ABSTRACT
The anti-tumor capacity of natural killer (NK) cells heavily relies on their ability to migrate towards their target cells. This process is based on dynamic actinrearrangement, so-called actin treadmilling, andis tightly regulated by proteins such as cofilin-1. The aim of the present study was to identify the role of cofilin-1 (CFL-1) in the migratory behavior of NK cells and to investigate a possible impact of an obesity-associated micromilieu on these cells, as it is known that obesity correlates with various impaired NK cell functions. CFL-1 was knocked-down via transfection of NK-92 cells with respective siRNAs. Obesity associated micromilieu was mimicked by incubation of NK-92 cells with adipocyte-conditioned medium from human preadipocyte SGBS cells or leptin. Effects on CFL-1 levels, the degree of phosphorylation to the inactive pCFL-1 as well as NK-92 cell motility were analyzed. Surprisingly, siRNA-mediated CFL-1 knockdown led to a significant increase of migration, as determined by enhanced velocity and accumulated distance of migration. No effect on CFL-1 nor pCFL-1 expression levels, proportion of phosphorylation and cell migratory behavior could be demonstrated under the influence of an obesity-associated microenvironment. In conclusion, the results indicate a significant effect of a CFL-1 knockdown on NK cell motility.
Introduction
Natural killer (NK) cells are granular immune cells and a subgroup of innate lymphoid cells with features of adaptive immunity (Beaulieu, Citation2018). NK cells exhibit two major effector functions. On the one hand, they are capable of recognizing and lysing virus-infected or malignant transformed cells without the need for prior sensitization. On the other hand, NK cells may link the innate to the adaptive immune system by secreting cytokines, e.g., interferon-γ, which are likely to activate adaptive immune cells (Vivier et al., Citation2008). Multiple clinical trials could prove the outstanding anti-cancer capacities of NKcells (Miller et al., Citation2005; Rosenberg et al., Citation1987; Ruggeri et al., Citation2002). Conversely, an 11-year follow-up study demonstrated that low NK cell activity leads to an increased cancer incidence (Imai et al., Citation2000).
In terms of their localization NK cells can be divided into a group of cells that circulate in the peripheral blood and a group of cells that reside in various tissues. Of course, this is only a very simplified subdivision, as it has been shown that there are several thousand different subtypes. What the vast majority of them have in common, however, is that under certain circumstances they can leave their habitual position and migrate through different tissues to a site of action (Castriconi et al., Citation2018).
Due to this natural NK cell behavior, there is particular clinical interest in using NK cells therapeutically. Chimeric antigen receptor (CAR) NK cells are currently the subject of intensive research in preclinical and clinical studies. It is hoped that CAR NK cells can be used effectively against solid tumors, an area in which CAR T cells show limited efficacy.However, for NK cell-based therapies to become established in cancer treatment, their efficiency must be further optimized, which in turn requires a broad understanding of the molecular basis of the various NK cell functions (Wang et al., Citation2024).
Cell movement is a fundamental process taking part in many physiological and pathophysiological contexts. While lymphocyte-mediated immune functions heavily rely on cellular migration, as mentioned above, the relocation of cells is also the basis of metastasis and invasion of malignancies (DesMarais et al., Citation2005; Mgrditchian et al., Citation2021; Mondal et al., Citation2021).
The actin cytoskeleton plays an important role in cell migration: by sensing a chemotactic stimulus the migratory cells become polarized and form a membrane protrusion, called lamellipodium, which is based on the organization of a branched F-actin network beneath the plasma membrane. The lamellipodium is formed at the leading edge during migration and points in the direction of cell movement. To keep this process active, actin monomers must be continuously recycled from the rear end of the cell to its leading edge where they polymerize again (Mitchison & Cramer, Citation1996). At this point, cofilin-1(CFL-1), a ubiquitous 19 kDa protein that belongs to the family of actin-binding proteins plays a central role. CFL-1 is capable of severing actin filaments close to the plasma membrane, thereby initiating actin repolymerization (McGough et al., Citation1997). Even more important, it creates the basis for actin treadmilling by accelerating actin monomer detachment (DesMarais et al., Citation2005; Svitkina & Borisy, Citation1999). Consequently, CFL-1 enables the high turn-over rate of actin dynamics that is essential for cell movement (Bamburg, Citation1999). The activity of CFL-1 is regulated by its upstream effectors: for example, CaMKII (Ca/calmodulin-dependent protein kinase II)-regulated LIMK1/2 (LIM domain kinase 1/2) phosphorylates CFL-1 on its serine 3 residue for inactivation (Ohashi, Citation2015; Shi et al., Citation2018).
Interestingly, previous in vitro and in vivo studies showed that adipokines affect NK cell functionality leading to impaired NK cell activity in diet-induced-obese (DIO) rats as well as in obese humans (Bahr et al., Citation2017; Huebner et al., Citation2013; Jahn et al., Citation2015; Lautenbach et al., Citation2009; Nave et al., Citation2008; O’Shea et al., Citation2010; Viel et al., Citation2017; Wrann et al., Citation2012). Obesity, defined as a body mass index (BMI) ≥ 30 kg/m2, is one of the fastest spreading global health threats nowadays. Since 1975 its worldwide prevalence has almost tripled, leading to 13% of adults being obese in 2016 (Organization, W.H, Citation2023). Beside common secondary diseases, like type 2 diabetes or cardiovascular diseases, excessive body fat leads to an enhanced susceptibility to infections and certain cancer types (e.g., colon- and esophagus-carcinoma, postmenopausal breast cancer) (Emerging Risk Factors et al., Citation2011; Mitsuhashi et al., Citation2017; Renehan et al., Citation2008). Obesity can be called a provoked state of immunodeficiency (Dixit, Citation2008). Especially white adipose tissue is a highly active endocrine organ that secretes several adipokines. These, mainly polypeptide molecules, act on both local and systemic levels, therefore enabling adipose tissue to interfere with biological procedures throughout the body, inter alia immunological processes (Kershaw & Flier, Citation2004). Whereas tumor necrosis factor α (TNF-α), interleukin (IL-) 6, leptin, visfatin and resistin act as pro-inflammatory cytokines (Yudkin et al., Citation1999), there are also adipokines, such as adiponectin, that mediate anti-inflammatory processes and protect against obesity associated pathologies (Kim et al., Citation2007; Tian et al., Citation2012; Yamauchi et al., Citation2001). Adiponectin plasma levels, however, are inversely correlated with the BMI (Arita et al., Citation1999). In contrast, plasma levels of leptin correlate with dimensions of energy available in the body, mostly stored in fat tissue. Obese individuals usually display elevated plasma levels of leptin, albeit physiological effects of leptin such as food intake regulation are significantly reduced (Shah et al., Citation2012). Leptin- (ob/ob) and leptin-receptor- (db/db) deficient mice exhibit not only severe obesity and insulin resistance, but also various defects in cell-mediated and humoral immunity (Busso et al., Citation2002; Fernandez-Riejos et al., Citation2010; Howard et al., Citation1999; Mandel & Mahmoud, Citation1978). Furthermore, a dose- and time-dependent influence of leptin on the filopodia length of NK cells as well as on the colocalization of actin and CFL-1 have been demonstrated which might indicate an alteration in the migratory behavior of these cells (Oswald et al., Citation2018). Migration can be regarded as one of the main capabilities that allows NK cells to intervene in the very early phase of tumor development and metastasis (Langers et al., Citation2012). The objective of this investigation was to elucidate the molecular mechanisms of NK cell motility, a crucial prerequisite for their migratory capacity, while simultaneously identifying potential modulatory factors.
