?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Pyrite, the most abundant sulfide mineral in base-metal deposits, presents significant challenges in mineral processing due to its deleterious effects when not properly managed. Its undesirable association with valuable minerals leads to low-quality concentrates, hindering smelting processes. Pyrite’s distinct textures and variable elemental compositions affect its floatability, with complex textures challenging to depress. Moreover, since multiple textures can be present within an orebody, their relative proportion in the ore could affect the overall flotation performance. Therefore, understanding the types of pyrite present, their abundance, and how they affect the flotation behavior of the ore is critical for an efficient depression and possible reclamation of pyrite. At the Mount Isa copper deposit, framboidal or ‘carbonaceous’ pyrite has been an ongoing challenge to the copper concentration operation, reducing the flotation circuit’s efficiency. Previous work by Yenial-Arslan et al. showed that high pyrite recoveries could be strongly linked to the prevalence of fine-grained pyrite. However, samples belonging to the coarse pyrite domain exhibited low natural floatability. This study further investigates the effect of the prevalence of pyrite textures on the flotation performance of copper orebodies at the Mount Isa deposit. The prevalence of pyrite textures was quantified and statistically linked to the natural floatability of pyrite, proving that fine-grained and framboidal pyrite greatly influence pyrite’s overall recovery. Furthermore, various mineral characterization techniques, such as synchrotron X-ray fluorescence microscopy (XFM) analysis, were used to explore how the natural floatability of pyrite was affected.
1. Introduction
Pyrite, the most abundant sulfide mineral worldwide, holds great economic and environmental importance in deposits containing base and precious metals. These mineral resources are mainly recovered by froth flotation (Mudd Citation2021, Citation2023; Mudd and Jowitt Citation2018). Depending on the flotation conditions, pyrite can be naturally floatable, accompanying valuable minerals in the flotation process, yielding a low-quality concentrate that hinders smelting operations (Finch, Rao, and Nesset Citation2007; Mu, Peng, and Lauten Citation2015; Wang and Forssberg Citation1996; Young et al. Citation2006). Once in the sulfide concentrate, pyrite undergoes decomposition at elevated temperatures, releasing sulfur vapor and sulfur dioxide. Subsequently, these byproducts can interact with atmospheric water vapor, forming sulfuric acid (commonly referred to as acid rain) (Dold Citation2008). Furthermore, as a gangue mineral, pyrite presents challenges in managing mining waste and storage facilities for tailings. This leads to environmental concerns such as acid rock drainage (ARD) and hampers efforts to restore the land after mine closure (Commonwealth of Australia Citation2016; Kuter Citation2013; Lottermoser Citation2010).
Challenges depressing pyrite have been documented in numerous greenfield and brownfield operations, mainly attributed to the presence of complex mineral textures (Akop Citation2014; Bakalarz Citation2021; Barker, Gerson, and Menuge Citation2014; Bulatovic Citation2007; Tayebi-Khorami Citation2018). Mineral textures, including crystal structures, grain sizes, and mineral associations, influence the liberation and recovery of valuable minerals during flotation (Bradshaw Citation2014; Dzingai et al. Citation2021; Ekmekçi et al. Citation2010; Jameson Citation2012; Vos Citation2017). Fine mineral grains tend to be less liberated and present a higher chance of complex mineral associations that can be detrimental to the valuable mineral flotation performance (Bakalarz Citation2021; Bradshaw Citation2014; Evans Citation2010; Mishra, Viljoen, and Mouri Citation2013; Sutherland Citation1989; Tungpalan, Wightman, and Evans Citation2019). Complex mineral associations and micro-inclusions of sulfide minerals and other naturally floatable minerals can diminish the effectiveness of flotation by decreasing its selectivity, ultimately resulting in lower metal recovery rates and grades owing to inadequate mineral liberation (Cropp, Goodall, and Bradshaw Citation2013; Dzingai et al. Citation2021; Grano et al. Citation1991). On the other hand, associations between pyrite and other sulfides can facilitate galvanic interactions between the two minerals, thus affecting their surface chemistry and flotation performance (Barker, Gerson, and Menuge Citation2014; Ekmekçi and Demirel Citation1997; Forbes, Smith, and Vepsalainen Citation2018; Rao and Finch Citation1988; Trahar, Senior, and Shannon Citation1994; Yang et al. Citation2022). Framboidal pyrite has a large surface area, which may increase galvanic interactions (Barker, Gerson, and Menuge Citation2014; Pugh, Hossner, and Dixon Citation1984). Pyrite has a higher rest potential than other sulfides; thus, in a mixed sulfide system, the other minerals will tend to preferentially oxidize (Forbes, Smith, and Vepsalainen Citation2018; Mu, Peng, and Lauten Citation2016; Peng, Wang, and Gerson Citation2012; Rao and Finch Citation1988). The oxidation of the minerals can be accompanied by the dissolution of the metal ions, which, once released, can be adsorbed into pyrite surfaces, enhancing their surface reactivity and floatability in a phenomenon known as metal ion activation of pyrite, widely studied for Cu and Pb ions (Cullinan et al. Citation1999; Mu, Peng, and Lauten Citation2016; Peng, Wang, and Gerson Citation2012). Furthermore, the presence of impurities in the pyrite crystal structure has been known to influence the electrochemical properties of the mineral and thus their floatability (Abraitis, Pattrick, and Vaughan Citation2004; Arrouvel and Eon Citation2018; John Citation2017; Moslemi, Shamsi, and Alimohammady Citation2012; Vaughan and Craig Citation1978). In synthetic pyrite studies (doped with As, Ni, and Co), the higher the concentration of impurities in pyrite grains, the higher the surface reactivity (Lehner et al. Citation2007; Lehner, Savage, and Ayers Citation2006; Li et al. Citation2011; Vaughan and Craig Citation1978). The presence of these impurities and concentration will not only affect the natural floatability of pyrite but also affect the interaction between the pyrite surface and xanthates (Babedi et al. Citation2023; Xian et al. Citation2012).
Pyrite can incorporate small amounts of other minor and trace elements in its crystal structure during formation, commonly As, Co, and Ni, which are likely to either occur as small inclusions of other minerals or as ion substitution in the crystal lattice (Craig, Vokes, and Solberg Citation1998; Osborne Citation2013). Extensive literature has been published reviewing the occurrence and concentration of trace elements in pyrite from a deposit interpretation and prospectivity perspective (Abraitis, Pattrick, and Vaughan Citation2004; Craig and Vaughan Citation1990; Craig, Vokes, and Solberg Citation1998; Huston et al. Citation1995; Jefferson et al. Citation2023; Steadman et al. Citation2021). A review of the literature suggests that fine and complex pyrite textures tend to have higher concentrations of impurities compared to coarser textures (Abraitis, Pattrick, and Vaughan Citation2004; Craig and Vaughan Citation1990; Craig, Vokes, and Solberg Citation1998; Jefferson et al. Citation2023). Since more than one pyrite texture can be present within an orebody, their relative proportion in the ore could affect the overall flotation behavior. However, prior literature has not documented a direct correlation between their relative proportions and the overall flotation behavior.
Although limited studies exist on the effect of specific pyrite textures on flotation performance, the existing literature points out that fine-grained textures and complex textures with a high surface area have higher flotation rates and recoveries (Barker, Gerson, and Menuge Citation2014; Can, Özçelik, and Ekmekçi Citation2021; Medina Citation2012). This effect is attributed to galvanic interactions between pyrite and other sulfides. It is important to note that in the mentioned studies, the prevalence of each pyrite texture in the test samples was not quantified.
