Abstract
Purpose: Hypertensive patients are at increased risk of atrial fibrillation (AF). Although low baseline high density lipoprotein (HDL) cholesterol has been associated with a higher risk of AF, this has not been verified in recent population-based studies. Whether changing levels of HDL over time are more strongly related to the risk of new AF in hypertensive patients has not been examined.
Material and methods: Incident AF was examined in relation to baseline and on-treatment HDL levels in 8267 hypertensive patients with no history of AF, in sinus rhythm on their baseline electrocardiogram, randomly assigned to losartan- or atenolol-based treatment. HDL levels at baseline and each year of testing were categorised into quartiles according to baseline HDL levels.
Results: During 4.7 ± 1.10 years of follow-up, 645 patients (7.8%) developed new AF. In univariate Cox analyses, compared with the highest quartile of HDL levels (>1.78 mmol/l), patients with on-treatment HDL in the lowest quartile (≤ 1.21 mmol/l) had a 53% greater risk of new AF. Patients with on-treatment HDL in the second and third quartiles had intermediate increased risks of AF. Baseline HDL in the lowest quartile was not a significant predictor of new AF (hazard ratio (HR): 1.14, 95% confidence interval (CI): 0.90–1.43). In multivariable Cox analyses adjusting for multiple baseline and time-varying covariates, the lowest quartile of on-treatment HDL remained associated with a nearly 54% increased risk of new AF (HR: 1.54, 95% CI: 1.16–2.05) whereas a baseline HDL≤ ⩽1.21 mmol/l was not predictive of new AF (HR: 1.01, 95% CI: 0.78–1.31).
Conclusion: Lower on-treatment HDL is strongly associated with risk of new AF. These findings suggest that serial assessment of HDL can estimate AF risk better than baseline HDL in hypertensive patients with left ventricular hypertrophy. Future studies may investigate whether therapies that increase HDL can lower risk of developing AF.
Clinical Trials Registration: http://clinicaltrials.gov/ct/show/NCT00338260?order=1
Introduction
Atrial fibrillation (AF) is a common arrhythmia [Citation1,Citation2] that is increasing in prevalence [Citation2]. The incidence of AF increases with age [Citation1] and is increased in patients with heart failure, left ventricular hypertrophy (LVH), coronary heart disease and hypertension [Citation3–10]. The higher risk of death [Citation3–Citation5], sudden cardiac death [Citation6], heart failure [Citation5] and stroke [Citation3,Citation7,Citation8] in patients with AF and the substantial risks associated with antithrombotic therapies aimed at decreasing the risk of embolic sequelae [Citation11], make prevention of new AF a major clinical and epidemiologic goal.
In addition to the traditional cardiac and non-cardiac conditions that predispose to AF [Citation1–10], there has been a growing appreciation of the potential relationship of blood lipid levels to AF risk [Citation12–20]. In contrast to the relationship of high levels of total and low density lipoprotein (LDL) cholesterol to atherosclerosis and coronary disease, high levels of LDL and total cholesterol have been associated with decreased risk of AF in some, but not all, studies [Citation15–20]. Although low levels of high density lipoprotein (HDL) cholesterol have been associated with a higher risk of AF in some analyses [Citation12–15], this has not been born out or not examined in other studies [Citation16–20]. However, HDL levels decrease with age and weight gain [Citation21] and often in response to increasing statin therapy. As a consequence, it is unclear if a single, baseline measurement of HDL will best stratify AF risk or whether changing levels of HDL over time would more strongly reflect the risk of AF. Therefore, the aim of the present study was to compare the predictive value of baseline and on-treatment HDL levels for development of AF. We also aimed to determine whether HDL remained associated with high risk of AF after multivariate adjusting for potential confounding effects of various known risk factors for AF, effect of statin therapy, randomised study treatment allocation, on-treatment non-HDL, systolic blood pressure, heart rate and electrocardiogram (ECG) LVH [Citation3,Citation9,Citation22,Citation23].
Material and methods
Data availability statement
The data that support the findings of this study are available from the corresponding author, P.M.O. upon reasonable request.
