ABSTRACT
Copper ores, end-of-life electric and electronic equipment and car electronics can contain, besides Cu, substantial amounts of geochemically scarce companion elements. Geochemically scarce elements have an upper crustal abundance of <0.025 (weight)%. In view of resource conservation and reduction of pollution there is a case for near-zero waste processing. Improving the generation of ore concentrates and use of kinetic and thermodynamic data regarding smelting and converting can increase the production of geochemically scarce elements in the pyrometallurgical processing of copper ores. Reprocessing of copper ore processing residues can serve the generation of geochemically scare elements and the clean-up of matrix materials. Modularization of products, closed-loop take-back, including deposit-refund, systems for end-of -life products, and changes in the pre-processing thereof can be conducive to improved recovery of geochemically scarce elements from end-of-life electric and electronic equipment and car electronics. A comparatively large variety of geochemically scarce elements originating in end-of-life products can be recovered when in smelting lead and copper serve as collectors. Substantial research and development work is needed to optimize the co-production of geochemically scarce elements by hydrotechnology from copper ores and (mined) end-of-life products and to assess the potential of solvochemistry. There is technical scope for significant progress in the direction of near-zero waste processing in processing copper ores and (mined) end-of-life products, but for the realization of near-zero waste processing there are hurdles to be overcome related to marketability of outputs, safe handling of hazardous elements and company behavior. Also, the techno-economic potential of hydrotechnology and solvochemistry in extracting copper ores, copper ore processing residues and end-of-life products is uncertain. In view thereof, the feasibility near-zero waste production of copper and its geochemically scarce companion elements from copper ores and end-of-life electric and electronic equipment and car electronics is uncertain.
1. Introduction
Spooren et al. (Citation2020) view near-zero waste processing as goal for handling low grade complex metal ores and wastes containing metals. They state that this goal is in line with current trends of process development. In view of the study of Spooren et al. (Citation2020) one might formulate the hypothesis that near-zero waste processing is a feasible goal for handling metal ores and wastes containing metals.
Spooren et al. (Citation2020) have discussed possibilities for near-zero waste processing for Polish and Greek laterites, complex ores that derive their economic value mainly from the presence of Ni, with scope for Co and Zn contributing to the future economic value of extracted metals. Spooren et al. (Citation2020) also considered the valorization of matrix materials which are present in co-outputs of laterite processing. They furthermore discussed the scope for near-zero waste processing in the secondary production of metals from production wastes containing a variety of geochemically scarce metals (with an average upper crustal abundance of <0.025 (weight)%), such as Co, Cr, Cu, Ni and Zn.
The present review extends the work of Spooren et al. (Citation2020) to copper ores and wastes containing copper. Copper in ores is often accompanied by other geochemically scarce elements (companion elements). shows geochemically scarce elements that may have a substantial presence in copper ores. Copper ores show a wide variety as to the presence of these elements. Geochemically scarce elements may also be added to copper during copper life cycles for alloying, joining and coating. presents these elements. shows geochemically scarce elements that can become companions to copper in electric and electronic equipment and electronic components of cars. In part these are elements used for alloying, coating and joining copper present in electric and electronic equipment and electronic components of cars, such as Ag, Au, Be, Bi, In, Ni, Sb and Sn (cf. )
Box 1. Geochemically scarce elements, other than Cu, that may have a substantial presence in copper ores (Ayres, Ayres and Rade Citation2002; Sracek et al. Citation2010; Mudd, Weng and Jowitt Citation2013a,b; Izatt et al. Citation2014; Chmielewski, Wawzczak and Brykala Citation2016; Weidenbach, Dunn and Tao Citation2016; González et al. Citation2017; Safarzadeh et al. Citation2018; Crespo et al. Citation2018; Makuei and Senanayake Citation2018; Mikoda et al. Citation2019a; Araya et al. Citation2021).
Box 2. Geochemically scarce elements, other than Cu, associated with electric and electronic equipment and electronic components of passenger cars (Andersson, Söderman and Sandén Citation2019; Buechler et al. Citation2020; Cesaro et al. Citation2018; Widmer et al. Citation2015).
Box 3. Subjects requiring major research and development efforts to narrow gaps in present knowledge.
Table 1. Geochemically scarce functional elements that may become associated with copper by alloying, coating and joining. (Samuels and Méranger Citation1984; Marshakov Citation2005; Karpagavalli and Balasubramaniam Citation2007; Chen and Bull Citation2008; Sarver and Edwards Citation2011; Jolly Citation2013; Ruzic et al. Citation2013; Garza-Montes-de-Oca et al. Citation2014; Imamura et al. Citation2015; Kanlayasiri and Ariga Citation2015; Laws et al. Citation2015; Young and Dunand Citation2015; Forsén, Aromaa and Lindström Citation2017; Guarino et al. Citation2017; Noor, Zuhallawati and Radzali Citation2016; Lee Citation2018; Nagel Citation2018; Ulman et al. Citation2018).
Pollution by copper and its geochemically scarce companion elements linked to the current handling of ores and wastes can impact the environment with consequences that may be detrimental for ecosystems and/or human health (e. g. Du et al. Citation2020; Fry et al. Citation2020; González-Castanedo et al. Citation2014; Kimball et al. Citation2016; Potysz et al. Citation2015; Rubinos et al. Citation2021; Sorooshian et al. Citation2012; Wiklund et al. Citation2018). Near zero-waste processing of ores and wastes might strongly reduce such pollution.
This paper will consider the question whether near-zero waste production of copper and its companion elements is a feasible goal for handling coper ores and waste electric and electronic equipment and car electronics. The focus of this paper will be on increasing geochemically scarce elements as product outputs and lowering of the presence of the same elements in production residues, which may improve the scope for valorization of matrix materials present in these residues and reduce pollution. In its focus on increasing geochemically scarce elements in product outputs and on the valorization of matrix materials present in ores, the present paper will be similar to the study of Spooren et al. (Citation2020). Differently from the review of Spooren et al. (Citation2020), the secondary production of metals from product wastes (specifically mined waste electric and electronic equipment and car electronics), rather than from production wastes, will be discussed. Due to the wide variety of geochemically scarce elements present in those wastes (cf. ) their processing is challenging from a near-zero waste perspective. The present review will add to the review of Spooren et al. (Citation2020), by considering the release of geochemically scarce elements from recycled matrix materials and proposals for preventing the release to the environment of hazardous companion elements, especially As, for which there is little demand. Also, hurdles to the feasibility of near-zero waste processing will be considered.
The co-production of Cu and other geochemically scarce elements from copper ores, and options for the improvement thereof, will be addressed in section 2. Both optimizing processing of copper ores and reprocessing of residues, to increase the recovery of copper and its geochemically scarce companion elements, will be discussed. This section will also deal with the valorization of matrix materials that are co-outputs of copper ore processing. The recovery of copper and its geochemically scarce companion elements from end-of-life electric and electronic equipment and car electronics, and options for the improvement thereof, will be addressed in section 3. Processing of mined end-of-life electric and electronic equipment and car electronics in secondary smelters, the pyrometallurgical co-processing thereof with copper ores, and the role of copper smelters will be discussed. Section 4 will consider hurdles for the feasibility of near-zero waste processing. These regard marketability of outputs, company behavior and safe handling of hazardous elements when potential supply exceeds demand. Section 5 will present the conclusions of this paper.
2. Production of geochemically scarce elements from copper ore and the valorization of matrix materials that are co-outputs of copper ore processing
The primary production of copper will be outlined in section 2.1. Technical options for increasing the production (recovery) of copper from ore will be presented, Section 2.1 also provides the necessary background for section 2.2. which considers the extraction of geochemically scarce companion elements from copper ores and options for the improvement thereof. Section 2.3 contains the concluding remarks regarding sections 2.1 and 2.2. Section 2.4 briefly discusses new technologies for copper extraction. Section 2.5 will consider the valorization of matrix materials that are co-outputs from copper ore processing.
2.1. Primary production of copper
Extraction of copper from ores is currently dominated by two types of processing: pyrometallurgical and hydrometallurgical, both combined with refining using electrometallurgy (electrolysis) (e. g. ICSG Citation2016; Norgate and Jahanshahi Citation2010). The main steps in both types of processing are in and 1b.
Figure 1. (a) Main steps in pyrometallurgical primary production of copper (cathodes). (b) Main steps in hydrometallurgical primary production of copper (cathodes).

Spooren et al. (Citation2020), focusing on near-zero waste metallurgy, would seem to prefer hydrometallurgy and leaching or extraction with non-aqueous solvents (solvometallurgy), as these extraction technologies have a low impact on the physicochemical properties of the matrix materials present in concentrates or ores. However, there is also scope for valorization of the matrix materials that end up in copper slags, an important co-output of pyrometallurgy (Kinnunen et al. Citation2020).
Copper production from mining in 2020 is estimated at about 21 × 109 kg (ICSG Citation2019), of which about 21% is relying on hydrometallurgical technology (ICSG (International Copper Study Group) Citation2016). Solvometallurgical leaching (leaching with non-aqueous solvents) of Cu from copper ores has been demonstrated, but commercial application thereof has not been reported (Binnemans and Jones Citation2017; Spooren et al. Citation2020). The application of electrochemistry in molten salts to the primary production of copper, in analogy to the primary production of aluminum in the Hall-Héroult electrolysis process, has been investigated (Allanore Citation2017; Daehn and Allanore Citation2020; Sahu, Chmielowiec and Allanore Citation2017; Sokhanvaran et al. Citation2016; Tan et al. Citation2016), but is as yet not commercially applied.
Pyrometallurgy regards sulfidic copper ores. Preceding pyrometallurgical processing, the copper content of ore-derived material is commonly increased. This is done by physico-mechanical treatment in concentrators that use crushing, grinding (milling) and flotation, occasionally combined with other technologies such as use of magnetic fields or centrifugation (Katwika et al. Citation2019; Lagos et al. Citation2018; Rönnlund et al. Citation2016; Spooren et al. Citation2020). Geometallurgical modeling may help in achieving improvement of copper recovery and copper concentrations in the concentrate (Boisvert et al. Citation2013; Rincon, Gaydardzhiev and Stamenov Citation2019). Electrical fragmentation, treatment with microwaves, sensor-based sorting, improved flocculation and machine learning have been suggested as technologies that can improve the composition of the concentrate (Spooren et al. Citation2020). Optimizing flotation time, particle size, pH and dosages of reagents such as collector and frother may improve copper recovery and/or copper grade (e. g. Asghari, Nakhaei and VandGhorbany Citation2018; Bakalarz Citation2019; Dhar, Thornhill and Kota Citation2019; Ghodrati et al. Citation2020). Copper ore concentrates may also serve hydrometallurgical processing (cf. section 2.2.3). The processing residues originating in concentrate production (tailings) are commonly dumped, landfilled or stored in dams and ponds. Worldwide, average Cu losses in mining and the generation of concentrates have been estimated at about 18% of the Cu input in primary production in 2010 (Glöser, Soulier and Espinoza Citation2013). Reducing this 18% loss may well be difficult when the downward trend of copper concentrations in ores continues (cf. Alcalde, Kelm and Vergara Citation2018). Increased exploitation of complex ores and increasing co-production of other geochemically scarce elements may add to the difficulty of reducing this percentage (e. g. Corin et al. Citation2017; Sousa et al. Citation2017). On the other hand, reprocessing of tailings from copper concentrate production may increase the recovery of Cu (e. g. Alcalde, Kelm and Vergara Citation2018; Bakalarz Citation2019; Brest et al. Citation2021; Drobe et al. Citation2021; Falagán, Grail and Johnson Citation2017; Lutandula and Maloba Citation2013; Shengo Citation2021).
In pyrometallurgy, firstly matte (containing substantial amounts of S and 45–85 (weight)% Cu) is generated in flash or bath smelters. In 2015 about 72% of copper ore smelting capacity regarded flash smelter-based production of matte (ICSG (International Copper Study Group) Citation2016). Flash smelting requires a dry input of particles <100 micrometer, whereas bath smelters can also process larger sized materials and have a less strict drying requirement (Forsén, Aromaa and Lindström Citation2017). A higher matte grade and a higher temperature in flash smelting increase solubility of Cu in slag (Forsén, Aromaa and Lindström Citation2017; Klaffenbach et al. Citation2021). Subsequently, through oxidative treatment in converters, matte is turned into blister copper with >90 (weight)% Cu (e. g. ICSG (International Copper Study Group) Citation2016). In the presence of tailored slag systems direct concentrate-to-blister copper smelting is an option (Voigt et al. Citation2017). Blister copper in turn is refined in an anode furnace (fire refining), to generate copper anodes with >99 (weight)% Cu (e. g. Dupont et al. Citation2016; Gregurek et al. Citation2018; Li et al. Citation2016). Subsequently, anode copper is subjected to electrolytic treatment. This implies dissolution of anode-Cu in the electrolyte and the generation of anode slime (mainly precipitates, also called anode mud). Cu is removed from the electrolyte, generating cathodes with high purity (>99.9 (weight)%) copper (Forsén, Aromaa and Lindström Citation2017; Norgate and Jahanshahi Citation2010; Rönnlund et al. Citation2016).
During smelting, converting and fire refining dusts and slags are generated. These dusts are partly discarded (Schreck Citation1999; Shibayama et al. Citation2010), but recovery of Cu from a part of smelter dusts by internal recycling is also often practiced commercially (Bakhtiari et al. Citation2011; Montenegro, Sano and Fujisawa Citation2013; Liu et al. Citation2018; Moats, Alagha and Awuah-Offei Citation2021; see also section 2.2.2.4). Furthermore, several primary smelters have been reported recover Cu from dusts by hydrometallurgy (Schlesinger et al. Citation2011). High-recovery extraction of Cu from dusts by bioleaching is also an option (e. g. Ebrahimpour et al. Citation2021): Smelting, converting and fire refining give rise to the co-production of slags. Published data about the Cu content of slags generated in matte production vary between about 0.3 and 2.74 (weight) % (Sridhar, Toguri and Simeonov Citation1997; Mikula et al. Citation2021; Tian et al. Citation2021). The partitioning of Cu to slags in converters is relatively high because the partial oxygen pressure in converters is relatively high, commonly leading to a Cu content in the order of 4–8 (weight)% (Bellemans et al. Citation2018). In an anode furnace, partial oxygen pressures are even higher than in converting: Selivanov et al. (Citation2013) reported a copper content of about 33% in anode furnace slag. Slags are partly discarded (Li et al. Citation2018b; Sanchez et al. Citation2004) and partly the object of copper recovery (Coursol et al. Citation2012; Cusano et al. Citation2017; Guo et al. Citation2018; Han, Yu and Cui Citation2016; Hughes Citation2000; Khalid et al. Citation2019; Mikoda, Potysz and Kmiecik Citation2019b). Recovery of copper from slags is possible by a variety of processes. These are: internal recycling of slag concentrate (generated by milling and flotation), leaching and treatment in a slag cleaning furnace under reducing conditions (Chun et al. Citation2016; Coursol et al. Citation2012; Cusano et al. Citation2017; Guo et al. Citation2018; Han, Yu and Cui Citation2016; Khalid et al. Citation2019; Mikoda, Potysz and Kmiecik Citation2019b). Recovery of Cu in concentrate may be enhanced by modifying molten copper slag (Guo et al. Citation2016). Internal recycling of slag concentrate, and treatment in slag cleaning furnaces are practiced commercially (Coursol et al. Citation2012; Cusano et al. Citation2017).
After internal recycling of slag concentrate or treatment in a slag cleaning furnace, reported Cu concentrations in slag tailings vary from 0.2 to 1.2 (weight)% and are often in the range of 0.7–1 (weight)% (Carranza et al. Citation2009; Coursol et al. Citation2012; Cusano et al. Citation2017; Holland et al. Citation2019; Mikoda et al. Citation2019a; Muravyov et al. Citation2012). Slag tailings are often landfilled or used for infrastructural applications without further treatment (Khalid et al. Citation2019; Holland et al. Citation2019; Mikoda et al. Citation2019a, Sibanda et al. Citation2020).
Glöser, Soulier and Espinoza (Citation2013) estimated the worldwide losses in 2010 of Cu to slags and dusts originating in primary copper smelting, converting and refining operations at about 3% of the Cu input in primary production. This percentage may be reduced by optimizing primary copper pyrometallurgy guided by thermodynamics and kinetics (e. g. Nakajima et al. Citation2011; Shihshin, Hayes and Jak Citation2018; Sineva et al. Citation2021) and by increasing the extraction of Cu from slags, slag tailings and dusts. There is technical scope for doing the latter by optimizing slag cleaning furnaces (e. g. Yang et al. Citation2018), by improving flotation of milled slag (Sibanda et al. Citation2020), by leaching slag tailings, whether or not after sulfation roasting (Carranza et al. Citation2009; Grudinsky et al. Citation2021; Mikoda, Potysz and Kmiecik Citation2019b; Muravyov et al. Citation2012) and by improved leaching of dusts whether or not after sulfation roasting (Liu et al. Citation2018; Priya et al. Citation2020; Sabzezari et al. Citation2019).
Current commercial hydrometallurgical processing mainly regards heap (bio)leaching of comminuted materials under acid conditions. This regards constructed heaps of low-grade sulfidic ores (oxidative acid bioleaching), and ores containing Cu-oxides and -carbonates (acid leaching) (e. g. Ghorbani, Franzidis and Petersen Citation2018; Petersen Citation2016; Scheffel, Guzman and Dreier Citation2016). For suitable copper ores (including chalcocite and covellite), and when effective measures preventing poor percolation (by improving agglomeration) and leakage of leachate are in place, copper recoveries of 60–90% have been reported (Domic Citation2007; Gentina and Acevedo Citation2016; Ghorbani, Franzidis and Petersen Citation2018; Norgate and Jahanshahi Citation2010; Petersen Citation2016; Ruan et al. Citation2013; Yin et al. Citation2018b). The optimization of heap (bio)leaching, which would be conducive to improved Cu recovery, has been discussed by Ghorbani, Franzidis and Petersen (Citation2018). Options for improved Cu recovery include better heap permeability and aeration (Ghorbani, Franzidis and Petersen Citation2018). The Cu recovery from the main copper ore chalcopyrite by heap bioleaching tends to be poor due to the formation of a passivation layer on the mineral (e. g. Pradhan et al. Citation2019; Tanne and Schippers Citation2019). Options for improved Cu recovery from chalcopyrite include: better redox control, improved mixtures of bioleaching organisms, addition of chloride, the use of thermoacidophiles and high temperatures (Ai et al. Citation2019; Ghorbani, Franzidis and Petersen Citation2018; Ma et al. Citation2017, Citation2018; Watling Citation2006; Zhao et al. Citation2019).
Leachate obtained by heap leaching is commonly treated by solvent extraction. Reported estimated Cu extraction efficiencies are about 95% (Panda et al. Citation2016 or >95% (Hosseinzadeh, Azizi and Hassanzadeh Citation2021). Recycling the extracted leachate (raffinate) is common practice (Liu et al. Citation2020). However, the raffinate contains residues of solvent that may negatively impact microorganisms conducive to bioleaching (Zhang et al. Citation2019a). Moreover, metals (Cd, Cr, Fe, Ni, Zn) and As tend to accumulate in the raffinate which in practice can be considered problematic for ongoing recycling of raffinate (Liu et al. Citation2020). Chemical precipitation of metals from raffinate followed by landfilling is commonly practiced (Liu et al. Citation2020).
The solvent extract is stripped by sulfuric acid, which can be done with an efficiency >99% (Hosseinzadeh, Azizi and Hassanzadeh Citation2021). The resulting copper sulfate solution is treated by electrowinning (electrolysis) generating copper cathodes. Ion exchange technology can be more efficient than solvent extraction at low Cu concentrations and can also have other environmental benefits (Izatt et al. Citation2015; Sole, Mooiman and Hardwick Citation2017). Hawker et al. (Citation2018) have proposed to precipitate copper from the leachate and to add the precipitate to the inputs for a copper smelter or- converter (the synergistic copper process). Hawker et al. (Citation2018) mentioned as advantages of the synergistic copper process that it may reduce copper losses in pyrometallurgy and extend the use of low-grade copper ores. However, they did not compare the efficiencies in processing of leachate in copper production by the synergistic process with conventional (bio)hydrometallurgy based on heap leaching (cf. ). It would seem uncertain whether the recovery of copper improves if compared with the conventional approach of solvent extraction, followed by stripping and electrowinning. Also, the exclusive focus of the synergistic copper process on copper production is at variance with developments in hydrometallurgy aimed at the co-production of geochemically scarce companion elements (discussed in section 2.2.3).
Heap leaching-based technologies for Cu extraction based on chloride chemistry (Ghorbani, Franzidis and Petersen Citation2018) and (bio)leaching in alkaline ammonia solutions (Yin et al. Citation2018a) have been demonstrated but their commercial application has not been reported.
In primary hydrometallurgical Cu production, there is furthermore commercial application of contained processes. These are: agitated tank leaching of copper ores and copper ore tailings and autoclave-based, pressurized oxidative acid leaching processes applied to processed copper ores. Agitated tank leaching of copper ores and copper ore tailings has been reported for Africa (Sole et al. Citation2019). In Cobre Las Cruces (Spain) an ore-derived input with 5.1% Cu is extracted with a 91.8% efficiency by pressurized oxidative acid leaching (Dreisinger Citation2015). In the Freeport plants in Arizona (USA) Cu is recovered from ore concentrates by pressurized oxidative leaching with an overall efficiency of about 98% (Dreisinger Citation2015; Gertenbach Citation2016; Green, Robertson and Marsden Citation2018). Pressurized oxidation and leaching of copper ore/pyrite mixture is practiced by Sepon Copper (Laos) (Dreisinger Citation2015). Solvent extraction and electrowinning are used to produce Cu from the leachate generated by pressurized oxidative leaching (Dreisinger Citation2015). Contained hydrometallurgy is also applied when, besides Cu, geochemically scarce companion elements are present in substantial concentrations (see section 2.2.3). Residues remaining after contained leaching can be reprocessed. This offers scope for increased recovery of Cu (Hubau et al. Citation2020; Shengo et al. Citation2021).
2.2. The co-production of Cu and geochemically scarce companion elements in copper ore processing
In primary production of copper from suitable ores, there is currently co-production of other geochemically scarce elements. Examples are the co-production of Cu and Mo (e. g. Izatt et al. Citation2014; Tabelin et al. Citation2021), Cu and precious elements (Schwartz et al. Citation2017) and Cu and Co (Sole et al. Citation2019). In the following part of this section the scope for increased co-production of geochemically scarce elements from copper ores will be discussed.
2.2.1. Geochemically scarce companion elements in copper ore concentration
Copper ore concentrators are often aiming at high concentrations of copper in concentrate for primary copper production while sacrificing as little copper recovery as possible (Alcalde, Kelm and Vergara Citation2018). A strategy optimized for Cu might well lead to relatively large losses of other valuable elements to concentrator residues or tailings (Agorhom et al. Citation2015; Zanin et al. Citation2009). For instance, in the case of Te present in copper ores, current worldwide average partitioning to tailings in concentrate production has been estimated at about 90% (Makuei and Senanayake Citation2018; Moats, Alagha and Awuah-Offei Citation2021). Similar losses to tailings are likely for Se and Bi (Moats, Alagha and Awuah-Offei Citation2021). Mikula et al. (Citation2021) reported as to concentrator residues originating in Turkish, Chinese and Polish mines oxide concentrations for Zn up to 2.8%, for Cr up to 0.12%, and for Co up to 0.2%. Araya, Kraslawski and Cisternas (Citation2020), (Citation2021)) found substantial amounts of Co, Nb, rare earths, Sb, and V in copper mine tailings from the Antofagasta region, Chile. Hazardous substances are likely to be mobilized from stored tailings and even more so after mine tailings dam failures (Araya, Kraslawski and Cisternas Citation2020; Falagán, Grail and Johnson Citation2017; Mikula et al. Citation2021; Rubinos et al. Citation2021).
By changing concentrator processes, the recovery of other elements than Cu may be improved (Zanin et al. Citation2009; Agorhom et al. Citation2015; Katwika et al. Citation2019; Tabelin et al. Citation2021). Geometallurgical modeling may help in achieving improvement (Boisvert et al. Citation2013; Rincon, Gaydardzhiev and Stamenov Citation2019), though recovery of all elements of interest at high efficiency is difficult, if possible at all (Mudd, Jowitt and Werner Citation2017). Further technologies that may improve recoveries are sensor-based sorting, machine learning and selective flocculation (Spooren et al. Citation2020):
Other valuable elements than Cu may be channeled into other concentrates than copper concentrate, with some loss of Cu to those other concentrates (e. g. Li et al. Citation2013; Manouchehri Citation2018; Yin et al. Citation2017). There are commercial concentrator processes generating more than one concentrate. At Boliden (Sweden) a complex copper ore is processed into three outputs: concentrates of Cu, Pb and Zn (Manouchehri Citation2018). At Norilsk (Russia) ores are processed into three concentrates, of Cu, Ni and Co respectively (Fröhlich et al. Citation2017). Flotation may generate Cu and Co concentrates when substantial amounts of these elements are present in ores (Sracek et al. Citation2010); such technology has been applied in the African Copperbelt (Sole et al. Citation2019). Concentrates derived from Cu-Mo-Au-Re porphyry ore deposits can be converted to copper concentrates and molybdenite concentrates, with the latter containing most of the Re (Izatt et al. Citation2014; Mudd, Jowitt and Werner Citation2017).