Notably, the Mount Isa Copper Operation has reported operational challenges due to the presence of framboidal pyrite in their ore, resulting in nonselective flotation and high reagent consumption (Grano Citation1990; Grano et al. Citation1991; Grano, Ralston, and Smart Citation1990; O’Donnell and Muller Citation2018). While the difficulties depressing framboidal pyrite has been traditionally attributed to naturally hydrophobic graphitic carbon rimming of the framboidal structures (Grano Citation1990; Grano et al. Citation1991), recent studies have demonstrated a lack of correlation between the presence of graphitic carbon and pyrite (Forbes et al. Citation2024), necessitating a revaluation of factors influencing flotation performance of such pyrite texture. Although the presence of framboidal pyrite in the Mount Isa orebodies has been documented since 1937 (Grondijs and Schouten Citation1937), their abundance in the orebodies has not been fully quantified due to limitations in the commercially available SEM-based mineral characterization techniques, which cannot accurately estimate grain sizes of pyrites made up of fine-grained clusters (Forbes et al. Citation2024; Jefferson et al. Citation2023, Citation2023; Yenial-Arslan et al. Citation2023).
This study aims to pioneer a quantitative analysis of pyrite texture prevalence in the Mount Isa deposit, establishing a statistical link between pyrite prevalence and its flotation behavior. For the first time, this research aims to systematically quantify the pyrite mineral textures within the Mount Isa copper orebodies, providing essential insights into their impact on flotation performance. Furthermore, the study seeks to explore the reasons behind the observed natural hydrophobicity of pyrite based on differences in pyrite’s elemental composition by texture using advanced mineralogical techniques such as synchrotron X-ray fluorescence microscopy and laser ablation inductively coupled plasma mass spectrometry.
2. Materials and methods
2.1. Sample selection
Samples were selected to namely represent different pyrite textures in the deposit. The samples were collected to represent the two major pyrite macro-texture domains that have been historically identified for the copper orebodies: Fine-grained pyrite (FGP) and coarse-grain pyrite (CGP). Drill core examples of FGP and CGP pyrite textures are shown in . Drill holes from two orebodies, dubbed OD01 and OD02, were sampled, visually representing the pyrite domains. The description of the selected samples is shown in , and the main element composition of the samples analyzed by x-ray fluorescence (XRF) is shown in .
Figure 1. Core photographs showing coarse-grained and fine-grained pyrite macro textures. Annotations: pyrite (py), shale (shale), dolomite (Dol), quartz (qz).
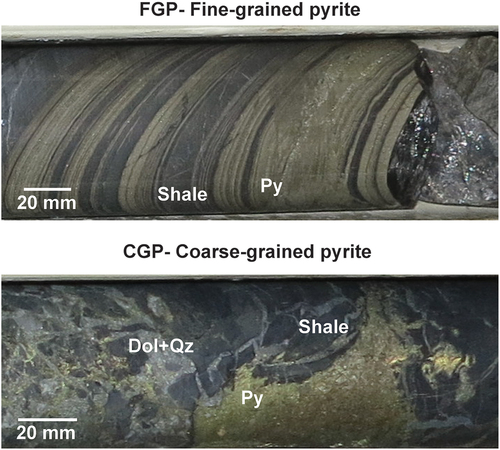
Table 1. Description of selected ore samples.
Table 2. Chemical composition of selected ore samples.
The geological and geochemical information and complete characterization of the selected samples have been detailed by Yenial-Arslan et al. (Citation2023).
2.2. Flotation experimental procedure
The experimental methodology comprised several stages. Initially, drill core samples were subdivided into sub-samples weighing approximately 1.6 ± 0.07 kg using a rotary splitter. These sub-samples were further categorized into seven size fractions: +1180 μm, −1180 + 425 μm, −425 + 212 μm, −212 + 106 μm, −106 + 53 μm, −53 + 38 μm, and −38 μm. Prior to any grinding, mineral liberation analysis (MLA) was performed on each fraction of each size to preserve the ore textures for subsequent identification. The complete mineralogical characterization testwork of the samples has been detailed by Forbes et al. (Citation2024).
For each specific test, 1.6 kg of the sample underwent crushing in a Boyd crusher, ensuring a fineness of 100% − 3.35 mm. This crushing procedure was executed in a closed circuit, encompassing multiple stages with varying gaps (4 mm, 3 mm, and 2 mm) to maintain consistency across the samples. Following the crushing phase, the freshly crushed ore was mixed with process water at a 60% solids ratio by weight. Subsequently, wet grinding occurred in a stainless-steel rod mill to attain a target P80 size of 106 μm, ensuring uniform liberation sizes among the ore samples. Before each test, the mill was thoroughly cleaned through a 10-minute grinding process with sand.
Following the grinding process, the ore underwent flotation in a 3 L modified Denver cell at 1200 rpm rotor speed. This modified flotation cell was equipped with an impeller driven from below to ensure consistent froth scraping. The flotation tests were carried out under natural pH conditions, conditioned with frother but without collector addition. Methyl isobutyl carbinol (MIBC) was added at a concentration of 20 ppm during the tests. The concentrate was collected for 3 minutes, with scraping conducted every 10 seconds. Each flotation test was replicated three times.
In the context of the overall study, the flotation tests aimed to assess the natural floatability of pyrite, serving as a reference point for comparing different ore domains within the overall study. The evaluations mirrored the pre-flotation circuit of the Mount Isa Mines Copper Operation (MIMCO), where gangue minerals (pyrite and talc) are naturally floated without a collector (Forbes et al. Citation2022, Citation2022). Any chalcopyrite recovery during this phase is considered a loss of valuable material, thus termed ‘chalcopyrite losses’ in this work. Entrainment calculations based on a steady-state modeling approach (Engelbrecht and Woodburn Citation1975; Forbes et al. Citation2022; Johnson Citation2005; Wang et al. Citation2015) were carried out to determine the true flotation component for pyrite and chalcopyrite. Extensive details of the experimental procedure, entrainment calculations, and results for the flotation testwork have been previously published by Yenial-Arslan et al. (Citation2023) and Forbes et al. (Citation2024).
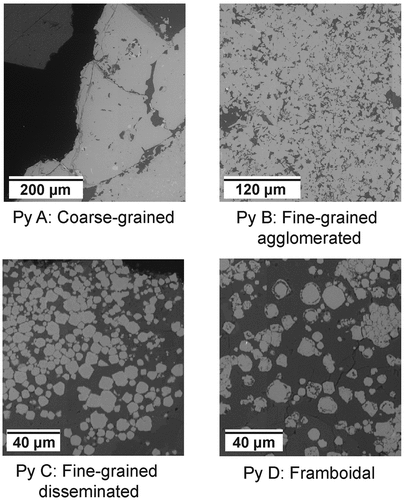
2.3. Texture quantification
Backscattered electron microscope (BSE) images were initially obtained and subsequently processed using the image recognition software eCognition for segmentation, generating distinct objects corresponding to individual minerals. Following segmentation, manual classification was undertaken to differentiate pyrite from all other minerals, further classifying pyrite into four separate texture classes. MLA image maps were used to validate the mineral species present, focusing on distinguishing between pyrite and other minerals (). The BSE image of each pyrite texture category is shown in . Notably, certain textures, characterized by very small grain sizes, resulted in objects that did not segregate individual grains but instead encompassed multiple grains of the same texture class. Therefore, the values obtained for the number of objects per class do not accurately reflect the number of individual pyrite grains.
