ABSTRACT
Introduction
Delirium is an acute alteration in attention, awareness, arousal, and cognition, precipitated by a sudden illness and highly prevalent in older, frail and acutely hospitalized patients. It is associated with poor outcomes, and few effective treatment alternatives. Non-pharmacological interventions and music show promising effects, warranting further research. This pilot randomized repeated measures trial aims to test feasibility of the trial methodology, acceptability, fidelity and safety of the music interventions, suitability of the effect-outcomes. and preliminary effectiveness.
Method
Acute geriatric patients with delirium or subsyndromal delirium will be randomized to Preferred Recorded Music (n = 30) or Preferred Live Music (n = 30), delivered for 30 minutes, over three consecutive days. Planned feasibility outcomes will comprise recruitment rate, retention and attrition rates, percentage of adherence, deviations rates, and success of treatment fidelity. Clinical outcomes will include: (a) trajectory of delirium symptoms: level of arousal as assessed by Observational Scale of Level of Arousal (OSLA) and modified Richmond Agitation Sedation Scale (mRASS); attention, assessed using backwards tests and digit span tests; orientation and short-term memory, assessed using recall tasks and orientation questions from Memorial Delirium Assessment Scale, (b) duration of delirium, (c) length of hospital stay, and (d) use of PRN medication (benzodiazepines and antipsychotics).
Discussion
The trial will provide results needed to design a subsequent sufficiently powered RCT, informing on the expected recruitment, feasibility and acceptability of the interventions and assessments and preliminary effectiveness
Introduction
Delirium is a neuropsychiatric syndrome characterized by an acute alteration in attention and consciousness, followed by cognitive dysfunction and psychomotor disturbances (5th ed.; DSM-5; American Psychiatric Association, Citation2013). The multifactorial aetiology of delirium involves an interplay between the predisposing factors and precipitating triggers such as acute illness or medical complications (Marcantonio, Citation2017; Ocagli et al., Citation2021; Wilson et al., Citation2020). In older individuals, delirium is a common and often early presenting symptom in COVID-19 (Shao et al., Citation2021; Tyson et al., Citation2022). Its psychomotor presentation is variable, involving hyperactive, hypoactive, or mixed features, and is usually fluctuating (Maldonado, Citation2017; Marcantonio, Citation2017). Delirium is undetected or misdiagnosed in 50–75% of the cases, often confused with other conditions with overlapping symptoms such as dementia, depression or psychosis (Kean & Ryan, Citation2008). Old age, dementia, acute illness, and hospitalization increase the risk, and the prevalence is thus highest in acutely hospitalized, older patients with dementia (50%) (Juliebø et al., Citation2009; Korevaar et al., Citation2005; Siddiqi et al., Citation2006), and in mechanically ventilated patients at intensive care units (ICUs) (80%) (Stollings et al., Citation2021). Delirium can last from a few days to weeks or even months (Maldonado, Citation2017; Wilson et al., Citation2020). The prognosis is often severe, with prolonged hospitalization (Gleason et al., Citation2015), increased need for long-term care (Krogseth et al., Citation2014; Witlox et al., Citation2010), onset of or accelerated progression of cognitive impairment (Gleason et al., Citation2015), and increased risk for mortality (Asghar et al., Citation2017; Wilson et al., Citation2020).
While early detection and treatment of underlying causes might reverse delirium (Bull et al., Citation2016), clinical management of its symptoms is necessary to prevent poor medical and functional outcomes and complications. Benzodiazepines and antipsychotic medication, commonly used for management of delirium, are not recommended because they are ineffective and can have serious side-effects (Agar et al., Citation2017; Evensen et al., Citation2019). Multicomponent approaches can prevent delirium (Asghar et al., Citation2017), decrease agitation (Marcantonio, Citation2017; Oh et al., Citation2017), and derive interest, pleasure and general well-being (O’Hanlon et al., Citation2014), however, more evidence for their effectiveness in delirium management is needed (Oh et al., Citation2017).
Music interventions (MIs), including both music therapy delivered by certified music therapists and music-based interventions facilitated by non-music therapists, have shown promise in regulating cognitive and behavioural symptoms in conditions like delirium (Brancatisano et al., Citation2020; O’Kelly et al., Citation2013; Sihvonen et al., Citation2017). Caregiver-facilitated music listening and music therapy interventions can: (a) improve disruptive behaviours, depressive symptoms, cognitive function and engagement in persons with dementia (Bian et al., Citation2021; Moreno-Morales et al., Citation2020; Ridder et al., Citation2013; van der Steen et al., Citation2018; Vink et al., Citation2014); (b) elicit favourable behavioural and physiological responses in patients with disorders of consciousness (Grimm & Kreutz, Citation2018; Li et al., Citation2020); and (c) reduce anxiety, respiratory rate and systolic blood pressure in ICU patients, highly predisposed to delirium (Bernatzky et al., Citation2011; Bradt & Dileo, Citation2014). In a recent systematic review (Golubovic et al., Citation2022), we found that despite high risk of bias and heterogeneity of studies, MIs delivered by certified music therapists and caregiver-facilitated music listening had positive effects on prevention and treatment of delirium. Further, adherence to the MIs was high. The meta-analysis showed 50% reduction in the risk of developing delirium after exposure to music in postsurgical, critically ill, and mechanically ventilated patients (Golubovic et al., Citation2022). Significant post-intervention improvements in delirium severity, mood, and engagement were also found in acute geriatric and long-term care patients (Browning et al., Citation2020; Cheong et al., Citation2016; Correâ et al., Citation2020). Future MI studies should incorporate comprehensive delirium assessments, better defined intervention protocols, correlations between intervention types, dosage, and different delirium symptoms, and evaluate treatment fidelity (Golubovic et al., Citation2022). The transient nature of delirium makes designing research on potential treatment alternatives challenging, as accurate delirium assessments, diagnosing, as well as inclusion of the participants may be difficult. Evaluating feasibility of assessment procedures and protocols is therefore essential for designing robust trials in the future.
Aims and objectives
As there are only a few published studies with poor methodological quality and scarce available evidence of the effectiveness of MIs in management of delirium, there is a need to implement robust trials. This feasibility and pilot randomized repeated measures trial aims to test pilot and test the feasibility of a RCT design for acceptability, intervention fidelity and safety of the MIs for the patients with delirium. In addition, it will test the suitability of the outcome measures and assess preliminary efficacy of the interventions.
The feasibility objectives are to examine:
Feasibility of recruitment procedures as determined by the proportion of eligible participants who gave informed consent from those screened as eligible, as well as the recruitment rate in a given period.
Feasibility of assessments and follow-up procedures, as assessed by the proportion of fully completed pre-post-intervention assessments.
Success of and fidelity adherence of interventions implementer by the therapist.
Interventions acceptability as determined by the number of the music sessions attended, refused, or not attended for other reasons.
Safety of the interventions determined by monitoring and registering minor and major adverse events potentially caused by the intervention, such as non-specific treatment effects, or other identifiable negative effects.
Sensitivity and suitability of the effect-outcomes (attention, cognition, arousal) to test the efficacy of the music interventions.
Clinical objectives are to (a) estimate preliminary efficacy of live and recorded MIs on severity of delirium symptoms, and (b) determine which specific delirium symptom domains are possibly most responsive to the MIs.
