Abstract
Purpose
An increased blood pressure variability (BPV) has been reported to be associated with older age and cognitive dysfunction; however, associations between increased BPV and rapid eye movement sleep behaviour disorder (RBD) has not been thoroughly investigated in patients without clinical Lewy body diseases.
Materials and methods
In frailty outpatient clinic, we evaluated ambulatory BP, RBD screening questionnaire (RBDSQ), and beat-to-beat heart rate variability during positional change from sitting to standing in 112 elderly hypertensive patients.
Results
The mean age was 81.2 ± 6.3 years (68% male). There were 15 patients who had probable RBD (RBDSQ scores ≥ 5). Patients with RBD had a greater body mass index, coefficient of variation (CV) in 24-h diastolic BP (23.5 ± 6.1 versus 18.7 ± 5.8, p = 0.005), awake diastolic BP (23.0 ± 7.7 versus 18.6 ± 6.2, p = 0.017), and nocturnal systolic BP (14.9 ± 5.5 versus 12.0 ± 4.4, p = 0.025) compared with those without RBD, while systolic BP, diastolic BP, and cognitive function did not differ significantly between patients with and without RBD. Patients with RBD exhibited larger orthostatic BP fall compared with patients without RBD (−4.9 ± 11.0 versus 7.5 ± 11.8, p = 0.009) and lower CV of R–R intervals while standing (1.3 ± 0.6 versus 2.4 ± 1.5, p = 0.039). Multiple regression analysis revealed that patients with RBD had significantly greater CV of nocturnal systolic BP independent of age, sex, BMI, history of diabetes and dyslipidaemia, and use of antihypertensive drugs (p = 0.008).
Conclusion
An increased BPV in ambulatory BP, associated with autonomic dysfunction, can be observed in patients with probable RBD even in elderly patients without clinical presentation of Lewy body diseases.
Introduction
An increased blood pressure variability (BPV) is one of the risk factors for cardiovascular mortality in previous results [Citation1], including elderly hypertensive population [Citation2]. There are several factors which increase BPV: older age, hypertension, chronic kidney diseases [Citation3], diabetes mellitus [Citation4], atherosclerosis [Citation5], obesity [Citation6], frailty [Citation7] and autonomic dysfunction [Citation8]. Additionally, there is growing evidence for associations between sleep disorders and increased BPV. Obstructive sleep apnoea syndrome has been studied extensively and was considered to be a prevalent causal factor of hypertension [Citation9] and increased BPV [Citation10]. Furthermore, impaired sleep quality, insufficient sleep, and insomnia are reported to be associated with elevated BP and an increased risk of hypertension [Citation11,Citation12]. Recent study indicated that altered BP control was involved in neurological sleep disorders such as restless leg syndrome [Citation13] or rapid eye movement (REM) sleep behaviour disorder (RBD) [Citation14], but evidence is currently limited [Citation15].
RBD is a type of parasomnia defined by intermittent loss of electromyographic atonia during REM sleep with emergence of complex and vigorous motor activity [Citation16]. Since RBD is a prodromal syndrome of Lewy body diseases, patients who present with RBD are likely to be diagnosed with Parkinson’s disease, multiple system atrophy, or dementia with Lewy bodies (DLB) after a period of time [Citation17].
An increased BPV associated with autonomic failure, such as orthostatic hypotension, supine hypertension, and postprandial hypotension, could be observed in clinical Lewy body diseases [Citation18,Citation19]. Aberrant autonomic cardiovascular function has been observed in patients with RBD, and one study reported that resting BP was not different between patients with RBD and healthy subjects [Citation20]. Therefore, it was unclear whether the relationship between RBD and BPV could be observed before presentation of clinical Parkinson’s disease or DLB.
The current study examined geriatric outpatients that were not diagnosed with Lewy body diseases to elucidate the association between BPV measured with ambulatory blood pressure monitoring (ABPM) and RBD.