Materials and methods
Cell culture
The human NK cell line NK-92, that originates from a patient with a rare NK-cell-lymphoma, was kindly provided by Prof. Dr. Roland Jacobs (Hannover Medical School, Hannover, Germany). NK-92 cells were cultured in 75 cm2 cell culture flasks containing RPMI 1640 growth medium supplemented with 10% fetal calf serum (FCS, both from Biochrom AG, Berlin, Germany), 100 µg/ml streptomycin, 100 U/ml penicillin (both from Sigma-Aldrich), 1 mM sodium pyruvate (SoPy, Biochrom AG), hereafter called RPMI, and 200 U/ml human interleukin-2 (IL-2, Novartis AG, Basel, Switzerland). The bottles were kept at 37°C in a humidified atmosphere with 5% CO2. NK cells were passaged every 48 to 72 hours. Trypan blue staining was used for routine evaluation of cell viability.
SGBS adipocyte-conditioned medium
Human Simpson-Golabi-Behmel-syndrome (SGBS) preadipocytes originate from the subcutaneous adipose tissue of an infant patient exhibiting the rare genetic condition SGBS and was kindly provided by Prof. Dr. Martin Wabitsch (Ulm University Medical Center, Ulm, Germany). This cell strain is widely used as in vitro model for human adipocyte differentiation because it displays a protein secretion pattern that is comparable to differentiating primary human preadipocytes (Wabitsch et al., Citation2001). Cells were grown to near confluence in DMEM/F12 medium (Biochrom AG), supplemented with 150 µM pantothenate and 300 µM biotin (Sigma Aldrich), 100 µg/ml streptomycin, 100 U/ml penicillin (0F-Medium) and 10% FCS (0F + 10% FCS medium).After trypsinization, the cells were washed in phosphate-buffered saline (PBS, Biochrom AG) and seeded into Quick-Diff medium (0F medium supplemented with 0.01 mg/ml transferrin, 20 nM insulin, 100 nM cortisol, 0.2 nM triiodothyronine (T3), 25 nM dexamethasone, 250 µM 3-isobutyl-1-methylxanthine (IBMX) (all from Sigma Aldrich) and 2 µM rosiglitazone (Cayman Chemicals, Michigan, USA)) to be incubated for 4 days in a humidified atmosphere (37°C, 5% CO2). Quick-Diff medium was then replaced by 3FC medium (0F + 10% FCS medium containing 0.01 mg/ml transferrin, 20 nM insulin, 100 nM cortisol and 0.2 nM T3) and cells were kept in a humidified atmosphere with 5% CO2 for another 10 days (Fischer-Posovszky et al., Citation2008). Supernatants were harvested on day 10 of adipogenesis. To obtain SGBS adipocyte-conditioned medium free of cellular debris, samples were centrifuged (1,500 rpm, 10 min, room temperature). Supernatants were then collected and pooled into a single falcon tube. The combined composition of the SGBS adipocyte-conditioned medium was analyzed using a multiplex immunoassay (HADCYMAG-61K, Merck Chemicals GmbH, Darmstadt, Germany) according to the manufacturers’ instructions. Adipocytokine levels were determined using the LiquiChipluminex 200 system (Qiagen, Hilden, Germany; ). The sample was then mixed well and aliquoted into 250 µl portions. SGBS adipocyte-conditioned medium was stored at −20°C before use.
Table 1. Concentrations of different adipokines measured in SGBS supernatants (IL, interleukin; TNF, tumor necrosis factor) used for stimulation, harvested on day 10 of adipogenesis. Analyses were performed using Thermo Fisher Scientific’s LPrecellys® technology.
Transfection
Two different CFL-1 siRNA constructs (#1 5’GGGAUCAAGCAUGAAUUGCtt3,’ ID #146652; #2 5’CCAGUAAGGGACCUUCGAUtt3,’ ID #146653) and one siRNA that is guaranteed to not target any human gene product (Ambion Silencer Negative Control, catalog #AM4611) were purchased at Ambion, Inc. (Austin, Texas, USA). As widely described, gene transfer into NK-92 cells is inefficient when conventional transfection protocols are applied. However, to perform siRNA-mediated CFL-1 knockdown we successfully used LipocalyxViromer®BLUE transfection reagent (Lipocalyx GmbH, Halle (Saale), Germany). In each case, 1 × 106 NK-92 cells per well were seeded into a 6-well plate containing 2 ml of RPMI and 200 U/ml IL-2. All required transfection reagents were prepared according to the manufacturer instructions. The resulting mixture was then carefully added to the particular well and incubated for 24 h at 37°C and 5% CO2. Transfected cells were used in the other assays as described.
Cell stimulation
NK-92 cells were stimulated with SGBS adipocyte-conditioned medium and harvested on day 10 of adipogenic differentiation. For short-term stimulation, 2 × 106 cells were seeded into 6-well plates, containing 5 ml of RPMI substituted with 250 µl of SGBS adipocyte-conditioned medium in various concentrations (pure; diluted 1:10, 1:100 and 1:1000 in RPMI) and 200 U/ml IL-2, whereas two wells treated with either 5 ml of buffer RPMI or 5 ml of 3FC medium, each supplemented with 200 U/ml IL-2, served as controls. Cells were cultivated for 0.5 h in a humidified atmosphere with 5% CO2. For long-term stimulation, 200,000 (24 h) or 100,000 (48 h, 72 h) NK-92 cells were seeded into 6-well plates containing 1 ml of RPMI supplemented with 250 µl of pure SGBS ACM and 200 U/ml IL-2. Controls were again incubated with 5 ml of RPMI or 3FC medium only supplemented with 200 U/ml IL-2. Cells were cultivated for 24, 48 or 72 h in a humidified atmosphere with 5% CO2. The supplemented medium was refreshed every 24 h to avoid medium exhaustion and to make sure that concentrations of SGBS adipocyte-conditioned medium components remained constant throughout the stimulation.Regarding leptin-stimulation, leptin (R&D Systems, Minneapolis, Minnesota, USA) was diluted in Tris-HCl buffer (stock concentration 10 µg/ml). 2 × 106 NK-92 cells were seeded into 6-well-plates containing 5 ml of RPMI and 200 U/ml IL-2 substituted with 50 µl leptin to achieve a total leptin concentration of 100 ng/ml. Cells treated with 50 µl sterile Tris-HCl buffer served as controls. The plates were kept for 0.5 h in a humidified atmosphere with 5% CO2.