Moreover, it is critical to recognize that, for a comparable area, the number of objects per class for coarse pyrite textures is expected to be considerably lower than for finely disseminated grains. Consequently, to mitigate these limitations and provide a more representative measure of texture abundance, this methodology employs pixel area per texture class as the indicator of the abundance of each pyrite texture. Utilizing pixel area accounts for both the spatial distribution and the fine-grained nature of certain textures, offering a more accurate and comprehensive representation of pyrite abundance in the analyzed samples.
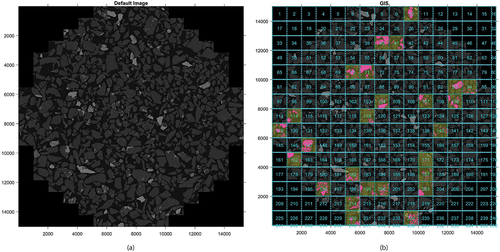
To facilitate the processing of images and reduce the computational power needs, the BSE images were tiled into 1,000 × 1,000 pixel and 500 × 500 pixel tiles as shown in . A total of 4,240 tiles containing mineral particles were created, of which 15% were randomly selected for further analysis.
Figure 3. BSE image and mineral map showing the main minerals present in the samples. Sample OD1-FGP. Deleterious groups tetrahedrite, enargite and arsenopyrite.
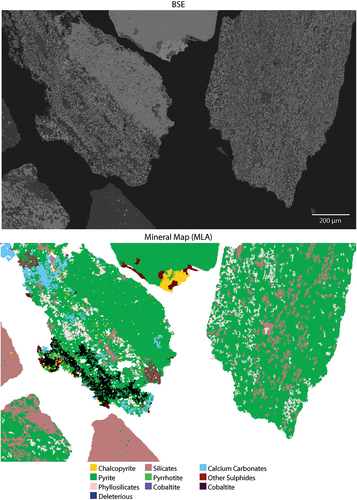
Figure 4. (a) Original BSE Image. (b) Tiled imaged. Yellow shows tiles classified by hand. Pink shows pyrite (Jefferson et al. Citation2023).
2.4. Mineral characterization
2.4.1. X-ray fluorescence microscopy
Five polished quartz-thin sections 28 × 48 mm were prepared from crushed ore 100% + 425 µm and five from pre-float concentrate. Elemental mapping of the ten quartz slides were carried out using the X-ray fluorescence microscopy (XFM) beamline at the Australian Synchrotron, Clayton, Australia. A monochromatic 18.5 KeV X-ray beam was focused to 1 μm using a Kirkpatrick-Baez mirror pair. Overview scans were run with 20 μm pixels, which were decreased to 1 μm for high-resolution maps captured by a 384-element Maia detector. Image data was analyzed using GeoPIXE to produce elemental maps of the experimental systems. The experimental settings for each scan are summarized in .The main goal was to detect the four pyrite textures of interest, determine the element composition of inclusions, and image a potential zonation within pyrite grains.
Table 3. Analytical settings for synchrotron X-ray fluorescence microscopy.
The high speed and resolution of the XFM beamline allowed for rapid scanning of the thin slides and identification of areas of interest. After carefully examining the five ore samples, all four pyrite textures (PyA, PyB, PyC and PyD) were observed in samples OD1-FGP and OD2-FGP. Within the samples, areas where PyD was spotted were highlighted as areas of interest and subsequently studied by SEM-EDS (SU3900).
2.4.2. Laser ablation inductively coupled plasma mass spectrometry
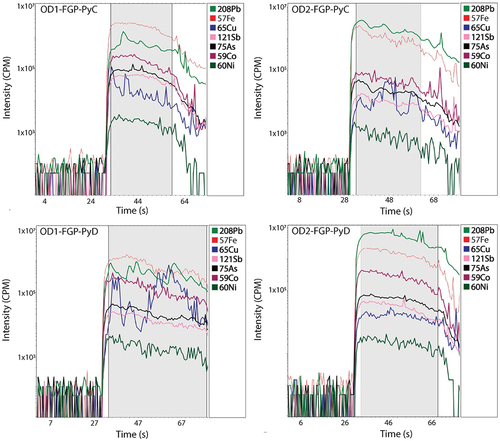
The concentration of major and minor elements in pyrite for samples OD2-FGP and OD1-FGP was determined by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) spot analyses. LA-ICP-MS analyses were undertaken using an Agilent 8900 ICP-MS instrument attached to a high-performance RESOlution 193 nm ArF excimer laser ablation system at CODES (University of Tasmania, Australia). A spot size of 20 µm was consistently used for all the analysis, which included between 20 to 30 spots per pyrite texture class. For each analysis, the background signal was recorded for 30 seconds, then the laser was turned on and the targeted mineral was ablated while the ICP-MS collects data for each element for ~60 seconds. The following isotopes were monitored: 23Na, 24Mg, 27Al, 29Si, 34S, 39K, 43Ca, 49Ti, 51V, 53Cr, 55Mn, 57Fe, 59Co, 60Ni, 65Cu, 66Zn, 75As, 77Se, 90Zr, 93Nb, 95Mo, 107Ag, 109Ag, 111Cd, 118Sn, 121Sb, 125Te, 157Gd, 178Hf, 181Ta, 182W, 195Pt, 197Au, 202Hg, 205Tl, 206Pb, 207Pb, 208Pb, 209Bi, 232Th, and238U.
During the spot analysis the material analyzed was predominantly pyrite, characterized by a strong Fe signal. Element signals that showed no significant changes and remained stable during the measurement period were considered part of the pyrite structure. On the other hand, elements that showed sudden changes in the laser signal unrelated to sharp chemical zoning were interpreted as inclusions. Small inclusions do not affect the signal of the target mineral; thus, they are referred to as micro-inclusions.
LADR software (Norris and Danyushevsky Citation2018) was used for data reduction. The internal standard element used for quantification in pyrite was Fe. Analyses were normalized to 100 wt.% total. The average of the signal during the time interval of interest within the sample grain was calibrated against reference standards, i.e. STDGL3 (a sulfur-rich glass made in CODES Analytical Laboratories; Belousov et al. (Citation2023)), USGS SRM GSD-1 G, and in-house pyrite.
2.5. Statistical analysis
All flotation experiments were conducted in triplicate, and throughout the manuscript, error bars denote the 95% confidence intervals of the mean values. The obtained flotation results were cross-referenced with the pyrite texture prevalence in each feed sample. For each case, correlation coefficients (R) and coefficients of determination (R2) were computed. The Student’s t distribution for each correlation was determined using Equation 1, where ‘n’ represents the number of tests per domain (Napier-Munn, Citation2014).
The resulting ‘t’ values were utilized to assess the statistical probability associated with each correlation. The criteria for significance were established as follows:
The probability of significance needed to surpass the 95% confidence limit.
The correlation had to be significant for both total recovery and recovery by true flotation values.
These rigorous significance conditions were selected to ensure that the observed trend accurately represented true flotation and was not influenced by entrainment contributions. Also, to establish that the correlation would remain valid even without a comprehensive entrainment analysis, which is frequently omitted in industrial geo-metallurgical programs.
3. Results
3.1. Flotation results
The flotation results presented in this work have been described in detail in previous publications, and only a brief overview will be presented here. A summary of chalcopyrite and pyrite recoveries and grades obtained in the flotation test are shown in .
Table 4. Flotation test results.
