Abstract
Haloperidol, a typical antipsychotic, is the most commonly prescribed medication for the treatment of mental health problems such as agitation and psychosis. We attempted to determine the effects of haloperidol treatment on the kidneys of female rats. In addition, we aimed to estimate the numerical density, total number, and height of renal glomeruli and the volume and volumetric fractions of the cortex, medulla, and whole kidneys, and tried to determine whether there was a change in these stereological parameters depending on haloperidol treatment. Both the qualitative and quantitative histological features of the kidney samples were analyzed with conventional histopathological and modern stereological methods at the light microscopic level. The total number of glomeruli and numerical density of glomerulus in the haloperidol-treated groups was not changed by increasing the dose in comparison to the control group. The mean height of the glomerulus significantly increased, especially in low-dose groups. In the haloperidol-treated groups, the volumetric fractions of the cortex to the whole kidney of the rats were significantly decreased by increasing the dose. The volumetric fractions of the medulla to the whole kidney of the rats were increased significantly in parallel by the given dose. In addition, we present quantitative findings showing that haloperidol is associated with many alterations in rat kidneys. It was shown that haloperidol may lead to undesirable changes in the kidney after chronic treatment with especially high doses.
INTRODUCTION
Haloperidol, a typical antipsychotic agent that is one of the most frequently prescribed neuroleptics, is clinically used for the treatment of psychiatric patients with psychoses and schizophrenia.[Citation1] These psychotic diseases require a long treatment period. Over such a long term, it is known that neuroleptics may lead to side effects in both the target and peripheral organs. Some of these side effects may be potentially life-threatening events, including parkinsonian symptoms, tardive dyskinesia, and neuroleptic malignant syndrome.[Citation2–4] These situations make it important to select the appropriate drug from all alternatives for treatment.
Haloperidol has been known to cause cell death in various cell lines, and mostly has been shown to be necrotic in nature with its oxidative value.[Citation5–7] Similar to these findings, in our previous study, we showed that haloperidol causes liver cell injury and vasoconstriction on basilar arteries associated with chronic and high-dose administration.[Citation8,Citation9]
Stereology is a set of methods designed to concretize attentive quantitative analysis of the size, shape, and number of objects. When properly used, stereology plays an important role in acceptable and popular experimental hypotheses in biological research. Stereology produces results that are accurate, efficient, and more reliable than other quantitative-based analyses. Stereology provides an important contribution to the progress in biological research by improving the consistency and dependability of quantitative analytical results produced in the laboratory and reported in scientific publications.[Citation10]
In addition, these methods have been used to quantify renal morphology.[Citation11,Citation12] Parameters including the kidney volume; surface area; volume of tubular structures; volume of mesangium, cortex, medulla, or glomerulus; number of glomeruli; and the length, surface area, and number of glomerular capillaries can be easily estimated. Methods for obtaining data for average glomeruli as well as individual glomeruli were described.[Citation13,Citation14]
In the present study, we have attempted to determine the effects of haloperidol treatment on the kidneys of female rats. Both the qualitative and quantitative histological features of the kidney samples were analyzed with conventional histopathological and modern stereological methods at the light microscopic level. In addition, we aimed to estimate the numerical density, total number, and height of renal glomeruli, as well as the volume and volumetric fractions of the cortex, medulla, and whole kidneys, and tried to determine whether there was any change in these stereological parameters related to haloperidol treatment in rats.
MATERIAL AND METHODS
Animals and Diets
Eighteen adult female Sprague Dawley (200 ± 10 g) rats were obtained from the Experimental Research and Application Center (Atatürk University, Erzurum, Turkey). The rats were housed in plastic cages (two animals per cage) and maintained under standardized conditions of light (12-h light/dark cycle) and room temperature (22 ± 2°C), with free access to food and tap water. The rats were fed a commercial rat diet. This study was given ethical approval by the Experimental Research and Application Center of Atatürk University.
Drugs and Chemicals
Haloperidol was obtained from Biosel Ilaç Sanayi ve Ticaret A.S., İstanbul, Turkey. All chemicals for histological processes were obtained from Merck Company (Merck Ilac, Ecza ve Kimya Ticaret Anonim Sirketi, İstanbul, Turkey).