Methods
Study design and participants
Among patients who were referred to the frailty clinic at Tokyo Metropolitan Geriatric Hospital from November 2019 to May 2021, we consecutively performed ABPM and the REM Sleep Behaviour Disorder Screening Questionnaire (RBDSQ) in hypertensive patients in the department of cardiology. Details of registration at the frailty outpatient clinic were reported previously [Citation21]. All patients underwent cognitive function tests, the hand grip strength test, walking ability measurement, and assessment of frailty. Information regarding medical history, present conditions, and antihypertensive agents were obtained from patients’ medical records. Patients who were diagnosed with Parkinson’s disease, DLB, or multiple system atrophy were excluded from the study (n = 1). The Ethics Committee of Tokyo Metropolitan Geriatric Hospital approved this study (R15-20, 19-03). All participants provided written informed consent.
Questionnaires
The RBDSQ contains 13 questions: items 1–4 measure the frequency and content of dreams and their relationship with nocturnal movements and behaviour; item 5 measures self-injuries and injuries to the patient’s bed partner; item 6 includes four sub-items that more specifically assess nocturnal motor behaviour, including questions on nocturnal vocalisation (6.1), sudden-limb movements (6.2), complex movements (6.3) or bedding items that fall down (6.4); items 7 and 8 measure nocturnal awakenings; item 9 measures general disturbances of sleep; and item 10 measures the presence of any neurological disorder. Each item can be answered ‘yes’ or ‘no’. RBDSQ scores ≥ 5 were considered consistent with probable RBD, based on previous reports [Citation22–24]. Family members could help patients to fill out the questionnaires.
ABPM
After visiting a frailty clinic, patients were scheduled to undergo ABPM measurements (TM2433 or 2441, A&D, Tokyo, Japan) within 2 weeks from the visit. Measurements were taken at 30-min intervals for 24 h. Daytime and night-time periods were defined according to the patient’s diary (daytime and asleep periods). Coefficient of variation (CV) values were calculated using standard deviation (SD)/BP.
BP and heart rate variability with positional change
BP, heart rate, and R–R interval measurements during the sitting and standing position were performed using ‘Kiritsu Meijin’ version 4 (Crosswell, Tokyo, Japan). After resting in a quiet room and taking five deep breaths, patients performed a simple standing test in which they sat for 2 min, then stood erect for 1 min, then sat for 1 min. During the test, brachial BP and pulse rate were measured every minute using Kiritsu Meijin. Power spectrum analysis was conducted using the MemCalc system, based on the variability of 1-min R–R intervals. The MemCalc method is a new technique for time series analysis. This approach is a combination of the maximum entropy method for spectral analysis and the non-linear least squares method for fitting analysis. Time domain analysis and spectral analyses of heart rate variability using the MemCalc system were performed for each 1-minute period during the standing test.
Other associated clinical factors
Information regarding demographic characteristics and associated factors was collected from medical records. Body mass index (BMI) values were calculated by dividing weight (in kilograms) by the square of height (in meters). Blood samples were collected for determination of haemoglobin A1c, brain natriuretic protein, albumin, and creatinine. Diabetes mellitus was defined as haemoglobin A1c ≥ 6.4% or active use of anti-diabetes drugs.
Statistical analysis
IBM SPSS Statistics Version 27 (IBM Corp., Armonk, NY) was used for statistical analysis. All values were expressed as mean ± SD or percentage. Significant differences between patients with and without RBD were determined using Student’s t-test, Mann–Whitney’s U test, or Chi-square test. Analysis of covariance was used to assess the independent relationship between patients with and without RBD after adjustment for the potential confounding effects of age, gender, BMI, history of hypertension, diabetes mellitus, dyslipidaemia, and use of antihypertensive drugs. Multiple regression analysis was performed to identify variables that determine the variability of blood pressure on ABPM. Independent variables included RBDSQ score, age, gender, BMI, history of diabetes and dyslipidaemia, and use of antihypertensive drugs. P-values less than 0.05 were considered significant.