Theoretical framework
Delirium pathophysiology and targeted features
Onset of delirium has been understood as a central neural integration failure, caused by dysregulation in neurotransmitters and disruption to brain network connectivity, necessary for processing and maintaining sensory, cognitive, and motor responses (Maldonado, Citation2017; Wilson et al., Citation2020). An altered level of arousal (LoA) affects inattention, disorientation, agitation, sleep disturbance, delusions, visual hallucinations, anxiety, irritability, and depression (American Psychiatric Association, Citation2013; Inouye et al., Citation2014; Maldonado, Citation2017; Marcantonio, Citation2017; Neerland et al., Citation2018; Wilson et al., Citation2020). External manifestations of LoA inform diagnosis of hypoactive and the hyperactive delirium subtypes (Chester et al., Citation2012), and detecting delirium superimposed on dementia (Richardson, Davis, Bellelli, et al., Citation2017). The impact of suboptimal LoA impacts delirium patients’ attention and orientation, which further impacts their capacity to participate in cognitive tests (Neerland et al., Citation2018).
Rationale for MIs for delirium
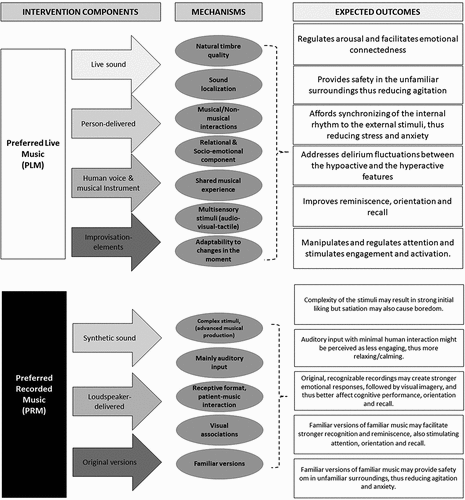
Our hypothesis is that Preferred Recorded Music (PRM) and Preferred Live Music (PLM) interventions, delivered by a certified music therapist (MT), may positively affect changes in arousal, attention, agitation, apathy, and cognitive performance. For PLM intervention, music attunement created through live music, and responsive non-musical interactions with the MT, may regulate LoA. Conversely, the synthetic sound delivered from the loud-speakers and original versions of the preferred music are the core component of the PRM intervention. There is currently no strong evidence supporting superiority of either MI type in treatment of delirium.
Shared therapeutic mechanisms of PRM and PLM
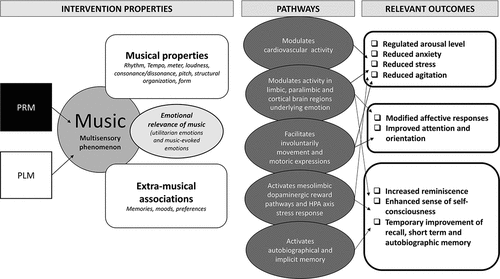
The multisensory nature of PRM and PLM simultaneously engage and modulate neurocognitive, perceptual, behavioural, physiological and psychosocial functions simultaneously (Hillecke et al., Citation2005; Schaefer, Citation2017). In doing so, they activate neuro-plastic and neurochemical processes, auditory-motor coupling, neural entrainment, arousal-mood pathways, autobiographical and implicit memory, and affect attunement (Brancatisano et al., Citation2020; Gold et al., Citation2019; Koelsch, Citation2014; O’Kelly et al., Citation2013; Park et al., Citation2016; Sihvonen et al., Citation2017; Vuilleumier & Trost, Citation2015). The interplay between the musical components regulates behavioural and psychological change (Ellsworth & Scherer, Citation2003; Koelsch, Citation2014; Sihvonen et al., Citation2017) (). The novelty, surprise, importance, anticipation, expectation, and predictability induce a process of tension, release and emotional contagion, and subsequently regulates attention and LoA (Schaefer, Citation2017; Thaut & Hoemberg, Citation2014) ().
Music regulates (a) cardiovascular activity, (b) limbic, paralimbic and cortical brain activity responsible for emotion (Koelsch, Citation2014; Schaefer, Citation2017; Sihvonen et al., Citation2017), (c) mesolimbic dopaminergic reward pathways, the hypothalamus-pituitary-adrenal axis stress response (Blood & Zatorre, Citation2001; Schaefer, Citation2017; Sihvonen et al., Citation2017), and (d) involuntary movement and motoric expressions (Grahn & Brett, Citation2007; Koelsch, Citation2014). Collectively, the activation of these mechanisms results in improved memory, reduced anxiety, stress (Baker, Citation2001; Bradt & Dileo, Citation2014), agitation (Baker, Citation2001), and improved attention and orientation to space, time and person (Baker, Citation2001), in older adults with neurological conditions similar to delirium (). Further, as music preferences can stimulate autobiographical recall, it holds promise in modifying affective responses (Baird & Samson, Citation2015), enhancing a sense of self-concept (Arroyo-Anlló et al., Citation2013), and temporarily improving cognitive functions (Thaut & Hoemberg, Citation2014) ().
Rationale for comparison
Music itself and the therapeutic relationship in which the musical interactions are situated are the main agents for change in the PLM intervention, which may thus be considered a music therapy intervention (Sihvonen et al., Citation2017). As a music therapist (MT) can respond moment to moment and adapt the PLM to the changing needs of participants, it may be better suited than PRM in responding to the fluctuating nature of delirium. Additionally, the shared musical interactions with the MT may lead to emotional connectedness, safety and agitation regulation (McDermott et al., Citation2014). Live performance and improvisation elements are engaging for patients with delirium (Cheong et al., Citation2016); synchronize the patients' internal physiological rhythm, and thus reduce anxiety and stress (Bush et al., Citation2021). Sound vibrations, live performance and the presence of the musical instrument(s) might provide additional sensory input, introduce a visual and sound-localization component, and thus impact on attention, orientation, reminiscence, and recall (Lee et al., Citation2021) ().
In the absence of a musical interaction, and fewer stimulating sensory element, the PRM may allow the participants to interact more directly with the music itself, and therefore be more calming and relaxing, for those with hyperactive delirium symptoms when compared with PLM. Recorded music has positive effects on cognition, orientation, recall, anxiety and aggression (Clare & Camic, Citation2020). Further, orientation and biographical recall may be stronger with the recognition of the unique “sound” of an original version of the song (Baker, Citation2001). However, habituation may result after prolonged exposure to the complexity of a music containing more than one instrument (Szpunar et al., Citation2004) ().
Method
This study protocol follows the Consolidated Standards of Reporting Trials (CONSORT) statement’s extended checklist for pilot and feasibility studies (Eldridge et al., Citation2016).
Study design
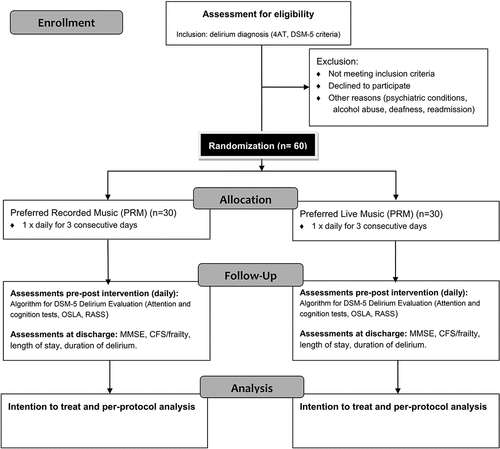
To evaluate the feasibility and preliminary effectiveness of the PLM and PRM interventions, a two-arm randomized repeated measures design will be implemented. Participants will receive either PRM or PLM, once a day for three consecutive days. Delirium symptoms will be assessed in participants pre and post each daily exposure to the allocated intervention.