The generation of more than one concentrate does not necessarily mean that the recovery of companion metals is optimized. There may be optimization detrimental to specific companion elements. In several cases of processing Cu-Ni-Co ores the presence of Cu and Ni in concentrates was reported to have been maximized to the detriment of Co (Mudd et al. Citation2013b).
As to the concentrator tailings there is a case for reprocessing, aiming at the generation of geochemically scarce elements and sufficiently clean matrix materials. In the case of old tailings with relatively high concentrations of other valuable geochemically scarce elements application of flotation technology may be useful in reprocessing, as shown by Parviainen, Soio and Caraballo (Citation2020) focusing on Au and Cu. For rare earths, Abaka-Wood, Addai-Mensah and Skinner (Citation2021) found that froth-flotation of Australian copper ore concentrator tailings might be conducive to a 90% recovery, and magnetic separation to a 86% recovery. (Bio)hydrometallurgy (cf. sections 2.1 and 2.2.3) is another option to exploit in reprocessing concentrator tailings. (Bio)hydrometallurgy applied to concentrator tailings can be conducive to the recovery of Ag, Au, Co, Cu, Ni, Pb, rare earths, Th, U, V and Zn, (Liu et al. Citation2007; Kondriat´eva et al. Citation2012; Lutandula and Maloba Citation2013; Chen et al. Citation2014; Chmielewski, Wawzczak and Brykala Citation2016; Falagán, Grail and Johnson Citation2017; Alcalde, Kelm and Vergara Citation2018; Altikaya et al. Citation2018; Shengo Citation2021; Sole et al. Citation2019; Araya, Kraslawski and Cisternas Citation2020; Fomchenko and Muravyov Citation2020; Drobe et al. Citation2021; Zhang et al. Citation2020). Particle sizes of the tailings may be at variance with successful conventional heap-leaching, which would imply heap leaching of agglomerated tailings or the use of relatively costly tank-based hydrometallurgy (e. g. Alcalde, Kelm and Vergara Citation2018; Ghorbani, Franzidis and Petersen Citation2018; Hao et al. Citation2017). Much research and development work would seem needed to optimize the production of geochemically scarce elements by reprocessing of concentrator tailings.
2.2.2. Distribution of Cu and other geochemically scarce elements during smelting and recovery of these elements
Applying thermodynamics and kinetics to smelting and converting can be used to optimize generating outputs of geochemically scarce elements by guiding the partitioning of geochemically scarce elements in smelting and converting (Chen, Zhang and Jahanshahi Citation2013; Jak et al. Citation2019; Nakajima et al. Citation2011; Shihshin, Hayes and Jak Citation2018; Shuva et al. Citation2016; Sineva et al. Citation2021; Van Schalkwyk et al. Citation2018).
In smelting, Cu and other geochemically scarce elements present in concentrates are distributed over de metal (matte) phase, the slag phase and the gas phase. Elements distributed to the gas phase largely partition to recoverable smelter dusts. Determinants of the distribution of Cu and other geochemically scarce elements over the metal, slag and gas phase are (Bellemans et al. Citation2018; Chen, Zhang and Jahanshahi Citation2013; Chen et al. Citation2006; Forsén, Aromaa and Lindström Citation2017; Moats, Alagha and Awuah-Offei Citation2021; Nakajima et al. Citation2011; Shihshin, Hayes and Jak Citation2018; Shuva et al. Citation2016; Sridhar, Toguri and Simeonov Citation1997; Wang et al. Citation2017b):
the characteristics of the smelting operation (bath- or flash smelting; parameters such as temperature and partial oxygen pressure),
the composition of concentrate and slagging materials, and
the matte grade, that is the (weight)% of Cu in matte.
Jak et al. (Citation2019) and Sukhomlinov et al. (Citation2019) modeled the distribution of Ag, As, Au, Bi, Co. Cu, Ni, Pb, Pt group metals Sb, Sn and Zn over blister copper, slag and dusts, depending on converting conditions.
2.2.2.1. Geochemically scarce elements other than Cu partitioning to the metal phase in the production of matte and blister copper
In matte production and converting, noble metals, such as Ag, Au and Pt-group metals, partition to a large extent to the metal phase (Avarmaa et al. Citation2015; Dupont et al. Citation2016; Forsén, Aromaa and Lindström Citation2017; Schlesinger et al. Citation2011; Sineva et al. Citation2021; Sukhomlinov et al. Citation2019). Slag composition may be varied to increase the relative amounts of these elements ending up in the metal phase (e. g. Chen and Wright Citation2016). For several other geochemically scarce elements than the noble metals the percentages estimated to partition to matte are in . The ranges reflect differences in the smelting operations, the inputs therein and the matte grade.
Table 2. Estimated percentage of smelter input partitioning to matte for several geochemically scarce companion elements of copper (Moats, Alagha and Awuah-Offei Citation2021; Schlesinger et al. Citation2011).
A higher matte grade in flash smelting was reported to be associated with lower partitioning of As, Bi, Pb, Sn and Zn to matte (Forsén, Aromaa and Lindström Citation2017; Klaffenbach et al. Citation2021; Sineva et al. Citation2021). A higher Fe/SiO2 ratio increases the partitioning of Pb, and lowers the partitioning of As, to matte (Klaffenbach et al. Citation2021). In bath smelting the partitioning of Bi, Ni and Sb to matte was estimated to be lower than in flash smelting (Moats, Alagha and Awuah-Offei Citation2021; Schlesinger et al. Citation2011). In bottom blown smelters (applying a variety of bath smelting), a higher matte grade is reportedly associated with lower partitioning to matte of Pb and Zn, and higher partitioning of As, Bi and Sb (Wang et al. Citation2017b). At Anglo Platinum, matte produced from ore concentrates, containing Cu, Ni and Pt group metals, is slowly cooled to form crystals containing Pt group metals and Cu-Ni alloy respectively. Thereafter, the matte is ground and subjected to magnetic separation (Fröhlich et al. Citation2017). The residue remaining after separation is treated to obtain Pt-group metals (Fröhlich et al. Citation2017). In flash converting of matte Se and Te decrease in the metal phase when temperature and CaO addition increase (Yu et al. Citation2021).
2.2.2.2. Geochemically scarce companion elements in anode slime and electrolyte
Ag, As, Au, Bi, Cu, Ni, Pb, platinum-group metals, Sb, Se, Sn and Te can be present in anode slime that originates in the electrolytic treatment of copper anodes generated in primary production (Dupont et al. Citation2016; Forsén, Aromaa and Lindström Citation2017; Liu et al. Citation2014). Anode slimes have been reported to contain the (weight) percentages for geochemically scarce elements presented in . The actual percentages are dependent on the characteristics of the pryrometallurgical process and the inputs therein.
Table 3. Reported (weight) percentages in anode slime for a variety of geochemically scarce companion elements (Hait, Jana and Sanyal Citation2009; Lu et al. Citation2015; Forsén, Aromaa and Lindström Citation2017; Jin, Hu and Hu Citation2018; Moats, Alagha and Awuah-Offei Citation2021).
A wide variety of technologies is available for recovering geochemically scarce elements from anode slime: hydrometallurgical, pyrometallurgical and combinations thereof. No comprehensive comparative evaluation regarding the resource efficiency of these technologies and combinations thereof could be found. Forsén, Aromaa and Lindström (Citation2017) suggest that typical anode slime treatment currently includes: dissolving Cu, Ni and Te by oxidative acid leaching, roasting to recover Se by leaching, followed by smelting of residue and sequential electrolysis to recover the noble metals.
Substantial amounts of Ag and Au are currently commercially generated by anode slime processing (Forsén, Aromaa and Lindström Citation2017; Hedjazi and Monhemius Citation2014). Anode slime is currently also important as a source of Se and Te (e. g. Li et al. Citation2017a; Moats, Alagha and Awuah-Offei Citation2021; Xu et al. Citation2020b). For Te extraction from anode slime efficiencies of >99% have been reported in laboratory settings (Ma et al. Citation2015). For Se recoveries of up to 98% have been noted in laboratory settings (Hait, Jana and Sanyal Citation2009; Li et al. Citation2017a; Lu et al. Citation2015). The average Se recovery reported by 52 copper refiners was about 50% (Lu et al. Citation2015), suggesting that there is technical scope for increased co-production. Sb may also be produced from anode slime (Forsén, Aromaa and Lindström Citation2017; Moats, Alagha and Awuah-Offei Citation2021), but no significant production from this source has been noted (e. g. Moats, Alagha and Awuah-Offei Citation2021). The same holds for Bi (Moats, Alagha and Awuah-Offei Citation2021).
Apart from Cu, the elements Bi, Co, Ni, and Sb can dissolve in the electrolyte (Forsén, Aromaa and Lindström Citation2017). These elements can be removed from the electrolyte (Alam et al. Citation2007; Arroyo-Torralvo et al. Citation2017; Forsén, Aromaa and Lindström Citation2017). Ni and Co are reported to be commercially recoverable from electrolyte (Forsén, Aromaa and Lindström Citation2017; Tabelin et al. Citation2021). Sb and Bi are commercially recovered from electrolyte by several companies using ion-exchange, whereas in the case of Bi also molecular recognition technology is used commercially (Moats, Alagha and Awuah-Offei Citation2021).
2.2.2.3. Geochemically scarce companion elements present in slags of primary copper pyrometallurgy
Nakajima et al. (Citation2011) and Sukhomlinov et al. (Citation2019) have shown that, dependent on flux composition, in converting matte, the elements B, Cr, Ga, Ge, In, W and Zn may mainly, and Cd, Co, Ni, Re and Se may partially, partition to slags. Estimates for several geochemically scarce companion elements regarding the percentage of input with copper ore concentrate partitioning to slags are in . presents reported concentrations of geochemically scarce companion elements in slags from smelters in a number of counties. The ranges reflect the differences in pyrometallurgical processing and the inputs therein.
Table 4. Estimates for several geochemically scarce companion elements as to the percentage of input with Cu concentrate partitioning to slags of primary copper pyrometallurgy (Ayres, Ayres and Rade Citation2002; Makuei and Senanayake Citation2018; Miganei et al. Citation2017; Moats, Alagha and Awuah-Offei Citation2021; Wang et al. Citation2017b).
Table 5. Reported concentrations of geochemically scarce companion elements in slags from primary copper smelters in Canada, Chile, China, the Democratic Republic of Congo, Germany, Japan, Italy, Turkey, Uzbekistan and Zambia (Mikula et al. Citation2021; Tian et al. Citation2021).
As, Bi, Mo, rare earths and V have also been found in slags from primary copper smelters (Holland et al. Citation2019; Mikoda et al. Citation2019a; Tian et al. Citation2021). Though in practice slags are often discarded (Guo et al. Citation2016; Sanchez et al. Citation2004; Tian et al. Citation2021), reported concentrations in slags can offer commercial scope for reprocessing aimed at the recovery of geochemically scarce elements. Reprocessing of slags, aimed at increasing Cu yields, is commercially practiced in industry using flotation technology and slag cleaning furnaces (see section 2.1). Measurements regarding tailings of commercial slag processing found concentrations that may offer commercial scope for reprocessing generating geochemically scarce elements. Data regarding smelters in Finland, Namibia, Poland and Russia showed that slag tailings contained up to 0.08 (weight)% Co, up to 0.63 (weight)% Cu, up to 0.14 (weight)% Ni, up to 0.9 (weight)% Pb and up to 3.11 (weight)% Zn, whereas the presence of Bi, Mo, rare earths and Sb was also reported (Grudinsky et al. Citation2021; Holland et al. Citation2019; Mikoda et al. Citation2019a).
Data about the co-production of geochemically scarce companion elements from copper slags are patchy. In Zambia slags from primary smelters containing 1.5 (weight)% Cu and 1–3 (weight)% Co have been noted to be melted in an electrical furnace to obtain a metal containing Cu and Co (Sanchez et al. Citation2004). In Zimbabwe a top submerged lance smelter has been reported to be commercially used for the extraction of metals from a slag containing Co, Cu and Ni (Sanchez et al. Citation2004). The production of copper concentrate from slags has been reported to be at variance with the recovery of the companion elements Co and Ni (Shen and Forssberg Citation2003), but can be combined with the recovery of Mo (Sanchez and Sudbury Citation2013). Treatment in a slag cleaning furnace can be conducive to the recovery of geochemically scarce companion metals partitioning to the metal phase (e. g. Hughes Citation2000). Reductive sulfurizing smelting of slag with added CaO has been found conducive to Co recovery (Li et al. Citation2018b). Treatment in slag cleaning furnaces does give rise to dust formation. Such dust is likely to be landfilled but can also be treated by pyrometallurgy or hydrometallurgy to recover Pb and Zn (Perez-Moreno et al. Citation2018). Reduction of slag followed by magnetic separation offers scope for the recovery of Pb and Zn (Tian et al. Citation2021). Grudinsky et al. (Citation2021) and Wan et al. (Citation2021) studied sulfation roasting, followed by extraction with water, of respectively tailings of copper slag processing and copper slags and found technical scope for the extraction of Cu and other metals such as Co, Ni and Zn. Wan et al. (Citation2021) concluded that a process using slag and waste sulfurdioxide might be commercially viable. If (bio)hydrometallurgy is applied to treatment of (sufficiently comminuted) slags or tailings from slag processing, there is scope for the recovery of Cd, Co, Cu, Mo, Ni, Pb, rare earths, V and Zn (Arslan and Arslan Citation2002; Banza, Gock and Kongolo Citation2001; Fomchenko and Muravyov Citation2020; He et al. Citation2021; Kaksonen et al. Citation2017; Kinnunen et al. Citation2020; Mikoda, Potysz and Kmiecik Citation2019b; Potysz and Kierczak Citation2019; Potysz, van Hullebusch and Kierczak Citation2018; Tian et al. Citation2021; Turan, Sari and Miller Citation2017). All in all, there is technical scope for substantially increasing the production of geochemically scarce elements from slags and slag tailings.
2.2.2.4. Geochemically scarce companion elements present in dusts of primary copper pyrometallurgy
5–10% of the mass entering smelters processing ore concentrates may end up in dusts from primary copper pyrometallurgy (Ha et al. Citation2015). Estimated partitioning of several geochemically scarce companion elements present in copper concentrates to dusts of primary copper pyrometallurgy is presented in . The ranges reflect differences in pyrometallurgical processing and the inputs therein.
Table 6. Estimated partitioning of several geochemically scarce companion elements present in copper concentrates to dusts of primary copper pyrometallurgy (Ayres, Ayres and Rade Citation2002; Chen, Zhang and Jahanshahi Citation2013; González-Castanedo et al. Citation2014; Wang et al. Citation2017b; Avarmaa et al. Citation2019; Makuei and Senanayake Citation2018; Moats, Alagha and Awuah-Offei Citation2021).
When matte is low-grade, more of Sb tends to end up in smelter dust (Forsén, Aromaa and Lindström Citation2017). Sukhomlinov et al. (Citation2020) have pointed out that Ag and Sn may to a large extent partition to dusts of anode furnaces. Dusts of primary copper pyrometallurgy have furthermore been reported to contain substantial amounts of Bi, Ge, In, Ni and Re (Ayres, Ayres and Rade Citation2002; Cusano et al. Citation2017; Dupont et al. Citation2016; Forsén, Aromaa and Lindström Citation2017; González et al. Citation2017; Moldabayeva et al. Citation2015; Montenegro, Sano and Fujisawa Citation2013; Selivanov et al. Citation2014). Some Li and Rb has also been found in dusts of primary copper pyrometallurgy (González et al. Citation2017). Best available technology (Cusano et al. Citation2017) should be used to prevent the emission of smelter dusts, as such dusts are a threat to human health (Fry et al. Citation2020; González-Castanedo et al. Citation2014; Sorooshian et al. Citation2012).
Published data about the current co-production of geochemically scarce elements from primary copper pyrometallurgy dusts are patchy. As pointed out in section 2.1, dusts can be internally recycled and such recycling is practiced in industry, to increase copper yields. However, dust recycling may lead to the accumulation of elements which may be considered unwanted such as As, Bi, Hg, Ni, Pb, Sb and Zn (Agorhom et al. Citation2015; Dupont et al. Citation2016; Ha et al. Citation2015; Montenegro, Sano and Fujisawa Citation2013). Efforts to prevent such accumulation have led to treatment of smelter dusts before recirculation (e. g. Montenegro et al. Citation2013; Liu et al. Citation2018). Dusts may be pretreated by roasting and centrifugation (e. g. Okanigbe et al. Citation2019). They can be treated by (bio)hydrometallurgy, pyrometallurgy and combinations thereof (Bakhtiari et al. Citation2011; Dupont et al. Citation2016; Gao et al. Citation2021; Grudinsky, Dyubanov and Kozlov Citation2019; Ha et al. Citation2015; Liu et al. Citation2018; Lucheva, Iliev and Kolev Citation2017; Montenegro, Sano and Fujisawa Citation2013; Morales et al. Citation2010; Sabzezari et al. Citation2019; Shibayama et al. Citation2010; Vitkova et al. Citation2011; Xu et al. Citation2020a; Zhang et al. Citation2019b). Such treatments provide technical scope for the co-production of Cu and companion elements such as Ag, Bi, Cd, Co, Ge, In, Li, Ni, Pb, Re, Sb, Se, Sn and Zn (Chen et al. Citation2012; Gao et al. Citation2020, Citation2021; González et al. Citation2017; Grudinsky, Dyubanov and Kozlov Citation2018, Citation2019; Ha et al. Citation2015; Morales et al. Citation2010; Ozberk et al. Citation1995; Paz-Gómez et al. Citation2020; Sabzezari et al. Citation2019; Vitkova et al. Citation2011; Xu et al. Citation2020a; Zhang et al. Citation2019b). Dusts may be treated at the same location (Government of Canada Citation2018; Ozberk et al. Citation1995) or be sold to facilities elsewhere, e. g. to smelters for Pb and/or Zn production (Cusano et al. Citation2017; Grudinsky, Dyubanov and Kozlov Citation2019; Ha et al. Citation2015). Re may be commercially produced from copper smelter dusts. One primary copper producer does this exploiting hydrometallurgy and ion exchange technology (Sole, Mooiman and Hardwick Citation2017). Sb may be commercially produced from copper smelter dusts too (Dupont et al. Citation2016; Dupont and Binnemans Citation2017). This is practiced at the Rönnskär plant of Boliden (Sweden) (Shuva et al. Citation2017), which has been reported to process an ore containing about 0.16 (weight)% Sb (Weidenbach, Dunn and Tao Citation2016). Such practice is so far apparently rare (Dupont and Binnemans Citation2017). There is technical scope for substantially increasing the generation of geochemically scarce elements from dusts of primary copper pyrometallurgy.
2.2.3. Geochemically scarce companion elements in hydrometallurgical processing of copper ores
Commercial heap leaching processes often focus on Cu recovery, and not on geochemically scarce co-products. Exceptions can be found in the African Copperbelt (Democratic Republic of Congo and Zambia), where both Cu and Co are reported to be recovered following heap leaching (Alexander et al. Citation2018; Sole et al. Citation2019). Co recovery from leachate is often by precipitation following the addition of hydroxides, but may also be by solvent extraction or (chelating, ion exchange) resins (Botelho, Dreisinger and Espinosa Citation2019; Sole et al. Citation2019). There is technical scope for the recovery of other companion elements. Rhenium (Re) can be found in heap leaching solutions (Sole, Mooiman and Hardwick Citation2017). Use of activated carbon and ion exchange have been proposed as extraction technologies for such Re (Sole, Mooiman and Hardwick Citation2017; Waterman Citation2013), but no commercial extraction of Re from heap leaching-based solutions has been reported (Sole, Mooiman and Hardwick Citation2017). Orrego, Hernández and Reyes (Citation2019) noted the presence of substantial amounts of Mo and U in Chilean copper leaching solutions but did not report the commercial recovery thereof. One would furthermore expect that Cd, Cr, Ni, rare earths, V and Zn may be mobilized by bioleaching (Liu et al. Citation2020; Mikoda, Potysz and Kmiecik Citation2019b). As noted before, the geochemically scarce metals Cd, Cr, Ni, Zn tend to accumulate during the recycling in heap leaching of raffinate remaining after solvent extraction of Cu (Liu et al. Citation2020). This is likely to reduce the costs for the recovery of such metals from recycled raffinate using e. g. ion exchange technology or solvent extraction, if compared to recovery from virgin leachate. Supported liquid membranes ionic solvents, supported ionic liquid phases, and biosorption may also be used for recovering geochemically scarce co-products from leachates (e. g. Spooren et al. Citation2020), but no current commercial application thereof could be identified
Contained hydrometallurgical processes which may extract more than one metal from ore concentrates or fines from crushed ores have been developed (e. g. Akbulut et al. Citation2018; Alexander et al. Citation2018; Halinen Citation2015; Sole et al. Citation2019; Spooren et al. Citation2020: Vardanyan et al. Citation2019). In Africa there is widespread application of agitated tank leaching to co-extract Cu and Co (Sole et al. Citation2019). Also, stirred tank-based leaching is applied to copper-gold ore concentrates (Dreisinger Citation2016). Contained reductive leaching of oxidized copper-cobalt ores, using SO2, is practiced in the African Copperbelt (Sole et al. Citation2019). Tshipeng, Tsamala-Kanaki and Kime (Citation2017) have discussed the optimization of such leaching. Furthermore, pressurized oxidative acid leaching to co-produce Co and Cu is applied in the African Copperbelt (Alexander et al. Citation2018; Sole et al. Citation2019). There is also technical scope for the recovery of other geochemically scarce elements. Pressurized oxidative acid leaching processes can generate soluble sulfates from base metals other than Cu and Co, such as Ni, Pb and Zn, and may produce a residue from which precious metals Ag and Au, and possibly also Pt group metals, can be recovered (Dreisinger Citation2015; Jorjani and Ghahreman Citation2017; Safarzadeh, Horton and Van Rythoven Citation2018). Sequential solvent extractions can be used when more base metals are present in the leachate (Hedrich et al. Citation2018).
Pressurized oxidative leaching of copper ore concentrates can furthermore lead to co-leaching of Se and Te (Mokmeli, Dreisinger and Wassink Citation2015). This appears to have triggered efforts to reduce concentrations of Se and Te in Cu-electrowinning solutions, rather than efforts to generate Se and Te as co-products (Mokmeli, Dreisinger and Wassink Citation2015). Re is also solubilized in pressurized oxidative acid leaching and can be recovered (Muruchi et al. Citation2019), but no commercial recovery from leachate has been reported. All in all, there would seem to be technical scope for a substantial increase in the generation of geochemically scarce elements in the hydrometallurgical processing of copper ores and copper ore concentrates.
There is also technical scope for extracting geochemically scare elements from residues of hydrometallurgical processing. Hubau et al. (Citation2020) found that stirred-tank bioleaching can extract Cu, Co, Ni and Zn from residues remaining after heap leaching at a sulfidic polymetallic mine in Finland. Shengo et al. (Citation2021) have shown that additional Cu and Co might be extracted by reprocessing residues from commercial agitated tank leaching of Cu and Co. Wu, Ahn and Lee (Citation2021) obtained Au by hydrometallurgical reprocessing of residue from commercial pressure oxidation extraction of Cu from chalcopyrite ore.
2.3. Concluding remarks regarding sections 2.1. and 2.2
There appears to be substantial technical scope for increasing the production of geochemically scarce elements from copper ores. Developing flexible minerals- and residues processing plants may help in optimizing the production of geochemically scarce elements (Reuter Citation2016). As it stands, technology for commercially producing geochemically scarce elements has been much better developed for pyrometallurgy-based processing than for (bio)hydrometallurgical processing of Cu ores (also: Izatt et al. Citation2014). A major research and development effort to widen the scope for commercial (bio)hydrometallurgical extraction technologies for geochemically scarce elements may help in optimizing (bio)hydrometallurgy-based processing technology (also: Guezennec et al. Citation2017; Mahmoud et al. Citation2017). This regards especially (bio)hydrometallurgy for the extraction of copper ores and tailings from copper ore concentrators and for the extraction of slag tailings and dusts, preferably at low cost.