Western Blot
Anti-CFL-1 rabbit monoclonal antibody D3F9 (mAb), anti-pCFL-1 rabbit mAb77G2 and anti-GAPDH rabbit mAb14C10 were all purchased from Cell Signaling Technology (Danvers, Massachusetts, USA)anti-beta-actin mouse mAb4C2 was purchased from Sigma-Aldrich (Darmstadt, Germany). After incubation, stimulated or transfected NK-92 cells were washed in ice-cold PBS, then resuspended in lysis buffer (10 ml 10× PBS, 1 ml Nonidet, 100 µl sodium dodecyl sulfate, 0.5 g deoxycholic acid sodium salt, 100 ml sterile H2O, 143 µl protease- and 100 µl phosphatase-inhibitors (Roche Deutschland Holding GmbH, Mannheim, Germany) and homogenized using VWR International’s Precellys® (VWR International, Pennsylvania, USA). After 30 min of incubation on ice, samples were centrifuged (13,000 rpm, 15 min, 4°C) and protein concentration of the harvested supernatants was analyzed using Micro BCA™ Protein Assay (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Portions of 15 µg of protein were mixed 6:1 with 5× loading buffer and boiled for 10 min at 70°C before being loaded into the pockets of a 4–20% tris-glycine-gradient-gel (NovexWedgeWell, Thermo Fisher Scientific) to be divided by size in a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) running at 100 V to 120 V for approximately 3 h. Proteins were then transferred onto a 0.2 µm nitrocellulose membrane (AmershamProtran, GE Healthcare, Little Chalfont, UK) and incubated with primary antibodies (overnight, 4°C). Membranes were washed with Tris-buffered saline (TBS, pH 7.4) and tris-buffered saline with Tween 20 (TBST; 0.1% Tween 20, Carl Roth GmbH + Co. KG, Karlsruhe, Germany) and incubated with a 1:12,000 dilution of HRP-conjugated secondary antibodies (Santa Cruz Biotechnology Inc., Texas, USA) at room temperature for 1 h. Membranes were incubated for 5 min at room temperature with enhanced chemiluminescence reagent (AmershamProtran, GE Healthcare). Protein bands were visualized using ChemiDoc™ Touch Imaging System (Bio-Rad Laboratories Inc., California, USA).
CytoSMARTTM
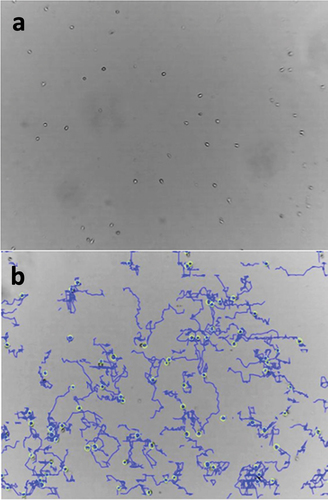
To evaluate migratory behavior of transfected and/or stimulated NK-92 cells the Lonza CytoSMARTTM live cell imaging technology (Lonza Group, Basel, Switzerland) was applied. NK-92 cells normally grow in suspension. However, cells need to become adherent to a surface before one can distinguish floating from migrating cells. To induce adherence in NK-92 cells, one well of a 12-well plate was coated with fibronectin (AMS Biotechnology Europe Ltd, Abingdon, United Kingdom) diluted 1:50 in PBS. The plate was coated in a humidified atmosphere (37°C, 5% CO2) for 1 h. Next, the coated well was carefully washed with PBS and seeded with 10,000 NK-92 cells of choice. The plate was then centrifuged (600 rpm, room temperature, 1 min) and kept in a humidified atmosphere (37°C, 5% CO2) for 3 h. Finally, CytoSMARTTM software was started to record the cells’ migratory activity (see video in supplemental material).
Regarding short-term stimulation 10,000 NK-92 cells were resuspended in RPMI and seeded into a fibronectin-coated well. The plate was centrifuged and kept in a humidified atmosphere for 3 h as described above. Then, the sample was carefully supplemented with 250 µl of either pure SGBS adipocyte-conditioned medium or a 1:100 dilution with RPMI. The video recording was started immediately after supplementation of SGBS . Regarding long-term stimulation and CFL-1 knockdown 10,000 NK-92 cells, either being incubated for 24 h in SGBS adipocyte-conditioned medium supplemented RPMI or being transfected using CFL-1 siRNA construct and LipocalyxViromer®BLUE transfection reagent were resuspended in 1 ml RPMI and seeded into the well. The plate was then centrifuged and kept in a humidified atmosphere for 3 h. After this time, recording was started without any other treatment. NK-92 cells treated with RPMI only served as a control in all cases (). Recorded data were analyzed using Gradientech’s Tracking ToolTM PRO software (Gradientech AB, Uppsala, Sweden)to evaluate velocity, overall direction of individual movement and distance covered by every tracked cell.
Statistical analysis
Statistical analyses were performed using one-way ANOVA with Bonferroni-test for post-hoc analysis and Student’s t-test. Differences were considered significant if p < .05 (* = p < .05; ** = p < .01). The software used was GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, USA).Data is presented as mean with indication of standard error of mean. The number of replicates is indicated under the figures.