Participants and setting
Participants will be recruited from an acute geriatric (AG) hospital ward (Ahmed, Citation2017) where there is a high prevalence of delirium and dementia comorbidities. The ward admits approximately 75 new patients per month with a patient average length of stay of 6.5 days (Ahmed, Citation2017).
Eligibility criteria
Patients admitted to the AG ward will be eligible if:
Aged ≥ 65
Diagnosed with delirium or subsyndromal delirium within the last 72 hours and is still present.
Appropriate informed consent is obtained.
Patients will not be excluded if they are under long-term care, have co-morbidities such as dementia or mild cognitive impairment, or if they have COVID-19. Patients will be excluded if:
Previously enrolled in the study and were readmitted during the study period,
Present with severe hearing impairments,
Present with severe psychiatric conditions other than delirium, or
Admitted due to severe alcohol or substance addiction,
Their musical preferences include orchestral or other kinds of music impossible to perform live by voice and the guitar ().
Recruitment procedures
Screening and enrolment
All the patients admitted to the AG ward will be routinely screened for delirium by the hospital nurses, using the 4AT which is a validated rapid (<2 min) screening tool for delirium (Shenkin et al., Citation2019). Patients with a 4AT score of ≥4 will be assessed by geriatricians according to the Diagnostic and Statistical Manual for Mental Disorders (DSM-5) criteria (American Psychiatric Association, Citation2013) and using a diagnostic algorithm applied in several other trials () (Neerland et al., Citation2015; Richardson, Davis, Stephan, et al., Citation2017; Tsui et al., Citation2022). Delirium subtypes will be determined using Delirium Motor Subtype Scale (DMSS-4) (Meagher et al., Citation2014). Geriatricians at the ward are responsible for obtaining informed consent from eligible patients or their legal representatives and will be masked to intervention allocation post randomization.
Table 1. Diagnostic algorithm for DSM-5 delirium evaluation.
Randomization
Consenting eligible patients will be randomly assigned to two different MIs using permuted block randomization 1:1, and the online randomization software, True Random Numbers (https://www.random.org/). The random allocation sequence will be generated by an independent researcher, and the participants will be enrolled and assigned to interventions by the music therapist. Randomized blocks of 10 participants aim to maintain even numbers of participants per study-arm.
Masking
Masking the therapist and the participants will not be possible. Masking assessors will be attempted. In case where allocation is revealed, the assessor will be replaced with a new assessor masked to allocation wherever possible. Success of masking will be verified for each of the post-intervention delirium assessments. The success of masking will be reported.
Interventions
Participants’ music preferences will be determined by legal guardians completing an adapted Norwegian version of the Assessment of Personal Music Preference (family version) tool (APMP) (Gerdner, Citation2021). A Music Assessment Tool (MAT), developed for the use with critically ill and mechanically ventilated patients (Chlan & Heiderscheit, Citation2009) will be used to assess music preferences directly from the participants. Assessment sessions will be performed by the certified MT prior to other baseline assessments, will not exceed 30 minutes, will be adapted to the participants’ cognitive functions and responsiveness, and will include observation of responses to musical pieces played within the assessment session. The MT will design PRM and PLM interventions and select the songs to be included, using the information derived from the APMP, MAT and preference assessment session.
Participants will receive the PRM or PLM intervention, once per day, for 30 minutes, for three consecutive days. The same songs in the same order will be used in each of the three daily sessions, to ensure familiarity, foster safety and minimize confusion. PLM intervention will involve singing preferred songs live with or without the guitar/tone-chimes accompaniment. MT will actively engage with the participants while singing and playing the selected songs by encouraging them to sing along, move to the music, and, if appropriate, offer small percussion instruments for them to play on. Elements of musical improvisation, both vocal and instrumental will be present, including techniques such as repetition, variation, and extension of the themes from the selected songs, as well as musical matching, mirroring or imitating the participants’ responses that occur. Eye-contact, and both verbal and physical interaction will be in focus. Music attunement created through such live musical interaction, and responsive non-musical interactions with the MT are, thus, the most important components of this intervention. During the PRM intervention, the MT will refrain from engaging with the participants during their listening session. However, the MT's implicit therapeutic skills might make it challenging to adhere to the protocol and refrain from interacting with the patient. Each interaction between the MT and the participants during the sessions will, thus, be considered a deviation from the protocol, and recorded post session.
In addition to the MIs, all the participants will receive usual care. The MIs will take place in the participants’ private rooms containing a single-bed, night table, chairs, wardrobe and a TV. As the bedrooms do not usually contain radio devices, we expect the participants’ music listening during the intervention days to be limited but will, nevertheless, attempt at recording it by talking to the caregivers and the nursing staff. Interventions will be discontinued or modified in response to participants’ request or worsening of their health condition.
Outcomes
Primary outcomes (feasibility outcomes)
Primary outcomes of the feasibility trial comprise: (a) recruitment rate, (b) retention and attrition rates, (c) percentage of adherence and deviations rates, and (d) success of treatment fidelity, to determine the extent to which the study design is replicable and its results generalizable (external validity), and that no other factors/variables caused the observed effect (reliability and internal validity) (Borrelli, Citation2011).
Secondary outcomes (clinical outcomes)
Clinical outcomes comprise: (a) trajectory of delirium symptoms: level of arousal as assessed by Observational Scale of Level of Arousal (OSLA) (Hall et al., Citation2020) and modified Richmond Agitation Sedation Scale (mRASS) (Chester et al., Citation2012); attention, using backwards tests and digit span tests; orientation and short-term memory, using recall tasks and orientation questions from Memorial Delirium Assessment Scale (Breitbart et al., Citation1997), (b) duration of delirium, (c) length of hospital stay, and (d) use of PRN medication (benzodiazepines and antipsychotics).
Data collection and study procedures
Background variables
Demographic data collected will include gender, age, marital status, education, accommodation, alcohol/tobacco use, etc. Clinical baseline data comprise height/weight, past diagnoses, comorbidities, and prescribed medication. Frailty status will be assessed using the Clinical Frailty Scale (CFS) (Rockwood et al., Citation2005) and cognitive status pre-admission will be assessed by asking the patients primary caregiver to complete the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) (Jorm, Citation1994). Severity of acute illness will be assessed using New Early Warning Score 2 (NEWS2) (Royal College of Physicians [RCoP], Citation2012). The Mini Mental Status Evaluation Norwegian version (MMSE-NR) (Arevalo-Rodriguez et al., Citation2015) will be performed at discharge for the purpose of general cognitive screening ().
Table 2. Schedule of clinical activities.
Delirium assessments
Pre-and post-intervention changes in delirium severity will be assessed after each music session, using the DSM-5 algorithm (described earlier). As DSM-5 only specifies criteria to be evaluated and not the tests that should be used, our algorithm includes validated attention and cognition tests, as well as the Observational Scale of Level of Arousal (OSLA) and Richmond Agitation and Sedation Scale (RASS).