2.4. New technologies for extraction of geochemically scarce companion elements of copper
The extraction of geochemically scarce companion elements from copper ores should be an important matter in the future development of new technologies for copper ore processing. As pointed out in section 2.1, extraction of copper from ores by non-aqueous solvents is under investigation. Research has been reported regarding manganese nodules investigating the recovery of copper, nickel and cobalt, using 5,8-diethyl-7-hydroxy-6 dodecanone oxime in kerosene as extractant (Binnemans and Jones Citation2017). Reported solvometallurgical recovery efficiencies after reductive treatment of manganese modules at 600°C were 69% for copper, 44% for Co and 71% for Ni under laboratory conditions (Binnemans and Jones Citation2017). Extraction of oxidized metals by deep eutectic solvents has been reported for Co, Cr, Ni, V and Zn, besides Cu, and also for Ag, Au and rare earths (Spooren et al. Citation2020). A major research and development effort would seem necessary to establish the techno-economical potential of solvometallurgical extraction of copper ores and copper ore processing residues. In research regarding electrolysis of CuS, MoS2 and ReS2 in molten sulfide salts Sahu, Chmielowiec and Allanore (Citation2017) found that phase-separated grains of Mo and Re in liquid copper can be generated. This suggests there may be technical scope for the co-production of these elements in molten salt technology.
2.5. Valorization of matrix materials that are co-outputs of copper ore processing
Applications considered for valorization of matrix materials that are co-outputs of copper ore processing mainly regard building and construction materials in which matrix materials are used as substitutes for conventional materials such as gravel and sand. Most research has been done on matrix materials in tailings from copper ore concentrators and slags.
Valorizations of copper ore concentrator tailings by their application in construction and building materials (cement, geopolymers, bricks, paving stones, road base materials) have been proposed (Ahmari and Zhang Citation2012, Citation2013: Ince et al. Citation2020; Jian et al. Citation2020; Lam et al. Citation2020; Manjarrez and Zhang Citation2018; Wang et al. Citation2021). As the tailings contain geochemically scarce elements that after release may have negative environmental impacts (cf. section 2.2.1), comprehensive testing is necessary under a variety of conditions reflecting the conditions to which applied materials can be exposed. Testing should not only regard newly produced materials but should also include aging, wear, tear and recycling of these materials (e. g. Engelsen, van der Sloot and Petkovic Citation2017; Kosson et al. Citation2004). Also, radioactivity should be tested (e. g. Kubissa et al. Citation2020: Wang, Wang and Hang Citation2020).
Unfortunately, only very limited testing of leaching from construction and building products containing copper ore concentrator tailings has been reported. Ahmari and Zhang (Citation2013) studied leaching from newly-produced bricks containing copper ore concentrator tailings-based geopolymer-bricks and found limited leaching of As, Co, Cu, Mo and Zn. Jian et al. (Citation2020) studied leaching of Cu, Cr, Mo and Zn from newly-produced cement clinker derived from copper ore concentrator tailings and found limited leaching of these elements. In both cases leaching conditions did not fully reflect the conditions to which these materials can be exposed. Nor were aging, wear, tear and recycling considered.
No studies could be found regarding the valorization of matrix material in residues remaining after hydrometallurgy of copper ores or copper ore concentrates. There probably is scope for valorization of such residues as Komnitsas et al. (Citation2019), (Citation2021) have shown that, after acid Ni and Co extraction, hydometallurgical residues of polymetallic saprolitic laterites might be applicable in the construction sector.
Slags from primary copper ore pyrometallurgy contain matrix materials derived from copper ores and can also contain substantial amounts of geochemically scarce elements, including radioactive elements (cf. section 2.2.2.3; also, Kinnunen et al. Citation2020; Kubissa et al. Citation2020). Valorizations of slags by application in or as building and construction materials are practiced on a substantial scale (e. g. Duester et al. Citation2016; Potysz et al. Citation2016). Proposals regarding the application of comminuted slags in construction and building materials include: cement (mortar), concrete, asphalt mixes and fire resistant geopolymers (Kinnunen et al. Citation2020; Mitiadis et al. Citation2020; Murani, Siddique and Jain Citation2015; Prem, Verma and Ambily Citation2018; Raposeiras et al. Citation2021; Siddique, Singh and Jain Citation2020; Wang, Wang and Hang Citation2020).
In view of the presence in copper slags of geochemically scarce elements that may have negative environmental impacts (e. g. Potysz et al. Citation2015, Citation2016; Duester et al. Citation2017, comprehensive testing of leaching would seem needed under a variety of conditions reflecting the conditions to which applied materials can be exposed, and not only of newly produced materials but also regarding aging, wear. tear and recycling of these materials (e. g.; Engelsen, van der Sloot and Petkovic Citation2017; Kosson et al. Citation2004). Also, radioactivity should be tested (Kubissa et al. Citation2020).
Leaching studies regarding slags from primary copper production showing significant releases of hazardous substances have been published (Duester et al. Citation2017; Potysz et al. Citation2015, Citation2016), but due to the absence of legal limits these have not impacted applications of such slags in hydraulic constructions (Duester et al. Citation2016). Only very limited testing results regarding building and construction materials including powdered copper slags have been published. Wang, Wang and Hang (Citation2020) found leaching of As, Cu, Ni, Pb and Zn from newly produced cement containing powdered slag from a copper ore smelter, and also found that high additions of slag powder might be linked to a radiological hazard. He et al. (Citation2021) studied newly produced ordinary Portland cement composites with copper slags and reported limited leaching of Cd, Cu, Ni, Pb and Zn. Aging, wear, tear and recycling were not included in the studies of Wang, Wang and Hang (Citation2020) and He et al. (Citation2021).
(Bio)hydrometallurgical leaching of slags and slag tailings from primary copper production can provide scope for the extraction of Cd, Co, Cu, Mo, Pb rare earths, V and Zn, cf. section 2.2.2.3. Kinnunen et al. (Citation2020) showed that acid leaching of ground slag can substantially reduce the concentrations of Cu, Co, Pb and Zn, which can be recovered, and that the leached material can be successfully included in cement. Kinnunen et al. (Citation2020) concluded on the basis of their experiments that complete valorization of copper slag is feasible. However, studies also including aging, wear, tear and recycling cement with leached slag under real-world conditions relevant to exposure of organisms would seem necessary to assess whether such valorization is also environmentally acceptable. Expanding studies on extracting geochemically scarce elements present in slags may provide technical scope for safe valorization of slag-derived materials.
Dusts from pyrometallurgical copper ore processing contain matrix materials (e. g. Caplan et al. Citation2021) and can be subjected to extraction of geochemically scarce elements (see section 2.2.2.4). However, no study could be found about the valorization of dusts from which geochemically scarce elements were extracted. Also, no study could be found regarding the environmental acceptability of solvometallurgically extracted matrix materials.
All in all, there is technical scope for the valorization of matrix materials but much research and development is needed as to the environmental acceptability of valorized materials.
3. Co-production of copper and other geochemically scarce elements from end-of-life products, especially end-of-life electric and electronic equipment and car electronics
In dealing with products containing copper that are considered for disposal the first choice for efficiently and functionally conserving copper and other geochemically scarce elements is to focus on options such as refurbishment, remanufacture, reuse and cascading use (Graedel et al. Citation2019). When such options are exhausted, processing end-of-life products for materials recycling can be conducive to the recovery of Cu and, where applicable, other useful substances (e. g. Knapp Citation2018; Sun et al. Citation2016).
The worldwide collection rate of end-of-life copper as copper input for materials recycling is estimated at about 45% (Glöser, Soulier and Espinoza Citation2013; Henckens and Worrell Citation2020). Copper used in transporting electricity and data commonly has high purity and may account for > 50% of copper in current use (Henckens and Worrell Citation2020). Such copper may be recovered from cables and wires with high efficiency (Catinean et al. Citation2021; Li et al. Citation2017b: Lu et al. Citation2019; Tanabe et al. Citation2019; Xu et al. Citation2019; Zablocka-Malicka, Rutkowski and Szczapaniak Citation2015). Copper recovered from cables and wires may be subjected to fire refining to obtain conductive copper rods (Li et al. Citation2017b). Dedicating facilities for secondary pyrometallurgy to the processing of copper from high purity applications can be conducive to closed loop recycling of copper applied in electricity and data transport.
As outlined in the Introduction, copper applied in products may become closely associated with other geochemically scarce elements. Increasingly important is the recovery of Cu and other geochemically scarce elements from end-of-life or waste electrical and electronic equipment (WEEE) (Sun et al. Citation2016; Tesfaye et al. Citation2017). Outputs derived from processing (mining) WEEE (e-wastes), such as waste printed circuit boards containing relatively large amounts of geochemically scarce elements (Priya and Hait Citation2018a), are rather often used for the recovery of Cu and other geochemically scarce elements (e. g. Andersson, Söderman and Sandén Citation2019; Hagelüken Citation2015; Priya and Hait Citation2018a).
Printed circuit boards are also present in end-of-life cars, but they are currently hard to recover and they tend to be left in cars that can be shredded (Andersson, Söderman and Sandén Citation2019; Hagelüken Citation2015). Modular redesign of cars to make electronics easily recoverable from end-of-life cars and design of such modules for recycling is a necessary step in the direction of near-zero waste processing. The current copper output fraction from automobile shredders is used for copper production and residues of automobile shredders have been considered for the recovery of geochemical scarce elements (e. g. Andersson, Söderman and Sandén Citation2019; Gunaratne et al. Citation2020a; Jordao, Sousa and Cavalho Citation2016; Kodier, Williams and Dallison Citation2018; Lewis et al. Citation2011; Restrepo et al. Citation2017; Singh and Lee Citation2016). There are major geographical differences in the amount of Cu that is recovered after shredding. Soo et al. (Citation2017) estimated the recovery of Cu in the copper output of vehicle shredders in Australia at 4.1% and in Belgium at 91.8%. This suggests that there is often scope for increased Cu recovery by vehicle shredders. When a fee is paid, there is a potential for application of residues from automobile shredders in a copper smelter, processing both primary and secondary raw materials, but the scope for such smelting can be limited by the presence of other metals (e. g. Al and Hg) (Gunaratne et al. Citation2020a). In practice, automobile shredder residues are often landfilled (Gunaratne et al. Citation2020a,b).
A major problem for recovering Cu and other geochemically scarce elements from end-of-life electric and electronic equipment is the collection thereof (Gorman and Dzombak Citation2020; Reck and Graedel Citation2012). It has for instance been estimated that that less than 10% of end-of-life mobile phones arrive at industrial recycling facilities (Navazo, Villalba Mendez and Talens Peiro Citation2014; Welfens, Nordman and Seibt Citation2016). Reck and Graedel (Citation2012) estimated a retrieval rate for end-of-life electric and electronic equipment in the European Union in the order of 25–40%. Closed-loop take-back, including deposit-refund, systems for end-of life products can improve collection rates (Gong et al. Citation2021) and may also be conducive to design improvements aimed at the recovery of geochemically scarce elements.
In the following the focus will be on formal recycling of end-of-life products containing Cu and other geochemically scarce elements, especially of end-of-life car electronics and electric and electronic equipment, which is legal processing as it is practiced in industrialized countries. Formal recycling may be assumed to be more efficient than informal recycling in recovering rare elements (e. g. Parajuly et al. Citation2018; Sthiannopkao and Wong Citation2013). Formal recycling of WEEE has also been recently reviewed by Tabelin et al. (Citation2021); here additional information is provided.
Often it is argued that in mining complex end-of-life products, physico-mechanical technologies should be applied, as in copper ore processing (e. g. Vidyadhar and Das Citation2013; Mäkinen et al. Citation2015; Kaya Citation2016; Ruan and Xu Citation2016; Zeghoul et al. Citation2016; Wang et al. Citation2017a; Jeon et al. Citation2018; Nekouei et al. Citation2018; Ding et al. Citation2019). Formal physico-mechanical pre-processing of WEEE and end-of-life cars is currently associated with substantial losses of geochemically scarce elements (Andersson, Söderman and Sandén Citation2017). The more materials are interlinked and particles are interwoven in WEEE, the worse are the results of physico-mechanical separation, and more sequential separations lead to larger losses (Hagelüken Citation2006a,b). Chancerel et al. (Citation2009) estimated that in formal pre-processing of WEEE (depollution, shredding and sorting) on average about 40% of Cu is lost. The recovery of rare earths from formal pre-processing has been estimated at less than 2% (Marra, Cesaro and Belgiorno Citation2018a). In current formal recycling of WEEE losses of about 30–88% have been reported for Ag, Au, Pd, Pt and Rh (Bigum, Brogaard and Christensen Citation2012; Marra, Cesaro and Belgiorno Citation2018a; Wellmer and Hagelüken Citation2015). Changing formal WEEE pre-processing by selectively removing components or modules from end-of-life products which are relatively rich in geochemically scarce elements, would be conducive to increased recovery of these elements. Additional improvement may come from designing those modules for recycling. Also, there is the option of forgoing shredding. For instance, the Umicore plant (Belgium) uses as an input for its operation, after removal of batteries, relatively small devices such as mobile telephones, digital cameras and laptop computers (Hagelüken Citation2006b). A benefit thereof is that the losses of geochemically scarce elements due to physico-mechanical pre-processing are avoided (Ding et al. Citation2019; Hagelüken Citation2006b). In the case of precious metals this benefit is not invalidated when trade-offs linked to the presence of other metals are considered (Hagelüken Citation2006b). The Boliden Rönnskär plant also processes ‘coarse´ WEEE (Lennartsson et al. Citation2018). On the other hand, the Dowa plant at Kosaka uses crushing and separation as pre-treatment for WEEE (Shuva et al. Citation2017). Similarly, the Mitsubishi Naoshima smelter has been reported to use crushed e-waste and WEEE shredder output (Ariizumi et al. Citation2016; Kawasaki Citation2014),
It has been argued that hydrometallurgy (including biohydrometallurgy) enables ‘sound, metal-selective and cost-effective processing to recover metals from WEEE’ (Isildar et al. Citation2018). Sethurajan et al. (Citation2019) discussing hydrometallurgical recovery of critical and precious elements from preprocessed e-waste stated that ‘leaching and recovery of heavy metals is the best solution to meet the growing critical raw materials demand and also to reduce the environmental impacts caused by the WEEEs in the environment.’ However, Li, Eksteen and Oraby (Citation2018a) have reviewed current hydrometallurgical recovery of metals from waste printed circuit boards. In view of their composition these are relatively promising objects for extracting geochemically scarce elements, including Cu. Li, Eksteen and Oraby (Citation2018a) found that extraction with acids, cyanide-based chemicals, thiourea, thiosulfate, thiocyanate, halides and ammonia did not meet economic, technical and environmental requirements. A comparative life cycle study about the recovery of Ag, Au, Cu and rare earths from electronic waste by current hydrometallurgy and pyrometallurgy suggested that there was no clear ‘winner’ as to the life cycle environmental burden (Li et al. Citation2019). So far, there is only limited application of hydrometallurgy, subsidiary to pyrometallurgy in the processing of outputs of mining complex end-of-life products (e. g. Karhu et al. Citation2018). However, further research and development in the field may improve the scope for its application.
There is a substantial amount of published laboratory-scale biohydrometallurgy research regarding the extraction of geochemically scarce elements from (comminuted) printed circuit boards by bacteria and fungi (e. g. Priya and Hait Citation2017; Kumar, Sain and Kumar Citation2018; Priya and Hait Citation2018b; Xia et al. Citation2018; Gu et al. Citation2019; Li, Eksteen and Oraby Citation2018a; Liu et al. Citation2019; Samal et al. Citation2019; Krishnamoorthy, Ramakrishnan and Dhandopani Citation2021). In some of these studies (Gu et al. Citation2019; Liu et al. Citation2019) biohydrometallurgy was combined with mechanical activation. The studies show that in principle, besides Cu, a substantial range of geochemically scarce elements may be extracted with varying recoveries. These include, Ag, Au, Cd, Co, Ni, Pb, rare earths, V and Zn. There is also limited research on biohydrometallurgical extraction of shredder dust (Marra et al. Citation2018b). Biohydrometallurgical studies have been judged encouraging, when reviewed by Priya and Hait (Citation2017) and this judgment remains defensible in view of the studies published after 2017. But a large concerted research and development effort would seem to be needed to assess whether industrial biohydrometallurgical production of a substantial range of geochemically scarce elements from mined complex end-of-life products is feasible (also: Priya and Hait Citation2017). Such an effort is also needed to assess the potential for the application of solvometallurgy.
Electrochemical extraction of Ag, Au, base metals and rare earths from electronic waste has been suggested. One process applies a mild oxidant (Fe3+) (Diaz, Clark and Lister Citation2017; Li et al. Citation2019). Another process is suspension electrolysis, generating metals (Yun et al. Citation2019). There is as yet no reported commercial application of such electrochemical technologies.
A further option is processing of mined WEEE and car electronics at elevated temperature. Substantial research has been published about treatment of printed circuit boards by pyrolysis (e. g. Williams Citation2010; Flandinet et al. Citation2012; Bidini et al. Citation2015; Riedewald and Sousa-Gallagher Citation2015; Kim et al. Citation2018; Khanna et al. Citation2018; Ulman et al. Citation2018). No commercial implementation of such pyrolysis-based treatments has been reported. Panda et al. (Citation2020) presented laboratory-scale pyrolysis of printed circuit boards, followed by roasting the metal-rich pyrolysis residue in the presence of excess NH4Cl, leading to the formation of metal chlorides to be dissolved in water. High extraction yields were reported for the base metals Cu, Ni, Pb and Zn, whereas the maximum recovery of Au and Ag was reported to be 42–43% (Panda et al. Citation2020). The relatively low recovery of the noble metals is a problem as the prices to be realized for extracted Ag and Au are important determinants of the commercial feasibility of a recovery process (Fizaine Citation2020).
Copper smelting at about 1200–1350 °C, followed by converting and fire refining, is widely used for the commercial recycling of geochemically scarce elements present in complex end-of-life electric and electronic equipment. The presence of relatively valuable elements (such as Au, Ag and Pt-group metals) in the outputs of mining complex end-of-life products can be conducive to the recovery of other geochemically scarce elements by pyrometallurgy (Zeng, Mathews and Li Citation2018). A potential trade-off between the recoveries in pyrometallurgy, when more than one element is recovered, has been reported for Pd and Ge (Shuva et al. Citation2017). Potential trade-offs also apply to Cu and the precious metals Ag and Au (Ghodrat et al. Citation2017) and to Au and Pd and on the other hand Ag (Avarmaa et al. Citation2019). There is presently no recycling technology that can simultaneously recover Ag and Ta (Ueberschaar et al. Citation2017). A solid basis of thermodynamic and kinetic data may help optimizing recoveries of geochemically scarce elements in pyrometallurgy (e. g. Shuva et al. Citation2017; Van Schalkwyk et al. Citation2018).
Outputs of mining complex end-of-life electric and electronic equipment containing copper can be fed to secondary smelters. The main steps in secondary pyrometallurgy of copper starting with end-of-life products containing copper are in . Secondary copper smelters generate a copper output with about 80 (weight)% Cu (‘black copper’) (Ghodrat et al. Citation2019; Schlesinger et al. Citation2011). Secondary smelters include electric furnaces, submerged lance furnaces and bath melting reactors (Steinacker and Antrekowitsch Citation2017; Tabelin et al. Citation2021). Black copper can be added to converters producing rough copper or blister copper (Forsén, Aromaa and Lindström Citation2017; Ghodrat et al. Citation2017; Schlesinger et al. Citation2011). Rough copper can be added to anode furnaces (e. g. Ghodrat et al. Citation2017; Gregurek et al. Citation2018). Copper anodes can be subjected to dissolution and electrolysis to generate copper cathodes, as described in section 2.1.
Figure 2. Main steps in pyrometallurgical secondary production of copper (cathodes) from end-of-life products containing copper.
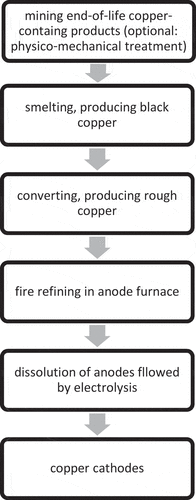
There are secondary copper smelters involved in co-producing a wide range of geochemically scarce elements (cf. ). Ag, Au, Bi, Cu, In, Ir, Ni, Pb, Pd, Pt, Rh, Ru, Sb, Se, Sn, and Te are recovered in standard runs by the Umicore (Belgium). This company processes industrial residues and (mined) end-of-life products. Umicore applies copper, lead and nickel smelting, electrochemistry and hydrometallurgy (Hagelüken Citation2015; Hagelüken and Corti Citation2010; Kaya Citation2016; Khaliq et al. Citation2014; Stamp, Althaus and Wäger Citation2013). The Umicore plant is flexible, as it can recover e. g. Co, Ga or rare earths in special runs (Hagelüken Citation2015). But the Umicore plant is e. g. unable to recover tantalum (Ta) (Hagelüken Citation2015). Aurubis (Germany), where primary and secondary copper production share a refinery and a lead smelter is also present, is reported to recover Ag, Au, Bi, Ni, Pb, platinum group metals, Sb, Se, Te and Zn as co-products, using pyrometallurgy, electrochemistry and hydrometallurgy (Aurubis Citation2017; Karhu et al. Citation2018; Rocchetti, Amato and Beolchini Citation2018; Zschiesche, Ayhan and Antrelkowitsch Citation2018).
Table 7. Reported relatively rare elements produced by plants co-processing mined complex end-of-life products containing copper (references in text).
Alternatively, outputs of mining complex end-of-life electric and electronic products can be fed to the large pyrometallurgical plants processing copper ore concentrates (Forsén, Aromaa and Lindström Citation2017). Here the focus will be on plants that generate a relatively large number of geochemically scarce elements.
The Boliden Rönnskär plant (Sweden) co-processes secondary sources of Cu, including coarse and fragmented WEEE (Lennartsson et al. Citation2018; Rocchetti, Amato and Beolchini Citation2018). At the Rönnskär plant, e-waste is first processed in a secondary smelter; black copper, with or without slag, is subsequently added to the converter of the primary copper smelter (Lennartsson et al. Citation2018). Also, at the Rönnskär plant a lead smelter serves the production of WEEE-derived geochemically scarce elements (Lennartsson et al. Citation2018). The Boliden Rönnskär plant is reported to recover Ag, Au, Cu, Ni, Pb, Pt-group metals, Se and Zn (Andersson, Söderman and Sandén Citation2019; Khaliq et al. Citation2014). Dust from this plant is processed to recover Sb, In and Cu (Shuva et al. Citation2017). A 2017 report about the Rönnskär smelter stated that 28% of Ag, 38% of Au, 27% of Cu and 72% of Zn produced by the smelter originated in secondary raw materials (Boliden Citation2017). The Dowa Kosaka plant (Japan), co-processing Cu ore concentrate and e-waste, is able to recover Ag, Au, Bi, Cu, Ni, Pb, Pt-group metals, Sb, Se, Sn and Te (Shuva et al. Citation2017). In the case of Dowa, Bi, Pb, Sb and Sn are outputs of a lead smelter and refinery processing copper smelter residues (Mitsune and Satoh Citation2007). The Glencore Horne copper pyrometallurgical plant, co-processing copper ore concentrates and outputs of mining end-of-life products containing copper (including e-waste), co-produces Ag, Au, Ni, Pt-group metals, Se and Te (Glencore Citation2018). The Naoshima smelter of Mitsubishi (Japan) has been reported to co-process copper concentrate, crushed e-scrap and WEEE shredder output, and to recover Ag, Au, Cu, Pb, Pt and Se (Ariizumi et al. Citation2016; Kawasaki Citation2014).
The Aurubis, Boliden and Dowa plants are characterized by the presence of both copper and lead processing, with copper and lead functioning as collectors for other geochemically scarce elements (also: UNEP Citation2013; Van Schalkwyk et al. Citation2018). Umicore in addition also uses a nickel. smelter. The systematic use of copper and lead as collectors for geochemically scarce elements may be expanded by cooperation. It is e. g. not uncommon that pyrometallurgical plants processing copper ore outsource processing of copper smelter dusts to lead smelters (see section 2.2.2.4). It is also not uncommon that co-outputs of lead smelters (crusts, dusts, matte, speiss) are processed in pyrometallurgical plants processing copper (e. g. Clement, Wettlaufer and Scott Citation1986; Ha et al. Citation2015; Ibragimov et al. Citation2019; Kotarski et al. Citation1999).