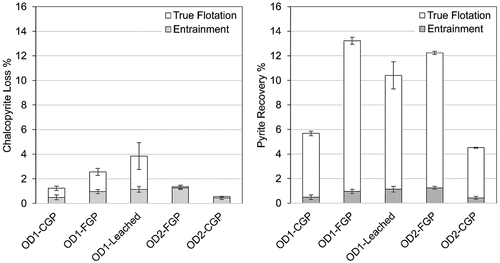
Additionally, entrainment calculations were carried out to define the true flotation component for pyrite and chalcopyrite recoveries. The full methodology for entrainment calculations for the current flotation results is published by Yenial-Arslan et al. (Citation2023). Approximately 90% of the achieved pyrite flotation recovery is associated with true flotation, and the highest entrainment is measured for the OD1-Leached ore domain, circa 11% ().
Figure 5. Entrainment and true flotation recoveries of (a) chalcopyrite and (b) pyrite. Adapted from Yenial-Arslan et al. (Citation2023).
3.2. Texture quantification
The pyrite prevalence or abundance per texture class was determined based on pixel area. A summary of the pyrite prevalence per sample is shown in .
Table 5. Pyrite texture prevalence estimates.
3.3. Mineral characterization
3.3.1. X-ray fluorescence microscopy
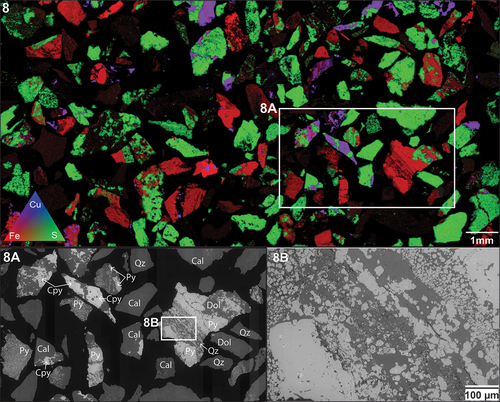
show occurrences and locations of framboidal pyrite (PyD) in samples OD2-FGP and OD1-FGP. In both instances, areas of transition between coarse-grained pyrite, fine-grained pyrite and framboidal pyrite can be observed.
Figure 6. XFM results. Ore sample OD2-FGP. Area of interest identified with distinct pyrite textures (10A). Framboidal pyrite textures (10B). Annotations: pyrite (py), chalcopyrite (cpy), dolomite (Dol), quartz (qz).
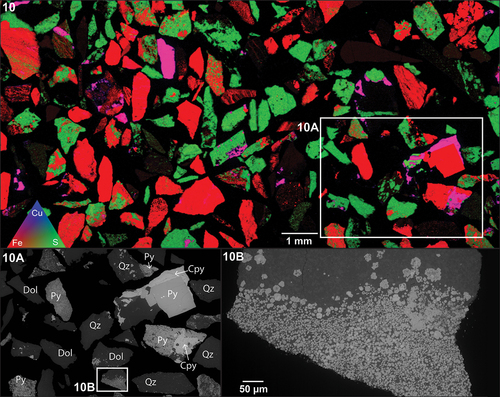
Figure 7. XFM results. Ore sample OD1-FGP. Area of interest identified with distinct pyrite textures (8A). Framboidal pyrite textures (8B). Annotations: pyrite (py), chalcopyrite (cpy), dolomite (Dol), calcite (cal), quartz (qz).
show the elemental distribution of various pyrite grains. The concentrations shown are semi-quantitative and are presented to show variations in elemental distribution rather than quantitative composition. The scales are based on wt. % concentration but calibrated to arbitrary values. The XFM semi-quantitative element maps for both samples () show that the pyrite grains contain substantial concentrations of As and Cu. The Co values reported by XFM are unreliable due to Co signal being overshadowed by the solid fluorescence of Fe in pyrite and chalcopyrite, thus not included in the results. However, the presence of Co within pyrite grains was later confirmed by LA-ICP-MS analysis.
Figure 8. Semi-quantitative XFM element fluorescence maps of ore sample OD2-FGP (10B) showing As, Ca, Cu, Fe, K, Pb, S and Zn concentrations. Minimum concentration shown in dark blue, and maximum concentration in bright yellow.
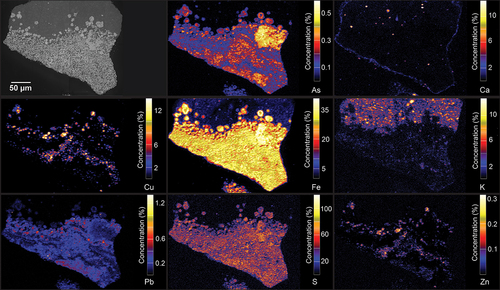
Figure 9. Semi-quantitative XFM element fluorescence maps of ore sample OD1-FGP (8B) showing As, Ca, Cu, Fe, K, Pb, S and Zn concentrations. Minimum concentration shown in dark blue, and maximum concentration in bright yellow.
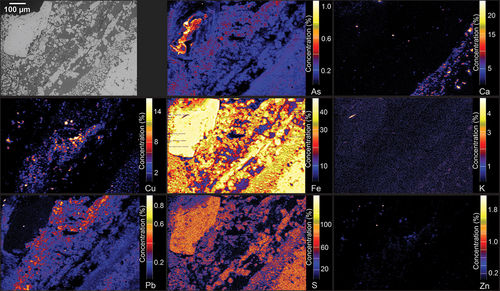
As shown in , framboidal pyrite grains contain Cu inclusions in the core of the framboids around 2 to 5 µm in size. The distribution of As seems to be spatially associated with the framboids’ rim. Arsenic zonation occurs in both coarse-grained and framboidal pyrites. For coarse grains, As is both present and depleted in different specific zones within the grains, whereas As is continuously present across all fine and framboidal pyrite grains. Lead appears to be relegated entirely to fine and framboidal pyrite, depleted in coarse-grained pyrite grains. Copper shows similar concentration characteristics, mainly present in the fine and framboidal grains at various concentrations.
3.3.2. Laser ablation inductively coupled plasma mass spectrometry
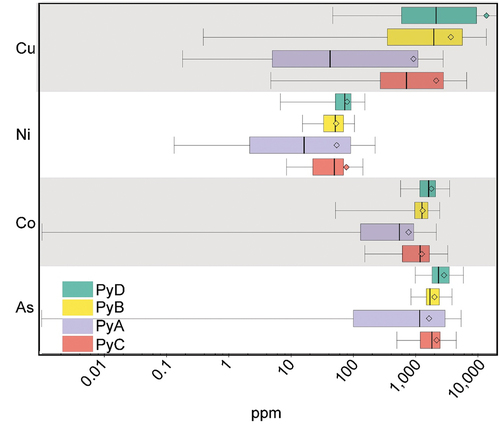
LA-ICP-MS time-resolved depth profiles for pyrite confirmed the presence of important concentrations of Co, As, and Cu in the pyrite grains. The results show a significant difference in the concentration of impurities by texture classes (). Fine-grained and framboidal pyrite show an increased concentration of impurities compared to coarse-grained pyrite. Important concentrations of Co have been detected in the framboidal pyrite, averaging 1780 ppm Co, whereas coarse pyrite grains contain about half that amount. All pyrite grains contain significant As, above 1000 ppm on average for all pyrite textures, with framboidal pyrite having the highest concentration with 2920 ppm As in average. Copper is the highest concentration impurity within fine-grained and framboidal pyrite, reaching on average up to 11,400 ppm compared to 714 ppm Cu for coarse pyrite.
Figure 10. Box and whisker plots of trace elements in pyrite grains by texture class, showing As, Co, Ni, and Cu concentrations in ppm. Framboidal (PyD), fine-grained agglomerated (PyB), coarse-grained (PyA), and fine-grained disseminated pyrite (PyC) are presented in the graph in that order.