Participants and treatment
The Losartan Intervention for End point reduction in hypertension (LIFE) study enrolled 9193 hypertensive patients with ECG LVH by Cornell voltage-duration product and/or Sokolow-Lyon voltage criteria on a screening ECG in a prospective, double-blind randomised study that compared cardiovascular morbidity and mortality with use of losartan- as opposed to atenolol-based treatment, as previously described in detail [Citation3,Citation9,Citation22–24]. The study was approved by all ethics committees concerned and all participants gave informed written consent. The 362 patients with a history of AF or AF on their ECG at study baseline [Citation3,Citation9,Citation22,Citation23] and 564 additional patients without AF who were missing baseline HDL levels were excluded, leaving 8267 patients who were at risk of developing new AF in the present post-hoc study. The 564 patients with missing baseline HDL levels were similar to the 8267 patients included in the study with respect to age, gender, randomised treatment allocation, baseline systolic blood pressure and severity of ECG LVH by Cornell product criteria (data not shown).
Blinded treatment was begun with losartan 50 mg or atenolol 50 mg daily and matching placebo of the other agent, with up-titration of study medication to 100 mg and addition of hydrochlorothiazide (HCTZ) and other antihypertensive therapies to achieve a pressure of ≤ 140/90 mm Hg as previously reported [Citation24].
Electrocardiography and lipid measurements
Study ECGs were obtained at baseline, six-months and yearly follow-up until study termination or patient death and were interpreted as previously reported [Citation3,Citation9,Citation22–24]. Cornell product >2440 mm × ms or Sokolow-Lyon voltage >38 mm was used to identify LVH [Citation25,Citation26].
Serum total cholesterol and HDL were measured in two central laboratories as previously reported [Citation27]. Low density lipoprotein cholesterol and triglycerides were not measured. Non-HDL cholesterol was calculated as total cholesterol minus HDL. Treatment of lipids was at the discretion of study investigators, but all treatment was reported [Citation27].
End point determination
New-onset AF was a pre-specified secondary endpoint in LIFE and was identified from protocol-mandated in-study ECGs undergoing Minnesota coding at the ECG core lab (n = 370) and/or by adverse event reports of AF by the investigators (n = 529) [Citation23]. In patients who had new AF by both criteria, the earliest onset of AF was taken as the time to new AF for this analysis.
Statistical analyses
Data management and analysis were performed with SPSS version 23.0 software (SPSS, Chicago, IL). Data are presented as mean ± standard deviation (SD) for continuous variables and proportions for categorical variables. Differences in prevalence were compared using χ2 analyses and of mean values using analysis of variance when comparing across quartiles of HDL and an unpaired t-test when comparing patients with and without new AF in supplemental data analyses.
The relation of new-onset AF to HDL was assessed using Cox proportional hazards models with patients categorised into quartiles according to HDL levels at baseline; the risk of new AF was calculated comparing each of the first three quartiles of HDL against the highest quartile of HDL. The predictive value of baseline HDL was determined using baseline quartiles of HDL entered as standard covariates in the Cox models; the predictive value of in-treatment levels of HDL was determined using baseline and in-treatment quartiles of HDL entered as time-varying covariates. Independence of the relationship of new-onset AF to baseline and on-treatment HDL was evaluated in multivariable Cox models that adjusted for randomised treatment, age, sex, race, prior antihypertensive treatment, history of myocardial infarction, ischaemic heart disease, heart failure, diabetes, baseline serum glucose and creatinine, urine albumin/creatinine ratio treated as standard covariates, incident myocardial infarction, incident heart failure, and on-treatment non-HDL cholesterol, heart rate, Cornell product left ventricular hypertrophy, diastolic and systolic pressure, and statin use treated as time-varying covariates. Baseline HDL was also included as a standard covariate in the multivariable Cox analyses examining on-treatment HDL. Analyses were also performed stratifying the population by sex, age, prior antihypertensive treatment, race, randomised treatment allocation and treatment with a statin at any time during the study, using on-treatment HDL entered as a continuous variable for simplicity of these analyses. For all tests, a two-tailed p < 0.05 was required for statistical significance.
The relationship of incident AF over time to changing quartiles of HDL during treatment was illustrated using a modified Kaplan-Meier method [Citation28] implemented in SAS release 8.2 on the WIN_PRO platform. Using this method, HDL quartile assignment is updated each year and patients may be variably included in one curve or another at different times during follow-up. These modified Kaplan-Meier curves illustrate the results of time-varying covariate analyses.