As indicated in , processes used by companies such as Aurubis (Germany), Boliden (Sweden), Dowa (Japan) and Umicore (Belgium) do relatively well in generating geochemically scarce elements from WEEE. They should be considered for wider application. They, however, are limited in their performance if compared with the range of companion elements in electric and electronic equipment and car electronics presented in . They also might be improved as to their emissions. For instance, Sutliff-Johansson et al. (Citation2021) found that processing of WEEE scrap by the Rönnskar plant of Boliden is associated with substantial Ta emissions. The Umicore plant processing WEEE in Belgium is a point source for As, Cd and Pb emissions to air which should be reduced (Vlaamse Milieu Maatschappij Citation2018).
All in all, there appears to be substantial technical scope for increasing the production of geochemically scarce elements from end-of-life electric and electronic equipment and car electronics.
Only a few studies could be found specifically addressing the extraction of geochemically scarce elements from residues generated in the secondary production of copper. Ahmed, Nayl and Daoud (Citation2016) and Xia et al. (Citation2020) found that much Cu and Zn might be recovered from smelting slag of waste brass by leaching with sulfuric acid and ammonium chloride respectively. Wijenayake and Sohn (Citation2020) studied the extraction of ZnO, for application in tires, from flue dust of a secondary smelter. Other geochemically scarce metals present in the flue dust (Cd 3.9 weight%, Cu 13.0 weight% and Pb 6.7 weight%) were not recovered in the study of Wijenayake and Sohn (Citation2020). Valorization of residues emerging from the treatments studied by Ahmed, Nayl and Daoud (Citation2016), Wijenayake and Sohn (Citation2020) and Xia et al. (Citation2020) was not addressed. In view thereof the valorization of residues from secondary copper smelters would require much additional research and development work.
4. Hurdles for the feasibility of near-zero waste production
Co-producing companion elements from copper ores and co-processing copper concentrates with wastes can have economic benefits. If there are substantial amounts of marketable companion elements present in copper ores (e. g. Mudd, Weng and Jowitt Citation2013a,b), there may be a financial case to consider ores containing copper ores as ores from which more geochemically scarce elements than copper may be extracted. Improving the case for such extraction may occur when copper concentrations in copper ores continue to decrease (Batterham Citation2013; Sverdrup, Olafsdottir and Ragnarsdottir Citation2019), and when interest in exploiting complex low-grade polymetallic ores increases (e. g. Mahmoud et al. Citation2017; Spooren et al. Citation2020). Co-production of companion elements from copper ores may have some protective effects against the fluctuations of individual metal prices (e. g. Afflerbach et al. Citation2014; Zemlick et al. Citation2017). Though there are, or can be, trade-offs as to yields in such co-production (e. g. Li et al. Citation2013; Reuter Citation2016; Shuva et al. Citation2016; Yin et al. Citation2017), financial benefits may well increase when more than one element is produced from copper ore (e. g. Dreisinger Citation2016; Lasheen et al. Citation2015; Sole et al. Citation2019). Pyrometallurgical co-processing of copper concentrates with mined end-of-life electric and electronic equipment can increase financial returns (Knapp Citation2018; Zeng, Mathews and Li Citation2018). Generating extracted matrix materials from ore processing residues which can be applied in construction and building materials may have financial benefits (e. g. Kinnunen et al. Citation2020; Spooren et al. Citation2020).
However, there are examples of company behavior that are at variance with progressing in the direction of near-zero waste primary copper production. Recoveries of geochemically scarce elements may well be lower than recoveries achievable by the application of best available commercial technologies (Mudd et al. Citation2013b; Pazik et al. Citation2016; Reuter and Kojo Citation2014; Sole et al. Citation2019). Furthermore, many smelters of copper ores restrict inputs of ‘penalty elements´. Such elements may cause hazardous outputs, copper anode passivation or excessive slag viscosity, or may negatively impact the properties of the final copper product (e. g. Agorhom et al. Citation2015; Dupont et al. Citation2016; Lane et al. Citation2016; Tabelin et al. Citation2021; Weidenbach, Dunn and Tao Citation2016). This, in turn, tends to lead to extraction and landfilling of penalty elements (e. g. Weidenbach, Dunn and Tao Citation2016). But the penalty elements Bi, Ni, Pb, Sb, Se, Te and Zn can also be marketable outputs of copper production. Handling companion elements such as Bi, Ni, Pb, Sb, Se, Te and Zn as potential co-products rather than as penalty elements, for instance by reprocessing extracted penalty elements, is likely to be conducive to the co-production of these elements.
A further problem for near-zero waste processing would seem that there may well be insufficient demand for potential product outputs of near-zero waste processing. Generating outputs that might be marketed entails costs. As shown in the previous sections there are cases in which the costs are such that the outputs can be commercially sold. This is not necessarily so for potential product outputs that are technologically possible within the framework of near-zero waste processing. In the following hurdles will be presented based on available studies regarding the following potential outputs: valuable geochemically scarce elements (section 4.1), outputs of matrix materials (section 4.2) and hazardous elements with potential supplies that exceed demand (section 4.3).
4.1. Valuable geochemically scarce elements
In view of life cycle losses, quantitatively important sources of geochemically scarce elements are concentrator tailings and complex end-of-life products, such as WEEE and end-of-life car electronic components. Geochemically scarce elements extracted from such sources are currently undifferentiated commodities: ´copper is copper and selenium is selenium´ (Casarin, Lazzarini and Vassolo Citation2020; Olvera Citation2021). So, to be commercially viable in current economic settings, production costs have to be competitive with those of conventionally produced elements. In practice this may be a hurdle. There are several cases in which Cu extraction by reprocessing of copper ore concentrator tailings was shown to be probably profitable at current copper prices (Chen et al. Citation2014; Drobe et al. Citation2021; Falagán, Grail and Johnson Citation2017). But there are also cases that commercial viability of the extraction of valuable elements from concentrator tailings has not been categorized as probable. Parviainen, Soio and Caraballo (Citation2020) concluded that in the case of reprocessing (old) Au-Cu tailings from the Haveri mine in Finland Au recovery would probably be commercially viable, but that commercial viability was uncertain for Cu and Co recovery. Araya, Kraslawski and Cisternas (Citation2020) suggested that heap leaching of the tailings in the Antofagasta region of Chile to recover V was likely to be commercially viable, whereas there would be a marginal commercial case for the recovery of rare earths (Araya, Kraslawski and Cisternas Citation2020). Araya, Kraslawski and Cisternas (Citation2020) also concluded and that recovery of Co, Nb and Sb was not commercially viable. In addition, Araya et al. (Citation2021) studied extraction of the rare earth Sc by hydrometallurgy with an estimated recovery of 80%. It was found that estimated capital costs of such extraction would probably be too high for the production of Sc at a competitive price. Araya et al. (Citation2021) concluded that changing extraction technology in a way that capital costs are lowered would be necessary to make extracted Sc a viable product.
Current prices used in establishing the commercial viability of generating geochemical scarce elements by reprocessing may be at variance with future prices. Both higher or lower prices may occur. Lower prices, which would negatively impact commercial viability, may originate in large increases of production linked to reprocessing. This may be exemplified by Se and Te. From data presented by Moats, Alagha and Awuah-Offei (Citation2021) one might estimate that extraction of Se and Te from copper ore concentrator tailings with an efficiency of about 80% may increase the worldwide production of Te by about a factor 100 and for Se by about a factor 50. In section 4.3 it will emerge that there is limited stockpiling for future use of As and Cd. It is conceivable to also stockpile Se end Te for future use. But in view of the wide disparity between potential supply and current demand the feasibility thereof is uncertain. Strongly increasing demand for Se and Te and cutting back the primary production of copper would be other options. But, again, the feasibility thereof is uncertain.
As to WEEE and electric and end-of-life car electronics, the concentrations of valuable geochemically scarce elements in mined wastes may well be too low for commercially viable recovery (Andersson, Söderman and Sandén Citation2017, Citation2019; Fizaine Citation2020). Improved recoveries of geochemically scarce elements from end-of-life electrical and electronic equipment, and cars, may be achieved by redesign of products and by selectively removing components or modules from end-of-life products which are relatively rich in specific geochemically scarce elements (e. g. Charles et al. Citation2020; Tabelin et al. Citation2021) and subjecting these to pyrometallurgical processing in smelters selected on the basis of component or module composition (also: Reuter, van Schaik and Ballester Citation2018). Such an approach may however still have its limitations, as it may well be that for specific elements (e. g. Ga, In, lanthanides, Ta, Y) concentrations remain too low (e. g. Andersson, Söderman and Sandén Citation2019; Liu and Keoleian Citation2020). This problem may perhaps be overcome by subjecting these components or modules to technologies that can upgrade and concentrate geochemically scarce elements, such as cryo-jacking, microwave ashing and treatment in solder baths (Charles et al. Citation2020), but applying these technologies adds to production costs which can negatively affect the competitive position of geochemically scarce elements generated from WEEE and electronic parts of end-of-life cars. If the valuable geochemically scarce elements produced from copper ores and end-of-life products remain undifferentiated commodities (Casarin, Lazzarini and Vassolo Citation2020; Olvera Citation2021), the hurdles presented in this section would seem formidable.
4.2. Matrix materials
As indicated in section 2.5, matrix materials derived from tailings and slags are substitutes for conventional construction and building materials such as sand and gravel. No characteristic of matrix materials could be found which would currently justify a relatively high price of matrix materials in their competition with conventional construction and building materials. Relatively low production costs of matrix materials would benefit their competitive position, but clean-up costs of matrix materials may well be at variance with low production costs. On the other hand, local scarcity of conventional construction and building materials would favor the competitive position of unconventional materials such as matrix materials (cf. Torres et al. Citation2021; Ulsen et al. Citation2021).
If compared with valuable elements as potential product outputs of processing copper ores, matrix materials are characterized by a much larger mass and by a relatively low price, with transport being a major factor in costs. Apart from exceptional cases such as the imports of construction and building materials by Dubai and Singapore, transport distances for gravel and sand are typically <100 km −300 km (Ioannidou et al. Citation2017; Torres et al. Citation2021; Ulsen et al. Citation2021). In view of such transport distances and the large volumes of matrix materials that are currently non-product outputs, large markets within 300 km would seem important for the viability of matrix materials as product outputs for application in construction and building. This is a substantial hurdle in cases that primary processing is remotely located and/or in thinly populated areas.
4.3. Hazardous elements
As and Cd are two hazardous elements that can occur in inputs used for the primary and secondary production of copper (Arslan, Djamgoz and Ukün Citation2016; Kammel, Göktepe and Oerlmann Citation1987; Sakoor et al. Citation2017; Schwartz, Omaynikova and Stocker Citation2017; Shi et al. Citation2020; Vermeulen et al. Citation2015; Yang et al. Citation2013). In both cases the potential supply of these elements as emerging from primary and secondary metallurgy is higher than demand (Karhu et al. Citation2018; Mudhoo et al. Citation2011; US Geological Survey Citation2020). Against this background in principle two options may be considered: stockpiling for future use and indefinite sequestration. Some stockpiling for future use is an option that has been chosen for Cd in the European Union (Karhu et al. Citation2018). When Cd is considered to be an element of strategic or critical importance for the economy (e. g. Randive and Jawadand Citation2019), this can be conducive to such stockpiling. Some stockpiling of As for future use has been reported for China (US Geological Survey Citation2020). In the case of sequestration in stable substances to be stored indefinitely in landfills, there is still a waste, but one might consider risk of storage in landfills to be removed. Long-term stability of sequestered cadmium has not been much studied. Tian et al. (Citation2020) found stability of Cd sequestration by suspended ferrous sulfide nanoparticles for 717 days under aerobic and anaerobic conditions with pH kept at 7. However, it is unlikely that the condition of pH 7 can be indefinitely met in landfills. Sequestration has been much discussed for As. However, finding stable substances to indefinitely sequester As appears far from easy. Traditionally, Fe3+ and arsenate were co-precipitated in pools for this purpose. Precipitates with a high Fe/As ratio are stable under oxidizing conditions at pH 4 and in the presence of heavy metals (Riveros, Dutizac and Spencer Citation2013). It is highly unlikely that such conditions can be indefinitely met in landfills. Li et al. (Citation2020) have advocated immobilization of As using copper slag. This gives rise to the formation of silica gel coated-FeAsO4. (Li et al. Citation2020). However, silica gel is not indefinitely stable when exposed to water (Sögaard et al. Citation2018). It has furthermore not been proven that the stabilization of As by interaction with sludge from a paper factory, as proposed by Morales et al. (Citation2010), provides sequestration of As in case of indefinite storage in landfills.
A crystalline octagonal variety of FeAsO4(.2 H2O), scorodite, is considered to have a greater thermodynamic stability than traditional Fe/arsenate co-precipitate, and has often been proposed as a candidate for the stable sequestration of As (e. g. Coudert et al. Citation2020; Okibe et al. Citation2014; Riveros, Dutizac and Spencer Citation2013; Rong et al. Citation2020; Schwartz, Omaynikova and Stocker Citation2017; Sun et al. Citation2018). However, exposure to natural waters or atmospheric weathering can degrade scorodite to goethite inducing the release of As. The stability of scorodite in landfills is limited to aerobic conditions and a pH of 2–5 (e. g. Nazari, Radzinski and Ghahreman Citation2017; Yuan et al. Citation2017). Under reducing condition and at a pH<2 or >5 there is substantial solubility of As from scorodite (Langmuir, Mahoney and Rowson Citation2006; Nazari, Radzinski and Ghahreman Citation2017; Paktunc and Bruggeman Citation2010; Yuan et al. Citation2017, Citation2016). Reducing conditions and pHs <2 or >5 may well occur over indefinite periods of storing scorodite in landfills. Moreover, As may be released from scorodite by iron reducing bacteria such as varieties of Shewanella and Desulfururomonas (Papassiopi, Vaxevanidou and Paspaliaris Citation2003; Revesz, Fortin and Pactunc Citation2015, Citation2016; Wang et al. Citation2016).
In view of the oxic conditions and the limited pH range required for scorodite stability, a variety of coatings have been proposed to reduce the release of As by scorodite under anaerobic conditions and at pH >5 and <2. These include coatings of ferric hydroxide sulfate, of Al or Ca phosphate, of Al (OH)3/AlOOH, and of silicate-derived gels (Adelman, Elouatik and Demopoulos Citation2015: Coudert et al. Citation2020; Guo and Demopoulos Citation2018; Ke and Liu Citation2018; Ke, Song and Liu Citation2018; Lagno et al. Citation2010; Leetma et al. Citation2016; Nazari, Radzinski and Ghahreman Citation2017). Silicate-derived gels are not indefinitely stable when exposed to water (Sögaard et al. Citation2018). Moreover, Adelman, Elouatik and Demopoulos (Citation2015) noted that the silicate-derived gel they studied (at pH 6) only marginally affected, or even increased, the release of As from scorodite due to ion exchange. The As containment by Al or Ca phosphate coatings may not remain protective in the long run due to loss of phosphate (Lagno et al. Citation2010; Leetma et al. Citation2016). Reduced leaching of As from scorodite coated with ferric hydroxide sulfate seems linked to preferential solution of the latter (Ke and Liu Citation2018), which strongly suggests that the reduction of As leaching from scorodite by this coating will not be indefinite. Coudert et al. (Citation2020) pointed out that the long-term effect of coating of scorodite with Al(OH)3/AlOOH should be further investigated. Arsenical natroalunite has been proposed as a more stable alternative to scorodite (Xu et al. Citation2020a). But testing such stability (Xu et al. Citation2020a) has so far been very limited and has not covered all conditions that may be associated with indefinite storage.
Other options for sequestration of As that have been suggested include vitrification, generating a glass, and cement-based solidification/stabilization technology. Solidification/stabilization technology is at risk of As release after long periods of time (ZZhao et al. Citation2017). Glass is metastable, subject to coarse-graining over time (e. g. Brito and Wyart Citation2006), and thus also appears to be at risk of As release over long time periods.
So far, the feasibility of indefinite sequestration of Cd and As for storage in landfills has not been demonstrated. A major research and development project aimed at the safe handling of hazardous element such as As and Cd would seem to be needed.
4.4. Valuing the hurdles
In view of the hurdles presented here, the feasibility near-zero waste production of copper and its geochemically scarce companion elements from copper ores, WEEE and end-of-life car electronics is uncertain.
5. Conclusions
This paper considered the feasibility of near-zero waste production of copper and its companion elements. It can be concluded that there is technical scope for significant progress in toward near-zero waste processing of copper ores and end-of-life electric and electronic equipment and car electronics. The generation of concentrates can be improved using e. g. geometallurgical modeling, sensor-based sorting, improved flotation and machine learning. Use of kinetic and thermodynamic data regarding smelting and converting can assist optimizing the co-production of geochemically scarce elements in the pyrometallurgical processing of copper ore. There is substantial technological scope for increasing the production of geochemically scare elements and cleaning-up matrix materials by improved processing and reprocessing of residues from concentrators and pyrometallurgical processing of copper ore. Developing flexible minerals- and residues processing plants may help in optimizing the co-production of companion elements. There is a case for narrowing gaps in knowledge which may be conducive to progress toward near-zero waste production. The research and development efforts to narrow these gaps are highlighted in . A major research and development effort to widen the scope for commercial (bio)hydrometallurgical extraction technologies may be conducive to optimizing (bio)hydrometallurgy-based primary processing technology producing geochemically scarce elements and cleaned-up matrix materials. This especially regards (bio)hydrometallurgy, at preferably low cost, for the extraction of copper ores, of copper ore tailings, and of dusts, slags and slag tailings from primary pyrometallurgy. Assessing the techno-economical scope for solvometallurgical processing of copper ores and copper ore processing residues for the production of a substantial range of geochemically scarce elements also requires a major research and development effort. Much research and development work is furthermore needed as to the environmental acceptability of valorized matrix materials. In developing new copper extraction technologies, the co-production of other geochemically scarce elements should be an important matter.
Well-considered modularization of products, design of modules for recycling and changes in the pre-processing of end-of-life products can help in improving the generation of geochemically scare elements from end-of life car electronics and electric and electronic equipment. A comparatively large variety of geochemically scarce elements may be recovered when both lead and copper serve as collectors in smelting mined car electronics and end-of life electric and electronic equipment. Process design aimed at obtaining outputs which can be efficiently processed by others into usable elements and guidance of smelting and converting by data about kinetics and thermodynamics can be conducive to improved recovery of geochemically scarce elements. The same holds for flexible smelters dealing with the outputs of mining WEEE and end-of-life electric car electronics. Slags and dusts from secondary pyrometallurgical copper production should be reprocessed for the extraction of geochemically scarce elements and the generation of sufficiently clean residual materials. This requires much additional research and development. A concerted research and development effort is needed to assess whether industrial (bio)hydrometallurgial and solvometallurgical production of a substantial range of geochemically scarce elements from mined complex end-of-life products is feasible.
Financial benefits of increasing the output of geochemically scarce elements and of matrix materials are known and the downward trend of Cu concentrations in copper ores and the increased consideration of exploiting polymetallic ores may be conducive to an increased interest of primary copper producers in the co-production of other geochemically scarce elements and of marketable matrix materials. However, to achieve substantial progress toward near-zero waste processing there are also hurdles to be overcome. Such hurdles regard marketability of outputs, the safe handling of hazardous elements and company behavior. In view of these hurdles and of the uncertainties about the techno-economical scope for improved processing, the feasibility near-zero waste production of copper and its geochemically scarce companion elements from copper ores and end-of-life car electronics and electric and electronic equipment is uncertain.
Acknowledgments
The comments by three anonymous reviewers are gratefully acknowledged.
Disclosure statement
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Funding
References
- Abaka-Wood, G. B., J. Addai-Mensah, and H. Skinner. 2021. The use of mining tailings as analog of rare earth elements resources: Part I- characterization and preliminary separation. Minerals Processing and Extractive Metallurgy Review 15pp. doi:10.1080/08827508.2021.1980410.
- Adelman, J. G., S. Elouatik, and G. P. Demopoulos. 2015. Investigation of sodium-silicate derived gels as encapsulants for hazardous materials-The case of scorodite. Journal of Hazardous Materials 292:108–17. doi:10.1016/j.jhazmat.2015.03.008.
- Afflerbach, P., G. Fridgen, R. Keller, A. W. Rathgeber, and F. Strobel. 2014. The by-product effect on metal markets – New insights to the price behavior of minor metals. Resources Policy 42:35–44. doi:10.1016/j.resourpol.2014.08.003.
- Agorhom, E. A., J. P. Lem, W. Skinner, and M. Zanin. 2015. Challenges and opportunities in the recovery/rejection of trace elements in copper flotation. Minerals Engineering 78:45–57. doi:10.1016/j.mineng.2015.04.008.
- Ahmari, S., and L. Zhang. 2012. Production of eco-friendly bricks from copper mine tailings through geopolymerization. Construction and Building Materials 29:323–31. doi:10.1016/j.conbuildmat.2011.10.048.
- Ahmari, S., and L. Zhang. 2013. Durability and leaching behavior of mine tailing-based geopolymer bricks. Construction and Building Materials 44:743–50. doi:10.1016/j.conbuildmat.2013.03.075.
- Ahmed, I. M., A. A. Nayl, and J. A. Daoud. 2016. Leaching and recovery of zinc and copper from brass slag by sulfuric acid. Journal of the Saudi Chemical Society 20:S280–S285. doi:10.1016/j.jscs.2012.11.003.
- Ai, C., Z. Yan, H. Chai, T. Gu, J. Wang, L. Chai, G. Qiu, and W. Zeng. 2019. Increased chalcopyrite bioleaching capabilities of extremely thermoacidophilic Metallosphaera sedula inocula by mixotrophic propagation. Journal of Industrial Biotechnology 46 (8):1113–27. doi:10.1007/s10295-019-02193-3.
- Akbulut, M., T. J. Webster, P. D. Eason, Z. Cheng, and D. Singh. 2018. NSC Hydrometallurgical pressure oxidation of combined copper and molybdenum concentrates. Journal of Powder Metallurgy and Mining 2 (3):1000115 (10 pp.).
- Alam, M. S., M. Tanaka, K. Koyama, T. Oishi, and J. C. Lee. 2007. Electrolyte purification in energy-saving monovalent electrowinning processes. Hydrometallurgy 87 (1–2):36–44. doi:10.1016/j.hydromet.2006.12.001.
- Alcalde, J., U. Kelm, and D. Vergara. 2018. Historical assessment of metal recovery from old mine tailings; a study case for porphyry copper tailings, Chile. Minerals Processing 127:334–38.
- Alexander, D., C. van der Merwe, R. Lumbule, and J. Kgomo. 2018. Innovative process design for copper-cobalt oxide ores in the Democratic Republic of Congo. Journal of the South African Institute of Mining and Metallurgy 118:1163–67.
- Allanore, A. 2017. Electrochemical engineering for commodity metals extraction. Electrochemical Society Interface. Accessed February 13, 2020. www.electrochem.org.
- Altikaya, P., J. Mäkinen, P. Kinnunen, E. Kolehmainen, M. Haapalainen, and M. Lundström. 2018. Effect of biological pretreatment on metal extraction from flotation tailings for chloride leaching. Minerals Engineering 129:47–53. doi:10.1016/j.mineng.2018.09.012.
- Andersson, M., M. L. Söderman, and B. A. Sandén. 2017. Are metals in cars functionally recycled? Waste Management 60:407–16. doi:10.1016/j.wasman.2016.06.031.
- Andersson, M., M. L. Söderman, and B. A. Sandén. 2019. Challenges of recycling multiple scarce metals. The case of Swedish ELV and WEEE recycling. Resources Policy 63 (10143):12pp. doi:10.1016/j.resourpol.2019.101403.
- Araya, N., A. Kraslawski, and L. A. Cisternas. 2020. Towards mine tailings valorization: Recovery of critical materials from Chilean mine tailings. Journal of Cleaner Production 263 (121555):(10. doi:10.1016/j.jclepro.2020.121555.
- Araya, N., Y. Ramírez, A. Kraslawski, and L. A. Cisternas. 2021. Feasibility of reprocessing mine tailings to obtain critical raw materials using real options analysis. Journal of Environmental Management 284:112060. (10 pp.). doi:10.1016/j.jenvman.2021.112060.
- Ariizumi, M., M. Takagi, O. Inoue, and N. Oguma 2016. Integrated processing of e-scrap at Naoshima smelter and refinery. Proceedings Copper 2016. Kobe (Japan), November 13-16, 2016 6: RW 1-2.
- Arroyo-Torralvo, E., A. Rodriguez-Almansa, I. Ruiz, I. Gonzalez, G. Rios, C. Fernandez-Pereira, and L. F. Vilches-Arenas. 2017. Optimizing operating conditions in anion-exchange column treatment applied to the removal of Sb and Bi impurities from an electrolyte of a copper electro-refining plant. Hydrometallurgy 171:285–97. doi:10.1016/j.hydromet.2017.06.009.
- Arslan, B., M. B. A. Djamgoz, and E. Ukün. 2016. Arsenic: A review on exposure, pathways, accumulation and transmission into the human food chain. Reviews of Environmental Contamination and Toxicology 243:27–51.