The spike LA-ICP-MS time-resolved signal spectra imply the presence of Cu-bearing micro-inclusions in the pyrite grains (). On the other hand, Co, Ni and As shows parallel LA-ICP-MS time-resolved signal profiles with Fe for fine and framboidal pyrite grains, suggesting a relatively homogeneous distribution within those grains.
4. Data analysis and discussion
4.1. Texture abundance and flotation
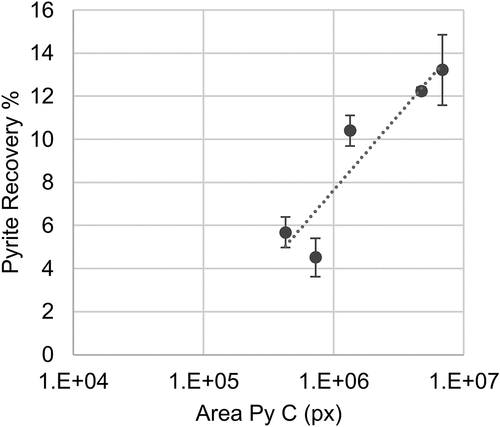
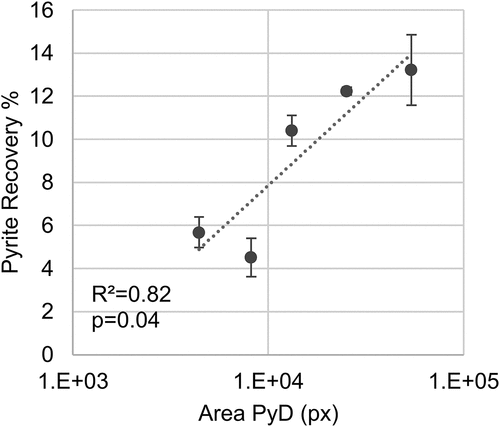
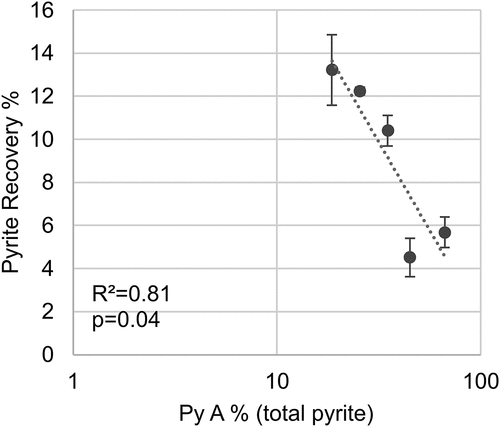
The correlations between the natural floatability of pyrite and associated chalcopyrite losses and the prevalence of pyrite textures for each sample were explored. summarizes the overall p values expressed in percentage, with significant relationships (p > 95%) highlighted in bold. The textural parameters evaluated included the pixel area per texture class as the indicator of the abundance of each pyrite texture and the relative proportion of each texture class (relative to the total pyrite pixel area). Additionally, logarithmic transformation of the previously mentioned features was included in the analysis to assess possible non-linear relationships that could be overlooked otherwise. The results show a lack of linear relationships between pyrite textures and pyrite and chalcopyrite recoveries. However, there are significant non-linear correlations between the abundance of fine (PyC) and framboidal (PyD) pyrite and pyrite recoveries as well as the prevalence of coarse grain pyrite (PyA), both for total and true flotation recoveries.
Table 6. Probability analysis of pyrite prevalence and natural floatability of pyrite and chalcopyrite.
No correlation between the chalcopyrite losses and the prevalence of pyrite textures could be established. On the other hand, higher pyrite floatability was observed at a higher abundance of fine disseminated pyrite (PyC) and framboidal pyrite (PyD), as shown in . Furthermore, the higher the coarse pyrite (PyA) percentage relative to the total pyrite area, the lower the pyrite floatability in the ore sample ().
Coarse euhedral pyrite textures have a lower concentration of minor and trace elements compared to other fine-grained or complex textures such as the ones described as PyC and PyD (Basori et al. Citation2018; Chen et al. Citation2020; Craig, Vokes, and Solberg Citation1998; Li et al. Citation2018; Ma, Jiang, and Frimmel Citation2022; Maslennikov et al. Citation2020). These textures have also been linked to increased surface reactivity and galvanic interactions caused by increased surface area and micro-inclusions of impurities, which can enhance pyrite floatability (Abraitis, Pattrick, and Vaughan Citation2004; Bulut, Arslan, and Atak Citation2004; Pugh, Hossner, and Dixon Citation1984; Xian et al. Citation2012).
4.2. Pyrite elemental composition by texture
Synchrotron XFM (SC XFM) and LA-ICP-MS analysis of the pyrite samples shows that fine-grained and framboidal textures have a higher content of impurities, particularly Cu, As and Pb, compared to coarse grains. Furthermore, these elements are only found in the sulfide minerals. The rock matrix hosting the sulfides does not contain significant Fe, S, Co, As, Cu, or Pb levels. Based on previous work by Forbes et al. (Citation2024), the non-sulfide gangue material present in the studied samples, where the pyrite is hosted, is dolomite and silicates (mainly quartz).
The findings are in accordance with the bulk of the literature, which suggests that coarse euhedral pyrite textures have a lower concentration of minor and trace elements than other fine-grained, subhedral or anhedral textures (Jefferson et al. Citation2023). For example, As is typically found in higher concentrations in framboidal pyrite, followed by fine-grained pyrite, and to a lesser extent in coarse-grained pyrite, particularly in low-temperature ores (Abraitis, Pattrick, and Vaughan Citation2004; Gregory et al. Citation2015; Maslennikov and Large Citation2021; Simmons Citation1997; Tesh Citation2020).
The increased impurities in the pyrite matrix and micro inclusions of different mineral species in fine-grained and framboidal pyrite could explain the enhanced floatability observed. In particular, zonations of Cu-rich pyrite within PyC and PyD grains have the potential to impact the floatability of pyrite by galvanic interactions (Ekmekçi and Demirel Citation1997; Forbes, Smith, and Vepsalainen Citation2018; Rao and Finch Citation1988), and their surface reactivity further accelerated by the increased surface area of those textures (Pugh, Hossner, and Dixon Citation1984). Furthermore, copper-containing minerals can release Cu ions during grinding and flotation, which have been shown to activate the pyrite surface, affecting its flotation behavior (Moslemi and Gharabaghi Citation2017; Peng et al. Citation2003; Peng, Wang, and Gerson Citation2012).
More testwork is required to prove the exact mechanism that drives the enhanced floatability of fine and framboidal pyrite. However, the SC XFM and LA-ICP-MS show that a main difference between the mentioned textures is their elemental distribution, indicating higher concentrations of Cu, As and Pb in PyC and PyD textures. The impurities in the pyrite grains have been shown to affect its electrochemical properties and surface reactivity. The variation in composition within the pyrite grains can affect the galvanic interactions between the minerals. Cu has been widely reported to influence pyrite floatability even at low concentrations. Therefore, it can be inferred that the Cu inclusions in PyC and PyD constitute a significant driver for the natural floatability of those pyrite types.