Results
During mean follow-up of 4.7 ± 1.2 years, new-onset AF developed in 645 patients (7.8%). Demographic and clinical characteristics according to quartiles of HDL at baseline are show in . Patients in the lowest quartile of baseline HDL were younger, less likely to be female, more likely to have a history of diabetes, ischaemic heart disease, myocardial infarction, heart failure, peripheral vascular disease and prior antihypertensive treatment, and more likely to be a current smoker; they were also more obese, had higher baseline serum glucose and creatinine levels, lower baseline total and HDL cholesterol levels and higher urine albumin to creatinine ratios. Demographic and clinical characteristics of patients according to development of new AF are compared in Supplemental Table 1. As previously reported [Citation9], patients who developed AF were older, less likely to be female or black, more likely to have a history of ischaemic heart disease, myocardial infarction, heart failure, stroke and to have prior antihypertensive treatment, had lower total cholesterol levels and higher urine albumin to creatinine ratios.
Table 1. Study baseline demographic and clinical characteristics in relation to quartiles of HDL cholesterol levels at baseline.
Blood pressure and ECG LVH measurements at baseline and changes in these measurements between baseline and last in-study determination or last measurement prior to development of AF in relation to baseline quartiles of HDL are shown in . Patients in the lowest quartile of HDL had lower baseline systolic pressures and both lower baseline and change in Sokolow-Lyon voltage; there were small differences in baseline heart rate across quartiles with a trend towards higher heart rates in the highest quartile of HDL. These measurements are compared according to development of new AF in Supplemental Table 2. As previously reported [Citation9], patients who developed AF had higher baseline systolic pressures, Cornell product and Sokolow-Lyon measures of LVH but lower baseline diastolic pressures and heart rates. Patients who developed new AF had greater decreases in systolic pressure, but smaller reductions in Cornell product and heart rate over time.
Table 2. Study baseline and change from study baseline to last in-study measurement of blood pressure, electrocardiographic left ventricular hypertrophy and heart rate in relation to quartiles of HDL cholesterol levels at baseline.
Table 3. Univariate and multivariable cox regression analyses to assess the relation of new onset atrial fibrillation to quartiles of baseline and on-treatment high density lipoprotein (HDL) cholesterol levels.
Changes in HDL at each year of treatment in relation to the development of new AF are shown in Supplemental Table 3. Changes in HDL were similar between groups at years 1 and 2, but from year 3 –5 development of new AF was associated with greater mean decreases in HDL over time.
Because statin therapy has been implicated in the risk of developing AF [Citation19], the relationship of baseline and on-treatment statin use to development of AF is examined in Supplemental Table 4. Statin therapy was relatively uncommon and similar in patients with and without new AF at baseline and year-1 of the study, became more common but similar between groups in years 2–4 and was slightly less frequent among patients who developed new AF in year 5 of the study.
The relationship of new-onset AF to quartiles of HDL cholesterol levels at baseline and during treatment is shown in and . In univariate Cox analyses, compared with the highest quartile of HDL levels (HDL >1.78 mmol/l), on-treatment HDL in the lowest quartile (≤ ⩽1.21 mmol/l) identified patients with a 53% higher risk of new AF; patients with baseline or on-treatment HDL in the second and third quartiles had intermediate increased risk of AF. In multivariable Cox analyses, the risk associated with being in the lowest quartile of on-treatment HDL was increased with 54% with intermediate increased risk of AF in the second and third quartiles. In contrast, baseline levels of HDL in the first quartile were not associated with an increased risk of new AF in either univariate or multivariable Cox models (). Of note in a parallel multivariable Cox model adjusting for the same variables, lower on-treatment HDL treated as a continuous variable remained strongly associated with new-onset AF, with 1 SD of the baseline mean lower on-treatment HDL (0.44 mmol/l) associated with a greater than three-fold higher adjusted risk of new AF (hazard ratio (HR): 3.07, 95% confidence interval (CI): 2.78–3.37, p < 0.001). On-treatment non-HDL cholesterol was not a significant predictor of new AF either in univariate (HR: 1.06, 95% CI: 0.98–1.14) per 1 SD of baseline mean (1.10 mmol/l) or multivariable Cox models (HR: 0.94, 95% CI: 0.86–1.03). Although statin use was associated with a statistically significant lower incidence of new AF in univariate Cox analysis (HR: 0.68, 95% CI: 0.56–0.84, p < 0.001), this association was no longer significant after adjusting for other predictors of new AF in the full multivariable model (HR: 0.83, 95% CI: 0.66–1.03, p = 0.092). The association between lower serum HDL and an increased risk of new AF was statistically similar in all subsets of the population ().