- Arslan, C., and F. Arslan. 2002. Recovery of copper, cobalt and zinc from copper smelter and converter slags. Hydrometallurgy 67 (1–3):1–7. doi:10.1016/S0304-386X(02)00139-1.
- Asghari, M., F. Nakhaei, and O. VandGhorbany. 2018. Copper recovery improvement in an industrial flotation circuit: A case study of Sarcheshmeh copper mine. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 41:761–78. doi:10.1080/15567036.2018.1520356.
- Aurubis. 2017. Environmental protection in the Aurubis Group. Environmental Statement 2017. Hamburg, Germany. Assessed December 20, 2019. www.aurubis.com.
- Avarmaa, K., H. O’Brien, H. Johto, and P. Taskinen. 2015. Equilibrium distribution of precious metals between slag and copper matte at 1250–1350 °C. Journal of Sustainable Metallurgy 1 (3):216–28. doi:10.1007/s40831-015-0020-x.
- Avarmaa, K., L. Klemettinen, H. O’Brien, and P. Taskinen. 2019. Urban mining of precious metals via oxidizing copper smelting. Minerals Engineering 133:95–102. doi:10.1016/j.mineng.2019.01.006.
- Ayres, R. U., L. W. Ayres, and I. Rade. 2002. The life cycle of copper, its coproducts and by-products. INSEAD (Fontainebleau, France): International Institute for Environment and Development & Chalmers University (Gothenburg Sweden).
- Bakalarz, A. 2019. Chemical and mineral analysis of flotation tailings from stratiform copper ore from Lubin concentrator plant (SW Poland). Minerals Processing and Extractive Metallurgy Review 40 (6):437–46. doi:10.1080/08827508.2019.1667778.
- Bakhtiari, F., H. Atashi, M. Zivdar, S. Seyedbagheri, and M. H. Fazaelipoor. 2011. Bioleaching kinetics of copper from copper smelting dust. Journal of Industrial Engineering and Chemistry 17 (1):29–35. doi:10.1016/j.jiec.2010.10.005.
- Banza, A. N., E. Gock, and K. Kongolo. 2001. Base metals recovery from copper smelter slag by oxidising leaching and solvent extraction. Hydrometallurgy 67:63–69. doi:10.1016/S0304-386X(02)00138-X.
- Batterham, R. J. 2013. Major trends in the mineral processing industry. BHM 158 (2):42–46.
- Bellemans, I., E. De Wilde, N. Moelans, and K. Verbeken. 2018. Metal losses in pyrometallurgical operations. Advances in Colloid and Interface Science 255:47–63. doi:10.1016/j.cis.2017.08.001.
- Bidini, G., F. Fantozzi, P. Bartocci, B. D’Alleandro, M. D’Amico, P. Laranci, F. Scozza, and M. Zaragoli. 2015. Recovery of precious metals from scrap printed circuit boards through pyrolysis. Journal of Analytical and Applied Pyrolysis 111:140–42. doi:10.1016/j.jaap.2014.11.020.
- Bigum, M., L. Brogaard, and T. H. Christensen. 2012. Metal recovery from high grade WEEE: A life cycle assessment. Journal of Hazardous Materials 207-208:8–14. doi:10.1016/j.jhazmat.2011.10.001.
- Binnemans, K., and P. T. Jones. 2017. Solvometallurgy: An emerging branch of extractive metallurgy. Journal of Sustainable Metallurgy 3 (3):570–300. doi:10.1007/s40831-017-0128-2.
- Boisvert, J. B., M. E. Rossi, K. Ehrig, and C. V. Deutsch. 2013. Geometallurgical modelling at Olympic Dam Mine, South Australia. Mathematical Geosciences 45 (8):901–25. doi:10.1007/s11004-013-9462-5.
- Boliden. 2017. Metals for sustainable value creation. GRI report, Stockholm p. 24. Accessed December 14, 2019. www.boliden.com.
- Botelho, A. B., Jr., D. B. Dreisinger, and D. C. R. Espinosa. 2019. A review of nickel, copper and cobalt recovery by chelating ion exchange resins from mining processes and mining tailings. Mining, Metallurgy & Exploration 36 (1):199–213. doi:10.1007/s42461-018-0016-8.
- Brest, K. K., M. M. Henock, N. Guellord, M. Kimpiab, and K. E. Kapiamba. 2021. Statistical investigation on flotation parameters for copper recovery from sulfide flotation tailings. Results in Engineering 9:100207. (5 pp.). doi:10.1016/j.rineng.2021.100207.
- Brito, C., and M. Wyart. 2006. On the rigidity of a hard sphere glass near random close packing. Europhysics Letters 76 (1):149–55. doi:10.1209/epl/i2006-10238-x.
- Buechler, D. T., N. N. Zyaykina, C. A. Spencer, E. Lawson, N. M. Ploss, and I. Hua. 2020. Comprehensive element analysis of consumer electronic devices: Rare earth, precious and critical elements. Waste Management 103:67–75. doi:10.1016/j.wasman.2019.12.014.
- Caplan, M., J. Trouba, C. Anderson, and C. Wang. 2021. Hydrometallurgical leaching of copper flash furnace electrostatic precipitator dust for the separation of copper from bismuth and arsenic. Metals 4:371 (18pp.).
- Carranza, F., N. Iglesias, A. Mazuelos, R. Romero, and O. Forcat. 2009. Ferric leaching of copper slag flotation tailings. Minerals Engineering 22 (1):107–10. doi:10.1016/j.mineng.2008.04.010.
- Casarin, A. A., S. C. Lazzarini, and R. S. Vassolo. 2020. The forgotten competitive arena: Strategy in the natural resources industry. Academy of Management Perspectives 34 (3):378–99. doi:10.5465/amp.2017.0158.
- Catinean, A., L. Dascalescu, M. Lungu, L. M. Dumitran, and A. Samuila. 2021. Improving the recovery of Cu from electric cable waste derived from automotive industry by corona-electrostatic separation. Particulate Science and Technology 39 (4):449–56. doi:10.1080/02726351.2020.1756545.
- Cesaro, A., A. Marra, K. Kuchta, V. Belgiorno, and E. D. van Hullebusch. 2018. WEEE management in a circular economy perspective: An overview. Global NEST Journal 20:743–50.
- Chancerel, P., C. E. M. Meskers, C. Hagelüken, and V. S. Rotter. 2009. Assessment of precious metal flows during preprocessing of waste electrical and electronic equipment. Journal of Industrial Ecology 13 (5):791–810. doi:10.1111/j.1530-9290.2009.00171.x.
- Charles, R. G., T. Douglas, M. Dowling, G. Liversage, and M. L. Davies. 2020. Towards increased recovery of critical raw materials from WEEE- evaluations of CRMs at component level and pre-processing methods for interface optimisation with recovery processes. Resources Conservation and Recycling 161:104923. (21 pp.). doi:10.1016/j.resconrec.2020.104923.
- Chen, C., L. Zhang, and S. Jahanshahi 2013. Application of MPE model to direct-to-blister flash smelting and deportment of minor elements. Proceedings of Copper 2013, Santiago, Chile, 857–71.
- Chen, C., L. Zhang, S. Wright, and S. Jahanshai. 2006. Thermodynamic modelling of minor elements in copper smelting process. Advanced Processing of Metals and Materials. Thermo and Physicochemical Principles 1:335–48.
- Chen, C., and S. Wright. 2016. Distribution of Bi between slags and liquid copper. Metallurgy and Materials Transactions B 47 (3):1681–89. doi:10.1007/s11663-016-0610-4.
- Chen, J., and S. J. Bull. 2008. The investigation of creep of electroplated Si and Ni-Sn coating on copper at room temperature by nanoindentation. Surface & Coatings Technology 203 (12):1609–17. doi:10.1016/j.surfcoat.2008.12.007.
- Chen, T., C. Lei, B. Yan, and X. Xiao. 2014. Metal recovery from copper sulfide tailing with leaching and fractional precipitation technology. Hydrometallurgy 147-148:178–82. doi:10.1016/j.hydromet.2014.05.018.
- Chen, Y., T. Liao, B. Chen, and X. Shi. 2012. Recovery of bismuth and arsenic from copper smelter flue dusts after copper and zinc extraction. Minerals Engineering 39:23–28. doi:10.1016/j.mineng.2012.06.008.
- Chmielewski, A. G., D. Wawzczak, and M. Brykala. 2016. Possibility of uranium and rare metal recovery in the Polish copper mining industry. Hydrometallurgy 159:12–18. doi:10.1016/j.hydromet.2015.10.017.
- Chun, T., C. Ning, H. Long, J. Li, and J. Yang. 2016. Mineralogical characterization of copper slag from Tongling Nonferrous Metals Group China. JOM 68 (9):2332–40. doi:10.1007/s11837-015-1752-6.
- Clement, T. P., J. R. Wettlaufer, and A. Scott 1986. Process for treating speiss. United States Patent 4891061A.
- Corin, K. C., M. Kalichini, C. T. O’Connor, and S. Simukanga. 2017. The recovery of oxide copper minerals from a complex copper ore by sulphidisation. Minerals Engineering 102:15–17. doi:10.1016/j.mineng.2016.11.011.
- Coudert, L., R. Bondu, T. V. Rakotonimaro, E. Rosa, M. Guitonny, and C. M. Neculita. 2020. Treatment of As-rich mine effluents and produced residues stability: Current knowledge and research priorities for gold mining. Journal of Hazardous Materials 386:121920 (19 pp.). doi:10.1016/j.jhazmat.2019.121920.
- Coursol, P., N. C. Valencia, P. Mackey, S. Bell, and B. Davis. 2012. Minimization of copper losses in copper smelting slag during electric furnace treatment. JOM 64 (11):1305–13. doi:10.1007/s11837-012-0454-6.
- Crespo, J., M. Reich, F. Barra, J.J. Verdugo, and C. Martinez. 2018. Critical metal particles in copper sulfides from supergiant Rio Blanco porphyry Cu-Mo deposit, Chile. Minerals 8: 519 (13pp) doi:10.3390/min8110519
- Cusano, G., M. R. Gonzalo, F. Farrell, R. Remus, S. Roudier, and L. D. Sancho 2017. Best Available Techniques (BAT) reference document for the non-ferrous metals industries. Report 28648 EN, Brussels: European Commission.
- Daehn, K., and A. Allanore. 2020. Electrolytic production of copper from chalcopyrite. Current Opinion in Electrochemistry 22:110–19. doi:10.1016/j.coelec.2020.04.011.
- Dhar, P., M. Thornhill, and H. R. Kota. 2019. Investigation of copper recovery from a new copper deposit (Nussir) in Northern Norway. Mineral Processing and Extractive Metallurgy Review 40 (6):380–89. doi:10.1080/08827508.2019.1635475.
- Diaz, L. A., G. G. Clark, and T. E. Lister. 2017. Optimization of the electrochemical extraction and recovery of metals from electronic waste using response surface methodology. Industrial & Engineering Chemistry Research 56 (26):7516–24. doi:10.1021/acs.iecr.7b01009.
- Ding, Y., S. Zhang, B. Liu, H. Zheng, C. Chang, and C. Ekberg. 2019. Recovery of precious metals from electronic waste and spent catalysts: A review. Resources Conservation and Recycling 141:284–98. doi:10.1016/j.resconrec.2018.10.041.
- Domic, E. M. 2007. A review of the development and current status of copper bioleaching operations in Chile; 25 years of successful commercial implementation. In Biomining, ed. D. E. Rawlings and D. B. Johnson, 81–95. Berlin: Springer Verlag.
- Dreisinger, D. B. 2015. New developments in the atmospheric and pressure leaching of copper ores and concentrates. Accessed November 15, 2019. www.convencionminera.com.
- Dreisinger, D. B. 2016. Case study flowsheets: Copper-gold concentrate treatment. In Biotechnology of Metals: Principles, Recovery Methods and Environmental Concerns, ed. K. A. Natarajan, 803–20. Amsterdam: Elsevier.
- Drobe, M., F. Haubrich, M. Gajardo, and H. Marbler. 2021. Processing tests, adjusted cost models and the economies of reprocessing copper mine tailings in Chile. Metals 11 (1):103 (21pp.). doi:10.3390/met11010103.
- Du, B., J. Zhou, B. Lu, C. Zhang, D. Li, J. Zhou, S. Jiao, K. Zhao, and H. Zhang. 2020. Environmental and human health risks from cadmium exposure near an active lead-zinc mine and a copper smelter, China. Science of the Total Environment 720:137585. (9 pp.). doi:10.1016/j.scitotenv.2020.137585.
- Duester, L., C. Brinkmann, T. A. Ternes, and P. Heininger. 2016. Commentary. Critical Reviews in Environmental Science and Technology 46 (4):434–37. doi:10.1080/10590501.2015.1131559.
- Duester, L., D.-S. Wahrendorf, C. Brinkmann, A. Fabricius, B. Meermann, J. Pelzer, D. Ecker, M. Renner, H. Schmid, T. A. Ternes, et al. 2017. A framework to evaluate the impact of armourstones on the chemical quality of surface water. PLOS One 12 (1):e0168926 (12pp). doi:10.1371/journal.pone.0168926.
- Dupont, D., and K. Binnemans. 2017. Preventing antimony from becoming the next rare earth. Recycling Today 1:18–20.
- Dupont, D., S. Arnout, P. T. Jones, and K. Binnemans. 2016. Antimony recovery from end-of-life products and industrial process residues: A critical review. Journal of Sustainable Metallurgy 2 (1):9–103. doi:10.1007/s40831-016-0043-y.
- Ebrahimpour, S., H. Abdollahi, M. Gharabaghi, Z. Manafi, and O. H. Tuovinen. 2021. Acid bioleaching of copper from smelter dust at incremental temperatures. Mineral Processing and Extractive Metallurgy Review (10 pp.). doi:10.1080/08827508.2021.1888726.
- Engelsen, C. J., H. A. van der Sloot, and G. Petkovic. 2017. Long term leaching from recycled aggregates applied as sub-base materials in road construction. Science of the Total Environment 587-588:94–101. doi:10.1016/j.scitotenv.2017.02.052.
- Falagán, C., B. M. Grail, and D. B. Johnson. 2017. New approaches for extracting and recovering metals from mine tailings. Minerals Engineering 106:71–78. doi:10.1016/j.mineng.2016.10.008.
- Fizaine, F. 2020. The economics of the recycling rate. New insights from waste electrical and electronic equipment. Resources Policy 67:101675. (14 pp.). doi:10.1016/j.resourpol.2020.101675.
- Flandinet, L., F. Tedjar, V. Ghetta, and J. Fouletier. 2012. Metals recovering from waste printed circuit boards (WPCBs) using molten salts. Journal of Hazardous Materials 213-214:485–90. doi:10.1016/j.jhazmat.2012.02.037.
- Fomchenko, N., and M. Muravyov. 2020. Sequential bioleaching of pyritic tailings and ferric leaching of nonferrous slags for metal recovery from mining and metallurgical wastes. Minerals 10 (12):1097 (15 pp.). doi:10.3390/min10121097.
- Forsén, O., J. Aromaa, and M. Lindström. 2017. Primary copper smelter and refinery as a recycling plant- a system integrated approach to estimate secondary raw material tolerance. Recycling 2 (4):19 (11 pp). doi:10.3390/recycling2040019.
- Fröhlich, P., T. Lorenz, G. Martin, B. Brett, and M. Bertau. 2017. Valuable metals-recovery processes, current trends and recycling strategies. Angewandte Chemie International Edition 56 (10):2544–80. doi:10.1002/anie.201605417.
- Fry, K. L., C. A. Wheeler, M. M. Gillings, A. R. Flegal, and M. P. Taylor. 2020. Anthropogenic contamination of residential environments from smelter As, Cu and Pb emissions: Implications for human health. Environmental Pollution 262 (114235):(11. doi:10.1016/j.envpol.2020.114235.
- Gao, J., Z. Huang, Z. Wang, and Z. Guo. 2020. Recovery of crown zinc and metallic copper from copper smelter dust by evaporation, condensation and super gravity. Separation and Purification Technology 231 (115925):(8. doi:10.1016/j.seppur.2019.115925.
- Gao, W., B. Xu, J. Yang, Y. Yang, Q. Li, B. Zhang, G. Liu, Y. Na, and T. Jang. 2021. Comprehensive recovery of valuable metals from copper smelting open-circuit dust with a clean and economical hydometallurgical process. Chemical Engineering Journal 124 (130411):(13.
- Garza-Montes-de-Oca, N. F., N. A. Garcia-Gomez, J. Alvarez-Elcoro, and R. Colás. 2014. Surface degradation of nickel-plated brass fittings. Engineering Failure Analysis 36:314–21. doi:10.1016/j.engfailanal.2013.10.019.
- Gentina, J. C., and F. Acevedo. 2016. Copper bioleaching in Chile. Minerals 6 (1):23 (9 pp.). doi:10.3390/min6010023.
- Gertenbach, D. D. 2016. Scale up of pressure oxidation processes. Minerals and Metallurgical Processing 33 (4):178–86. doi:10.19150/mmp.6839.
- Ghodrat, M., B. Samali, M. A. Rhamdhani, and G. Brooks. 2019. Thermodynamic-based exergy analysis of precious metal recovery out of printed circuit board through black copper smelting process. Energies 12 (7):1313 (20 pp). doi:10.3390/en12071313.
- Ghodrat, M., M. A. Rhamdhani, G. Brooks, M. Rashidi, and B. Samali. 2017. A thermodynamic-based life cycle assessment of precious metal recycling out of waste printed circuit boards through secondary copper smelting. Environmental Development 24:36–49. doi:10.1016/j.envdev.2017.07.001.
- Ghodrati, S., F. Fardis Nakhaei, O. VandGhorbany, and M. Hekmani. 2020. Modeling and optimization of chemical reagents to improve copper flotation performance using response surface methodology. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 42 (13):1633–48. doi:10.1080/15567036.2019.1604874.
- Ghorbani, Y., J. Franzidis, and J. Petersen. 2018. Heap leaching technology – Current state, innovations, and future directions: A review. Minerals Processing and Extractive Metallurgy Review 37:73–119.
- Glencore. 2018. Who we are & recycling services FAQ. Accessed December 5, 2019. www.glencore.ca.
- Glöser, S., M. Soulier, and L. A. T. Espinoza. 2013. Dynamic analysis of global copper flows: Global stocks, postconsumer material flows: Recycling indicators and uncertainty evaluation. Environmental Science & Technology 47 (12):6564–72. doi:10.1021/es400069b.
- Gong, Y., M. Chen, Z. Wang, and J. Zhan. 2021. With or without deposit-refund system for a network platform-led electronic closed-loop supply system. Journal of Cleaner Production 231:125356. (14 pp). doi:10.1016/j.jclepro.2020.125356.
- González, A., O. Font, N. Moreno, X. Querol, N. Arancibia, and R. Navia. 2017. Copper flash smelting flue dust as a source of germanium. Waste and Biomass Valorization 8 (6):2121–29. doi:10.1007/s12649-016-9725-8.
- González-Castanedo, Y., T. Moreno, R. Fernández-Camcho, A. M. Sanchez De La Camp, A. Alastuey, X. Querol., and J. De La Rosa. 2014. Emitted smelter dusts with As, Cd and Pb represent a risk to human health. Atmospheric Environment 98:271–82. doi:10.1016/j.atmosenv.2014.08.057.
- Gorman, M., and D. Dzombak. 2020. Stocks and flows of copper in the US; analysis of circularity 1970-2015. Resources Conservation and Recycling 153:104542. (15 pp.). doi:10.1016/j.resconrec.2019.104542.
- Government of Canada. 2018. Accessed December 21, 2019. https://ec.gc.ca/plan2-p2plan/
- Graedel, T. E., B. K. Reck, L. Ciacci, and F. Passarini. 2019. On the spatial dimension of the circular economy. Resources 8 (1):32 (10 pp.). doi:10.3390/resources8010032.
- Green, C., J. Robertson, and J. O. Marsden. 2018. Pressure leaching of copper concentrates at Morenci, Arizona −10 years of experience. Minerals and Metallurgical Processing 35 (3):109–16. doi:10.19150/mmp.8459.
- Gregurek, D., J. Schmidl, K. Reinharter, V. Reiter, and A. Spanring. 2018. Copper anode furnace: Chemical, mineralogical and thermochemical considerations of refractory wear mechanisms. JOM 70 (11):2428–34. doi:10.1007/s11837-018-3089-4.
- Grudinsky, P. I., E. E. Zhiltsova, D. D. Grigorieva, and V. G. Dyubanov. 2021. Experimental study of the sulphatizing roasting of flotation tailings from copper slag processing using iron sulfates. IOP Conference Series of Earth and Environmental Science 666 (2):022046 (7 pp.). doi:10.1088/1755-1315/666/2/022046.
- Grudinsky, P. I., V. G. Dyubanov, and P. A. Kozlov. 2018. Distillation of copper-smelting dusts with primary recovery of lead. Russian Metallurgy 1:7–13. doi:10.1134/S003602951801007X.
- Grudinsky, P. I., V. G. Dyubanov, and P. A. Kozlov. 2019. Copper smelter dust is a promising material for the recovery of nonferrous metals by the Waelz process. Inorganic Materials: Applied Research 10 (2):496–501. doi:10.1134/S2075113319020175.
- Gu, W., J. Bai, L. Lu, X. Zhuang, J. Zhao, W. Yuan, C. Zhang, and J. Wang. 2019. Improved bioleaching efficiency of metals from waste printed circuit boards by mechanical activation. Waste Management 98:21–28. doi:10.1016/j.wasman.2019.08.013.
- Guarino, S., N. Ucciardello, S. Venettacci, and S. Genna. 2017. Life cycle assessment of new graphene-based electrodeposition process on copper components. Journal of Cleaner Production 165:520–29. doi:10.1016/j.jclepro.2017.07.168.
- Guezennec, A., S. Hedrich, A. Schippers, A. Kamradt, S. Matys, R. Michels, C. Joulian, and F. Bodenan. 2017. Bioleaching in stirred tank reactors to process Kupferschiefer-type ore: Current status and perspectives. HAL: 16ème Congrès de la Societè Francaise de Gènie des Procèdès. Jul 2017. Nancy, France Hal Archives Ouvertes –01501238 (3 pp.).
- Gunaratne, T., J. Krook, H. Andersson, and M. Eklund. 2020b. Guiding future research in the valorisation of shredder fine residues: A review of four decades of research. Detritus 9:150–64
- Gunaratne, T., J. Krook, H. Andersson, and M. Eklund. 2020a. Potential valorisation of shredder fines: Towards integrated processes for material upgrading and resource recovery. Resources Conservation and Recycling 154:104590 (12 pp.). doi:10.1016/j.resconrec.2019.104590.
- Guo, F., and G. P. Demopoulos. 2018. Development of an encapsulation process to extend the stability of scorodite under wider pH and redox potential range conditions. In Extraction 2018, The Minerals, Metals and Materials Series, ed. B. Davis, 1411–20. Cham: Springer Verlag.
- Guo, Z., D. Zhu, J. Pan, T. Wu, and F. Zhang. 2016. Improving beneficiation of copper and iron from copper slag by modifying the molten copper slag. Metals 6 (86):(17. doi:10.3390/met6040086.
- Guo, Z., D. Ziu, J. Pan, F. Zhang, and C. Yang. 2018. Industrial tests to modify copper slag for improvement of copper recovery. JOM 70 (4):533–38. doi:10.1007/s11837-017-2671-5.
- Ha, T. K., B. H. Kwon, K. S. Park, and D. Mohapatra. 2015. Selective leaching and recovery of bismuth as Bi2O3 from copper smelter converter dust. Separation and Purification Technology 142:116–22. doi:10.1016/j.seppur.2015.01.004.
- Hagelüken, C. 2006a. Improving metal returns and eco-efficiency in electronic recycling. Proceedings of the 2006 IEEE International Symposium on Electronics and the Environment, 218–23
- Hagelüken, C. 2006b. Recycling of electronic scrap at Umicore’s integrated metals smelter and refinery. Erzmetall 59:152–61.
- Hagelüken, C. 2015. Closing the loop for rare metals used in consumer products: Opportunities and challenges. Natural Resources Management and Policy 46:103–19.
- Hagelüken, C., and C. W. Corti. 2010. Recycling gold from electronics: Cost effective use through ‘design for recycling´. Gold Bulletin 43 (3):209–20. doi:10.1007/BF03214988.
- Hait, J., R. K. Jana, and S. K. Sanyal. 2009. Processing of copper electrorefining anode slime: A review. Minerals Processing and Extractive Metallurgy 118 (4):240–52. doi:10.1179/174328509X431463.