Results
Transfection of a CFL-1 siRNA construct into NK-92 cells led to significantly lower protein expression levels of CFL-1, but not pCFL-1
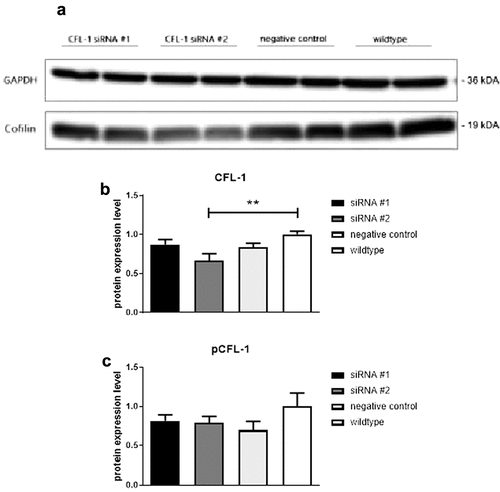
Transfection with siRNA construct #2 using Viromer®BLUE led to a statistically significant reduction of CFL-1 expression levels in transfected NK-92 cells compared to RPMI-incubated control NK-92 cells as is evident from Western Blotting analyses, whereas transfection with construct #1 did not significantly alter CFL-1 expression levels (). Construct #2 was therefore chosen to be used in further experiments. Levels of inactive pCFL-1 were not affected by any of the used siRNA constructs, implicating that only the pool of active CFL-1 was significantly reduced in NK-92 cells transfected with siRNA construct #2. Cells transfected with a silencer negative control siRNA (negative control) and untransfected cells (wildtype) were used as controls.
Figure 2. a) Representative Western Blot analysis of GAPDH and CFL-1 after the transfection of CFL-1 siRNA constructs into NK-92 cells using LipocalyxViromer®BLUE. Protein levels of CFL-1 (b), pCFL-1 (c) after Viromer®BLUE-mediated CFL-1 knockdown. NK-92 cells were incubated with Viromer®BLUE reagents and either CFL-1 siRNA construct #1, CFL-1 siRNA construct #2 or silencer negative control siRNA for 24 hours (negative control). NK-92 cells incubated with RPMI only were considered as controls. All data represent means and standard error of means (±SEM). Data was pooled from four independent replicates.
Migratory pattern of NK-92 changes after introducing a CFL-1 siRNA construct
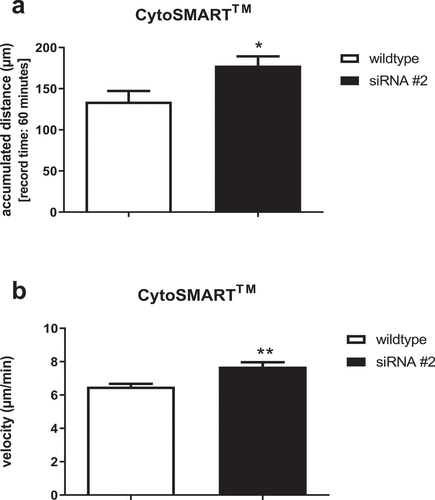
siRNA-mediated CFL-1 knockdown had significant effects on the migratory behavior of NK-92 cells. Interestingly, both velocity and covered accumulated distance increased with impaired expression of CFL-1 ().
Figure 3. Accumulated distance in µm (a) and mean velocity in µm/min (b) shown by transfected NK-92 cells that exhibited significantly reduced CFL-1 expression levels. Migration activity was recorded for 60 minutes (1 picture per minute) via CytoSmart analyses in control cells and CFL-1 siRNA #2 transfected cells. All data represent means ±SEM. Data was pooled from four independent replicates.
Short-term stimulation of NK-92 cells with SGBS adipocyte-conditioned medium does not alter the expression levels of CFL-1 and pCFL-1
SGBS adipocyte-conditioned medium harvested on day 10 of adipocyte differentiation was used to mimic an in vitro obese microenvironment ().
To differentiate, SGBS cells need special growth mediums supplemented with several hormonal additives (see method section for details), e.g., cortisol which is widely known for its immunosuppressive effects. To make sure that outcomes were not accidentally caused by supplements, an extra medium control group (3FC-medium with supplements) was implemented. As no statistically significant difference between RPMI and 3FC medium (data not shown) controls was detected, solely the RPMI control is shown (). To investigate whether an obesity associated microenvironment would have any effect on CFL-1 expression levels in NK cells, NK-92 cells were cultured for 0.5 h (short-term) with RPMI supplemented with serial dilutions of 250 µl of SGBS adipocyte-conditioned medium, starting with pure adipocyte-conditioned medium at 250 µl.
Figure 4. Protein levels of beta-actin (a), CFL-1 (b) and inactive pCFL-1 (c) as well as ratios of pCFL-1/CFL-1 (d) after short-term SGBS adipocyte-conditioned medium stimulation analyzed by Western Blot. NK-92 cells were incubated for 0.5 hours with serial dilutions of SGBS adipocyte-conditioned medium (harvested on day 10 of adipogenesis, pure or diluted 1:10, 1:100 and 1:1000 in RPMI, each with 250 µl). NK-92 cells incubated with RPMI only served as controls. All data represent means ± SEM. Data was pooled from three independent replicates.
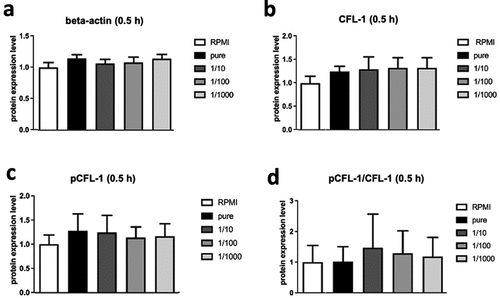
No significant changes regarding the protein levels of CFL-1 or its inactive phosphorylated form pCFL-1 in NK-92 cells was measured in Western Blot analyses. Additionally, expression levels of beta-actin, a main component of the cytoskeleton, remained also unchanged ().
NK-92 cells do not show a modified migration pattern after short-term or long-term stimulation with SGBS ACM
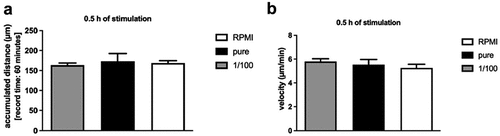
In order to study whether an obese microenvironment would have an influence on the migratory behavior of NK cells, NK-92 cells were stimulated with pure or 1:100 diluted SGBS ACM for 0.5 hours and simultaneously, cell movement was recorded for 60 minutes using the CytoSMARTTM live-cell-imaging system. The analysis revealed no significant alterations in the migratory behavior regarding both accumulated distance and velocity of NK-92 cells ().In accordance with the short-term stimulation experiments, NK-92 cells that were stimulated with RPMI supplemented with SGBS ACM for 24 hours did not show altered migration patterns in Tracking Tool Pro TM analyses (see supplemental material).
Figure 5. Accumulated distance in µm (a) and mean velocity in µm/min (b) of NK-92 cells after short-term stimulation with SGBS ACM adipocyte-conditioned medium. Adherent NK-92 cells were incubated with RPMI supplemented with 250 µl of either pure or 1:100 diluted in RPMI SGBS adipocyte-conditioned medium (harvested on day 10 of adipogenesis). Migration activity was recorded for 60 minutes (1 frame per minute) via CytoSmart analyses. NK-92 cells treated with RPMI only served as controls. All data represent means ± SEM. Data were pooled from three independent replicates.