Figure 1. Survival curves illustrating the rate of new-onset atrial fibrillation in relation to quartiles of on-treatment HDL cholesterol levels.
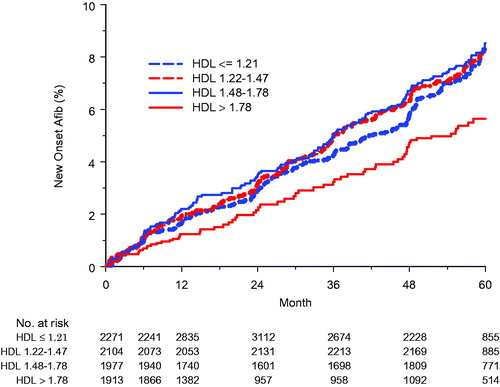
Table 4. Multivariable Cox analyses to assess the predictive value of on-treatment high density lipoprotein (HDL) for new-onset atrial fibrillation in relevant subgroups of the study population.
Discussion
These findings demonstrate that lower on-treatment levels of HDL during antihypertensive therapy are strongly associated with increased risk of new-onset atrial fibrillation. In contrast, baseline levels of HDL were not associated with AF risk. The increased risk of AF with lower on-treatment HDL levels was not attenuated in multivariable models that adjusted for other known and potential risk factors for AF, the possible impact of concurrent therapy with statins [Citation19], and the previously demonstrated relationship of AF incidence to losartan vs atenolol therapy, and to on-treatment heart rate, systolic blood pressure and ECG LVH in this population [Citation3,Citation9,Citation22,Citation23]. These findings support the value of serial measurement of HDL to better estimate AF risk in hypertensive patients.
Prior work has found inconsistent relationships between HDL and the risk of AF in a variety of populations and settings [Citation12–20]. These findings [Citation12–20] are discussed in detail in the supplemental material.
The predictive value of on-treatment HDL for new-onset AF was similar in all subgroups examined (). Particularly of note, lower on-treatment HDL had statistically similar predictive value in groups defined by age, randomised treatment allocation to either losartan or atenolol, and by race, despite the strong association of lower age, losartan therapy and black race with lower AF incidence in this population [Citation3,Citation29]. In addition, there was no significant interaction of on-treatment HDL with statin use in this study, suggesting that neither the potential impact of statins on HDL levels nor the possible relationship of incidence AF to statin use [Citation13,Citation30,Citation31] significantly contributes to the impact of low HDL on AF risk. Of note, the absence of a significant relationship between non-randomised statin use and incident AF in the current study supports previous findings among hypertensive patients in ALLHAT randomised to pravastatin versus usual care [Citation13] and the lack of a statin effect in a collaborative meta-analysis of over 100,000 patients [Citation31], but stands in contrast to the 27% decreased incidence of new AF among patients selected to have higher baseline levels of inflammation on the basis of elevated C-reactive protein levels randomised to rosuvastatin in a post-hoc analysis from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial [Citation31], which may reflect more on the increased baseline inflammatory state of these patients.
There are a number of possible mechanisms via which low levels of HDL could increase the risk of developing AF, potentially mediated via the decreased anti-inflammatory effects of lower HDL levels [Citation32]. There is increasing evidence that inflammation may play a significant role in the initiation of AF [Citation31,Citation33–35] and that patients with AF have increased levels of inflammatory markers, such as C-reactive protein (CRP) and interleukin-6 [Citation33–Citation35]. Supporting this hypothesis, comparison of 29 relatively young male patients with paroxysmal AF and 27 controls of similar age who were referred for ablation of supraventricular tachycardia demonstrated that the AF group had 16% lower HDL levels, higher CRP levels and higher levels of oxidised species of and advanced glycated end products of all lipoproteins [Citation33]. Moreover, among 17,120 participants in the JUPITER trial with no prior history of arrhythmia, selected for underlying inflammation based on a baseline CRP ≥2.0 mg/L [Citation31], each increasing tertile of baseline CRP was associated with a 36% increased risk of incident AF [Citation31].