- Halinen, A. 2015. Heap bioleaching of low-grade multimetal sulphidic ore in Boreal conditions. PhD thesis. Tampere University of Technology. Publication 1347
- Han, F., F. Yu, and Z. Cui. 2016. Industrial metabolism of copper and sulfur in a copper-specific eco-industrial park in China. Journal of Cleaner Production 133:459–66. doi:10.1016/j.jclepro.2016.05.184.
- Hao, X., Y. Liang, H. Yin, H. Liu, W. Zeng, and X. Liu. 2017. Thin-layer heap bioleaching of copper flotation tailings containing high levels of fine grains and microbial community analysis. International Journal of Minerals, Metallurgy and Materials 24 (4):360–68. doi:10.1007/s12613-017-1415-4.
- Hawker, W., J. Vaughan, E. Jak, and P. C. Hayes. 2018. The synergistic copper process concept. Mineral Processing and Extractive Metallurgy 127 (4):210–20. doi:10.1080/03719553.2017.1375768.
- He, R., S. Zhang, X. Zhang, Z. Zhang, Y. Zhao, and H. Ding. 2021. Copper slag: The leaching of heavy metals and its applicability as a supplementary cementitious material. Journal of Environment and Chemical Engineering 9 (2):105132 (12pp.). doi:10.1016/j.jece.2021.105132.
- Hedjazi, F., and A. J. Monhemius. 2014. Copper-gold or processing with ion exchange and SART technology. Minerals Engineering 64:120–25. doi:10.1016/j.mineng.2014.05.025.
- Hedrich, S., R. Kermer, T. Aubel, M. Martin, A. Schippers, D. B. Johnson, and E. Janneck. 2018. Implementation of biological and chemical techniques to recover metals from copper-rich leach solutions. Hydrometallurgy 179:274–81. doi:10.1016/j.hydromet.2018.06.012.
- Henckens, M. L. C. M., and E. Worrell. 2020. Reviewing the availability of copper and nickel for future generations. The balance between production growth, sustainability and recycling rates. Journal of Cleaner Production 264:121460 (12 pp.). doi:10.1016/j.jclepro.2020.121460.
- Holland, K., R. H. Eric, P. Taskinen, and A. Jokilaakso. 2019. Upgrading copper slag cleaning tailings for reuse. Minerals Engineering 133:35–42. doi:10.1016/j.mineng.2018.12.026.
- Hosseinzadeh, M., A. Azizi, and A. Hassanzadeh. 2021. Solvent extraction and kinetic studies of copper from a heap leach liquor using CuPRO MEX-3302. Separation Science and Technology (18pp). doi:10.1080/01496395.2021.1922445.
- Hubau, A., A. Guezennec, C. Joulian, C. Falagán, D. Dew, and K. A. Hudson-Edwards. 2020. Bioleaching to reprocess sulfidic polymetallic primary mining residues: Determination of metal leaching mechanisms. Hydrometallurgy 197:105484 (14 pp.). doi:10.1016/j.hydromet.2020.105484.
- Hughes, S. 2000. Applying Ausmelt technology to recover Cu, Ni, and Co from slags. JOM 52 (8):30–33. doi:10.1007/s11837-000-0170-5.
- Ibragimov, R. M., O. G. Bernayaev, S. A. Kazakov, and G. V. Skopov. 2019. Processing of the silver-zinc crust of the product of refining raw lead in a copper-smelting converter. Metallurgist 63 (5–6):529–33. doi:10.1007/s11015-019-00853-4.
- ICSG (International Copper Study Group). 2016. The World Copper Factbook 2016. Lisbon, Portugal. Accessed May 10, 2019. www.icsg.org.
- ICSG (International Copper Study Group). 2019. Copper market forecast 2019/2020. Lisbon, Portugal. Press release 23.10.2019.
- Imamura, Y., K. Ikeda, J. Piao, and T. Takemoto 2015. Solder alloy, solder paste, and electronic circuit board. US Patent 9221132B2.
- Ince, C., S. Derogar, K. Gurkaya, and R. J. Ball. 2020. Properties, durability and efficiency of cement and hydrated lime mortars reusing copper mine tailings of Lefke-Xeroos in Cyprus. Construction and Building Materials 268:121070 (17 pp.).
- Ioannidou, D., G. Meylan, G. Sonnemann, and G. Habert. 2017. Is gravel becoming scarce? Evaluating the local criticality of construction materials. Resources Conservation and Recycling 126:25–33. doi:10.1016/j.resconrec.2017.07.016.
- Isildar, A., E. R. Rene, E. D. van Hullebusch, and P. N. L. Lens. 2018. Electronic waste as a secondary source of critical materials: Management and recovery technologies. Resources Conservation and Recycling 136:296–314. doi:10.1016/j.resconrec.2017.07.031.
- Izatt, R. M., S. R. Izatt, N. E. Izatt, K. E. Krakowiak, R. L. Bruening, and L. Navarro. 2015. Industrial applications of molecular recognition technology to separations of platinum group metals and selective removal of metal impurities from process streams. Green Chemistry 17 (4):2236–45. doi:10.1039/C4GC02188F.
- Izatt, R. M., S. R. Izatt, R. L. Bruening, N. E. Izatt, and B. A. Moyer. 2014. Challenges to achievement of metal sustainability in our high-tech society. Chemical Society Reviews 43 (8):2451–75. doi:10.1039/C3CS60440C.
- Jak, E., T. Hidayat, D. Shishin, P. J. Mackey, and P. C. Hayes. 2019. Modelling of liquid phases and metal distributions in copper converters: Transferring process fundamentals to plant practice. Minerals Processing and Extractive Metallurgy Review 128 (1–2):74–107. doi:10.1080/25726641.2018.1506273.
- Jeon, S., M. Ito, C. B. Tabelin, K. Pongsumrankul, N. Kitajima, I. Park, and N. Hiroyoshi. 2018. Gold recovery from shredder light fraction E-waste recycling plant by flotation-ammonium thiosulfate leaching. Waste Management 77:195–202. doi:10.1016/j.wasman.2018.04.039.
- Jian, S., W. Gao, Y. Lv, H. Tan, X. Li, B. Li, and W. Huang. 2020. Potential utilization of copper tailings in the preparation of low heat cement clinker. Construction and Building Materials 252 (119130):(9. doi:10.1016/j.conbuildmat.2020.119130.
- Jin, W., M. Hu, and J. Hu. 2018. Selective and efficient electrochemical recovery of dilute copper and tellurium from acid chloride solutions. ACS Sustainable Chemistry & Engineering 6 (10):13378–84. doi:10.1021/acssuschemeng.8b03150.
- Jolly, J. L. 2013. The US Copper-based scrap industry and its by-products. New York, USA: Copper Development Association Inc.
- Jordao, H., A. J. Sousa, and M. T. Cavalho. 2016. Optimization of wet shaking table process using response surface methodology applied to the separation of copper and aluminum from the fine fraction of shredder ELVs. Waste Management 48:366–73. doi:10.1016/j.wasman.2015.10.006.
- Jorjani, E., and A. Ghahreman. 2017. Challenges with elemental sulfur removal during the leaching of copper and zinc sulfdes, and from the residues; a review. Hydrometallurgy 171:333–43. doi:10.1016/j.hydromet.2017.06.011.
- Kaksonen, A. H., S. Särjijävi, E. Peuraniemi, S. Junnikkala, J. A. Puhakka, and O. H. Tuovinen. 2017. Metal biorecovery in acid solutions from a copper smelter slag. Hydrometallurgy 168:135–40. doi:10.1016/j.hydromet.2016.08.014.
- Kammel, R., M. Göktepe, and H. Oerlmann. 1987. Zinc electrowinning from flue dust at a secondary copper smelter and connected adhesion problems of the metal deposits. Hydrometallurgy 19 (1):11–24. doi:10.1016/0304-386X(87)90038-7.
- Kanlayasiri, K., and T. Ariga. 2015. Physical properties of Sn58Bi-xNi lead-free solder and its interfacial reaction with copper substrate. Materials & Design 86:371–78. doi:10.1016/j.matdes.2015.07.108.
- Karhu, M., J. Kotnis, Y. Yang, P. Mener, A. G. Uriarte, K. Kaunisto, E. Huttunen-Saarvita, E. Yli-Rantala, M. Karhu, L. S. Okvist, et al. 2018. Circular economy and zero waste aspects and business models of production. Solutions for Critical Raw Materials, 206 pp. Brussels: European Union.
- Karpagavalli, R., and R. Balasubramaniam. 2007. Development of novel brasses to resist dezincification. Corrosion Science 49 (3):963–79. doi:10.1016/j.corsci.2006.06.024.
- Katwika, C., M. Kime, P. N. M. Kalenga, B. I. Mbuya, and T. R. Mwilen. 2019. Application of Knelson concentrator for beneficiation of copper-cobalt ore tailings. Minerals Processing and Extractive Metallurgy Review 40 (1):35–45. doi:10.1080/08827508.2018.1481057.
- Kawasaki, M. 2014. Innovative system for e-scrap treatment at Naoshima smelter and refinery. presented at Workshop 2014 of the Asian network for prevention of illegal transboundary movement of hazardous wastes. Accessed April 3, 2019. https://www.env.gov.jp/.
- Kaya, M. 2016. Recovery of metals from electronic waste by physical and chemical recycling processes. International Journal of Chemical, Molecular, Nuclear, Materials and Metallurgical Engineering 10:232–43.
- Ke, P., K. Song, and Z. Liu. 2018. Encapsulation of scorodite using crystalline polyferric sulphate precipitated from SO42–O2-H2O system. Hydrometallurgy 180:78–87. doi:10.1016/j.hydromet.2018.07.011.
- Ke, P., and Z. Liu. 2018. Fe (III)-As(V) precipitates obtained from a sulfate system and their leach stability. Canadian Metallurgical Quarterly 57 (3):304–11. doi:10.1080/00084433.2018.1460441.
- Khalid, M. K., J. Hamuyuni, V. Agarwal, J. Pihlasalo, M. Haapalainen, and M. Lundström. 2019. Sulfuric acid leaching for capturing value from copper-rich converter slag. Journal of Cleaner Production 215:1005–13. doi:10.1016/j.jclepro.2019.01.083.
- Khaliq, A., M. A. Rhadhani, G. Brooks, and S. Masood. 2014. Metal extraction processes for electronic waste and existing industrial routes: A review and Australian perspective. Resources 3 (1):153–79. doi:10.3390/resources3010152.
- Khanna, R., G. Ellamparuthy, B. Cayumil, S. K. Mishra, and P. S. Mukherjee. 2018. Concentration of rare earth elements during high temperature pyrolysis of waste printed circuit boards. Waste Management 78:602–10. doi:10.1016/j.wasman.2018.06.041.
- Kim, J. W., A. S. Lee, S. Yu, and J. W. Han. 2018. En masse pyrolysis of flexible printed circuit board wastes quantitatively yielding environmental resources. Journal of Hazardous Materials 342:51–57. doi:10.1016/j.jhazmat.2017.08.010.
- Kimball, B. E., A. L. Forster, R. R. Seal, N. M. Piatak, S. M. Webb, and J. M. Hammarstrom. 2016. Copper speciation in variably toxic sediments at the Ely copper mine, Vermont, USA. Environmental Science & Technology 50 (3):1126–36. doi:10.1021/acs.est.5b04081.
- Kinnunen, P., J. Mäkinen, M. Salo, R. Soth, and K. Komnitsas. 2020. Efficiency of chemical and biological leaching for the recovery of metals and valorisation of the leach residue as raw material in cement production. Minerals 10 (8):654 (19 pp.). doi:10.3390/min10080654.
- Klaffenbach, E., S. Mostaghel, M. Guo, and B. Blanpain. 2021. Thermodynamic analysis of copper smelting, considering the impact of minor elements behavior on slag application options and Cu recovery. Journal of Sustainable Metallurgy (20 pp.). doi:10.1007/s40831-021-00345-2.
- Knapp, F. L. 2018. The birth of the flexible mine: Changing geographies of mining and e-waste commodity frontier. Environmental Planning A 48:1885–909.
- Kodier, A., K. Williams, and N. Dallison. 2018. Challenges around automobile shredder residue production and disposal. Waste Management 73:566–73. doi:10.1016/j.wasman.2017.05.008.
- Komnitsas, K., E. Petrakis, G. Bartzas, and V. Karnali. 2019. Column leaching of low-grade saprolitic laterites and valorization of leaching residues. Science of the Total Environment 665:347–57. doi:10.1016/j.scitotenv.2019.01.381.
- Komnitsas, K., G. Bartzas, V. Karnali, and E. Petrakis. 2021. Factors affecting alkali activation of laterite acid leaching residues. Environments 8 (4):(21. doi:10.3390/environments8010004.
- Kondriat´eva, T. F., T. A. Pivovarova, A. G. Bulaev, V. S. Melamud, A. Muravyov, and E. A. Vasil´ev. 2012. Percolation bioleaching of copper and zinc and gold from flotation tailings of the sulfide complex ores of the Ural region, Russia. Hydrometallurgy 111-112:81–86.
- Kosson, D. S., H. A. van der Sloot, F. Sanchez, and A. C. Garrabrants. 2004. An integrated framework for evaluating leaching in waste management and utilization of secondary materials. Environmental Engineering Science 19 (3):159–204. doi:10.1089/109287502760079188.
- Kotarski, J., J. Stec, T. Niemiec, J. Czernecki, and R. Parjsnar. 1999. Progress in processing of lead bearing materials from Polish copper smelters. Erzmetall 52:49–54.
- Krishnamoorthy, S., G. Ramakrishnan, and B. Dhandopani. 2021. Recovery of valuable metals from waste printed circuit boards using organic acids synthesised by Aspergillus niveus. IET Nanotechnology 15 (2):212–20. doi:10.1049/nbt2.12001.
- Kubissa, W., R. Jaskulski, D. Gil, and I. Wilinska. 2020. Holistic analysis of waste copper slag-based concrete by means of EIPI method. Buildings 10 (1):(14.
- Kumar, A., H. S. Sain, and S. Kumar. 2018. Bioleaching of gold and silver from waste printed circuit boards by Pseudomonas balearica SAE1 isolated from an e-waste recycling facility. Current Microbiology 75 (2):194–201. doi:10.1007/s00284-017-1365-0.
- L. Liu, and G.A.Keoleian 2020. LCA of rare earth and critical metal recovery and replacement decisons for commercial lighting waste management. Resources, Conservation and Recycling 159: 104846 (12pp) doi:10.1016/j.resconrec.2020.104846
- Lagno, F., S. D. F. Rocha, S. Chryssoulos, and G. F. Demopoulos. 2010. Scorodite encapsulation by controlled deposition of aluminum phosphate coating. Journal of Hazardous Materials 181 (1–3):526–34. doi:10.1016/j.jhazmat.2010.05.046.
- Lagos, G., D. Peters, A. Videla, and J. J. Jara. 2018. The effect of mine aging on the evolution of environmental footprint indicators in Chilean copper mining industry 2001-2005. Journal of Cleaner Production 174:389–400. doi:10.1016/j.jclepro.2017.10.290.
- Lam, E. J., V. Zetola, Y. Ramirez, I. L. Montofré, and F. Perreira. 2020. Making paving stones from copper mine tailings as aggregates. International Journal of Environmental Research and Public Health 17 (7):2448 (14 pp.). doi:10.3390/ijerph17072448.
- Lane, D. J., N. J. Cook, S. R. Grano, and K. Ehrig. 2016. Selective leaching of penalty elements from copper concentrates. A review. Minerals Egineering 98:110–21. doi:10.1016/j.mineng.2016.08.006.
- Langmuir, D., J. Mahoney, and J. Rowson. 2006. Stability of amorphous ferric arsenate and crystalline scorodite (FeAsO4.2H2O) and their application to arsenic behavior in buried mine tailings. Geochimica et Cosmochimica Acta 70 (12):2942–56. doi:10.1016/j.gca.2006.03.006.
- Lasheen, T. A., M. E. El-Ahmadi, H. B. Hassib, and A. S. Helal. 2015. Molybdenum metallurgy review: Hydrometallurgical routes to recovery of molybdenum from ores and mineral raw materials. Minerals Processing and Extractive Metallurgy Review 36 (3):145–73. doi:10.1080/08827508.2013.868347.
- Laws, K. J., C. Crosby, A. Sridhar, P. Conway, L. S. Koloadin, M. Zhao, S. Aron-Dire, and L. C. Bossman. 2015. High entropy brasses and bronzes – Microstructure, phase evolution and properties. Journal of Alloys and Compounds 650:949–61. doi:10.1016/j.jallcom.2015.07.285.
- Lee, D. 2018. Experimental investigation of laser ablation characteristics on nickel-coated beryllium-copper. Metals 8 (4):211 (14 pp.). doi:10.3390/met8040211.
- Leetma, K., F. Guo, L. Becze, M. A. Gomez, and G. P. Demopoulos. 2016. Stabilization of iron arsenate solids by encapsulation with aluminum hydroxyl gels. Journal of Chemical Technology and Biotechnology 91 (2):408–15. doi:10.1002/jctb.4590.
- Lennartsson, A., F. Engström, C. Samuelsson, B. Björkman, and J. Petersson. 2018. Large-scale WEEE recycling integrated in an ore-based Cu extraction system. Journal of Sustainable Metallurgy 4 (2):222–32. doi:10.1007/s40831-018-0157-5.
- Lewis, G., S. Gaydardzhiev, D. Bastin, and P. Bareel. 2011. Bio hydrometallurgical recovery of metals from fine shredder residues. Minerals Engineering 24 (11):1166–71. doi:10.1016/j.mineng.2011.03.025.
- Li, D., X. Guo, Z. Xu, R. Xu, and Q. Feng. 2016. Metal values separation from residue generated in alkali fusion-leaching of copper anode slime. Hydrometallurgy 165:290–94. doi:10.1016/j.hydromet.2016.01.021.
- Li, G., W. Weng, W. Weng, T. Zuo, T. Zuo, and T. Zuo. 2017b. Overview of recycling technology for copper containing cables. Resources Conservation and Recycling 126:132–40. doi:10.1016/j.resconrec.2017.07.024.
- Li, H., J. Eksteen, and E. Oraby. 2018a. Hydrometallurgical recovery of metals from waste printed circuit board (WPCBs). Current status and perspectives - a review. Resources Conservation and Recycling 139:122–39. doi:10.1016/j.resconrec.2018.08.007.
- Li, L., Z. Cao, H. Zhong, M. Wang, G. Liu, S. Wang, and X. Cao. 2013. The selective leaching and separation of molybdenum from complex molybdenite concentrate containing copper. Minerals Metallurgical Processing 30:233–37.
- Li, X., H. Yang, Z. Jin, L. Tong, and F. Xiao. 2017a. Selenium leaching from copper anode slimes using a nitric acid-sulfuric acid mixture. Metallurgist 61 (3–4):348–56. doi:10.1007/s11015-017-0500-2.
- Li, Y., S. Yang, C. Tang, Y. Chen, J. He, and M. Tang. 2018b. Reductive–sulfurizing treatment of smelter slag for copper and cobalt recovery. Journal of Mining and Metallurgy B 54 (1):73–79. doi:10.2298/JMMB160315049L.
- Li, Y., X. Zhu, Y. Qi, B. Shu, X. Zhang, K. Li, Y. Wie, and H. Wang. 2020. Removal and immobilization of arsenic from copper smelter using copper slag by in situ encapsulation in silica gel. Chemical Engineering Journal 394:124833 (10 pp.). doi:10.1016/j.cej.2020.124833.
- Li, Z., L. A. Diaz, Z. Yang, H. Jin, T. E. Lister, E. Vahidi, and F. Zhao. 2019. Comparative life cycle analysis for value recovery of precious metals and rare earth elements from electronic waste. Resources Conservation and Recycling 149:20–30. doi:10.1016/j.resconrec.2019.05.025.
- Liu, K., J. Yang, H. Hou, S. Liang, Y. Chen, J. Wang, B. Liu, K. Xiao, J. Hu, and H. Deng. 2019. Facile and cost-effective approach for copper recovery from waste printed circuit boards via a sequential mechanochemical/leaching/recrystallization process. Environmental Science & Technology 53 (5):2748–57. doi:10.1021/acs.est.8b06081.
- Liu, W., X. Fu, T. Yang, D. Zhang, and L. Chen. 2018. Oxidation leaching of copper smelting dust by controlling potential. Transactions of the Nonferrous Metals Society China 28 (9):1854–61. doi:10.1016/S1003-6326(18)64830-7.
- Liu, W., Z. Yang, D. Zhang, D. Chen, and Y. Liu. 2014. Pretreatment of copper anode slime with alkaline pressure oxidative leaching. International Journal of Minerals Processing 128:48–54. doi:10.1016/j.minpro.2014.03.002.
- Liu, X., X. Ke, H. Zhu, R. Chen, X. Chen, X. Zhang, S. Jin, and B. van der Bruggen. 2020. Treatment of raffinate generated via copper ore hydrometallurgical processing using bipolar membrane electrodialysis system. Chemical Engineering Journal 382:122956 (10 pp.). doi:10.1016/j.cej.2019.122956.
- Liu, Y., M. Zhou, G. Zeng, X. Li, W. Xu, and T. Fan. 2007. Effect of solids concentration on the removal of heavy metals from mine tailings via bioleaching. Journal of Hazardous Materials 141 (1):202–08. doi:10.1016/j.jhazmat.2006.06.113.
- Lu, D., Y. Chang, H. Yang, and F. Xie. 2015. Sequential removal of selenium and tellurium from copper anode slime with high nickel content. Transactions of the Nonferrous Metals Society China 25 (4):1307–14. doi:10.1016/S1003-6326(15)63729-3.
- Lu, J., J. Xu, S. Kumagai, T. Kameda, Y. Saito, and T. Yashioka. 2019. Separation mechanism of polyvinylchloride and copper components from swollen electric cables by mechanical agitation. Waste Management 93:54–62. doi:10.1016/j.wasman.2019.05.024.
- Lucheva, B., I. Iliev, and D. Kolev. 2017. Hydro-pyrometallurgical treatment of copper converter flue dust. Journal of Chemical Technology and Metallurgy 52:320–25.
- Lutandula, M. S., and B. Maloba. 2013. Recovery of cobalt and copper through reprocessing of tailings from flotation of oxidised ore. Journal of Environmental Chemistry and Engineering 1 (4):1085–90. doi:10.1016/j.jece.2013.08.025.
- Ma, L., X. Wang, X. Feng, Y. Liang, Y. Xiao, X. Han, H. Yin, H. Liu, and X. Liu. 2017. Co-culture microorganisms with different initial proportions reveal the mechanism of chalcopyrite bioleaching coupling with microbial community succession. Bioresource Technology 223:121–30. doi:10.1016/j.biortech.2016.10.056.
- Ma, L., X. Wang, X. Liu, S. Wang, and H. Wang. 2018. Intensified bioleaching of chalcopyrite by communities with enriched ferrous or sulphur oxidizers. Bioresource Technology 268:415–24. doi:10.1016/j.biortech.2018.08.019.
- Ma, Z., H. Yang, S. Huang, Y. Lu, and L. Xiong. 2015. Ultra-fast microwave-assisted leaching for the recovery of copper and tellurium from copper-anode slime. International Journal of Minerals, Metallurgy and Materials 22 (6):582–88. doi:10.1007/s12613-015-1110-2.
- Mahmoud, A., P. Cezac, A. F. A. Hoadley, and F. Contamine. 2017. A review of sulfide minerals microbially assisted leaching in stirred tank reactor. International Biodeterioration Biodegradation 119:118–46.
- Mäkinen, J., J. Bacher, T. Kaartinen, M. Wahlström, and J. Saklminen. 2015. The effect of flotation parameters for bioleaching of printed circuit boards. Minerals Engineering 75:26–31. doi:10.1016/j.mineng.2015.01.009.
- Makuei, F. M., and G. Senanayake. 2018. Extraction of tellurium from lead and copper bearing feed materials and interim metallurgical product- a short review. Minerals Engineering 115:79–87. doi:10.1016/j.mineng.2017.10.013.
- Manjarrez, L., and L. Zhang. 2018. Utilization of copper mine tailings as road base construction material through geopolymerization. Journal of Materials for Civil. Engineering 30 (9):04018201. doi:10.1061/(ASCE)M.T.1943-5533.00052397.
- Manouchehri, H. B. 2018. Magnetic conditioning of sulfide minerals to improve recoveries of fines in flotation- a plant practice. Minerals Metallurgy Processing 36:46–54. doi:10.19150/mmp.8057.
- Marra, A., A. Cesaro, E. R. Rene, V. Belgiorno, and P. N. L. Lens. 2018b. Bioleaching of metals from WEEE shredding dust. Journal of Environmental Management 210:180–90. doi:10.1016/j.jenvman.2017.12.066.