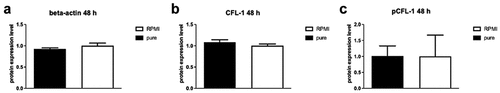
Short-term and long-term stimulated NK-92 cells do not show altered expression levels of CFL-1 and pCFL-1
NK-92 cells were stimulated with 250 µl of pure SGBS ACM for 0.5, 24, 48 and 72 hours to mimic obese conditions. Subsequently, protein expression levels of CFL-1 and pCFL-1 were determined performing Western Blot. Neither short term, nor long-term incubation did lead to significant changes regarding the expression levels of CFL-1 or its inactive phosphorylated form pCFL-1. Representative results of the 48-hour stimulation are depicted in . Short-time incubation of 0.5 hours was also performed using high doses of leptin (100 ng/ml), without any effect on the expression levels of CFL-1 or pCFL-1 (see supplemental material).
Figure 6. Protein levels of ß-actin (A), CFL-1 (B) and inactive pCFL-1(C) after long-term SGBS adipocyte-conditioned medium stimulation. NK-92 cells were incubated for 48 hours with RPMI supplemented with 250 µl pure SGBS adipocyte-conditioned medium (harvested on day 10 of adipogenesis). NK-92 cells incubated with RPMI only served as controls. All data represent means ± SEM. Data was pooled from three independent replicates.
Discussion
CFL-1 is a known modulator of the actin polymerization-depolymerization cycle, which in turn is the molecular basis for NK cell motility. The CFL-1 knockdown by siRNA performed in order to investigate the role of CFL-1 in NK cell motility, however, led to unexpected results.
Transfected cells, exhibited an increase in both velocity and accumulated distance, which is rather paradoxical because one would expect a slowdown in cell movement, not an acceleration. Introducing a CFL-1 siRNA into Jurkat T-cells led to a statistically significant reduction in chemotaxis as well as chemokinesis. However, Nishitaet al. investigated directed cell migration, while the herein used assay measured general motility (Nishita et al., Citation2005). It might therefore be interesting to perform other specific migration assays that would allow evaluating whether chemotaxis-induced directed movement of NK-92 cells is altered by CFL-1 knockdown. Nevertheless, one could assume that a decrease in depolymerization of actin (mediated by reducing levels of active CFL-1) would consequentially lead to a depletion of available G-actin-monomers in the cell while filamentous F-actin would accumulate. Hence, the affected cell would eventually become “paralyzed,” thereby being unable to perform any further movement. On the other hand, the CFL-1 knockdown achieved in NK-92 cells might simply not be strong enough to mediate the expected effects.Genetic manipulation of NK cells is challenging and often associated with low efficiency or significant loss of cell viability (Rezvani et al., Citation2017). Techniques other than the herein used Viromer®-based transfection, like viral transduction or electroporation are frequently used for the generation of CAR-NK cells. It is quite possible that the aforementioned methods could lead to a more pronounced CFL-1 knockdown and thus divergent effects with respect to NK cell motility (Glienke et al., Citation2015). The hypothesis of inadequate CFL-1-knockdown is supported by the observation that pCFL-1 protein levels did not significantly decrease after CFL-1 siRNA transfection, but total CFL-1 levels did, indicating a net loss of active CFL-1.Huet et al. have shown that in fibroblasts low levels of G-actin lead to CFL-1 dephosphorylation via phosphatase actin regulator 4 (Phactr4) and protein phosphatase 1, thereby preventing complete depletion of G-actin (Huet et al., Citation2013). It is very likely that a similar mechanism exists in NK cells, although experimental evidence for this is pending.If the siRNA-mediated loss of active CFL-1 were to happen to an extent relevant to the cell, one would expect a significant drop in pCFL-1 levels due to compensatory dephosphorylation.
However, the paradoxical increase in NK cell motility we observed after knockdown of CFL-1 can hardly be explained by the fact that there was too little decrease in CFL-1 levels. Andrianantoandro et al. challenged the idea of a linear correlation between cofilin concentration and the rate of G-actin-monomer cleavage. They could show that lower concentrations of cofilin increase the separation of actin monomers from F-actin while higher concentrations induce actin nucleation (Andrianantoandro & Pollard, Citation2006). Given the nonlinear dose-response relationship, it can be suggested that the siRNA-induced reduction in CFL-1 concentration leads to a more efficient interaction between CFL-1 and actin and thus to a more rapid actin treadmilling.
Alternatively, it has to be considered that other proteins mediating disassembly of F-actin, such as destrin, coronin 1A or any other members of the actin-depolymerizing factors (ADF)/cofilin family are possibly compensating for the lack of active CFL-1 (Bamburg, Citation1999). A previous study of Mace and Orange for instance could show that coronin 1A plays a crucial role in NK cell cytotoxicity by promoting lytic granule secretion via remodeling F-actin that can be found at the immunological synapse (Mace & Orange, Citation2014). It is possible that coronin 1A is also required for other processes involving actin-depolymerization, e.g., migration. The potential influences that ADF apart from CFL-1 might have on cytoskeleton-related processes in NK cells have not been studied yet. It might therefore be interesting to investigate the changes in both quantity and activity of several ADF in CFL-1 knockdown NK-92 cells to find out what exactly induces the paradoxical rise in migration activity in these cells. Furthermore, it would be interesting to compare the effects of CFL-1 knockdown on NK-92 cells and on primary NK cells to confirm the transferability of the presented data and to examine its effects on NK cell mediated cancer cell killing in co-culture or tissue migration assays (Spielmann et al., Citation2017).
The secondary aim of the present study was to examine whether an obesity-like microenvironment (simulated by adjusting levels of adipokines) affects NK cell motility by altering CFL-1 expression levels or activity. In order to reach conditions as physiological as possible while performing stimulations, a mixture of adipokines (SGBS adipocyte-conditioned medium) was used.
Our investigations show that stimulation of NK-92 cells using serial dilutions of SGBS adipocyte-conditioned medium does not alter CFL-1 levels or phosphorylation ratios and does not affect the cells’ motility. We considered that the measured concentrations of leptin in SGBS adipocyte-conditioned medium could be too low to promote a noticeable effect but the use of supraphysiological doses of leptin did not result in significant changes of CFL-1 expression or pCFL-1/CFL-1 ratios either. In line, another study performed by our group could show that cells’ migratory behavior remained unchanged after short-term leptin treatment (unpublished data). In contrary to these findings, previous studies reported that leptin induces chemotaxis as well as chemokinesis in primary neutrophils and monocytes (Caldefie-Chezet et al., Citation2003; Krinninger et al., Citation2014; Montecucco et al., Citation2006).