There are several other mechanisms by which the potential link between HDL and, inflammation could increase AF risk [Citation10,Citation36–39]. These mechanisms [Citation10,Citation36–39] are discussed in detail in the Online Supplement.
Study limitations
First, inclusion criteria of hypertension, age 55–80 years and ECG LVH by either Cornell product or Sokolow-Lyon voltage increased the risk of new-onset AF in the population; as a consequence, our findings may not be representative of other lower-risk populations or representative for untreated hypertensive populations.
Second, the absence of measurements of CRP and other inflammatory markers in the LIFE study does not allow determination whether on-treatment HDL levels would remain predictive of AF after adjusting for the demonstrated predictive value of elevated CRP for AF [Citation31].
Third, because incident AF was only ascertained on study ECGs and at study visits [Citation23], cases of paroxysmal AF may have been missed. However, missing some AF endpoint would rather weaken than strengthen the type of findings we report.
Implications
Our findings suggest that monitoring HDL levels over time may provide important insights into the risk of developing AF in middle-aged and elderly hypertensive patients with ECG LVH. Perhaps greater attention should be placed on screening such hypertensive patients with low HDL levels for incident or paroxysmal AF or high risk of AF.
Further research may be necessary to investigate whether low HDL levels are solely a marker of increased inflammation and atrial arrhythmia frequency that independently mediate the increased risk of AF in these patients. Future study may also be of interest to determine whether therapies aimed at raising HDL levels could be of clinical value in reducing the risk of AF.
Online_Suppl._BP-2020-OR-0013_rev._HDL_and_new_Afib._Kjeldsen_29.5.20.docx
Download MS Word (24.9 KB)Disclosure statement
Dr. Okin, Dr. Wachtell and Dr. Julius have no disclosures. Ms. Hille is employed by Merck & Co., Inc. Dr. Kjeldsen has received honoraria from Merck KGaA, Sanofi and Takeda. Dr. Devereux has received support from Merck & Co. Inc.
Additional information
Funding
References
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults. National implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042–1046.
- Wachtell K, Lehto M, Gerdts E, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol. the Losartan Intervention for End point reduction in hypertension (LIFE) study. J Am Coll Cardiol. 2005;45(5):712–719.
- Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the framingham heart study. Circulation. 1998;98(10):946–952.
- Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98(5):476–484.
- Okin PM, Bang CN, Wachtell K, et al. Relationship of sudden cardiac death to new-onset atrial fibrillation in hypertensive patients with left ventricular hypertrophy. Circ Arrhythm Electrophysiol. 2013;6(2):243–251.
- Marini C, De Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke. 2005;36(6):1115–1119.
- Verdecchia P, Reboldi R, Bentivoglio M, et al. Atrial fibrillation in hypertension: predictors and outcome. Hypertension. 2003;41(2):218–223.
- Okin PM, Hille D, Larstorp ACK, et al. Effect of lower on-treatment systolic blood pressure on the risk of atrial fibrillation in hypertensive patients . Hypertension. 2015;66(2):368–373.
- Andrade J, Khairy P, Dobrev D, et al. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114(9):1453–1468.
- Hylek EM, Chang YC, Skates SJ, et al. Prospective study of the outcomes of ambulatory patients with excessive warfarin anti-coagulation. Arch Intern Med. 2000;160(11):1612–1617.
- Annoura M, Ogawa M, Kumagai K, et al. Cholesterol paradox in patients with paroxysmal atrial fibrillation. Cardiology. 1999;92(1):21–27.
- Haywood LJ, Ford CE, Crow RS, et al. Atrial fibrillation at baseline and during follow-up in ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial). J Am Coll Cardiol. 2009;54(22):2023–2031.
- Barkas F, Elisaf M, Korantzopoulos P, et al. The CHADS2 and CHA2DS2-VASc scores predict atrial fibrillation in dyslipidemic individuals: role of incorporating low high-density lipoprotein cholesterol levels. Int J Cardiol. 2017;241:194–199.