Results
Baseline characteristics and BP in patients with and without probable RBD
The data of 112 patients are shown in . Patients’ mean age was 81.2 ± 6.3 years and 68% of patients were male. There were 15 patients with RBDSQ scores ≥ 5 points, and 97 patients had scores of < 5 points. Patients with RBD had higher BMI values, and higher CV values in 24-h diastolic BP, awake diastolic BP, and nocturnal systolic BP. There were no significant differences in systolic or diastolic BP between patients with and without RBD. Cognitive function measured with the Mini-Mental State Examination and Montreal Cognitive Assessment scales did not differ significantly according to RBDSQ scores. Most patients fulfilled the criteria for mild cognitive impairment.
Table 1. Baseline characteristics of patients with and without RBD.
BP variability and RBDSQ scores
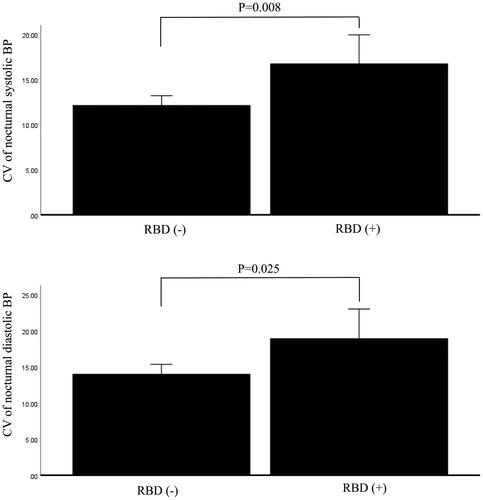
There was a significant difference in CV of nocturnal systolic BP between patients with and without RBD after adjustment for age, gender, BMI, history of hypertension, diabetes mellitus, dyslipidaemia, and use of antihypertensive drugs (p = 0.008). In addition, patients with and without RBD exhibited significantly different CV values for nocturnal diastolic BP (p = 0.025) ().
Figure 1. Difference in CV of blood pressure between patients with and without probable RBD after adjusting for potential confounding factors: age, gender, BMI, history of hypertension, diabetes mellitus, dyslipidaemia, and use of antihypertensive drugs. The analyses were performed by analysis of covariance. 1-1 Difference in CV of nocturnal systolic blood pressure. 1-2 Difference in CV of nocturnal diastolic blood pressure.
Postural changes in BP and RBDSQ scores
Postural changes in BP, HR, and autonomic function are shown in . BP decreased from rest to standing after 1 min in patients with RBD (130.8 ± 22.1/78.0 ± 13.3 to 122.3 ± 21.4/74.9 ± 11.3). In contrast, BP increased in patients without RBD (125.1 ± 18.7/75.5 ± 10.3/132.2 ± 19.4). This BP change was significant between the two groups (− 4.9 ± 11.0 versus 7.5 ± 11.8, p = 0.009). Coefficients of variation of R-R intervals (CVRR) after standing for 1 min were significantly decreased in patients with RBD compared with those without RBD (1.3 ± 0.6 versus 2.4 ± 1.5, p = 0.039).
Table 2. Postural change of blood pressure and relation between RBDSQ score.
Predictors of BPV
We performed multiple regression analysis to identify the predictors of BPV measured with ABPM (). RBDSQ scores of ≥ 5 were independently associated with CV of nocturnal systolic BP (p = 0.008), but this association was not significant for CV of 24-h diastolic BP and awake diastolic BP.
Table 3. Multiple regression analysis of determinant of CV of nocturnal systolic blood pressure.
Discussion
In the current study of outpatients at a frailty clinic, higher RBDSQ scores were associated with higher BPV measured by ABPM. Patients with probable RBD exhibited higher CV values for nocturnal systolic BP, and this association was significant after adjustment for confounding factors. In this population, the main determinant of the CV values of nocturnal systolic BP was an RBDSQ score of ≥ 5, and the main determinant of CV values for 24-h diastolic BP and awake diastolic BP was age. Autonomic responses expressed by CVRR was lower and BP decreased after standing up in patients with probable RBD. The current findings revealed probable RBD was associated with an increased BPV in ABPM who had no clinical symptoms of motor dysfunction or dementia.