The validated Observational Scale of Level of Arousal (OSLA) involves assessing LoA through observation of eye opening, eye contact, posture and movement (Hall et al., Citation2020). The test is performed by trained geriatricians, takes under one minute to complete, and requires no verbal interaction with the patient. Total scores range from 0 to 15, with a cut-off of ≥ 2 or ≥ 3 already indicating abnormal arousal (Hall et al., Citation2020). For a cut-off ≥ 2 OSLA scale has sensitivity of 0.87 (95% CI [0.84, 0.93]), and specificity of 0.53 (95% CI [0.48, 0.58]). OSLA is sensitive to within-patient fluctuations in delirium status, and thus recommended for monitoring and assessing changes in LoA and delirium severity over time (Hall et al., Citation2020).
Richmond Agitation and Sedation Scale (RASS) is a validated observational scale that takes less than 20 seconds to administer, and measures sedation and agitation – the main components of consciousness, and strong indicators of the hypoactive and hyperactive delirium subtypes (Chester et al., Citation2012; Han et al., Citation2015). Changes in agitation/sedation are recorded by observing the duration of eye contact following verbal and physical stimulation, with level of agitation ranging from “combative” to “calm”, and level of sedation ranging from “alert” to “comatose” (Ely et al., Citation2003). Scores range from −5 (comatose state) to +5 (combativeness) (Han et al., Citation2015). A modified version of RASS scale incorporating attention assessments (mRASS) is recommended for use with geriatric and critically ill patients (Chester et al., Citation2012). mRASS has a sensitivity of 0.64, and specificity of 0.93, using a cut-off ≥ 3 (Chester et al., Citation2012) ().
Cut-off scores for the attention tests, recall tasks and orientation and short-term memory questions from Memorial Delirium Assessment Scale (Breitbart et al., Citation1997) are described in .
Length of hospital stay, duration of delirium during hospital stays and the use of psychopharmacological medications per patient during the hospitalization will be assessed from electronic medical journals before discharge from the ward ().
Assessments of feasibility outcomes
Recruitment procedures will be evaluated, and recruitment rate calculated by dividing the total number of the eligible patients randomized per month by the total number of months that the trial recruited for.
Retention is defined as trial completion on study intervention and will be calculated by dividing the number of participants completing the study by the total number of participants recruited. Attrition rate is defined as the percentage of the participants that did not complete the study and will be calculated by dividing the number of the participants who withdrew by the total number recruited.
Adherence to the study protocol relates to compliance with the described study protocol and procedures, and protocol deviations to any change or divergence from the protocol for each participant. Overall adherence rate will be estimated by calculating the percentage of the music sessions and assessments completed from those described in the protocol by dividing the number of completed sessions/assessments by the number that was planned. The percentage of participants who had received all three sessions as per protocol will also be estimated. Deviation rates will be measured by counting the deviations per participant during their participation in the study.
The level of treatment fidelity will be determined according to the National Institute of Health Behavioural Change Consortium (NIH BCC) recommendations (Bellg et al., Citation2004; Borrelli, Citation2011; Borrelli et al., Citation2005), directly, by observing the recorded sessions with randomly selected 10% of the participants, and indirectly by self-reporting via MT’s logs and checklists (Supplementary material S1). Only two of the NIH BCC features will be assessed: (a) whether the design of the trial has been defined and described prior to implementation in such way that it can answer the proposed research question and be replicated and (b) whether the treatments are delivered as intended (Bellg et al., Citation2004), with the general goal of standardizing delivery, and ensuring adherence to the intervention protocols (Bellg et al., Citation2004; Borrelli et al., Citation2005). A full description of the fidelity strategy can be found in Supplementary material S2.
Participant retention and withdrawal
Participant retention and withdrawal and reasons for doing so will be tracked. These include early discharge, participant/guardian withdrawal of consent, safety concerns identified by the geriatrician, and an inability/low compliance with the study protocols and procedures. All participants who had received at least one session will be included in an intention-to-treat analysis.
Analytical methods
Statistical considerations
Changes in OSLA, mRASS, attention and cognitive status scores assessed pre and post session will be recorded for three consecutive days until the end of the intervention. Changes in response pre- and post-intervention within the participants and between the two participant samples will be compared each day and across the three consecutive days. The between groups effect will be estimated in mixed linear regression models for each outcome with adjustment for their respective pre-intervention score and participant ID included as a random-effect. For outcomes measured only at discharge (length of stay, duration of delirium, PRN medication), the Independent Samples t-test for the normally distributed, or Mann-Whitney test for the skewed data, will be used to compare intervention groups for continuous variables, and a Chi-squared test for binary variables. Any participants who are discharged prior to completion of three music sessions, or have not been able to receive the intervention as per protocol for other reasons, will be included in the analysis under the intention-to-treat principle. We will also attempt per-protocol analysis, for the participants that have received all the interventions as per protocol.
Sample size and test power
This feasibility study is not intended to be adequately powered to draw conclusive findings on the effects on delirium symptoms. We aim to recruit the sample of 60 participants in accordance with CONSORT recommendations. The sample size of n = 60 participants will enable us to examine the main objectives of the study, and also allow for dropouts, which may be expected in the context of acute-geriatric medicine, considering the frailty and sensitivity of the patients, as well as the challenging and fluctuating nature of delirium. This sample size will allow for collecting sufficient data to inform a robust, more conclusive RCT in the future.
Ethical considerations
Research ethics approval and consent
Ethical approval has been obtained from the Regional Ethics Committee in Norway (REK 457017). The trial is registered at Clinical Trials (NCT05398211). Most eligible participants in this study are expected to have reduced ability to give consent, and each patient’s ability to give consent will be individually evaluated. Experienced physicians will be obtaining consents from the patients directly and supplement or replace them by the consent from the legally assigned representatives (LAR) when necessary, using three different consent forms. Consents will be obtained primarily in written format. However, the use of verbal consent was also approved by REK in cases where the LAR is not present physically. The verbal consent will then be obtained via phone-call, and the written confirmation will be obtained at the first possible occasion.
Data management
Appropriate permission for personal-data storage has been obtained from Data Protection Authorities at Oslo University Hospital (OUS) and a data-management plan has been created. The data in this study will be collected both using paper forms, and electronically, and all the data collection will take place at the participating site, where the data originated. The data registered during the study will be indirectly personally identifiable (de-identified/coded) and will be stored safely on the research servers at OUH, and in locked cabinets. The participants will be thoroughly informed about the personal data collected about them, as well as that the data will be unidentifiable, through the informed consent forms.
Risk management
The potential risks associated with this study are slow recruitment, poor fidelity and acceptability of the interventions, low reliability of clinical outcomes, poor adherence to assessment procedures, and high percentage of dropouts and/or adverse events due to the challenging and fluctuating nature of delirium. As our primary aim is investigating feasibility, most risks will be viewed as relevant findings. We will not be able to influence recruitment rate, drop-outs or adverse events. To mitigate the risk of poor adherence to the interventions, we developed a detailed intervention manual with accompanying treatment fidelity protocol checklist for PRM and PLM interventions (Supplementary material S1 and S2).
To reduce the risk of low reliability of the clinical outcome measures we will report delirium by symptom domains rather than present/absent, using continuous variables rather than dichotomous (Tieges et al., Citation2021). We chose mRASS and OSLA scales as they are highly correlated, have high interrater reliability (k = 0.91), can reliably assess changes in delirium severity over time (Chester et al., Citation2012; Hall et al., Citation2020) and when used in combination, increase the accuracy of diagnosis.