- Marra, A., A. Cesaro, and V. Belgiorno. 2018a. Separation efficiency of valuable and critical metals in WEEE mechanical treatments. Journal of Cleaner Production 186:490–98. doi:10.1016/j.jclepro.2018.03.112.
- Marshakov, I. K. 2005. Corrosion resistance in dezincing of brasses. Protection of Metals 41 (3):205–10. doi:10.1007/s11124-005-0031-2.
- Miganei, L., E. Gock, M. Achimovicova, L. Koch, H. Zobel, and J. Kähler. 2017. New residue-free processing of copper slag from smelter. Journal of Cleaner Production 164:534–42. doi:10.1016/j.jclepro.2017.06.209.
- Mikoda, B., A. Potysz, and E. Kmiecik. 2019b. Bacterial leaching of critical metal values from Polish copper metallurgical slags using Acidithiobacillus thiooxidans. Journal of Environmental Management 236:436–45. doi:10.1016/j.jenvman.2019.02.032.
- Mikoda, B., H. Kucha, A. Potysz, and E. Kmiecik. 2019a. Metallurgical slags from Cu production and Pb recovery in Poland - their environmental stability and resource potential. Applied Geochemistry 101:63–74.
- Mikula, K., G. Izydorczyk, D. Skzypzak, K. Moustakas, A. Wirtek-Kowiak, and K. Chojnacka. 2021. Value-added strategies for the sustainable handling, disposal or value-added use of copper smelter and refinery wastes. Journal of Hazardous Materials 403:123602 (13 pp.). doi:10.1016/j.jhazmat.2020.123602.
- Mitiadis, S. K., I. Giannopoulou, M. F. M. Tatur, M. F. Hashin, and D. Padias. 2020. Upgrading copper slags to added value fire resistant geopolymers. Waste and Biomass Valorization 11 (7):3811–20. doi:10.1007/s12649-019-00666-1.
- Mitsune, Y., and S. Satoh. 2007. Lead smelting and refining at Kosaka smelter. Journal of MMIJ 123 (12):630–33. doi:10.2473/journalofmmij.123.630.
- Moats, M., L. Alagha, and K. Awuah-Offei. 2021. Towards resilient and sustainable supply of critical elements from copper supply chain. Journal of Cleaner Production 307:127207 (14 pp.). doi:10.1016/j.jclepro.2021.127207.
- Mokmeli, M., D. Dreisinger, and B. Wassink. 2015. Modeling of selenium and tellurium removal from copper electrowinning solution. Hydrometallurgy 53:12–20. doi:10.1016/j.hydromet.2015.01.007.
- Moldabayeva, G. Z., S. K. Akilbekova, K. K. Mamyrbayeva, and B. Mishra. 2015. Electrosmelting of lead-containing dusts from copper smelters. Journal of Sustainable Metallurgy 1 (4):286–96. doi:10.1007/s40831-015-0025-5.
- Montenegro, V., H. Sano, and T. Fujisawa. 2013. Recirculation of high arsenic content copper smelting dust to smelting and converting processes. Minerals Engineering 48:184–89. doi:10.1016/j.mineng.2010.03.020.
- Morales, A., M. Cruells, A. Roc, and R. Bergo. 2010. Treatment of flash smelter flue dusts for copper and zinc extraction and arsenic stabilization. Hydrometallurgy 105 (1–2):148–54. doi:10.1016/j.hydromet.2010.09.001.
- Mudd, G. M., S. M. Jowitt, and T. T. Werner. 2017. The world’s by-product and critical metal resources part I: Uncertainties, current reporting practices, implications and grounds for optimism. Ore Geology Reviews 86:924–38. doi:10.1016/j.oregeorev.2016.05.001.
- Mudd, G. M., Z. Weng, and S. M. Jowitt. 2013a. A detailed assessment of global Cu reserve and resource trends and worldwide Cu endowments. Economic Geology 108 (5):1163–83. doi:10.2113/econgeo.108.5.1163.
- Mudd, G. M., Z. Weng, S. M. Jowitt, J. D. Turnbull, and T. E. Graedel. 2013b. Quantifying the recoverable resources of by-product metals: The case of cobalt. Ore Geology Reviews 55:87–98. doi:10.1016/j.oregeorev.2013.04.010.
- Mudhoo, A., S. K. Sharma, V. K. Garg, and C. Tseng. 2011. Arsenic: An overview of applications, health and environmental concerns and removal processes. Critical Reviews in Environmental Science and Technology 41 (5):435–519. doi:10.1080/10643380902945771.
- Murani, K., R. Siddique, and K. K. Jain. 2015. Use of copper slag: A sustainable material. Journal of Material Cycles and Waste Management 17 (1):13–26. doi:10.1007/s10163-014-0254-x.
- Muravyov, M. I., N. V. Fomchenko, A. V. Usoltsev, E. A. Vasilyev, and T. F. Kondrat’eva. 2012. Leaching of copper and zinc from copper converter flotation tailings using H2SO4 and biologically generated Fe2(SO4)3. Hydrometallurgy 119-120:40–46. doi:10.1016/j.hydromet.2012.03.001.
- Muruchi, L., N. Schaeffer, H. Passos, C. M. N. Mendonca, J. A. P. Coutinho, and Y. P. Jimenez. 2019. Sustainable extraction and separation of rhenium from model copper mining effluents using a polymeric aqueous two-phase system. ACS Sustainable Chemistry & Engineering 7 (1):1178–785. doi:10.1021/acssuschemeng.8b05759.
- Nagel, N. 2018. Beryllium and copper-beryllium alloys. ChemBioEng Reviews 3:30–33. doi:10.1002/cben.201700016.
- Nakajima, K., O. Takeda, T. Miki, K. Matsubae, and T. Nagasaka. 2011. Thermodynamic analysis for the controllability of elements in the recycling process of metals. Environmental Science & Technology 45 (11):4929–36. doi:10.1021/es104231n.
- Navazo, J. M. V., G. Villalba Mendez, and L. Talens Peiro. 2014. Material flow and energy requirements of mobile phone material recovery processes. International Journal of Life Cycle Assessment 19 (3):567–79. doi:10.1007/s11367-013-0653-6.
- Nazari, A. M., R. Radzinski, and A. Ghahreman. 2017. Review of arsenic metallurgy: Treatment of arsenical minerals and the immobilization of arsenic. Hydrometallurgy 174:258–81.
- Nekouei, R. K., F. Pahlevani, R. Rajarao, R. Gohmohammadzadeh, and V. Sahajwalla. 2018. Two-step pre-processing enrichment of waste printed circuit boards: Mechanical milling and physical separation. Journal of Cleaner Production 184:1113–24. doi:10.1016/j.jclepro.2018.02.250.
- Noor, E. E. M., H. Zuhallawati, and O. Radzali. 2016. Low temperature In-Bi-Zn solder alloy on copper substrate. Journal of Materials Science and Materials Electronics 27 (2):1408–15. doi:10.1007/s10854-015-3904-4.
- Norgate, T., and S. Jahanshahi. 2010. Low grade ores –smelt, leach or concentrate?”. Minerals Engineering 23 (2):65–73. doi:10.1016/j.mineng.2009.10.002.
- Okanigbe, D. O., A. P. I. Popoola, A. A. Adeleke, I. O. Otunniyi, and O. M. Popoola. 2019. Investigating impact of pretreating a waste copper smelter dust for likely higher recovery of copper. Procedia Manufacturing 35:430–35. doi:10.1016/j.promfg.2019.05.062.
- Okibe, N., M. Koga, S. Morishita, M. Tanaka, S. Heguri, S. Asano, K. Sasaki, and T. Hirajima. 2014. Microbial formation of crystalline scorodite for the treatment of As(III)-bearing copper refinery process solution using Acidianus brierleyi. Hydrometallurgy 143:34–41. doi:10.1016/j.hydromet.2014.01.008.
- Olvera, B. C. 2021. Innovation in mining: What are the challenges and opportunities along the value chain for Latin American suppliers? Mineral Economics (18 pp.). doi:10.1007/s13563-021-00251-w.
- Orrego, P., J. Hernández, and A. Reyes. 2019. Uranium and molybdenum recovery from copper leaching solutions using ion exchange. Hydrometallurgy 184:116–22. doi:10.1016/j.hydromet.2018.12.021.
- Ozberk, E., W. A. Jankola, M. Vecchiarelli, and B. D. Krysa. 1995. Commercial operations of the Sherritt zinc pressure leach process. Hydrometallurgy 39 (1–3):49–52. doi:10.1016/0304-386X(95)00047-K.
- Paktunc, D., and K. Bruggeman. 2010. Solubility of nanocrystalline scorodite and amorphous ferric arsenate for stabilization of arsenic in mine wastes. Applied Geochemistry 25 (5):674–83. doi:10.1016/j.apgeochem.2010.01.021.
- Panda, R., P. R. Jadhao, K. K. Pani, S. N. Naik, and T. Bhaskar. 2020. Eco-friendly recovery of metals from waste mobile printed circuit boards using low temperature roasting after pyrolysis. Journal of Hazardous Materials 395:122642 (11 pp.). doi:10.1016/j.jhazmat.2020.122642.
- Panda, S., G. Mishra, C. K. Sarangi, K. Sanjay, T. Subbaiah, S. K. Das, K. Sarangi, M. K. Ghosh, N. Pradhan, and B. K. Mishra. 2016. Reactor and column leaching studies for extraction of copper from two low grade resources. A comparative study. Hydrometallurgy 165:111–17. doi:10.1016/j.hydromet.2015.10.005.
- Papassiopi, N., K. Vaxevanidou, and J. Paspaliaris. 2003. Investigating the use of iron reducing bacteria for the removal of arsenic from contaminated soils. Water, Air, and Soil Pollution 3 (3):81–90. doi:10.1023/A:1023905128860.
- Parajuly, K., K. Thapa, C. Cimpan, and H. Wenzel. 2018. Electronic waste and informal recycling in Kathmandu, Nepal: Challenges and opportunities. Journal of Material Cycles and Waste Management 20 (1):656–66. doi:10.1007/s10163-017-0610-8.
- Parviainen, A., F. Soio, and M. A. Caraballo. 2020. Revalorization of Haveri Au-Cu mine tailings (SWE Finland) for potential reprocessing. Journal of Geochemical Exploration 218:106614 (9 pp.). doi:10.1016/j.gexplo.2020.106614.
- Paz-Gómez, D. C., S. M. Pérez-Moreno, I. Ruiz-Oria, G. Rios, and J. P. Bolivar. 2020. Characterization of two sludges from a pyrometallurgical copper smelting complex for designing a Pb and Se recovery proposal. Waste and Biomass Valorization 12 (5):2739–55. doi:10.1007/s12649-020-01197-w.
- Pazik, P. M., T. Chmielewski, H. J. Glass, and P. B. Kowalczuk. 2016. World production and possible recovery of cobalt from the Kupferschiefer stratiform copper ore. E3S Web of Conferences 8:01063 (9 pp.). doi:10.1051/e3sconf/20160801063.
- Perez-Moreno, S. M., M. J. Gasquez, I. Ruiz-Oria, G. Rios, and J. P. Bolivar. 2018. Diagnose for valorisation of reprocessed slag cleaning furnace flue dust from copper smelting. Journal of Cleaner Production 194:383–95. doi:10.1016/j.jclepro.2018.05.090.
- Petersen, J. 2016. Heap leaching as a key technology for recovery of values from low grade ores – A brief overview. Hydrometallurgy 165:201–12. doi:10.1016/j.hydromet.2015.09.001.
- Potysz, A., E. D. van Hullebusch, and J. Kierczak. 2018. Perspective regarding use of metallurgical slags as secondary metal resource. A review of bioleaching approaches. Journal of Environmental Management 219:128–52. doi:10.1016/j.jenvman.2018.04.083.
- Potysz, A., E. D. van Hullebusch, J. Kierczak, M. Grybos, P. N. L. Leens, and G. Guibaud. 2015. Copper metallurgical slags- current knowledge and fate: A review. Critical Reviews in Environmental Science and Technology 45 (22):2423–88. doi:10.1080/10643389.2015.1046769.
- Potysz, A., E. D. van Hullebusch, J. Kierczak, M. Grybos, P. N. L. Leens, and G. Guibaud. 2016. Response to comment on “Copper metallurgical slags- current knowledge and fate: A review. Critical Reviews in Environmental Science and Technology 46:438–40. doi:10.1080/10643389.2015.1131560.
- Potysz, A., and J. Kierczak. 2019. Prospective (bio)leaching of historical copper slags as an alternative to their disposal. Minerals 9 (9):542 (24 pp.). doi:10.3390/min9090542.
- Pradhan, D., D. J. Kim, L. B. Sukla, A. Pattanaik, and D. P. K. Samal. 2019. Bacterial leaching of chalcopyrite concentrates using Acidithiobacillus ferrooxidans. Inglomayor C 16:1–9.
- Prem, P. R., M. Verma, and P. S. Ambily. 2018. Sustainable cleaner production of concrete with high volume copper slag. Journal of Cleaner Production 173:43–58. doi:10.1016/j.jclepro.2018.04.245.
- Priya, A., and S. Hait. 2017. Comparative assessment of metallurgical recovery of metals from electronic waste with special emphasis on bioleaching. Environmental Science and Pollution Research 24 (8):6989–7008. doi:10.1007/s11356-016-8313-6.
- Priya, A., and S. Hait. 2018a. Comprehensive characterization of printed circuit boards of various end-of-life electrical and electronic equipment for beneficiation investigation. Waste Management 75:103–23. doi:10.1016/j.wasman.2018.02.014.
- Priya, A., and S. Hait. 2018b. Extraction of metals from high grade waste printed circuit board by conventional and hybrid bioleaching using Acidithiobacillus ferrooxidans. Hydrometallurgy 127:132–39. doi:10.1016/j.hydromet.2018.03.005.
- Priya, J., N. S. Randhawa, J. Hait, N. Bordoloi, and J. N. Patel. 2020. High purity copper recycled from smelter dust by sulfation roasting, water leaching and electrorefining. Environmental Chemistry Letters 18 (6):2133–39. doi:10.1007/s10311-020-01047-0.
- Randive, K., and S. Jawadand. 2019. Strategic minerals in India: Present status and future challenges. Mineral Economics 32 (3):337–52. doi:10.1007/s13563-019-00189-0.
- Raposeiras, A. C., D. Movilla-Quesada, O. Munoz-Cáseres, V. Andrés-Valeri, and M. Lagos-Varas. 2021. Production of asphalt mixes with copper industry wastes: Use of copper slags as raw material replacement. Journal of Environmental Management 293:112867 (8 pp.). doi:10.1016/j.jenvman.2021.112867.
- Reck, B. K., and T. E. Graedel. 2012. Challenges in metal recycling. Science 337 (6095):690–95. doi:10.1126/science.1217501.
- Restrepo, E., A. Lovik, P. Wäger, R. Widmer, R. Lonka, and D. B. Müller. 2017. Stocks, flows and distribution of critical metals in embedded electronics in passenger vehicles. Environmental Science & Technology 51 (3):1129–39. doi:10.1021/acs.est.6b05743.
- Reuter, M. A. 2016. Digitalizing the circular economy. Metallurgy and Materials Transactions B 47 (6):3194–220. doi:10.1007/s11663-016-0735-5.
- Reuter, M. A., A. van Schaik, and M. Ballester. 2018. Limits of the circular economy: Fairphone modular design pushing the limits. Erzmetall 71:68–78.
- Reuter, M. A., and I. V. Kojo. 2014. Copper: A key enabler of resource efficiency. Erzmetall 67:46–53.
- Revesz, E., D. Fortin, and D. Pactunc. 2015. Reductive dissolution of scorodite in the presence of Shewanella sp. CN 32 and Shewanella sp. ANA-3. Applied Geochemistry 63:347–56. doi:10.1016/j.apgeochem.2015.09.022.
- Revesz, E., D. Fortin, and D. Pactunc. 2016. Reductive dissolution of arsenical ferrihydrite by bacteria. Applied Geochemistry 66:129–39. doi:10.1016/j.apgeochem.2015.12.007.
- Riedewald, F., and M. Sousa-Gallagher. 2015. Novel waste printed circuit board recycling process with molten salt. Methods X 2:100–06.
- Rincon, J., S. Gaydardzhiev, and L. Stamenov. 2019. Investigation on the flotation recovery of copper sulphosalts through an integrated mineralogical approach. Minerals Engineering 130:36–47. doi:10.1016/j.mineng.2018.10.006.
- Riveros, P. A., J. E. Dutizac, and P. Spencer. 2013. Arsenic disposal practices in the metallurgical industry. Canadian Metallurgical Quarterly 40:395–420. doi:10.1179/cmq.2001.40.4.395.
- Rocchetti, L., A. Amato, and F. Beolchini. 2018. Printed circuit board recycling: A patent review. Journal of Cleaner Production 178:814–32. doi:10.1016/j.jclepro.2018.01.076.
- Rong, Z., X. Tang, L. Wu, X. Chen, W. Dang, and Y. Wang. 2020. A novel method to synthesize scorodite using ferrihydrite and its role in the removal and immobilization of arsenic. Journal of Materials Research and Technology 9 (3):5848–57. doi:10.1016/j.jmrt.2020.03.112.
- Rönnlund, I., M. Reuter, S. Horn, J. Aho, M. Aho, M. Päällysaho, L. Yilmäki, and T. Pursula. 2016. Eco-efficiency indicator framework implemented in the metallurgical industry: Part 2 – A case study from the copper industry. International Journal of Life Cycle Assessment 21 (12):1719–48. doi:10.1007/s11367-016-1123-8.
- Ruan, J., and Z. Xu. 2016. Constructing environment-friendly return road of metals from e-waste: Combination of physical separation technologies. Renewable and Sustainable Energy Reviews 54:745–60. doi:10.1016/j.rser.2015.10.114.
- Ruan, R., G. Zou, S. Zhong, Z. Wu, B. Chan, and D. Wang. 2013. Why Zijinshan copper bioheap leaching plant works efficiently at low microbial activity – Study on leaching kinetics of copper sulfides and its implications. Minerals Engineering 48:36–43. doi:10.1016/j.mineng.2013.01.002.
- Rubinos, D. A., O. Jerez, G. Forghani, M. Edraki, and U. Kelm. 2021. Geochemical stability of potentially toxic elements in porphyry copper-mine tailings from Chile as linked to ecotoxicological and human health risk assessment. Environmental Science and Pollution Research. doi:10.1007/s11356-021-12844.7.
- Ruzic, J., J. Stasic, V. Rajkovic, and D. Božić. 2013. Strengthening effects in precipitation and dispersion hardened powder metallurgy copper alloys. Materials & Design 49:746–54. doi:10.1016/j.matdes.2013.02.030.
- Sabzezari, B., S. M. J. Koleini, S. Ghassa, R. Shahbazi, and S. C. Chelani. 2019. Microwave-leaching of copper smelting dust for Cu and Zn extraction. Materials 12 (1822):(18. doi:10.3390/ma12111822.
- Safarzadeh, M. S., M. Horton, and A. D. Van Rythoven. 2018. Review of recovery of platinum group metals from copper leach residues and other resources. Minerals Processing and Extractive Metallurgy Review 39 (1):1–17. doi:10.1080/08827508.2017.1323745.
- Sahu, S. K., B. Chmielowiec, and A. Allanore. 2017. Electrolytic extraction of copper. molybdenum and rhenium from molten sulfide electrolyte. Electrochimica Acta 243:382–89. doi:10.1016/j.electacta.2017.04.071.
- Sakoor, M. B., R. Nawaz, E. Hussain, M. Raza, S. Ali, M. Rizwan, S. Oh, and S. Ahmad. 2017. Human health implications. Risk assessment and remediation of As contaminated water: A critical review. Science of the Total Environment 601-602:756–64. doi:10.1016/j.scitotenv.2017.05.223.
- Samal, D. P. K., L. B. Sukla, A. Pattanaik, and D. Pradhan. 2019. Extraction of gold from electronic scraps: A biohydrometallurgical process overview. Biointerface Research and Applied Chemistry 9:4362–67.
- Samuels, E. R., and J. C. Méranger. 1984. Preliminary studies on the leaching of some trace metals from kitchen faucets. Water Research 18 (1):75–80. doi:10.1016/0043-1354(84)90049-6.
- Sanchez, M., F. Parada, R. Parra, F. Marquez, R. Jara, J. C. Carrasco, and J. Palacios. 2004. Management of copper pyrometallurgical slags giving additional value to copper smelting industry. In Management of copper metallurgical slags, 543–50. South African Institute of Mining and Metallurgy, Johannesburg.
- Sanchez, M., and M. Sudbury. 2013. Reutilization of primary metallurgical wastes: Copper slag as a source of copper, molybdenum, and iron – Brief review of test work and the proposed way forward. In Proceedings of the Third International Slag Valorisation Symposium, 19-20 March, ed. A. Malfliet, P. T. Jones, K. Binnemans, O. Cizer, J. Fransaer, F. Yan, Y. Pontikes, M. Guo, and B. Blanpain, 135–46. Leuven (Belgium): KU Leuven.
- Sarver, E., and M. Edwards. 2011. Effects of flow, brass location, tube materials and temperature on corrosion of brass plumbing devices. Corrosion Science 53 (5):1813–24. doi:10.1016/j.corsci.2011.01.060.
- Scheffel, R. E., A. Guzman, and J. E. Dreier. 2016. Development of metallurgy guidelines for copper heap-leach. Minerals and Metallurgical Processing 33 (4):187–99. doi:10.19150/mmp.6840.
- Schlesinger, M. E., M. J. King, K. C. Sole, and W. G. Davenport. 2011. Chemical metallurgy of copper recycling. In Extractive metallurgy of copper, 389–96. Fifth ed. Oxford: Elsevier.
- Schreck, P. 1999. Flue dust from copper shale smelting in central Germany: Environmental pollution and its prevention. IMWA Proceedings, 163–67 Sevilla (Spain).
- Schwartz, D. M., V. Y. Omaynikova, and S. K. Stocker 2017. Environmental benefits of the CESL process for the treatment of high-arsenic copper-gold concentrates Hydroprocess ICMSE 2017, (9 pp.). Accessed April 21, 2021. www.teck.com
- Selivanov, E. N., G. V. Skopov, R. I. Gulyaeva, and A. V. Matveev. 2014. Material composition of the dust from electrostatic precipitators of a Vanyukov furnace in the middle Ural copper smelter. Metallurgist 58 (5–6):431–35. doi:10.1007/s11015-014-9928-9.
- Selivanov, E. N., I. Popov, N. I. Selmenskikh, and A. B. Lebed. 2013. Oxide inclusions of copper during its fire refining. Nonferrous Metals 13:19–22.
- Sethurajan, M., E. D. van Hullebusch, D. Fontana, A. Akcil, H. Deveci, B. Batinic, J. P. Leal, J. P. Gasche, M. A. Kurucer, K. Kuchta, et al. 2019. Recent advances on hydrometallurgical recovery of critical and precious elements from end-of-life electronic wastes – Review. Critical Reviews in Environmental Science and Technology 49 (3):212–75. doi:10.1080/10643389.2018.1540760.
- Shen, H., and E. Forssberg. 2003. An overview of recovery of metals from slags. Waste Management 23 (10):933–49. doi:10.1016/S0956-053X(02)00164-2.
- Shengo, L. M. 2021. Potentially exploitable reprocessing routes for recovering copper and cobalt retained in flotation tailings. Journal of Sustainable Metallurgy 7 (1):60–77. doi:10.1007/s40831-020-00325-z.
- Shengo, L. M., B. K. Kitungwa, C. W. N. Mutiti, J. L. M. Mulumba, and F. P. Ilunga. 2021. Recovery of copper metal through reprocessing of residues from a hydrometallurgy plant: An advance study. Advanced Aspects of Engineering Research 13:33–47.
- Shi, J., Y. Shi, Y. Feng, Q. Li, W. Chen, W. Zhang, and H. Li. 2020. Anthropogenic cadmium cycle and emissions in Mainland China 1990-2015. Journal of Cleaner Production 230:256–65.
- Shibayama, A., Y. Takasaki, T. William, A. Yamatodani, Y. Higuchi, S. Sunagawa, and E. Ono. 2010. Treatment of smelting residue for arsenic removal and recovery of copper using pyro-hydrometallurgical process. Journal of Hazardous Materials 181 (1–3):1016–23. doi:10.1016/j.jhazmat.2010.05.116.
- Shihshin, D., P. C. Hayes, and E. Jak. 2018. Multicomponent thermodynamic databases for complex non-ferrous pyrometallurgical processes. In Extraction 2018, The Minerals, Metals & Materials Series, ed. B. Davies, 853–68. Cham: Springer Verlag.