Associations between RBD and autonomic function disorders have been reported in several previous studies. Postuma et al. used polysomnography to measure R-R intervals and obtained data using spectral analysis [Citation25]. The results revealed that subjects with RBD exhibited a lack of REM-related cardiac and respiratory responses [Citation25]. Ferini-Strambi reported that heart rate variability while asleep was reduced in subjects with RBD and that this phenomenon was related to both vagal and sympathetic nerve activity dysfunction [Citation26]. The current finding that BP and CVRR decreased while standing in patients with RBD is in line with the results of previous studies. However, BP alteration was not thoroughly assessed in these studies. One previous study performed ABPM and orthostatic testing in non-medicated or mild Parkinson’s disease patients, and reported no differences in dipping status, frequency of nocturnal hypertension, or frequency of supine hypertension between patients with and without RBD [Citation14]. Data regarding BPV by SD or CV was not reported. However, orthostatic hypotension was related to RBD in association with cardiac sympathetic denervation. The current results clearly indicated that BPV was larger in patients with probable RBD independent of BP level.
In Parkinson’s disease, orthostatic hypotension is a common symptom that lowers patients’ quality of life and ability to perform daily activities [Citation27]. Other abnormalities in BP include reverse dipping and nocturnal hypertension evaluated using ABPM [Citation28–31]. These BP fluctuation including BP lability and orthostatic hypotension are caused by autonomic failure which at least three pathophysiologic mechanisms are involved: loss of cardiac sympathetic nerves, extra- cardiac noradrenergic denervation, and arterial baroreflex failure [Citation32]. According to Braak’s model of staging in Parkinson’s disease [Citation33], the process of Lewy body pathology begins in the lower brainstem in the dorsal motor nucleus of the vagus nerve, as well as anterior olfactory structures, then ascends to the midbrain and forebrain and into the cerebral cortex. Therefore, symptoms associated with autonomic failure often occurs in early stage of Parkinson’s disease before motor dysfunction or cognitive decline are apparent [Citation27,Citation34]. A previous study reported that among patients with Lewy body diseases, changes in day-night blood pressure were observed only in patients that exhibited autonomic failure [Citation35]. In the present study, autonomic function testing was limited to performing the simple sitting to standing-up test. However, the finding that CVRR and BP significantly decreased while standing up in patients with RBD suggests that these patients exhibit autonomic dysfunction and higher CV compared to those without RBD is presumed to be related to autonomic nervous system. The difference in the current results between CV of diastolic BP and systolic BP should be considered in the context of previous reports that systolic BP variability reflects arterial stiffness or ageing whereas diastolic BP variability is mainly due to autonomic function or endothelial dysfunction [Citation36].
The results of the present study suggest that RBD is another contributing factor in increased diurnal BPV. Cognitive function is another factor affected by fluctuations of BP. Short-term BPV measured using ABPM [Citation37] and long-term variability assessed by visit-to-visit BPV [Citation38] are both associated with cognitive decline. In the current study, cognitive function of patients with RBD was not significantly impaired compared with controls and was evaluated as mild cognitive impairment. This finding supports the notion that these patients were in a preclinical state in which overt cognitive decline had not yet appeared.
Patients with RBD will develop neurodegenerative symptoms in high frequency of 75–90% [Citation39,Citation40] indicating motor dysfunction or dementia may appear after several years has elapsed. Observation of clinical symptoms of these patients and additional diagnostic imaging, such as metaiodobenzylguanidine scanning and dopamine transporter scintigraphy, are important.
Limitations
The current study involved several limitations that should be considered: (1) the sample size was relatively small; (2) autonomic function was not tested thoroughly using the head up tilt test or the Valsalva manoeuvre; (3) diagnosis of RBD was only performed using a questionnaire and polysomnographic data were not measured, although a recent study suggested that questionnaire assessment was not inferior to polysomnography for detecting RBD [Citation41]; (4) although sleep apnoea syndrome, insufficient sleep quality, and restless leg syndrome causes BPV, we were not able to exclude it accurately; (5) short-term BPV was measured using ABPM, however, long-term BPV including seasonal changes or visit-to-visit variability was not evaluated.