Assessing LoA, combined with monitoring cognitive status and attention, is the most efficient approach to evaluating short-term post-intervention changes in delirium severity. Combined arousal-attention assessments (e.g. OSLA and SAVEHAART attention test), are, thus, more efficient and diagnostically accurate, particularly for detecting delirium in patients with dementia, and diagnosing delirium superimposed on dementia (Quispel-Aggenbach et al., Citation2018; Richardson, Davis, Bellelli, et al., Citation2017). Adherence to assessment procedures is protected by training the assessors in using the specially developed assessment-algorithm. Potential adverse events and unintended effects of the interventions will also be monitored and documented using treatment fidelity checklists.
Dissemination
Findings of this trial will be published in relevant scientific journals, and presented at international conferences.
Discussion
Relevance, benefits, and implications
While music interventions (MIs) are moderately used in hospital settings and with various neurological conditions, their use in management of delirium is mainly unexplored, both in the Norwegian context and internationally. The existing research is scarce, with small samples, poor designs, and heterogeneous effects (Golubovic et al., Citation2022). The proposed randomized feasibility study is designed to provide necessary knowledge for improving the design of future research, particularly the standardization of intervention protocols, relevance of effect-outcomes, validity of delirium-assessments, as well as the power calculation and optimal recruitment strategies. Measuring intervention effects will enable us to identify and evaluate correlations between different MIs and changes in the targeted delirium symptoms and evaluate sensitivity and accuracy of delirium tools and assessment procedures for measuring post-intervention effects. The results are expected to contribute to developing generalizable knowledge on the appropriateness and preliminary effectiveness of MIs in delirium management in acutely ill older patients.
By involving a music therapist (MT) in delivery of both PLM and PRM interventions, rather than having PRM delivered by a non-music therapist, we wish to isolate live and recorded music as main variables without introducing an additional facilitator variable. While we view the involvement of the MT important for the music preference assessments and designing of both interventions, we do acknowledge that PRM intervention may also be delivered by a non-music therapist if the study should be replicated in the future. However, the PLM intervention should be facilitated by a certified music therapist, due to its complexity and the therapeutic techniques demanding music therapeutic expertise.
Strengths and limitations
A strength of this study are our comprehensive music preference assessments, which will allow us to individualize the interventions. As people with delirium may not be able to voice their music preferences, MTs are specially trained and experienced in conducting interactive music preference assessments with people who have reduced cognitive abilities and physical functions. Our comprehensive, validated algorithm for assessing changes in delirium severity, which includes both continuous and categorical variables, is a strength because it offers a more nuanced picture of the changes in delirium after exposure to the MIs.
The main limitation of this feasibility study protocol is the lack of a control group, which would make it possible to compare the changes in severity of delirium symptoms between patients receiving usual care and the two MIs offered. However, as the recruitment-rate, feasibility of recruitment and assessment-procedures, as well as the feasibility, suitability and acceptability of the MIs is still widely unknown, we have considered it appropriate to omit the control group, and rather focus on exploring and determining feasibility using a pre-post measures design.
Conclusion
In conclusion, this study is expected to contribute to extending and strengthening the interdisciplinary collaboration between the fields of geriatric and acute medicine, neuroscience, nursing, music therapy and music medicine and drive changes in the care for older adults with delirium. The generated knowledge might indirectly contribute to an increased implementation of MIs in management of delirium across the clinical settings and levels of care, and thus open new research areas of high relevance for the public health.
Supplemental Material
Download MS Word (71.5 KB)Acknowledgements
We wish to acknowledge Oslo University Hospital (OUH), for being the main sponsor and the host of the project, and Norwegian Academy of Music (NMH) for being the collaborating institution and providing funding for JG’s PhD project of which this feasibility study is a part. Our gratitude extends to Prof. Torgeir Bruun Wyller, Prof. Leiv Otto Watne, from Akershus University Hospital (AHUS) and Oslo Delirium Research Group, as well as Dr. Marc Ahmed and Dr. Nina Ommundsen, at the Acute Geriatric Section (OUH), for their generous feedbacks concerning the study design. We also wish to acknowledge Centre for Research in Music and Health (CREMAH) at NMH, and the Director, Prof. Karette Stensæth, as well as the music therapists Kristi Stedje and Runa Bosnes Engen, for valuable discussions in the process of developing the intervention protocols.
Disclosure statement
The authors report no conflict of interest.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/08098131.2023.2192759.
Additional information
Funding
Notes on contributors
Jelena Golubovic
Jelena Golubovic is a certified music therapist, pianist and PhD Research Fellow affiliated with the Centre for Research in Music and Health and Oslo Delirium Research Group. Jelena’s professional focus is related to music therapy with older population, and areas of dementia, delirium and neuro-rehabilitation.
Felicity A. Baker
Professor Felicity Ann Baker is Associate Dean (Research) for the Faculty of Fine Arts and Music, and Director, International Research Partnerships for the Creative Arts and Music Therapy Research Unit. She is former Australia Research Council Future Fellow (2011–2015) in the area of music therapy and during this fellowship built models of song writing as practiced through the lenses of different orientations.
Melanie R. Simpson
Melanie Rae Simpson, PhD, is a study programme leader at the Office for Medical Education (OME), and associate professor in medical statistics at the Department of Public Health and Nursing.
Bjørn Erik Neerland
Bjørn Erik Neerland, PhD, is a medical doctor, specialist in internal medicine and geriatrics, and consultant and postdoctoral researcher at Oslo University hospital. Neerland has special interests in acute geriatric medicine and delirium, and is a member of Oslo Delirium Research Group.
References
- Agar, M. R., Lawlor, P. G., Quinn, S., Draper, B., Caplan, G. A., Rowett, D., Sanderson, C., Hardy, J., Le, B., Eckermann, S., McCaffrey, N., Devilee, L., Fazekas, B., Hill, M., & Currow, D. C. (2017). Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: A randomized clinical trial. JAMA Internal Medicine, 177(1), 34–42. https://doi.org/10.1001/jamainternmed.2016.7491
- Ahmed, M. (2017). Akuttgeriatri - pleietung avdeling i teknologitungt sykehus: Hvordan bevare personale, glød og entusiasme? Den Sjuende Norske Kongressen i Geriatri. https://www.legeforeningen.no/contentassets/523b04fe9c27460e91689eaa12b0fdc7/akuttgeriatri-pleietung-avdeling-i-teknologitungt-sykehus-ved-marc-ahmed.pdf
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). https://doi.org/10.1176/appi.books.9780890425596
- Arevalo-Rodriguez, I., Smailagic, N., Roqué, I. F. M., Ciapponi, A., Sanchez-Perez, E., Giannakou, A., Pedraza, O. L., Bonfill Cosp, X., & Cullum, S. (2015). Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database of Systematic Reviews, 2015(3), CD010783. https://doi.org/10.1002/14651858.CD010783.pub2
- Arroyo-Anlló, E. M., Díaz, J. P., & Gil, R. (2013). Familiar music as an enhancer of self-consciousness in patients with Alzheimer’s disease. BioMed Research International, 2013, 752965. https://doi.org/10.1155/2013/752965
- Asghar, A., Siddiqui, K. M., Ahsan, K., & Chughtai, S. (2017). Postoperative delirium after cardiac surgery; incidence, management and prevention. Anaesth Pain & Intensive Care, 21(1), 109–111.