5. Summary and conclusions
Understanding and quantifying pyrite textures is vital for optimizing flotation and beneficiation processes. Natural pyrite exhibits significant texture and elemental composition variability, affecting its electrochemical properties and flotation performance. Since multiple pyrite textures are present in an orebody, their relative prevalence in the ore affects the flotation performance. This study focuses on Mount Isa copper orebodies, revealing a notable correlation between the prevalence of fine and framboidal pyrite textures and pyrite flotation recoveries. The findings of this study can be summarized as follows:
Pyrite floatability appears independent of chalcopyrite floatability, suggesting the potential to incorporate a pre-flotation stage to remove the problematic pyrite.
The abundance of fine-grained and framboidal pyrite was robustly associated with pyrite recovery. Samples exhibiting higher fine and framboidal pyrite content demonstrated elevated pyrite recoveries, ranging from approximately 5% to 15%.
Elemental composition variations among pyrite textures were identified. Fine-grained and framboidal pyrite had an increased concentration of impurities, especially Cu and As, compared to coarse-grained pyrite. Framboidal pyrite exhibited a 2.3 times higher concentration of As and a 16 times greater concentration of Cu than coarse-grained pyrite.
The difference between Cu and As concentrations in fine and framboidal pyrite potentially accounts for their increased floatability. While As in pyrite is known to enhance surface reactivity and influence flotation behavior, the significant presence of Cu in framboidal pyrite suggests a more pronounced influence. Cu content influences pyrite recoveries due to Cu activation and increased surface reactivity through galvanic interaction with Cu-rich minerals.
The data provided herein do not offer sufficient evidence to conclusively assign galvanic interactions as the primary mechanism for pyrite recoveries for these ore systems. Further study would be required to definitively demonstrate the true drivers behind the variability in pyrite recoveries. Nonetheless, the robust association between fine-grained and framboidal pyrite and pyrite recovery provides evidence of the importance of pyrite texture quantification in flotation performance studies.
While the testwork was conducted on Mount Isa Copper orebodies, the understanding of pyrite mineral characteristics and how it behaves in flotation is transferable to other deposits with similar pyrite formations.
This study is the first to quantify pyrite textures for Mount Isa copper bodies, providing statistically significant evidence linking fine and framboidal pyrite prevalence to flotation performance. It introduces a novel perspective by identifying elemental composition variations as a potential cause for the observed effects. Additionally, the observed abundance of fine and framboidal pyrite in the FGP domains, compared to CGP, suggests a tangible link to macro-textures visually observed in the core. This connection reinforces the prospect of a geometallurgical approach, hinting at the potential to explore ore behavior at a macro-scale. Importantly, this work introduces the foundation for a valuable geometallurgical tool for performance prediction, emphasizing the predictive value of pyrite texture quantification in orebodies. Predicting pyrite floatability through texture quantification could enable mining engineers to anticipate and manage variations in pyrite floatability, ultimately leading to more efficient and cost-effective processing.
Acknowledgments
The authors would like to thank the staff at the XFM beamline at the Australian Synchrotron and the staff at CODES (University of Tasmania) for excellent support during the analytical work. Electron microscopy was performed at the Centre for Microscopy and Microanalysis (CMM) at the University of Queensland. The authors acknowledge the financial support of the Queensland Government and Glencore through the Advance Queensland Industrial Research Fellowship and the Australian Nuclear Science and Technology Organization (ANSTO).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abraitis, P. K., R. A. D. Pattrick, and D. J. Vaughan. 2004. Variations in the compositional, textural and electrical properties of natural pyrite: A review. International Journal of Mineral Processing 74 (1–4):41–59. doi:10.1016/j.minpro.2003.09.002
- Akop, C. 2014. Developing a bulk circuit suitable for chalcopyrite-pyrite ores with elevated pyrite content in copper-gold ore treatment. The University of Queensland.
- Arrouvel, C., and J.-G. Eon. 2018. Understanding the surfaces and crystal growth of pyrite FeS 2. Materials Research 22 (1). doi:10.1590/1980-5373-mr-2017-1140
- Babedi, L., M. Tadie, B. P. von der Heyden, and D. A. Chareev. 2023. A rest potential study of impurity (as, Au, Ni and Co) bearing synthetic pyrite in alkaline flotation conditions. Minerals Engineering 202:108277. doi:10.1016/j.mineng.2023.108277
- Bakalarz, A. 2021. An analysis of copper concentrate from a kupferschiefer-type Ore from Legnica-Glogow copper basin (SW Poland). Mineral Processing and Extractive Metallurgy Review 42 (8):552–64. doi:10.1080/08827508.2021.1971663
- Barker, G. J., A. R. Gerson, and J. F. Menuge. 2014. The impact of iron sulfide on lead recovery at the giant Navan Zn–pb orebody, Ireland. International Journal of Mineral Processing 128:16–24. doi:10.1016/j.minpro.2014.02.001
- Basori, M. B. I., S. Gilbert, R. R. Large, and K. Zaw. 2018. Textures and trace element composition of pyrite from the bukit botol volcanic-hosted massive sulphide deposit, Peninsular Malaysia. Journal of Asian Earth Sciences 158:173–85. doi:10.1016/j.jseaes.2018.02.012
- Belousov, I., L. Danyushevsky, K. Goemann, S. Gilbert, P. Olin, J. Thompson, E. Lounejeva, and D. Garbe-Schönberg. 2023. STDGL3, a Reference Material for Analysis of Sulfide Minerals by laser ablation ICP-MS: An Assessment of Matrix Effects and the impact of laser wavelengths and pulse widths. Geostandards and Geoanalytical Research 47 (3):493–508. doi:10.1111/ggr.12512
- Bradshaw, D. 2014. The role of process mineralogy in improving the process performance of complex sulphide ores. pp. 1–23, Santiago, Chile.
- Bulatovic, S. M. 2007 Handbook of flotation reagents - chemistry, theory and practice, volume 1 - flotation of sulfide ores. Ontario, Canada: Elsevier.
- Bulut, G., F. Arslan, and S. Atak. 2004. Flotation behaviors of pyrites with different chemical compositions. Mining, Metallurgy & Exploration 21 (2):86–92. doi:10.1007/BF03403308
- Can, İ. B., S. Özçelik, and Z. Ekmekçi. 2021. Effects of pyrite texture on flotation performance of copper sulfide ores. Minerals 11 (11):1218. doi:10.3390/min11111218
- Chen, Y., Y. Fan, T.-F. Zhou, B. Fu, Y.-N. Liu, B. Wang, and Q. Liu. 2020. Pyrite textures and compositions in Jiangshan gold deposit, Bengbu Uplift, southeastern North China Craton: Implications for ore genesis. Ore Geology Reviews 122:103512. doi:10.1016/j.oregeorev.2020.103512
- Commonwealth of Australia. 2016. Preventing acid and metalliferous drainage. Commonwealth of Australia.
- Craig, J. R., and D. J. Vaughan 1990. Sulphide deposits—their origin and processing. pp. 1–16, Springer.
- Craig, J. R., F. M. Vokes, and T. N. Solberg. 1998. Pyrite: Physical and chemical textures. Mineralium Deposita 34 (1):82–101. doi:10.1007/s001260050187
- Cropp, A. F., W. R. Goodall, and D. J. Bradshaw 2013. The influence of textural variation and gangue mineralogy on recovery of copper by flotation from porphyry ore–a review. pp. 279–91, AusIMM, Brisbane, Australia.