Conclusions
BPV measured by CV of ABPM was higher in patients with probable RBD, independent of age, sex, and BP level. RBD was significantly related to CV of nocturnal systolic BP. The current findings support the involvement of autonomic dysfunction in RBD. Further studies will be needed in a larger sample size and cognitive function of these patients should be monitored.
Acknowledgments
The authors thank Benjamin Knight, MSc., from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Disclosure statement
There are no conflicts of interest.
References
- Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098.
- Chowdhury EK, Owen A, Krum H, Second Australian National Blood Pressure Study Management Committee, et al. Systolic blood pressure variability is an important predictor of cardiovascular outcomes in elderly hypertensive patients. J Hypertens. 2014;32(3):525–533.
- Sarafidis PA, Ruilope LM, Loutradis C, et al. Blood pressure variability increases with advancing chronic kidney disease stage: a cross-sectional analysis of 16 546 hypertensive patients. J Hypertens. 2018;36(5):1076–1085.
- Chau NP, Bauduceau B, Chanudet X, et al. Ambulatory blood pressure in diabetic subjects. Am J Hypertens. 1994;7(6):487–491.
- Scuteri A, Rovella V, Alunni Fegatelli D, et al. An operational definition of SHATS (systemic hemodynamic atherosclerotic syndrome): role of arterial stiffness and blood pressure variability in elderly hypertensive subjects. Int J Cardiol. 2018;263:132–137.
- Tadic M, Cuspidi C, Pencic B, et al. The interaction between blood pressure variability, obesity, and left ventricular mechanics: findings from the hypertensive population. J Hypertens. 2016;34(4):772–780.
- Zhu Y, Chen X, Geng S, et al. Association between ambulatory blood pressure variability and frailty among older hypertensive patients. J Clin Hypertens (Greenwich). 2020;22(9):1703–1712.
- Liu Q, Han L, Chang F, et al. The relationship between the autonomic nervous function and early renal dysfunction in elderly patients with mild-to-moderate essential hypertension. Clin Exp Hypertens (New York, NY: 1993). 2018;40(2):136–140.
- Van Ryswyk E, Mukherjee S, Chai-Coetzer CL, et al. Sleep disorders, including sleep apnea and hypertension. Am J Hypertens. 2018;31(8):857–864.
- Hoshide S, Kario K, Chia YC, et al. Characteristics of hypertension in obstructive sleep apnea: an Asian experience. J Clin Hypertens (Greenwich). 2021;23(3):489–495.
- Jarrin DC, Alvaro PK, Bouchard MA, et al. Insomnia and hypertension: a systematic review. Sleep Med Rev. 2018;41:3–38.
- Lo K, Woo B, Wong M, et al. Subjective sleep quality, blood pressure, and hypertension: a meta-analysis. J Clin Hypertens. 2018;20(3):592–605.
- Van Den Eeden SK, Albers KB, Davidson JE, et al. Risk of cardiovascular disease associated with a restless legs syndrome diagnosis in a retrospective cohort study from Kaiser Permanente Northern California. Sleep. 2015;38(7):1009–1015.
- Kim JS, Park HE, Oh YS, et al. Orthostatic hypotension and cardiac sympathetic denervation in Parkinson disease patients with REM sleep behavioral disorder. J Neurol Sci. 2016;362:59–63.
- Mansukhani MP, Covassin N, Somers VK. Neurological sleep disorders and blood pressure: current evidence. Hypertension. 2019;74(4):726–732.
- Schenck CH, Bundlie SR, Ettinger MG, et al. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9(2):293–308.
- Howell MJ, Schenck CH. Rapid eye movement sleep behavior disorder and neurodegenerative disease. JAMA Neurol. 2015;72(6):707–712.
- Palma JA, Kaufmann H. Orthostatic hypotension in Parkinson disease. Clin Geriatr Med. 2020;36(1):53–67.
- Loew F, Gauthey L, Koerffy A, et al. Postprandial hypotension and orthostatic blood pressure responses in elderly Parkinson's disease patients. J Hypertens. 1995;13(11):1291–1297.