Although an obesity-like microenvironment as described in our study may not lead to reduced CFL-1 activity or impaired NK cell movement, there might be other side effects of overweight that could possibly affect migration activity significantly. In the study of Michelet et al. the effect of a high-fat diet on murine NK cells was investigated (Michelet et al., Citation2018). The NK cells of overfed mice presented with an accumulation of lipid droplets in their cytosol which led to a downregulation of metabolic processes such as oxidative phosphorylation and aerobic glycolysis. Consequently, the NK cells’ cytotoxicity was significantly restricted. Taking this into account, one has to consider that the high turnover rates that are crucial for dynamic reorganization of actin monomers – thereby promoting the remodeling of the cytoskeleton – are substantially dependent on ATP hydrolysis (Marelli-Berg & Jangani, Citation2018). Hence, it seems quite possible that the metabolic arrest shown in NK cells of high-fat diet mice would not only affect cytotoxicity but also migratory activity. In the present study an NK cell line was used, which has only partial overlap with the functional behavior of primary NK cells. It would therefore be interesting to examine primary NK cells of obese and normal weight individuals with respect to their migratory behavior.
In conclusion, the results of this study indicate a significant effect of a CFL-1 knockdown on the general mobility of human NK cells. Furthermore, the stimulation with adipokines (in the applied concentrations) was ineffective concerning CFL-1 levels or the impact on migration parameters.
Author contributions
Conceptualization, H.K.,M.H. and B.G.; methodology, M.H., J.O., J.F., T.F., M.B., D.Q., I.B. and S.J.B.; analysis, M.H., T.F., M.B., D.Q., I.B., B.G. and S.J.B.; resources, H.K.; project administration, H.K.; writing-original draft preparation, M.H.; writing-review and editing, H.K., I.B.-W. and B.G. All authors have read and agreed to the actual version of the manuscript.
Supplemental Material
Download PDF (707.1 KB)Acknowledgments
The authors are grateful to S. Möschter and F. Knöfel for technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/08820139.2024.2327327.
Additional information
Funding
References
- Andrianantoandro, E., & Pollard, T. D. (2006). Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Molecular Cell, 24(1), 13–23. https://doi.org/10.1016/j.molcel.2006.08.006
- Arita, Y., Kihara, S., Ouchi, N., Takahashi, M., Maeda, K., Miyagawa, J.-I., Hotta, K., Shimomura, I., Nakamura, T., Miyaoka, K., Kuriyama, H., Nishida, M., Yamashita, S., Okubo, K., Matsubara, K., Muraguchi, M., Ohmoto, Y., Funahashi, T., & Matsuzawa, Y. (1999). Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochemical and Biophysical Research Communications, 257(1), 79–83. https://doi.org/10.1006/bbrc.1999.0255
- Bahr, I., Goritz, V., Doberstein, H., Hiller, G. G. R., Rosenstock, P., Jahn, J., Pörtner, O., Berreis, T., Mueller, T., Spielmann, J., & Kielstein, H. (2017). Diet-induced obesity is associated with an impaired NK cell function and an increased colon cancer incidence. Journal of Nutrition and Metabolism, 2017, 1–14. https://doi.org/10.1155/2017/4297025
- Bamburg, J. R. (1999). Proteins of the ADF/cofilin family: Essential regulators of actin dynamics. Annual Review of Cell and Developmental Biology, 15(1), 185–230. https://doi.org/10.1146/annurev.cellbio.15.1.185
- Beaulieu, A. M. (2018). Memory responses by natural killer cells. Journal of Leukocyte Biology, 104(6), 1087–1096. https://doi.org/10.1002/JLB.1RI0917-366R
- Busso, N., So, A., Chobaz-Péclat, V., Morard, C., Martinez-Soria, E., Talabot-Ayer, D., & Gabay, C. (2002). Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. Journal of Immunology, 168(2), 875–882. https://doi.org/10.4049/jimmunol.168.2.875
- Caldefie-Chezet, F., Poulin, A., & Vasson, M. P. (2003). Leptin regulates functional capacities of polymorphonuclear neutrophils. Free Radical Research, 37(8), 809–814. https://doi.org/10.1080/1071576031000097526
- Castriconi, R., Carrega, P., Dondero, A., Bellora, F., Casu, B., Regis, S., Ferlazzo, G., & Bottino, C. (2018). Molecular mechanisms directing migration and retention of natural killer cells in human tissues. Frontiers in Immunology, 9, 2324. https://doi.org/10.3389/fimmu.2018.02324
- DesMarais, V., Ghosh, M., Eddy, R., & Condeelis, J. (2005). Cofilin takes the lead. Journal of Cell Science, 118(1), 19–26. https://doi.org/10.1242/jcs.01631
- Dixit, V. D. (2008). Adipose-immune interactions during obesity and caloric restriction: Reciprocal mechanisms regulating immunity and health span. Journal of Leukocyte Biology, 84(4), 882–92. https://doi.org/10.1189/jlb.0108028
- Emerging Risk Factors Collaboration. (2011). Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet, 377(9771), 1085–1095. https://doi.org/10.1016/S0140-6736(11)60105-0
- Fernandez-Riejos, P., Najib, S., Santos-Alvarez, J., Martín-Romero, C., Pérez-Pérez, A., González-Yanes, C., & Sánchez-Margalet, V. (2010). Role of leptin in the activation of immune cells. Mediators of Inflammation, 2010, 1–8. https://doi.org/10.1155/2010/568343
- Fischer-Posovszky, P., Newell, F. S., Wabitsch, M., & Tornqvist, H. E. (2008). Human SGBS cells – a unique tool for studies of human fat cell biology. Obesity Facts, 1(4), 184–189. https://doi.org/10.1159/000145784
- Glienke, W., Esser, R., Priesner, C., Suerth, J. D., Schambach, A., Wels, W. S., Grez, M., Kloess, S., Arseniev, L., & Koehl, U. (2015). Advantages and applications of CAR-expressing natural killer cells. Frontiers in Pharmacology, 6, 21. https://doi.org/10.3389/fphar.2015.00021