- Shuva, M. A. H., M. A. Rhamdhani, G. A. Brooks, S. Masood, M. A. Reuter, and M. Firdaus. 2017. Analysis for optimum conditions for recovery of valuable metals from e-waste through black copper smelting. In 8th International Symposium on High Temperature Metallurgical Processing, The Minerals, Metals and Material Series, ed. J. Y. Hwang, 419–27. Cham: Springer Verlag.
- Shuva, M. A. H., M. A. Rhamdhani, G. A. Brooks, S. Masood, and M. A. Reuter. 2016. Thermodynamics data of valuable elements relevant to e-waste processing and secondary copper production. Journal of Cleaner Production 131:795–809. doi:10.1016/j.jclepro.2016.04.061.
- Sibanda, V., E. Sipunga, G. Danha, and T. A. Mamvura. 2020. Enhancing the flotation recovery of copper minerals in smelter slags from Namibia prior to disposal. Heliyon 6 (1):e03135 (11 pp.). doi:10.1016/j.heliyon.2019.e03135.
- Siddique, R., M. Singh, and M. Jain. 2020. Recycling copper slag in steel fibre concrete for sustainable construction. Journal of Cleaner Production 221:122559 (10 pp.).
- Sineva, S., M. Shevchenko, D. Shishin, T. Hidayat, J. Chen, P. C. Hayes, and E. Jak. 2021. Phase equilibria distributions in complex copper/slag/matte systems. JOM 72 (10):3401–09. doi:10.1007/s11837-020-04326-x.
- Singh, J., and B. Lee. 2016. Recovery of precious metals from low-grade automobile shredder residue: A novel approach to the recovery of nanozero-valent copper particles. Waste Management 48:353–65. doi:10.1016/j.wasman.2015.10.019.
- Sögaard, C., J. Funching, M. Gregoric, and Z. Abbas. 2018. The long-term stability of silica nanoparticulate gels in waters of different ionic compositions and pH values. Colloids and Surfaces A 554:127–30. doi:10.1016/j.colsurfa.2018.02.020.
- Sokhanvaran, S., S. Lee, G. Lambotte, and A. Allanore. 2016. Electrochemistry of molten sulfides: Copper extraction from BaS-Cu2S. Journal of the Electrochemical Society 163 (3):D115–D120. doi:10.1149/2.0821603jes.
- Sole, K. C., J. Parker, P. M. Cole, and M. B. Mooiman. 2019. Flowsheet options for cobalt recovery in African copper-cobalt hydrometallurgy circuits. Minerals Processing and Extractive Metallurgy Review 40 (3):194–206. doi:10.1080/08827508.2018.1514301.
- Sole, K. C., M. B. Mooiman, and E. Hardwick. 2017. Ion exchange in hydrometallurgical processing; an overview and selected applications. Separation and Purification Review 60:1–20.
- Soo, V. K., J. Peeters, P. Compiston, M. Doolan, and J. R. Duflou. 2017. Comparative study of end-of-life vehicle recycling in Australia and Belgium. Procedia CRIP 61:269–74. doi:10.1016/j.procir.2016.11.222.
- Sorooshian, A., J. Csavina, T. Shingler, S. Dey, F. J. Brechtel, A. E. Sáez, and E. Betterton. 2012. Hygroscopic and chemical properties of aerosols collected near a copper smelter: Implications for public and environmental health. Environmental Science & Technology 46 (17):9473–80. doi:10.1021/es302275k.
- Sousa, R., A. Futuro, C. S. Pires, and M. M. Leite. 2017. Froth flotation of Aljustrel sulphide complex ore. Physicochemical Problems of Mineral Processing 53:758–60.
- Spooren, J., K. Binnemans, J. Björkmalm, K. Breeemersch, Y. Dams, K. Folens, M. González-Moya, L. Horckmans, K. Komitsas, V. Kurylak, et al. 2020. Near-zero waste processing of low grade, complex primary ores and secondary raw materials in Europe: Technology development trends. Resources Conservation and Recycling 160:104919 (18 pp.). doi:10.1016/j.resconrec.2020.104919.
- Sracek, O., F. Veselovsky, B. Kribek, B. Malec, and J. Jehlicka. 2010. Geochemistry, mineralogy and environmental impact of precipitated efflorescent salts at the Kabwe Cu-Co chemical leaching plant in Zambia. Applied Geochemistry 25 (12):1815–24. doi:10.1016/j.apgeochem.2010.09.008.
- Sridhar, R., J. M. Toguri, and S. Simeonov. 1997. Copper losses and thermodynamic considerations in copper smelting. Metallurgical and Materials Transactions B 28 (2):191–200. doi:10.1007/s11663-997-0084-5.
- Stamp, A., H. Althaus, and P. A. Wäger. 2013. Limitation to applying life cycle assessment to complex co-product systems: The case of an integrated precious metals smelter-refinery. Resources Conservation and Recycling 80:85–96. doi:10.1016/j.resconrec.2013.09.003.
- Steinacker, S.-R., and J. Antrekowitsch. 2017. Treatment of residues from the copper industry with an alternative approach to electric furnace slag. BHM 162 (7):252–57.
- Sthiannopkao, S., and M. H. Wong. 2013. Handling e-waste in developed and developing countries: Initiatives, practices, and consequences. Science of the Total Environment 463-464:1147–53. doi:10.1016/j.scitotenv.2012.06.088.
- Sukhomlinov, D., K. Avarmaa, O. Virtanen, P. Taskinen, and A. Jokilaakso. 2020. Slag-copper equilibria of selected trace elements in black-copper smelting. Part II. Trace element distributions. Minerals Processing and Extractive Metallurgy Review 41 (3):171–77. doi:10.1080/08827508.2019.1634561.
- Sukhomlinov, D., L. Klemettinen, K. Avarmaa, H. O´Brien, P. Taskinen, and A. Jokilaakso. 2019. Distribution of Ni, Co, precious and platinum group metals in copper making process. Metallurgical and Materials Transactions B 50 (4):1752–65. doi:10.1007/s11663-019-01576-2.
- Sun, Y., Q. Yao, N. Li, N. Li, Z. Hao, Z. Hao, and Z. Hao. 2018. Insight into mineralizer modified and tailored scorodite crystal characteristics and leachability for arsenic-rich smelter wastewater stabilization. RSC Advances 9 (35):19560–69. doi:10.1039/C8RA01721B.
- Sun, Z., X. Y. Agterhuis, H. Sietsma, and J. Yang. 2016. Recycling metals from urban mines – Strategic evaluation. Journal of Cleaner Production 112:2977–87. doi:10.1016/j.jclepro.2015.10.116.
- Sutliff-Johansson, S., S. Pontér, E. Engström, I. Rodushkin, P. Peltola, and A. Widerlund. 2021. Tracing anthropogenic sources of tantalum and niobium in Bothnian Bay sediments, Sweden. Journal of Soils and Sediments 21 (3):1488–503. doi:10.1007/s11368-020-02852-4.
- Sverdrup, H. U., A. H. Olafsdottir, and K. V. Ragnarsdottir. 2019. On the long-term sustainability of copper, zinc and lead supply using a system dynamics model. Resources Conservation and Recycling X 4:100007 (21 pp.).
- Tabelin, C. B., I. Park, T. Phengsaart, S. Jeo, M. Villacorte-Tabelin, D. Alonzo, K. Yoo, M. Ito, and N. Hiroyoshi. 2021. Copper and critical metal production from porphyry ores and E-wastes. A review of resource availability. Processing/recycling challenges, socio-environmental aspects, and sustainability issues. Resources Conservation and Recycling 170:105610 (35 pp.).
- Tan, M., R. He, Y. Yuan, Z. Wang, and X. Jinn. 2016. Electrochemical sulfur removal from chalcopyrite in molten NaCl-KCl. Electrochimica Acta 213:148–54. doi:10.1016/j.electacta.2016.07.088.
- Tanabe, E. H., R. M. Silva, D. L. Olivera Jr, and D. A. Bertuol. 2019. Recovery of valuable metals from waste cables employing mechanical processing followed by sprouted bed elutriation. Particulogy 45:71–80. doi:10.1016/j.partic.2018.12.002.
- Tanne, C. K., and A. Schippers. 2019. Electrochemical investigation of chalcopyrite (bio)leaching residues. Hydrometallurgy 187:8–17. doi:10.1016/j.hydromet.2019.04.022.
- Tesfaye, F., D. Lindberg, J. Hmayuni, P. Taskinen, and L. Hupa. 2017. Improving urban mining practices for optimal recovery of resources from e-waste. Minerals Engineering 111:209–21. doi:10.1016/j.mineng.2017.06.018.
- Tian, H., Z. Guo, J. Pan, D. Zhu, C. Yang, Y. Xue, S. Li, and D. Wang. 2021. Comprehensive review on metallurgical recycling and cleaning of copper slag. Resources Conservation and Recycling 168:105366 (22 pp.). doi:10.1016/j.resconrec.2020.105366.
- Tian, S., Y. Gong, H. Ji, J. Duan, and D. Zhao. 2020. Efficient removal and long-term sequestration of cadmium from aqueous solution using ferrous sulfide nanoparticles: Performance, mechanisms and long-term stability. Science of the Total Environment 704:135402 (10 pp.). doi:10.1016/j.scitotenv.2019.135402.
- Torres, A., M. U. Simoni, J. K. Kleiding, D. B. Müller, S. O. S. E. zu Ermgassen, J. Liu, J. A. G. Jaeger, M. Winter, and E. F. Lambin. 2021. Sustainability of the global sand system in the Anthropocene. One Earth 4 (5):639–50. doi:10.1016/j.oneear.2021.04.011.
- Tshipeng, S. Y., A. Tsamala-Kanaki, and M. Kime. 2017. Effect of addition points of reducing agents on the extraction of copper and cobalt from oxidized copper-cobalt ores. Journal of Sustainable Metallurgy 3 (4):823–28. doi:10.1007/s40831-017-0149-x.
- Turan, M. D., Z. A. Sari, and J. D. Miller. 2017. Leaching of blended copper slag in microwave oven. Transactions of the Nonferrous Metals Society China 27 (6):1404–10. doi:10.1016/S1003-6326(17)60161-4.
- Ueberschaar, M., D. D. Jalapoor, N. Korf, and V. S. Rotter. 2017. Potentials and barriers for tantalum recovery from waste electric and electronic equipment. Journal of Industrial Ecology 21 (3):700–14. doi:10.1111/jiec.12577.
- Ulman, K., S. Maoufi, S. Bhattacharyya, and V. Sahajwalla. 2018. Thermal transformation of printed circuit boards at 500 °C for synthesis of a copper-based product. Journal of Cleaner Production 198:1485–93. doi:10.1016/j.jclepro.2018.07.140.
- Ulsen, C., J. L. Antoniassi, I. M. Martins, and H. Kahn. 2021. High quality recycled sand from mixed CDW - is that possible? Journal of Materials Research and Technology 12:27–42. doi:10.1016/j.jmrt.2021.02.057.
- UNEP. 2013. Report of the working group on the global metal flows to the international resource panel. Accessed June 22, 2020. http://www.resourcepanel.org/reports.
- US Geological Survey. 2020. Minerals commodity summaries 2020. US Government Publishing Office, Washington DC. Virginia.
- Van Schalkwyk, R. F., M. A. Reuter, J. Gutzmer, and M. Stelter. 2018. Challenges in digitalizing the circular economy. Assessment of the state-of-the art metallurgical carrier metal platform for lead and its associated technology elements. Journal of Cleaner Production 186:585–601. doi:10.1016/j.jclepro.2018.03.111.
- Vardanyan, N., G. Svoyan, T. Navasardy, and A. Vardanyan. 2019. Recovery of valuable metals from polymetallic mine tailings by natural microbial consortium. Environmental Technology 40 (26):3467–72. doi:10.1080/09593330.2018.1478454.
- Vermeulen, A., W. Müller, K. D. Matson, B. I. Tieleman, L. Bervoets, and M. Eens. 2015. Sources of variation in innate immunity in great tit nestlings living along a metal pollution gradient: An individual-based approach. Science of the Total Environment 508:297–306. doi:10.1016/j.scitotenv.2014.11.095.
- Vidyadhar, A., and A. Das. 2013. Enrichment implication of froth flotation kinetics in separation and recovery of metal values from printed circuit boards. Separation and Purification Technology 118:305–302. doi:10.1016/j.seppur.2013.07.027.
- Vitkova, M., V. Ettler, J. Hyks, T. Astrup, and B. Kribek. 2011. Leaching of metals from copper smelter flue dust (Mufulira, Zambian Copperbelt). Applied Geochemistry 26:S263–S266. doi:10.1016/j.apgeochem.2011.03.120.
- Vlaamse Milieu Maatschappij. 2018. Luchtkwaliteit in Hoboken. Focus op de periode 2016-2018. (Air quality in Hoboken. Focus on the 2016-2018 period). Aalst (103 pp.).
- Voigt, P., A. Burrows, N. Somerville, and C. Chen. 2017. Direct-to-blister copper smelting with the ISASMELTTM Process. In 8th International Symposium on High-Temperature Metallurgical Processing, The Minerals, Metals & Materials Series, ed. J. Y. Hwang, 261–67. Cham: Springer Verlag.
- Wan, X., P. Taskinen, J. Shi, and A. Jokilaakso. 2021. A potential industrial waste-waste co-treatment process of utilizing waste SO2 gas and residue heat to recover Co, Ni and Cu from copper melting slag. Journal of Hazardous Materials 414:125541 (14 pp.). doi:10.1016/j.jhazmat.2021.125541.
- Wang, D., Q. Wang, and Z. Hang. 2020. Reuse of copper slag as a supplementary cementitious material: Reactivity and safety. Resources Conservation and Recycling 162:105037 (9 pp.). doi:10.1016/j.resconrec.2020.105037.
- Wang, F., Y. Zhao, T. Zhang, G. Zhang, X. Yang, Y. He, L. Wang, and C. Duan. 2017a. Metals recovery from dust derived from recycling line of waste printed circuit board. Journal of Cleaner Production 165:452–57. doi:10.1016/j.jclepro.2017.07.112.
- Wang, Q., X. Guo, Q. Tian, T. Jiang, M. Chen, and B. Zhao. 2017b. Effects of matte grade on the distribution of minor elements (Pb, Zn, As, Sb and Bi) in the bottom blown copper smelter process. Metals 7 (11):502 (11 pp.). doi:10.3390/met7110502.
- Wang, R. W., Q. Shi, Y. Li, Z. Cao, and Z. Si. 2021. A critical review of the use of copper slag (CS) as substitute constituent in concrete. Construction and Building Materials 292:123371 (21 pp.). doi:10.1016/j.conbuildmat.2021.123371.
- Wang, Y., X. Liu, Y. Si, and R. Wang. 2016. Release and transformation of arsenic from As-bearing iron minerals by Fe-reducing bacteria. Chemical Engineering Journal 293:29–38. doi:10.1016/j.cej.2016.03.027.
- Waterman, B. T. 2013. Methods and systems for recovering rhenium from a copper leach. US patent 8491700 B2
- Watling, H. R. 2006. The bioleaching of sulphide minerals with emphasis on copper sulphides – A review. Hydrometallurgy 84 (1–2):81–108. doi:10.1016/j.hydromet.2006.05.001.
- Weidenbach, M., G. Dunn, and Y. Y. Tao 2016. Removal of impurities from copper sulfide mineral concentrates. ALTA 2016 Nickel-Cobalt-Copper Proceedings, 21-28 May (17 pp). Perth (Australia). Accessed May 23, 2021. www.orway.com.au.
- Welfens, M. J., J. Nordman, and A. Seibt. 2016. Drivers and barriers to return and recycling mobile phones. Case studies of communication and collection campaigns. Journal of Cleaner Production 132:108–21. doi:10.1016/j.jclepro.2015.11.082.
- Wellmer, F., and C. Hagelüken. 2015. The feedback control cycle of mineral supply, increase of raw material efficiency, and sustainable development. Minerals 5 (4):815–36. doi:10.3390/min5040527.
- Widmer, R., X. Du, O. Heag, E. Restrepo, and A. Wager. 2015. Scarce metals in conventional passenger vehicles and end-of-life shredder output. Environmental Science & Technology 49 (7):4591–99. doi:10.1021/es505415d.
- Wijenayake, J. J., and H. S. Sohn. 2020. The synthesis of tire grade ZnO from top submerged lance (TSL) furnace flue dust generated in Cu recycling industries. Hydrometallurgy 198:105466 (8 pp.). doi:10.1016/j.hydromet.2020.105466.
- Wiklund, J. A., J. L. Kirk, D. C. G. Muir, J. Carrier, A. Gleason, F. Yang, M. Evans, and J. Keating. 2018. Widespread atmospheric tellurium contamination in industrial and remote regions of Canada. Environmental Science & Technology 52 (11):6137–45. doi:10.1021/acs.est.7b06242.
- Williams, P. T. 2010. Valorization of printed circuit boards from waste electrical and electronic equipment by pyrolysis. Waste and Biomass Valorization 1 (1):107–20. doi:10.1007/s12649-009-9003-0.
- Wu, J., J. Ahn, and J. Lee. 2021. Gold deportment and leaching study from a pressure oxidation residue of chalcopyrite concentrate. Hydometallurgy 201:105583 (9 pp.).
- Xia, M., P. Bao, A. Liu, M. Wang, L. Shen, B. Yu, Y. Liu, M. Chen, H. Li, X. Wu, et al. 2018. Bioleaching of low-grade waste printed circuit boards by mixed fungal culture and its community structure analysis. Resources Conservation and Recycling 136:267–75. doi:10.1016/j.resconrec.2018.05.001.
- Xia, Z., X. Zhang, X. Huang, S. Yang, Y. Chen, and L. Ye. 2020. Hydrometallurgical stepwise recovery of Cu and Zn from smelting slag of waste brass in ammonium chloride solution. Hydrometallurgy 157:105475 (7 pp.).
- Xu, B., W. Goa, R. Jiang, R. Jiang, R. Jiang, R. Jiang, and T. Jiang. 2020a. Comprehensive recovery of valuable elements from copper smelting open circuit dust and arsenic treatment. JOM 72 (11):3860–75. doi:10.1007/s11837-020-04242-0.
- Xu, J., S. Kumagai, T. Kameda, Y. Saito, K. Takahashi, H. Hayashi, and T. Yashioka. 2019. Separation of copper and polyvinylchloride from thin waste electric cables: A combined swelling and centrifugal approach. Waste Management 89:27–36. doi:10.1016/j.wasman.2019.03.049.
- Xu, L., Y. Yiong, Y. Song, G. Zhang, F. Zhang, Y. Yang, Z. Hua, Y. Tian, J. You, and Z. Zhao. 2020b. Recycling of copper telluride from copper anode slime processing: Toward efficient recovery of tellurium and copper. Hydrometallurgy 196:105436 (10 pp.). doi:10.1016/j.hydromet.2020.105436.
- Yang, B., G. L. Zhang, W. Deng, and J. Ma. 2013. Review of arsenic pollution and treatment progress in nonferrous metallurgy industry. Advanced Materials Research 634-638:3239–49.
- Yang, H., J. Wolters, P. Pischke, H. Soltner, S. Eckert, G. Natour, and J. Fröhlich. 2018. Modelling and simulation of a copper slag cleaning process improved by magnetic stirring. IOP Conference Series: Materials Science and Engineering 228:01204 (11 pp.).
- Yin, S., L. Wang, A. Wu, E. Kabwe, C. Chen, and R. Yan. 2018a. Copper recycle from sulfide tailings using combined leaching of ammonia solution and alkaline bacteria. Journal of Cleaner Production 189:746–53. doi:10.1016/j.jclepro.2018.04.116.
- Yin, S., L. Wang, A. Wu, M. L. Free, and E. Kabwe. 2018b. Enhancement of copper recovery by acid leaching of high-mud copper oxides: A case study at Yangla copper mine, China. Journal of Cleaner Production 202:321–31. doi:10.1016/j.jclepro.2018.08.122.
- Yin, Z., W. Sun, Y. Hu, J. Zhai, and G. Qingjun. 2017. Evaluation of replacement of NaCN with depressant mixtures in the separation of copper-molybdenum sulphide ore by flotation. Separation and Purification Technology 173:9–16. doi:10.1016/j.seppur.2016.09.011.
- Young, M. L., and D. C. Dunand. 2015. Comparing composition of modern cast bronze sculptures: Optical emission spectroscopy versus X-ray fluorescence spectroscopy. JOM 67 (7):1646–58. doi:10.1007/s11837-015-1445-1.
- Yu, F., Z. Liu, F. Ye, L. Xia, and A. Jokilaakso. 2021. A study of selenium and tellurium distribution behavior, taking the copper matte flash converting process as the background. JOM 73 (2):694–702. doi:10.1007/s11837-020-04517-6.
- Yuan, Z., D. Zhang, S. Wang, L. Xu, K. Wang, Y. Song, F. Xiao, and Y. Jia. 2016. Effect of hydroquinone-induced iron reduction of the stability of scorodite and arsenic mobilization. Hydrometallurgy 164:228–37. doi:10.1016/j.hydromet.2016.06.001.
- Yuan, Z., S. Wang, X. Ma, X. Wang, G. Zhang, J. Jia, and W. Zheng. 2017. Effect of iron reduction by enolic hydoxyl groups on the stability of scorodite in hydrometallurgical industries and arsenic mobilization. Environmental Science and Pollution Research 24 (34):26534–44. doi:10.1007/s11356-017-0016-0.
- Yun, L., A. Goyal, V. P. Singh, L. Gao, X. Peng, X. Niu, C. Wang, and A. Garg. 2019. Experimental coupled predictive modelling-based recycling of waste printed circuit boards for maximum extraction of copper. Journal of Cleaner Production 218:763–71. doi:10.1016/j.jclepro.2019.01.027.
- Zablocka-Malicka, M., P. Rutkowski, and W. Szczapaniak. 2015. Recovery of copper from PVC multiwire cable waste by steam gasification. Waste Management 46:488–96. doi:10.1016/j.wasman.2015.08.001.
- Zanin, M., I. Ametov, S. Grano, L. Zhou, and W. Skinner. 2009. A study of mechanisms affecting molybdenite recovery in a bulk copper/molybdenum flotation circuit. International Journal of Minerals Processing 93 (3–4):256–66. doi:10.1016/j.minpro.2009.10.001.
- Zeghoul, T., S. Touhami, G. Richard, M. Miloudi, O. Dahou, and L. Dascalescu. 2016. Optimal operation of a plate-type corona-electrostatic separator for the recovery of metals and plastics from granular wastes. IEEE Transactions Industrial Applications 52 (3):2506–12. doi:10.1109/TIA.2016.2533482.
- Zemlick, K., B. M. Thomson, J. Cheermak, and V. C. Tidwell. 2017. Modeled impacts of economics and policy on historic uranium mining operations in New Mexico. New Mexico Geology 39:11–24.
- Zeng, X., J. A. Mathews, and J. Li. 2018. Urban mining of e-waste is becoming more cost-effective than virgin mining. Environmental Science & Technology 52 (8):4835–41. doi:10.1021/acs.est.7b04909.
- Zhang, H., X. Liu, C. Su, and Y. Liu. 2019a. Effect of copper solvent extraction reagent on metabolism and leaching efficiency of bioleaching bacteria. IOP Conference Series: Earth and Environmental Science 237:052009 (6 pp.). doi:10.1088/1755-1315/237/5/052009.
- Zhang, R., S. Hedrich, E. Römer, D. Goldmann, and A. Schippers. 2020. Bioleaching of cobalt from Cu/Co-rich sulfidic mine tailings from the polymetallic Rammelsberg mine, Germany. Hydrometallurgy 197:105443 (8 pp.). doi:10.1016/j.hydromet.2020.105443.
- Zhang, Y., B. Jin, Y. Huang, Q. Song, and C. Wang. 2019b. Two-stage leaching of zinc and copper from arsenic-rich copper smelting hazardous dusts after alkali leaching or arsenic. Separation and Purification Technology 220:250–58. doi:10.1016/j.seppur.2019.03.067.
- Zhao, H., Y. Zhang, X. Zhang, L. Qian, M. Sun, Y. Yang, Y. Zhang, J. Wang, H. Kim, and G. Qiu. 2019. The dissolution and passivation mechanism of chalcopyrite in bioleaching.: An overview. Minerals Engineering 136:140–54. doi:10.1016/j.mineng.2019.03.014.
- Zhao, Z., L. Chai, B. Peng, Y. J. Liang, Y. He, and Z. Yam. 2017. Arsenic vitrification by copper-based glass: Mechanism and stability studies. Journal of Non-Crystalline Solids 466-467:21–28. doi:10.1016/j.jnoncrysol.2017.03.039.
- Zschiesche, C., M. Ayhan, and J. Antrelkowitsch. 2018. Challenges and opportunities of lead smelting process for complex feed mixture. In Extraction 2018, The Minerals, Metals and Materials Series, ed. B. Davis, 325–38. Cham: Springer Verlag.