- Baird, A., & Samson, S. (2015). Chapter 11 - Music and dementia. In E. Altenmüller, S. Finger, & F. Boller (Eds.), Progress in brain research (Vol. 217, pp. 207–235). Elsevier. https://doi.org/10.1016/bs.pbr.2014.11.028
- Baker, F. (2001). Rationale for the effects of familiar music on agitation and orientation levels of people in posttraumatic amnesia. Nordic Journal of Music Therapy, 10(1), 32–41. https://doi.org/10.1080/08098130109478015
- Bellg, A. J., Borrelli, B., Resnick, B., Hecht, J., Minicucci, D. S., Ory, M.Ogedegbe, G., Orwig, D., Ernst, D. & Czajkowski, S. Treatment Fidelity Workgroup of the NIH Behavior Change Consortium. (2004). Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH behavior change Consortium. Health Psychology, 23(5), 443–451. https://doi.org/10.1037/0278-6133.23.5.443
- Bernatzky, G., Presch, M., Anderson, M., & Panksepp, J. (2011). Emotional foundations of music as a non-pharmacological pain management tool in modern medicine. Neuroscience and Biobehavioral Reviews, 35(9), 1989–1999. https://doi.org/10.1016/j.neubiorev.2011.06.005
- Bian, X., Wang, Y., Zhao, X., Zhang, Z., & Ding, C. (2021). Does music therapy affect the global cognitive function of patients with dementia? A meta-analysis. NeuroRehabilitation, 48(4), 553–562. https://doi.org/10.3233/nre-210018
- Blood, A. J., & Zatorre, R. J. (2001). Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proceedings of the National Academy of Sciences, 98(20), 11818–11823. https://doi.org/10.1073/pnas.191355898
- Borrelli, B. (2011). The assessment, monitoring, and enhancement of treatment Fidelity in public health clinical trials. Journal of Public Health Dentistry, 71(s1), S52–63. https://doi.org/10.1111/j.1752-7325.2011.00233.x
- Borrelli, B., Sepinwall, D., Ernst, D., Bellg, A. J., Czajkowski, S., Breger, R., DeFrancesco, C., Levesque, C., Sharp, D. L., Ogedegbe, G., Resnick, B., & Orwig, D. (2005). A new tool to assess treatment fidelity and evaluation of treatment fidelity across 10 years of health behavior research. Journal of Consulting and Clinical Psychology, 73(5), 852–860. https://doi.org/10.1037/0022-006x.73.5.852
- Bradt, J., & Dileo, C. (2014). Music interventions for mechanically ventilated patients. Cochrane Database of Systematic Reviews, 2014(12), Cd006902. https://doi.org/10.1002/14651858.CD006902.pub3
- Brancatisano, O., Baird, A., & Thompson, W. F. (2020). Why is music therapeutic for neurological disorders? The Therapeutic music capacities model. Neuroscience and Biobehavioral Reviews, 112, 600–615. https://doi.org/10.1016/j.neubiorev.2020.02.008
- Breitbart, W., Rosenfeld, B., Roth, A., Smith, M. J., Cohen, K., & Passik, S. (1997). The memorial delirium assessment scale. Journal of Pain and Symptom Management, 13(3), 128–137. https://doi.org/10.1016/s0885-3924(96)00316-8
- Browning, S. G., Watters, R., & Thomson-Smith, C. (2020). Impact of therapeutic music listening on intensive care unit patients: A pilot study. The Nursing Clinics of North America, 55(4), 557–569. https://doi.org/10.1016/j.cnur.2020.06.016
- Bull, M. J., Boaz, L., & Jermé, M. (2016). Educating family caregivers for older adults about delirium: A systematic review. Worldviews on Evidence-Based Nursing, 13(3), 232–240. https://doi.org/10.1111/wvn.12154
- Bush, H. I., LaGasse, A. B., Collier, E. H., Gettis, M. A., & Walson, K. (2021). Effect of live versus recorded music on children receiving mechanical ventilation and sedation. American Journal of Critical Care, 30(5), 343–349. https://doi.org/10.4037/ajcc2021646
- Cheong, C. Y., Tan, J. A., Foong, Y. L., Koh, H. M., Chen, D. Z., Tan, J. J.Ng, C. J., & Yap, P. (2016). Creative music therapy in an acute care setting for older patients with delirium and dementia. Dementia and Geriatric Cognitive Disorders Extra, 6(2), 268–275. https://doi.org/10.1159/000445883
- Chester, J. G., Beth Harrington, M., & Rudolph, J. L. (2012). Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. Journal of Hospital Medicine, 7(5), 450–453. https://doi.org/10.1002/jhm.1003
- Chlan, L., & Heiderscheit, A. (2009). A tool for music preference assessment in critically ill patients receiving mechanical ventilatory support. Music Therapy Perspectives, 27(1), 42–47. https://doi.org/10.1093/mtp/27.1.42
- Clare, A., & Camic, P. M. (2020). Live and recorded group music interventions with active participation for people with dementias: A systematic review. Arts & Health, 12(3), 197–220. https://doi.org/10.1080/17533015.2019.1675732
- Correâ, L., Caparrol, A. J. D. S., Martins, G., Pavarini, S. C. I., & Grataõ, A. C. M. (2020). Effects of music on body and facial expressions and psychological and behavioral symptoms of older adults. Brazilian Journal of Occupational Therapy, 28(2), 539–553. https://doi.org/10.4322/2526-8910.CTOAO1889
- Eldridge, S. M., Chan, C. L., Campbell, M. J., Bond, C. M., Hopewell, S., Thabane, L., P. C. G., Lancaster, G. A., & on behalf of the PAFS consensus group.(2016). CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. Pilot and Feasibility Studies, 2(1), 64. https://doi.org/10.1186/s40814-016-0105-8
- Ellsworth, P. C., & Scherer, K. R. (2003). Appraisal processes in emotion. In R. J. Davidson & K. R. Scherer (Eds.), Handbook of affective sciences (pp. 572–595). Oxford University Press.