- Alonso A, Yin X, Roetker NS, et al. Blood lipids and the incidence of atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis and the Framingham Heart Study. J Am Heart Assoc. 2014;3:e001211.
- Li X, Gao L, Wang Z, et al. Lipid profile and incidence of atrial fibrillation: a prospective cohort study in China. Clin Cardiol. 2018;41(3):314–320.
- Lopez FL, Agarwal SK, MacLehose RF, et al. Blood lipid levels, lipid-lowering medications, and the incidence of atrial fibrillation: the atherosclerosis risk in communities study. Circ Arrhythm Electrophysiol. 2012;5(1):155–162.
- Allan V, Honarbakhsh S, Casas JP, et al. Are cardiovascular risk factors also associated with the incidence of atrial fibrillation? A systematic review and field synopsis of 23 factors in 32 population-based cohorts of 20 million participants. Thromb Haemost. 2017;117(05):837–850.
- Mora S, Akinkuolie AO, Sandhu RK, et al. Paradoxical association of lipoprotein measures with incident atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7(4):612–619.
- Magnussen C, Niiranen TJ, Ojeda FM, et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation. 2017;136(17):1588–1597.
- Ferrara A, Barrett-Connor E, Shan J. Total, LDL, and HDL cholesterol decrease with age in older men and women: the Rancho Bernardo Study 1984-1994. Circulation. 1997;96(1):37–43.
- Okin PM, Wachtell K, Kjeldsen SE, et al. Incidence of atrial fibrillation in relation to changing heart rate over time in hypertensive patients: the LIFE Study. Circ Arrhythm Electrophysiol. 2008;1(5):337–343.
- Okin PM, Wachtell K, Devereux RB, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA. 2006;296(10):1242–1248.
- Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003.
- Okin PM, Roman MJ, Devereux RB, et al. Electrocardiographic identification of increased left ventricular mass by simple voltage-duration products. J Am Coll Cardiol. 1995;25(2):417–423.
- Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37(2):161–186.
- Olsen MH, Wachtell K, Beevers G, et al. Effects of losartan compared with atenolol on lipids in patients with hypertension and left ventricular hypertrophy: the Losartan Intervention For Endpoint reduction in hypertension study. J Hypertens. 2009;27:567–574.
- Snappin SM, Jiamg Q, Iglewicz B. Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimate. Am Statistician. 2005;59:301–307.
- Okin PM, Wachtell K, Hille DA, et al. Racial differences in incident atrial fibrillation among hypertensive patients during antihypertensive therapy. Am J Hypertens. 2014;27(7):966–972.
- Rahimi K, Emberson J, McGale P, et al. Effect of statins on atrial fibrillation: collaborative meta-analysis of published and unpublished evidence from randomised controlled trials. Br Med J. 2011;342(2):d1250–d1250.
- Peña JM, MacFadyen J, Glynn RJ, et al. High-sensitivity C-reactive protein, statin therapy, and risks of atrial fibrillation: an exploratory analysis of the JUPITER trial. Eur Heart J. 2012;33(4):531–537.
- Barter PJ, Nicholls S, Rye KA, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95(8):764–772.
- Kim SM, Kim JM, Shin DG, et al. Relation of atrial fibrillation (AF) and change of lipoproteins: male patients with AF exhibited severe pro-inflammatory and pro-atherogenic properties in lipoproteins. Clin Biochem. 2014;47(10-11):869–875.
- Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108(24):3006–3010.
- Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104(24):2886–2891.
- Asselbergs FW, Moore JH, van den Berg MP, et al. A role for CETP TaqIB polymorphism in determining susceptibility to atrial fibrillation: a nested case control study. BMC Med Genet. 2006;7(1):39.
- He F, Xu X, Yuan S, et al. Oxidized low-density lipoprotein (ox-LDL) cholesterol induces the expression of miRNA-223 and L-type calcium channel protein in atrial fibrillation. Sci Rep. 2016;6:30368.
- Okin PM, Hille DA, Wiik BP, et al. In-treatment HDL cholesterol levels and development of new diabetes mellitus in hypertensive patients: the LIFE Study. Diabet Med. 2013;30(10):1189–1197.
- Conen D, Adam M, Roche F, et al. Premature atrial contractions in the general population: frequency and risk factors. Circulation. 2012;126(19):2302–2308.