- Rocchi C, Placidi F, Liguori C, et al. Daytime autonomic activity in idiopathic rapid eye movement sleep behavior disorder: a preliminary study. Sleep Med. 2018;52:163–167.
- Tamura Y, Ishikawa J, Fujiwara Y, et al. Prevalence of frailty, cognitive impairment, and sarcopenia in outpatients with cardiometabolic disease in a frailty clinic. BMC Geriatr. 2018;18(1):264.
- Stiasny-Kolster K, Mayer G, Schäfer S, et al. The REM sleep behavior disorder screening questionnaire–a new diagnostic instrument. Mov Disord. 2007;22(16):2386–2393.
- Miyamoto T, Miyamoto M, Iwanami M, et al. The REM sleep behavior disorder screening questionnaire: validation study of a Japanese version. Sleep Med. 2009;10(10):1151–1154.
- Li K, Li SH, Su W, et al. Diagnostic accuracy of REM sleep behaviour disorder screening questionnaire: a meta-analysis. Neurol Sci: Off J Italian Neurol Soc Italian Soc Neurol Sci. 2017;38(6):1039–1046.
- Postuma RB, Montplaisir J, Lanfranchi P, et al. Cardiac autonomic denervation in Parkinson's disease is linked to REM sleep behavior disorder. Mov Disord. 2011;26(8):1529–1533.
- Ferini-Strambi L, Oldani A, Zucconi M, et al. Cardiac autonomic activity during wakefulness and sleep in REM sleep behavior disorder. Sleep. 1996;19(5):367–369.
- Goldstein DS. Orthostatic hypotension as an early finding in Parkinson's disease. Clin Auton Res. 2006;16(1):46–54.
- Milazzo V, Di Stefano C, Vallelonga F, et al. Reverse blood pressure dipping as marker of dysautonomia in Parkinson disease. Parkinsonism Relat Disord. 2018;56:82–87.
- Chen SW, Wang YK, Dou RH, et al. Characteristics of the 24-h ambulatory blood pressure monitoring in patients with Parkinson's disease – the SFC BP multicentre study in China. J Hypertens. 2020;38(11):2270–2278.
- Vallelonga F, Di Stefano C, Merola A, et al. Blood pressure circadian rhythm alterations in alpha-synucleinopathies. J Neurol. 2019;266(5):1141–1152.
- Oka H, Umehara T, Nakahara A, et al. Comparisons of cardiovascular dysautonomia and cognitive impairment between de novo Parkinson's disease and de novo dementia with *lewy bodies. BMC Neurol. 2020;20(1):350.
- Jain S, Goldstein DS. Cardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesis. Neurobiol Dis. 2012;46(3):572–580.
- Braak H, Del Tredici K, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197–211.
- Kaufmann H, Nahm K, Purohit D, et al. Autonomic failure as the initial presentation of Parkinson disease and dementia with Lewy bodies. Neurology. 2004;63(6):1093–1095.
- Plaschke M, Trenkwalder P, Dahlheim H, et al. Twenty-four-hour blood pressure profile and blood pressure responses to head-up tilt tests in Parkinson's disease and multiple system atrophy. J Hypertens. 1998;16(10):1433–1441.
- Bilo G, Parati G. Blood pressure variability and kidney disease: another vicious circle? J Hypertens. 2018;36(5):1019–1021.
- Sakakura K, Ishikawa J, Okuno M, et al. Exaggerated ambulatory blood pressure variability is associated with cognitive dysfunction in the very elderly and quality of life in the younger elderly. Am J Hypertens. 2007;20(7):720–727.
- Rouch L, Cestac P, Sallerin B, for the SAGES Investigators, et al. Visit-to-visit blood pressure variability is associated with cognitive decline and incident dementia: the SAGES cohort. Hypertension (1979). 2020;76(4):1280–1288.
- Iranzo A, Fernández-Arcos A, Tolosa E, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One. 2014;9(2):e89741.
- Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and *parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019;142(3):744–759.
- Chahine LM, Daley J, Horn S, et al. Questionnaire-based diagnosis of REM sleep behavior disorder in Parkinson's disease. Mov Disord. 2013;28(8):1146–1149.