- Howard, J. K., Lord, G. M., Matarese, G., Vendetti, S., Ghatei, M. A., Ritter, M. A., Lechler, R. I., & Bloom, S. R. (1999). Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. Journal of Clinical Investigation, 104(8), 1051–1059. https://doi.org/10.1172/JCI6762
- Huebner, L., Engeli, S., Wrann, C. D., Goudeva, L., Laue, T., & Kielstein, H. (2013). Human NK cell subset functions are differentially affected by adipokines. Public Library of Science ONE, 8(9), e75703. https://doi.org/10.1371/journal.pone.0075703
- Huet, G., Rajakylä, E. K., Viita, T., Skarp, K.-P., Crivaro, M., Dopie, J., & Vartiainen, M. K. (2013). Actin-regulated feedback loop based on Phactr4, PP1 and cofilin maintains the actin monomer pool. Journal of Cell Science, 126(2), 497–507. https://doi.org/10.1242/jcs.113241
- Imai, K., Matsuyama, S., Miyake, S., Suga, K., & Nakachi, K. (2000). Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet, 356(9244), 1795–1799. https://doi.org/10.1016/S0140-6736(00)03231-1
- Jahn, J., Spielau, M., Brandsch, C., Stangl, G. I., Delank, K.-S., Bähr, I., Berreis, T., Wrann, C. D., & Kielstein, H. (2015). Decreased NK cell functions in obesity can be reactivated by fat mass reduction. Obesity (Silver Spring), 23(11), 2233–2241. https://doi.org/10.1002/oby.21229
- Kershaw, E. E., & Flier, J. S. (2004). Adipose tissue as an endocrine organ. The Journal of Clinical Endocrinology and Metabolism, 89(6), 2548–56. https://doi.org/10.1210/jc.2004-0395
- Kim, J. Y., van de Wall, E., Laplante, M., Azzara, A., Trujillo, M. E., Hofmann, S. M., Schraw, T., Durand, J. L., Li, H., Li, G., Jelicks, L. A., Mehler, M. F., Hui, D. Y., Deshaies, Y., Shulman, G. I., Schwartz, G. J., & Scherer, P. E. (2007). Obesity-associated improvements in metabolic profile through expansion of adipose tissue. Journal of Clinical Investigation, 117(9), 2621–2637. https://doi.org/10.1172/JCI31021
- Krinninger, P., Ensenauer, R., Ehlers, K., Rauh, K., Stoll, J., Krauss-Etschmann, S., Hauner, H., & Laumen, H. (2014). Peripheral monocytes of obese women display increased chemokine receptor expression and migration capacity. The Journal of Clinical Endocrinology and Metabolism, 99(7), 2500–2509. https://doi.org/10.1210/jc.2013-2611
- Langers, I., Renoux, V.M., Thiry, M., Delvenne, P., & Jacobs, N. (2012). Natural killer cells: Role in local tumor growth and metastasis. Biologics, 6, 73–82. https://doi.org/10.2147/BTT.S23976
- Lautenbach, A., Wrann, C. D., Jacobs, R., Müller, G., Brabant, G., & Nave, H. (2009). Altered phenotype of NK cells from obese rats can be normalized by transfer into lean animals. Obesity (Silver Spring), 17(10), 1848–1855. https://doi.org/10.1038/oby.2009.140
- Mace, E. M., & Orange, J. S. (2014). Lytic immune synapse function requires filamentous actin deconstruction by Coronin 1A. Proceedings of the National Academy of Sciences, 111(18), 6708–13. https://doi.org/10.1073/pnas.1314975111
- Mandel, M. A., & Mahmoud, A. A. (1978). Impairment of cell-mediated immunity in mutation diabetic mice (db/db). Journal of Immunology, 120(4), 1375–1377. https://doi.org/10.4049/jimmunol.120.4.1375
- Marelli-Berg, F. M., & Jangani, M. (2018). Metabolic regulation of leukocyte motility and migration. Journal of Leukocyte Biology, 104(2), 285–293. https://doi.org/10.1002/JLB.1MR1117-472R
- McGough, A., Pope, B., Chiu, W., & Weeds, A. (1997). Cofilin changes the twist of F-actin: Implications for actin filament dynamics and cellular function. The Journal of Cell Biology, 138(4), 771–781. https://doi.org/10.1083/jcb.138.4.771
- Mgrditchian, T., Sakalauskaite, G., Müller, T., Hoffmann, C., & Thomas, C. (2021). The multiple roles of actin-binding proteins at invadopodia. International Review of Cell and Molecular Biology, 360, 99–132.
- Michelet, X., Dyck, L., Hogan, A., Loftus, R. M., Duquette, D., Wei, K., Beyaz, S., Tavakkoli, A., Foley, C., Donnelly, R., O’Farrelly, C., Raverdeau, M., Vernon, A., Pettee, W., O’Shea, D., Nikolajczyk, B. S., Mills, K. H. G., Brenner, M. B., Finlay, D., & Lynch, L. (2018). Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nature Immunology, 19(12), 1330–1340. https://doi.org/10.1038/s41590-018-0251-7
- Miller, J. S., Soignier, Y., Panoskaltsis-Mortari, A., McNearney, S. A., Yun, G. H., Fautsch, S. K., McKenna, D., Le, C., Defor, T. E., Burns, L. J., Orchard, P. J., Blazar, B. R., Wagner, J. E., Slungaard, A., Weisdorf, D. J., Okazaki, I. J., & McGlave, P. B. (2005). Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood, 105(8), 3051–3057. https://doi.org/10.1182/blood-2004-07-2974
- Mitchison, T. J., & Cramer, L. P. (1996). Actin-based cell motility and cell locomotion. Cell, 84(3), 371–379. https://doi.org/10.1016/S0092-8674(00)81281-7
- Mitsuhashi, K., Hashimoto, Y., Tanaka, M., Toda, H., Matsumoto, S., Ushigome, E., Asano, M., Yamazaki, M., Oda, Y., & Fukui, M. (2017). Combined effect of body mass index and waist-height ratio on incident diabetes; a population based cohort study. Journal of Clinical Biochemistry and Nutrition, 61(2), 118–122. https://doi.org/10.3164/jcbn.16-116
- Mondal, C., DiMartino, J. S., & Bravo-Cordero, J. J. (2021). Actin dynamics during tumor cell dissemination. International Review of Cell and Molecular Biology, 360, 65–98. https://doi.org/10.1016/bs.ircmb.2020.09.004
- Montecucco, F., Bianchi, G., Gnerre, P., Bertolotto, M., Dallegri, F., & Ottonello, L. (2006). Induction of neutrophil chemotaxis by leptin: Crucial role for p38 and Src kinases. Annals of the New York Academy of Sciences, 1069(1), 463–471. https://doi.org/10.1196/annals.1351.045
