2133–2138.
- Nilsson PM. Early vascular aging (EVA): consequences and prevention. Vasc Health Risk Manag. 2008;4:547–552.
- Laucyte-Cibulskiene A, Petraviciute M, Gudynaite M, et al. Mismatch between stiffness in elastic and muscular arteries as a predictor of vascular calcification in dialysis patients. Aging Clin Exp Res 2018;30:375–382.
- Heijden-Spek JJ, van der Staessen JA, Fagard RH, et al. Effect of age on brachial artery wall properties differs from the aorta and is gender dependent: a population study. Hypertension. 2000;35:637–642.
- Fortier C, Sidibé A, Desjardins M-P, et al. Aortic-brachial pulse wave velocity ratio: a blood pressure-independent index of vascular aging. Hypertens Dallas Tex. 2017;69:96–101.
- Fortier C, Mac-Way F, Desmeules S, et al. Aortic-brachial stiffness mismatch and mortality in dialysis population. Hypertens Dallas Tex 1979. 2015;65:378–384.
- Niiranen TJ, Kalesan B, Larson MG, et al. Aortic-brachial arterial stiffness gradient and cardiovascular risk in the community: The Framingham Heart Study. Hypertens Dallas Tex 1979. 2017;69:1022–1028.
- London GM, Safar ME, Pannier B. Aortic aging in ESRD: structural, hemodynamic, and mortality implications. J Am Soc Nephrol. 2016;27:1837–1846.
- Covic A, Siriopol D. Pulse wave velocity ratio: the new “gold standard” for measuring arterial stiffness. Hypertens Dallas Tex 1979. 2015;65:289–290.
- Cardoso CRL, Salles GF. Aortic stiffness as a surrogate endpoint to micro- and macrovascular complications in patients with type 2 diabetes. Int J Mol Sci. 2016;17:2044. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5187844/.
- Bai B, Teliewubai J, Lu Y, et al. Comparison of pulse wave velocity and pulse pressure amplification in association with target organ damage in community-dwelling elderly: The Northern Shanghai Study. Hypertens Res. 2018;41:372–381.
- Benetos A, Gautier S, Labat C, et al. Mortality and cardiovascular events are best predicted by low central/peripheral pulse pressure amplification but not by high blood pressure levels in elderly nursing home subjects: the PARTAGE (Predictive Values of Blood Pressure and Arterial Stiffness in Institutionalized Very Aged Population) study. J Am Coll Cardiol. 2012;60:1503–1511.
- Hillege HL, Janssen WM, Bak AA, et al. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med. 2001;249:519–526.
- Kohara K, Tabara Y, Tachibana R, et al. Microalbuminuria and arterial stiffness in a general population: the Shimanami Health Promoting Program (J-SHIPP) study. Hypertens Res. 2004;27:471–477.
- Gottsäter M, Östling G, Persson M, et al. Non-hemodynamic predictors of arterial stiffness after 17 years of follow-up: the Malmö Diet and Cancer study. J Hypertens. 2015;33:957–965.
- Sedaghat S, Mattace-Raso FUS, Hoorn EJ, et al. Arterial stiffness and decline in kidney function. Clin J Am Soc Nephrol. 2015;10:2190–2197.
- CV Physiology | Mean Arterial Pressure [Internet]. [cited 2017 Aug 24]. Available from: http://www.cvphysiology.com/Blood%20Pressure/BP006
- Barnes JN, Hart EC, Curry TB, et al. Aging enhances autonomic support of blood pressure in women. Hypertens Dallas Tex 1979. 2014;63:303–308.
- Scuteri A, Morrell CH, Orrù M, et al. Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertens Dallas Tex 1979. 2014;64:1219–1227.
- Meyer C, Milat F, McGrath BP, et al. Vascular dysfunction and autonomic neuropathy in Type 2 diabetes. Diabet Med J Br Diabet Assoc. 2004;21:746–751.
- Kim J-M, Kim T-H, Lee H-H, et al. Postmenopausal hypertension and sodium sensitivity. J Menopausal Med. 2014;20:1–6.
- Stachenfeld NS. Hormonal changes during menopause and the impact on fluid regulation. Reprod Sci. 2014;21:555–561.
- He FJ, Li J, Macgregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane DB Syst Rev. 2013;CD004937.
- Jablonski KL, Fedorova OV, Racine ML, et al. Dietary sodium restriction and association with urinary marinobufagenin, blood pressure, and aortic stiffness. CJASN. 2013;8:1952–1959.
- Scuteri A, Cunha PG, Rosei EA, et al. Arterial stiffness and influences of the metabolic syndrome: a cross-countries study. Atherosclerosis. 2014;233:654–660.