- Cullinan, V. J., S. R. Grano, C. J. Greet, N. W. Johnson, and J. Ralston. 1999. Investigating fine galena recovery problems in the lead circuit of Mount Isa Mines lead/Zinc concentrator part 1: Grinding media effects. Minerals Engineering 12 (2):147–63. doi:10.1016/S0892-6875(98)00128-9
- Dold, B. 2008. Sustainability in metal mining: From exploration, over processing to mine waste management. Reviews in Environmental Science and Bio/technology 7 (4):275–85. doi:10.1007/s11157-008-9142-y
- Dzingai, T., B. McFadzean, M. Tadie, and M. Becker. 2021. Decoupling the effects of alteration on the mineralogy and flotation performance of Great Dyke PGE ores. Journal of the South African Institute of Mining and Metallurgy 121 (121):475–86. doi:10.17159/2411-9717/1487/2021
- Ekmekçi, Z., M. Becker, E. B. Tekes, and D. Bradshaw. 2010. The relationship between the electrochemical, mineralogical and flotation characteristics of pyrrhotite samples from different Ni ores. Journal of Electroanalytical Chemistry 647 (2):133–43. doi:10.1016/j.jelechem.2010.06.011
- Ekmekçi, Z., and H. Demirel. 1997. Effects of galvanic interaction on collectorless flotation behaviour of chalcopyrite and pyrite. International Journal of Mineral Processing 52 (1):31–48. doi:10.1016/S0301-7516(97)00050-1
- Engelbrecht, J., and E. Woodburn 1975. The effects of froth height, aeration rate, and gas precipitation on flotation.
- Evans, C. 2010. Development of a methodology to estimate flotation separability from ore microtexture. The University of Queensland.
- Finch, J. A., S. R. Rao, and J. E. Nesset 2007. Iron control in mineral processing. Canadian Institute of Mining, Metallurgy and Petroleum. Montreal, Canada.
- Forbes, E., S. Britoe Abreu, K. Tungpalan, R. Sashigunan, K. Runge, and R. O’Donnell. 2022. Effect of residual reagents on chalcopyrite losses at Mount Isa Mines copper operation: Part I – evaluation of mineral recoveries. Minerals Engineering 185:107706. doi:10.1016/j.mineng.2022.107706
- Forbes, E., S. B. E Abreu, K. Tungpalan, R. Sashigunan, K. Runge, and R. O’Donnell. 2022. Effect of residual reagents on chalcopyrite losses at Mount Isa Mines copper operation: Part II - evaluation of flotation mechanisms. Minerals Engineering 185:107687. doi:10.1016/j.mineng.2022.107687
- Forbes, E., M. Jefferson, U. Yenial-Arslan, C. Curtis-Morar, and R. O’Donnell. 2024. Solving the mystery of natural pyrite flotation – a mineralogy-based approach. Minerals Engineering 207:108544. doi:10.1016/j.mineng.2023.108544
- Forbes, E., L. Smith, and M. Vepsalainen. 2018. Effect of pyrite type on the electrochemistry of chalcopyrite/pyrite interactions. Physicochemical Problems of Mineral Processing 54 (4):1116–29.
- Grano, S. R. 1990. The influence of pyrite and pyrrhotite on the selective flotation of Mount Isa mines copper and lead/zinc ores/by Stephen Grano. Thesis (Master of Applied Science in Chemical Technology), South Australian Institute of Technology.
- Grano, S., J. Ralston, and R. S. C. Smart. 1990. Influence of electrochemical environment on the flotation behaviour of Mt. Isa copper and lead-zinc ore. International Journal of Mineral Processing 30 (1):69–97. doi:10.1016/0301-7516(90)90067-9
- Grano, S. R., L. F. Griffin, N. W. Johnson, S. T. Smart, and R. C. Ralston 1991. Treatment of naturally hydrophobic gangue minerals at the Cooper Concentrator of Mt Isa mines limited. pp. 10–14, AusIMM.
- Gregory, D. D., R. R. Large, J. A. Halpin, E. L. Baturina, T. W. Lyons, S. Wu, L. Danyushevsky, P. J. Sack, A. Chappaz, V. V. Maslennikov, et al. 2015. Trace element content of sedimentary pyrite in black Shales*. Economic Geology 110 (6):1389–410. doi:10.2113/econgeo.110.6.1389
- Grondijs, H. F., and C. Schouten. 1937. A study of the mount isa ores [Queensland, Australia]. Economic Geology 32 (4):407–50. doi:10.2113/gsecongeo.32.4.407
- Huston, D. L., S. H. Sie, G. F. Suter, D. R. Cooke, and R. A. Both. 1995. Trace elements in sulfide minerals from eastern Australian volcanic-hosted massive sulfide deposits; part I, Proton microprobe analyses of pyrite, chalcopyrite, and sphalerite, and part II, selenium levels in pyrite; comparison with delta 34 S values and implications for the source of sulfur in volcanogenic hydrothermal systems. Economic Geology 90 (5):1167–96.
- Jameson, G. J. 2012. The effect of surface liberation and particle size on flotation rate constants. Minerals Engineering 36-38:132–37. doi:10.1016/j.mineng.2012.03.011
- Jefferson, M., G. Forbes, E. Brown, C. Curtis-Morar, A. Parbhakar-Fox, and E. Forbes 2023. An object-based image recognition approach methodology for pyrite texture quantification in flotation performance studies, procemin 2023, Chile.
- Jefferson, M., U. Yenial-Arslan, C. Evans, C. Curtis-Morar, R. O’Donnell, A. Parbhakar-Fox, and E. Forbes. 2023. Effect of pyrite textures and composition on flotation performance: A review. Minerals Engineering 201:108234. doi:10.1016/j.mineng.2023.108234
- John, J. 2017. Differential oxidation of iron sulfides to modify the Au: S ratio in the flotation concentrate product at Lihir. The University of Queensland.
- Johnson, N. 2005. A review of the entrainment mechanism and its modelling in industrial flotation processes.
- Kuter, N. 2013. Advances in landscape architecture. IntechOpen.
- Lehner, S., K. Savage, M. Ciobanu, and D. E. Cliffel. 2007. The effect of As, Co, and Ni impurities on pyrite oxidation kinetics: An electrochemical study of synthetic pyrite. Geochimica et Cosmochimica Acta 71 (10):2491–509. doi:10.1016/j.gca.2007.03.005
- Lehner, S. W., K. S. Savage, and J. C. Ayers. 2006. Vapor growth and characterization of pyrite (FeS2) doped with Co, Ni, and As: Variations in semiconducting properties. Journal of Crystal Growth 286 (2):306–17. doi:10.1016/j.jcrysgro.2005.09.062
- Li, X.-H., H.-R. Fan, K.-F. Yang, P. Hollings, X. Liu, F.-F. Hu, and Y.-C. Cai. 2018. Pyrite textures and compositions from the Zhuangzi Au deposit, southeastern North China Craton: Implication for ore-forming processes. Contributions to Mineralogy and Petrology 173 (9):1–20. doi:10.1007/s00410-018-1501-2
- Li, Y.-Q., J.-H. Chen, Y. Chen, and J. Guo. 2011. Density functional theory study of influence of impurity on electronic properties and reactivity of pyrite. Transactions of Nonferrous Metals Society of China 21 (8):1887–95. doi:10.1016/S1003-6326(11)60946-1
- Lottermoser, B. 2010. Mine wastes (third edition): Characterization, treatment and environmental impacts. Springer Berlin Heidelberg, Berlin, Heidelberg.