- Ely, E. W., Truman, B., Shintani, A., Thomason, J. W. W., Wheeler, A. P., Gordon, S. … , Francis, J., Speroff, T., Gautam, S., Margolin, R., Sessler, C. N., Dittus, R. S., & Bernard, G. R. (2003). Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA, 289(22), 2983–2991. https://doi.org/10.1001/jama.289.22.2983
- Evensen, S., Saltvedt, I., Ranhoff, A. H., Myrstad, M., Myrstad, C., Mellingsæter, M., Wang-Hansen, M. S., & Neerland, B. E. (2019). Delirium og kognitiv svikt blant eldre i norske akuttmottak. Tidsskrift for Den norske legeforening. https://doi.org/10.4045/tidsskr.18.0578
- Gerdner, L. (2021). Evidence-based guideline: Individualized music for persons with dementia. (7th Ed.). https://doi.org/10.1097/01.NAJ.0000737292.96068.18
- Gleason, L. J., Schmitt, E. M., Kosar, C. M., Tabloski, P., Saczynski, J. S., Robinson, T., Cooper, Z., Rogers, S. O. Jr., Jones, R. N., Marcantonio, E. R., & Inouye, S. K. (2015). Effect of delirium and other major complications on outcomes after elective surgery in older adults. JAMA Surgery, 150(12), 1134–1140. https://doi.org/10.1001/jamasurg.2015.2606
- Gold, C., Eickholt, J., Assmus, J., Stige, B., Wake, J. D., Baker, F. A., Tamplin, J., Clark, I., Lee, Y. C., Jacobsen, S. L., Ridder, H. M. O., Kreutz, G., Muthesius, D., Wosch, T., Ceccato, E., Raglio, A., Ruggeri, M., Vink, A., Zuidema, S. … Geretsegger, M. (2019). Music Interventions for Dementia and Depression in ELderly care (MIDDEL): Protocol and statistical analysis plan for a multinational cluster-randomised trial. BMJ Open, 9(3), e023436. https://doi.org/10.1136/bmjopen-2018-023436
- Golubovic, J., Neerland, B. E., Aune, D., & Baker, F. A. (2022). Music interventions and delirium in adults: A systematic literature review and meta-analysis. Brain Sciences, 12(5), 568. https://doi.org/10.3390/brainsci12050568
- Grahn, J. A., & Brett, M. (2007). Rhythm and beat perception in motor areas of the brain. Journal of Cognitive Neuroscience, 19(5), 893–906. https://doi.org/10.1162/jocn.2007.19.5.893
- Grimm, T., & Kreutz, G. (2018). Music interventions in disorders of consciousness (DOC) - a systematic review. Brain Injury, 32(6), 704–714. https://doi.org/10.1080/02699052.2018.1451657
- Hall, R., Stíobhairt, A., allerhand, M., MacLullich, A. M. J., & Tieges, Z. (2020). The Observational Scale of Level of arousal: A brief tool for assessing and monitoring level of arousal in patients with delirium outside the ICU. International Journal of Geriatric Psychiatry, 35(9), 1021–1027. https://doi.org/10.1002/gps.5324
- Han, J. H., Vasilevskis, E. E., Schnelle, J. F., Shintani, A., Dittus, R. S., Wilson, A., & Ely, E. W. (2015). The diagnostic performance of the Richmond Agitation Sedation Scale for detecting delirium in older emergency department patients. Academic Emergency Medicine: Official Journal of the Society for Academic Emergency Medicine, 22(7), 878–882. https://doi.org/10.1111/acem.12706
- Hillecke, T., Nickel, A., & Bolay, H. V. (2005). Scientific perspectives on music therapy. Annals of the New York Academy of Sciences, 1060(1), 271–282. https://doi.org/10.1196/annals.1360.020
- Inouye, S. K., Westendorp, R. G., & Saczynski, J. S. (2014). Delirium in elderly people. The Lancet, 383(9920), 911–922. https://doi.org/10.1016/s0140-6736(13)60688-1
- Jorm, A. F. (1994). A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Development and cross-validation. Psychological Medicine, 24(1), 145–153. https://doi.org/10.1017/s003329170002691x
- Juliebø, V., Bjøro, K., Krogseth, M., Skovlund, E., Ranhoff, A. H., & Wyller, T. B. (2009). Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. Journal of the American Geriatrics Society, 57(8), 1354–1361. https://doi.org/10.1111/j.1532-5415.2009.02377.x
- Kean, J., & Ryan, K. (2008). Delirium detection in clinical practice and research: Critique of current tools and suggestions for future development. Journal of Psychosomatic Research, 65(3), 255–259. https://doi.org/10.1016/j.jpsychores.2008.05.024
- Koelsch, S. (2014). Brain correlates of music-evoked emotions. Nature Reviews Neuroscience, 15(3), 170–180. https://doi.org/10.1038/nrn3666
- Korevaar, J. C., van Munster, B. C., & de Rooij, S. E. (2005). Risk factors for delirium in acutely admitted elderly patients: A prospective cohort study. BMC Geriatrics, 5(1), 6. https://doi.org/10.1186/1471-2318-5-6
- Krogseth, M., Wyller, T. B., Engedal, K., & Juliebø, V. (2014). Delirium is a risk factor for institutionalization and functional decline in older hip fracture patients. Journal of Psychosomatic Research, 76(1), 68–74. https://doi.org/10.1016/j.jpsychores.2013.10.006
- Lee, Y., Lee, J., Kim, J., & Jung, Y. (2021). Non-pharmacological nursing interventions for prevention and treatment of delirium in hospitalized adult patients: Systematic review of randomized controlled trials. International Journal of Environmental Research and Public Health, 18(16). https://doi.org/10.3390/ijerph18168853
- Li, X., Li, C., Hu, N., & Wang, T. (2020). Music interventions for disorders of consciousness: A systematic review and meta-analysis. Journal of Neuroscience Nursing, 52(4), 146–151. https://doi.org/10.1097/jnn.0000000000000511
- Maldonado, J. R. (2017). Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Critical Care Clinics, 33(3), 461–519. https://doi.org/10.1016/j.ccc.2017.03.013
- Marcantonio, E. R. (2017). Delirium in hospitalized older adults. The New England Journal of Medicine, 377(15), 1456–1466. https://doi.org/10.1056/NEJMcp1605501
- McDermott, O., Orrell, M., & Ridder, H. M. (2014). The importance of music for people with dementia: The perspectives of people with dementia, family carers, staff and music therapists. Aging & Mental Health, 18(6), 706–716. https://doi.org/10.1080/13607863.2013.875124
- Meagher, D., Adamis, D., Leonard, M., Trzepacz, P., Grover, S., Jabbar, F., Meehan, K., O'Connor, M., Cronin, C., Reynolds, P., Fitzgerald, J., O'Regan, N., Timmons, S., Slor, C., de Jonghe, J., de Jonghe, A., van Munster, B. C., de Rooij, S. E., & Maclullich, A. (2014). Development of an abbreviated version of the Delirium Motor Subtyping Scale (DMSS-4). International Psychogeriatrics, 26(4), 693–702. https://doi.org/10.1017/S1041610213002585
- Moreno-Morales, C., Calero, R., Moreno-Morales, P., & Pintado, C. (2020). Music therapy in the treatment of dementia: A systematic review and meta-analysis. Frontiers in Medicine, 7, 160. https://doi.org/10.3389/fmed.2020.00160
- Neerland, B. E., Hov, K. R., Bruun Wyller, V., Qvigstad, E., Skovlund, E., MacLullich, A. M., & Bruun Wyller, T. (2015). The protocol of the Oslo Study of Clonidine in Elderly Patients with Delirium; LUCID: A randomised placebo-controlled trial. BMC Geriatrics, 15(1), 7. https://doi.org/10.1186/s12877-015-0006-3
- Neerland, B. E., Krogseth, M., & Wyller, T. B. (2018). Hvordan beskrive delirium? How to describe delirium? Tidsskrift for den Norske Laegeforening, 138(5). https://doi.org/10.4045/tidsskr.18.0087
- Ocagli, H., Bottigliengo, D., Lorenzoni, G., Azzolina, D., Acar, A. S., Sorgato, S., Stivanello, L., Degan, M., & Gregori, D. A. (2021). A machine learning approach for investigating delirium as a multifactorial syndrome. International Journal of Environmental Research and Public Health, 18(13), 7105. https://doi.org/10.3390/ijerph18137105