- Nave, H., Mueller, G., Siegmund, B., Jacobs, R., Stroh, T., Schueler, U., Hopfe, M., Behrendt, P., Buchenauer, T., Pabst, R., & Brabant, G. (2008). Resistance of janus kinase-2 dependent leptin signaling in natural killer (NK) cells: A novel mechanism of NK cell dysfunction in diet-induced obesity. Endocrinology, 149(7), 3370–3378. https://doi.org/10.1210/en.2007-1516
- Nishita, M., Tomizawa, C., Yamamoto, M., Horita, Y., Ohashi, K., & Mizuno, K. (2005). Spatial and temporal regulation of cofilin activity by LIM kinase and slingshot is critical for directional cell migration. The Journal of Cell Biology, 171(2), 349–359. https://doi.org/10.1083/jcb.200504029
- Ohashi, K. (2015). Roles of cofilin in development and its mechanisms of regulation. Development, Growth & Differentiation, 57(4), 275–90. https://doi.org/10.1111/dgd.12213
- Organization, W.H. (2023). Fact Sheet: Obesity and Overweight. July 31, https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- O’Shea, D., Cawood, T. J., O’Farrelly, C., & Lynch, L. (2010). Natural killer cells in obesity: Impaired function and increased susceptibility to the effects of cigarette smoke. Public Library of Science ONE, 5(1), e8660. https://doi.org/10.1371/journal.pone.0008660
- Oswald, J., Büttner, M., Jasinski-Bergner, S., Jacobs, R., Rosenstock, P., & Kielstein, H. (2018). Leptin affects filopodia and cofilin in NK-92 cells in a dose- and time-dependent manner. European Journal of Histochemistry: EJH, 62(1), 2848. https://doi.org/10.4081/ejh.2018.2848
- Renehan, A. G., Tyson, M., Egger, M., Heller, R. F., & Zwahlen, M. (2008). Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet, 371(9612), 569–578. https://doi.org/10.1016/S0140-6736(08)60269-X
- Rezvani, K., Rouce, R., Liu, E., & Shpall, E. (2017). Engineering natural killer cells for cancer immunotherapy. Molecular Therapy: The Journal of the American Society of Gene Therapy, 25(8), 1769–1781. https://doi.org/10.1016/j.ymthe.2017.06.012
- Rosenberg, S. A., Lotze, M. T., Muul, L. M., Chang, A. E., Avis, F. P., Leitman, S., Linehan, W. M., Robertson, C. N., Lee, R. E., Rubin, J. T., Seipp, C. A., Simpson, C. G., & White, D. E. (1987). A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. The New England Journal of Medicine, 316(15), 889–897. https://doi.org/10.1056/NEJM198704093161501
- Ruggeri, L., Capanni, M., Urbani, E., Perruccio, K., Shlomchik, W. D., Tosti, A., Posati, S., Rogaia, D., Frassoni, F., Aversa, F., Martelli, M. F., & Velardi, A. (2002). Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science, 295(5562), 2097–2100. https://doi.org/10.1126/science.1068440
- Shah, N. R., Braverman, E. R., & Nizami, Q. (2012). Measuring adiposity in patients: The utility of body mass index (BMI), percent body fat, and leptin. Public Library of Science ONE, 7(4), e33308. https://doi.org/10.1371/journal.pone.0033308
- Shi, C., Cai, Y., Li, Y., Li, Y., Hu, N., Ma, S., Hu, S., Zhu, P., Wang, W., & Zhou, H. (2018). Yap promotes hepatocellular carcinoma metastasis and mobilization via governing cofilin/F-actin/lamellipodium axis by regulation of JNK/Bnip3/SERCA/CaMKII pathways. Redox Biology, 14, 59–71. https://doi.org/10.1016/j.redox.2017.08.013
- Spielmann, J., Hanke, J., Knauf, D., Ben-Eliyahu, S., Jacobs, R., Stangl, G. I., Bähr, I., & Kielstein, H. (2017). Significantly enhanced lung metastasis and reduced organ NK cell functions in diet-induced obese rats. BMC Obesity, 4(1), 24. https://doi.org/10.1186/s40608-017-0161-5
- Svitkina, T. M., & Borisy, G. G. (1999). Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. The Journal of Cell Biology, 145(5), 1009–26. https://doi.org/10.1083/jcb.145.5.1009
- Tian, L., Luo, N., Zhu, X., Chung, B.-H., Garvey, W. T., & Fu, Y. (2012). Adiponectin-AdipoR1/2-APPL1 signaling axis suppresses human foam cell formation: Differential ability of AdipoR1 and AdipoR2 to regulate inflammatory cytokine responses. Atherosclerosis, 221(1), 66–75. https://doi.org/10.1016/j.atherosclerosis.2011.12.014
- Viel, S., Besson, L., Charrier, E., Marçais, A., Disse, E., Bienvenu, J., Walzer, T., & Dumontet, C. (2017). Alteration of natural killer cell phenotype and function in obese individuals. Clinical Immunology (Orlando, Fla), 177, 12–17. https://doi.org/10.1016/j.clim.2016.01.007
- Vivier, E., Tomasello, E., Baratin, M., Walzer, T., & Ugolini, S. (2008). Functions of natural killer cells. Nature Immunology, 9(5), 503–510. https://doi.org/10.1038/ni1582
- Wabitsch, M., Brenner, R. E., Melzner, I., Braun, M., Möller, P., Heinze, E., Debatin, K.-M., & Hauner, H. (2001). Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity, 25(1), 8–15. https://doi.org/10.1038/sj.ijo.0801520
- Wang, K., Wang, L., Wang, Y., Xiao, L., Wei, J., Hu, Y., Wang, D., & Huang, H. (2024). Reprogramming natural killer cells for cancer therapy. Molecular Therapy: The Journal of the American Society of Gene Therapy. https://doi.org/10.1016/j.ymthe.2024.01.027
- Wrann, C. D., Laue, T., Hübner, L., Kuhlmann, S., Jacobs, R., Goudeva, L., & Nave, H. (2012). Short-term and long-term leptin exposure differentially affect human natural killer cell immune functions. American Journal of Physiology Endocrinology and Metabolism, 302(1), E108–16. https://doi.org/10.1152/ajpendo.00057.2011
- Yamauchi, T., Kamon, J., Waki, H., Terauchi, Y., Kubota, N., Hara, K., Mori, Y., Ide, T., Murakami, K., Tsuboyama-Kasaoka, N., Ezaki, O., Akanuma, Y., Gavrilova, O., Vinson, C., Reitman, M. L., Kagechika, H., Shudo, K., Yoda, M., … Froguel, P. (2001). The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Medicine, 7(8), 941–946. https://doi.org/10.1038/90984
- Yudkin, J. S., Stehouwer, C. D. A., Emeis, J. J., & Coppack, S. W. (1999). C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arteriosclerosis, Thrombosis, and Vascular Biology, 19(4), 972–978. https://doi.org/10.1161/01.ATV.19.4.972