- Ma, Y., S.-Y. Jiang, and H. E. Frimmel. 2022. Deciphering multiple ore-forming processes of the shuangqishan orogenic gold deposit, Southeast China by in situ analysis of pyrite. Ore Geology Reviews 142:104730. doi:10.1016/j.oregeorev.2022.104730
- Maslennikov, V. V., G. Cherkashov, D. A. Artemyev, A. Firstova, R. R. Large, A. Tseluyko, and V. Kotlyarov. 2020. Pyrite varieties at Pobeda Hydrothermal Fields, Mid-Atlantic ridge 17°07′–17°08′ N: LA-ICP-MS data deciphering. Minerals 10 (7):622. doi:10.3390/min10070622
- Maslennikov, V. V., and R. R. Large. 2021. Editorial for special issue “pyrite varieties and LA-ICP-MS geochemistry in Ore genesis and exploration”. Minerals 11 (2):131. doi:10.3390/min11020131
- Medina, J. F. 2012. Liberation-limited grade/recovery curves for auriferous pyrite ores as determined by high resolution x-ray microtomography. University of Utah.
- Mishra, G., K. S. Viljoen, and H. Mouri. 2013. Influence of mineralogy and ore texture on pentlandite flotation at the Nkomati nickel mine, South Africa. Minerals Engineering 54:63–78. doi:10.1016/j.mineng.2013.04.009
- Moslemi, H., and M. Gharabaghi. 2017. A review on electrochemical behavior of pyrite in the froth flotation process. Journal of Industrial and Engineering Chemistry 47:1–18. doi:10.1016/j.jiec.2016.12.012
- Moslemi, H., P. Shamsi, and M. Alimohammady. 2012. Electrochemical properties of pyrite, pyrrhotite, and steel: Effects on grinding and flotation processes. Journal of the Southern African Institute of Mining and Metallurgy 112 (10):883–90.
- Mudd, G. M. 2021. Assessing the availability of Global Metals and Minerals for the Sustainable Century: From aluminium to zirconium. Sustainability 13 (19):10855. doi:10.3390/su131910855
- Mudd, G. M. 2023. A comprehensive dataset for Australian mine production 1799 to 2021. Scientific Data 10 (1):391. doi:10.1038/s41597-023-02275-z
- Mu, Y., Y. Peng, and R. A. Lauten. 2015. Electrochemistry aspects of pyrite in the presence of potassium amyl xanthate and a lignosulfonate-based biopolymer depressant. Electrochimica Acta 174:133–42. doi:10.1016/j.electacta.2015.05.150
- Mu, Y., Y. Peng, and R. A. Lauten. 2016. The depression of pyrite in selective flotation by different reagent systems – a literature review. Minerals Engineering 96-97:143–56. doi:10.1016/j.mineng.2016.06.018
- Mudd, G. M., and S. M. Jowitt. 2018. Growing global copper resources, reserves and production: Discovery is not the only control on supply. Economic Geology 113 (6):1235–67. doi:10.5382/econgeo.2018.4590
- Napier-Munn, T. J.2014. Statistical methods for mineral engineers - how to design experiments and analyse data, 627. Queensland, Australia: Julius Kruttschnitt Mineral Research Centre.
- Norris, A., and L. Danyushevsky 2018. Towards estimating the complete uncertainty budget of quantified results measured by LA-ICP-MS. Boston, MA, USA.
- O’Donnell, A. R., and M. L. Muller 2018. Selectively targeting hydrophobic gangue minerals at the Mount Isa copper concentrator. pp. 413–23, AUSIMM, Brisbane, Australia.
- Osborne, O. D. 2013. South Australia: Flinders University.
- Peng, Y., S. Grano, D. Fornasiero, and J. Ralston. 2003. Control of grinding conditions in the flotation of chalcopyrite and its separation from pyrite. International Journal of Mineral Processing 69 (1–4):87–100. doi:10.1016/S0301-7516(02)00119-9
- Peng, Y., B. Wang, and A. Gerson. 2012. The effect of electrochemical potential on the activation of pyrite by copper and lead ions during grinding. International Journal of Mineral Processing 102:141–49. doi:10.1016/j.minpro.2011.11.010
- Pugh, C. E., L. R. Hossner, and J. B. Dixon. 1984. Oxidation rate of iron sulfides as affected by surface area, morphology, oxygen concentration, and autotrophic bacteria. Soil Science 137 (5):309–14. doi:10.1097/00010694-198405000-00003
- Rao, S. R., and J. A. Finch. 1988. Galvanic interaction studies on sulphide minerals. Canadian Metallurgical Quarterly 27 (4):253–59. doi:10.1179/cmq.1988.27.4.253
- Simmons, G. L. 1997. Flotation of Auriferous Pyrite Using Santa Fe Pacific Gold’s N 2TEC flotation process. Preprints-Society of Mining Engineers of AIME.
- Steadman, J. A., R. R. Large, P. H. Olin, L. V. Danyushevsky, S. Meffre, D. Huston, A. Fabris, V. Lisitsin, and T. Wells. 2021. Pyrite trace element behavior in magmatic-hydrothermal environments: An LA-ICPMS imaging study. Ore Geology Reviews 128:103878. doi:10.1016/j.oregeorev.2020.103878
- Sutherland, D. N. 1989. Batch flotation behaviour of composite particles. Minerals Engineering 2 (3):351–67. doi:10.1016/0892-6875(89)90004-6
- Tayebi-Khorami, M. 2018. Arsenic in complex orebodies. AusIMM, Brisbane, QLD.
- Tesh, D. 2020. Liberation of different pyrite types in refractory gold ores. The University of Queensland.
- Trahar, W. J., G. D. Senior, and L. K. Shannon. 1994. Interactions between sulphide minerals—the collectorless flotation of pyrite. International Journal of Mineral Processing 40 (3–4):287–321. doi:10.1016/0301-7516(94)90049-3
- Tungpalan, K., E. Wightman, and C. Evans 2019. Key ore textures influencing separation behaviour of ores. pp. 1524–27, Society for Geology Applied to Mineral Deposits (SGA).
- Vaughan, D. J., and J. R. Craig 1978. Mineral chemistry of metal sulfides. Cambridge University Press, Cambridge Eng New York.
- Vos, C. F. 2017. The effect of mineral grain textures at particle surfaces on flotation response. The University of Queensland.
- Wang, L., Y. Peng, K. Runge, and D. Bradshaw. 2015. A review of entrainment: Mechanisms, contributing factors and modelling in flotation. Minerals Engineering 70:77–91. doi:10.1016/j.mineng.2014.09.003
- Wang, X. H., and K. S. E. Forssberg. 1996. The solution electrochemistry of sulfide-xanthate-cyanide systems in sulfide mineral flotation. Minerals Engineering 9 (5):527–46. doi:10.1016/0892-6875(96)00041-6
- Xian, Y.-J., S.-M. Wen, X.-M. Chen, J.-S. Deng, and J. Liu. 2012. Effect of lattice defects on the electronic structures and floatability of pyrites. International Journal of Minerals, Metallurgy, and Materials 19 (12):1069–76. doi:10.1007/s12613-012-0672-5
- Yang, L., X. Zhou, H. Yan, H. Zhang, X. Liu, and T. Qiu. 2022. Effects of galvanic interaction between chalcopyrite and monoclinic pyrrhotite on their flotation separation. Minerals 12 (1):39. doi:10.3390/min12010039
- Yenial-Arslan, U., M. Jefferson, C. Curtis-Morar, and E. Forbes. 2023. Pathway to prediction of pyrite floatability from Copper Ore Geological Domain Data. Minerals 13 (6):801. doi:10.3390/min13060801
- Young, M. F., K. E. Barnes, G. S. Anderson, J. D. Pease, and X. Zinc 2006. Jameson Cell: The ‘‘comeback’’ in base metals applications using improved design and flow sheets. pp. 311–22, Ottawa, Ontario, Canada.