- O’Hanlon, S., O’Regan, N., Maclullich, A. M., Cullen, W., Dunne, C., Exton, C., & Meagher, D. (2014). Improving delirium care through early intervention: From bench to bedside to boardroom. Journal of Neurology, Neurosurgery, and Psychiatry, 85(2), 207–213. https://doi.org/10.1136/jnnp-2012-304334
- Oh, E. S., Fong, T. G., Hshieh, T. T., & Inouye, S. K. (2017). Delirium in older persons: Advances in diagnosis and treatment. JAMA, 318(12), 1161–1174. https://doi.org/10.1001/jama.2017.12067
- O’Kelly, J., James, L., Palaniappan, R., Taborin, J., Fachner, J., & Magee, W. L. (2013). Neurophysiological and behavioral responses to music therapy in vegetative and minimally conscious states. Frontiers in Human Neuroscience, 7, 7. https://www.frontiersin.org/article/10.3389/fnhum.2013.00884
- Park, S., Williams, R. A., & Lee, D. (2016). Effect of preferred music on agitation after traumatic brain injury. Western Journal of Nursing Research, 38(4), 394–410. https://doi.org/10.1177/0193945915593180
- Quispel-Aggenbach, D. W. P., Holtman, G. A., Zwartjes, H., Zuidema, S. U., & Luijendijk, H. J. (2018). Attention, arousal and other rapid bedside screening instruments for delirium in older patients: A systematic review of test accuracy studies. Age and Ageing, 47(5), 644–653. https://doi.org/10.1093/ageing/afy058
- Richardson, S. J., Davis, D. H. J., Bellelli, G., Hasemann, W., Meagher, D., Kreisel, S. H., MacLullich, A. M. J., Cerejeira, J., & Morandi, A. (2017). Detecting delirium superimposed on dementia: Diagnostic accuracy of a simple combined arousal and attention testing procedure. International Psychogeriatrics, 29(10), 1585–1593. https://doi.org/10.1017/S1041610217000916
- Richardson, S. J., Davis, D. H. J., Stephan, B., Robinson, L., Brayne, C., Barnes, L., Parker, S., & Allan, L. M. (2017). Protocol for the Delirium and Cognitive Impact in Dementia (DECIDE) study: A nested prospective longitudinal cohort study. BMC Geriatrics, 17(1), 98. https://doi.org/10.1186/s12877-017-0479-3
- Ridder, H. M., Stige, B., Qvale, L. G., & Gold, C. (2013). Individual music therapy for agitation in dementia: An exploratory Randomized controlled trial. Aging & Mental Health, 17(6), 667–678. https://doi.org/10.1080/13607863.2013.790926
- Rockwood, K., Song, X., MacKnight, C., Bergman, H., Hogan, D. B., McDowell, I., & Mitnitski, A. (2005). A global clinical measure of fitness and frailty in elderly people. CMAJ: Canadian Medical Association Journal, 173(5), 489–495. https://doi.org/10.1503/cmaj.050051
- Royal College of Physicians, (2012). National Early Warning Score (NEWS): Standardising the assessment of acute-illness severity in the NHS. Report of working party. Royal College of Physicians.
- Schaefer, H.-E. (2017). Music-evoked emotions-current studies. Frontiers in Neuroscience, 11, 600. https://doi.org/10.3389/fnins.2017.00600
- Shao, S. C., Lai, C. C., Chen, Y. H., Chen, Y. C., Hung, M. J., & Liao, S. C. (2021). Prevalence, incidence and mortality of delirium in patients with COVID-19: A systematic review and meta-analysis. Age and Ageing, 50(5), 1445–1453. https://doi.org/10.1093/ageing/afab103
- Shenkin, S. D., Fox, C., Godfrey, M., Siddiqi, N., Goodacre, S., Young, J.Anand, A., Gray, A., Hanley, J., MacRaild, A., Steven, J., Black, P. J., Tieges, Z., Boyd, J., Stephen, J., Weir, C. J., & MacLullich, A. M. J. (2019). Delirium detection in older acute medical inpatients: A multicentre prospective comparative diagnostic test accuracy study of the 4AT and the confusion assessment method. BMC Medicine, 17(1), 138. https://doi.org/10.1186/s12916-019-1367-9
- Siddiqi, N., House, A. O., & Holmes, J. D. (2006). Occurrence and outcome of delirium in medical in-patients: A systematic literature review. Age and Ageing, 35(4), 350–364. https://doi.org/10.1093/ageing/afl005
- Sihvonen, A. J., Särkämö, T., Leo, V., Tervaniemi, M., Altenmüller, E., & Soinila, S. (2017). Music-based interventions in neurological rehabilitation. Lancet Neurology, 16(8), 648–660. https://doi.org/10.1016/s1474-4422(17)30168-0
- Stollings, J. L., Kotfis, K., Chanques, G., Pun, B. T., Pandharipande, P. P., & Ely, E. W. (2021). Delirium in critical illness: Clinical manifestations, outcomes, and management. Intensive Care Medicine, 47(10), 1089–1103. https://doi.org/10.1007/s00134-021-06503-1
- Szpunar, K. K., Schellenberg, E. G., & Pliner, P. (2004). Liking and memory for musical stimuli as a function of exposure. Journal of Experimental Psychology Learning, Memory, and Cognition, 30(2), 370. https://doi.org/10.1037/0278-7393.30.2.370
- Thaut, M., & Hoemberg, V. (2014). Handbook of neurologic music therapy. Oxford University Press.
- Tieges, Z., Quinn, T., MacKenzie, L., Davis, D., Muniz-Terrera, G., MacLullich, A. M. J., & Shenkin, S. D. (2021). Association between components of the delirium syndrome and outcomes in hospitalised adults: A systematic review and meta-analysis. BMC Geriatrics, 21(1), 162. https://doi.org/10.1186/s12877-021-02095-z
- Tsui, A., Searle, S. D., Bowden, H., Hoffmann, K., Hornby, J., Goslett, A., Weston-Clarke, M., Howes, L. H., Street, R., Perera, R., Taee, K., Kustermann, C., Chitalu, P., Razavi, B., Magni, F., Das, D., Kim, S., Chaturvedi, N., Sampson, E. L. … Davis, D. (2022). The effect of baseline cognition and delirium on long-term cognitive impairment and mortality: A prospective population-based study. The Lancet Healthy Longevity, 3(4), e232–241. https://doi.org/10.1016/S2666-7568(22)00013-7
- Tyson, B., Shahein, A., Erdodi, L., Tyson, L., Tyson, R., Ghomi, R., & Agarwal, P. (2022). Delirium as a presenting symptom of COVID-19. Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology, 35(2), 123–129. https://doi.org/10.1097/WNN.0000000000000305
- van der Steen, J. T., Smaling, H. J., van der Wouden, J. C., Bruinsma, M. S., Scholten, R. J., & Vink, A. C. (2018). Music-based therapeutic interventions for people with dementia. Cochrane Database of Systematic Reviews, 7(7), CD003477. https://doi.org/10.1002/14651858.CD003477.pub4
- Vink, A. C., Zuidersma, M., Boersma, F., de Jonge, P., Zuidema, S. U., & Slaets, J. P. (2014). Effect of music therapy versus recreational activities on neuropsychiatric symptoms in elderly adults with dementia: An exploratory randomized controlled trial. Journal of the American Geriatrics Society, 62(2), 392–393. https://doi.org/10.1111/jgs.12682
- Vuilleumier, P., & Trost, W. (2015). Music and emotions: From enchantment to entrainment. Annals of the New York Academy of Sciences, 1337(1), 212–222. https://doi.org/10.1111/nyas.12676
- Wilson, J. E., Mart, M. F., Cunningham, C., Shehabi, Y., Girard, T. D., MacLullich, A. M. J. Slooter, A. J. C., & Ely, E. W. (2020). Delirium. Nature Reviews Disease Primers, 6(1), 90. https://doi.org/10.1038/s41572-020-00223-4
- Witlox, J., Eurelings, L. S., de Jonghe, J. F., Kalisvaart, K. J., Eikelenboom, P., & van Gool, W. A. (2010). Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: A meta-analysis. JAMA, 304(4), 443–451. https://doi.org/10.1001/jama.2010.1013