Experimental Design and Application of the Drug
In the present study, 18 adult female rats were divided into three groups, six rats in each group. The animals were intraperitoneally treated once a day (at 09:00 h) with saline or haloperidol in different doses for six weeks according to the following schedule:
haloperidol 0.4 mg/kg, i.p. (low-dose group);
haloperidol 0.8 mg/kg, i.p. (high-dose group), which was chosen as equivalent to the highest dosage used in humans[Citation15]; and
saline vehicle, i.p. (control group), where haloperidol was dissolved in normal saline (0.9% NaCl).[Citation16]
At the end of the sixth week, the rats were killed by an overdose of a general anesthetic (thiopental sodium, 50 mg/kg Pental Sodium®, İ.E. Ulagay; Turkey), and the livers were dissected immediately. These were transferred into 10% formaldehyde and buffered 3% glutaraldehyde in 0.1 M phosphate solution for light microscopy.
Microscopy
The kidney samples for light microscopic examination were fixed in 10% formaldehyde, dehydrated in a graded alcohol series, and cleared in xylol. After dehydration, specimens were embedded in fresh paraffin (Agar, Cambridge, UK). Sections were cut using a microtome (Leica, Germany). Each paraffin block was serially cut into 5 μm-thick sections. Approximately 100 sections were obtained from each tissue block. The sections were stained with hematoxylin-eosin (H-E) for light microscopic examination and stereological analysis.
Volumes of Cortex and Medulla
We used 15–20 sections obtained for application of the Cavalieri method to estimate the volume of the cortex and medulla.[Citation17] For this aim, a point-counting test grid was used (see ). The point density of the point-counting grids is designed to hit a minimum of 1000 points per interested subject (cortex and medulla) for each animal and was appropriate for obtaining a significant CE.[Citation18,Citation19] Coefficients of error and the coefficient of variation (CV) were estimated according to Gundersen and Jensen's formula.[Citation20] The grid with a systematic array was randomly placed on the PC screen; the hitting point on the grid on all interesting subjects was counted. Sampled areas were chosen in a systematic random manner by means of a motorized stage. The total number of points that was superimposed on each area of interest was counted.
Figure 1. (A) A light microscopic image from a selected liver section. (B) Light microscopic image at (A) after superimposing a point-counting grid on it. (C and D) Stereological procedures for sampling and applying the physical dissector. C reference and D are look-up sections.*Section profiles of glomeruli in the reference and look-up sections. The black arrow was counted as a dissector particle if its profile was not seen in the look-up section. The arrow with a white filling sign profile of the particle in the look-up section was not seen in the reference section.
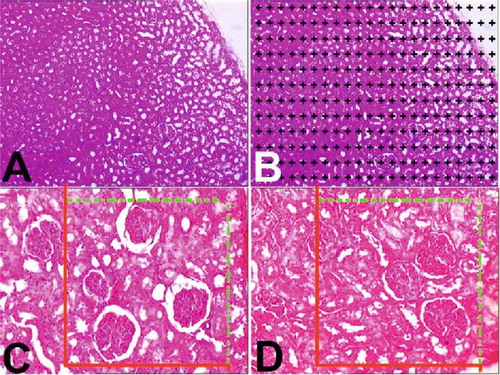
Subsequently, the volumes of the interesting structures in each section were estimated from the following equation:where V is the volume of the interesting objects (cortex and medulla) in one section plane, t is the section thickness, kxk (a/p) is the interpoint area, and ΣP the number of points hitting the interesting object in that section. After the same formula was applied for entry on other sections, the total volume that needs to be estimated was obtained from:
The volume fractions for each region were estimated by dividing the total point number that is superimposed on the related region by the sum of points superimposed on the whole kidney, by the following formula:where P is the number of points hitting the interesting region or whole kidney in that section.
Numerical Density and Total Number of Glomeruli
The selection of the physical dissector pairs was done as described by Sterio.[Citation21] Based on the findings obtained from a pilot study, the first chosen section and its adjacent section (called a dissector pair) were separated by a distance of 30 μm (7-section thickness: 35-μm distance) from each other as a rule of physical dissector. According to this rule, the distance between section pairs must be about 30–40% of the average projected height of the interesting object to be estimated (see ); in this way, approximately 15–20 section pairs were obtained and evaluated. This number is in an acceptable range for stereological analysis.[Citation22,Citation23] Two consecutive sections were mounted on each slide. Photographs of adjacent sections were taken with a digital camera at a magnification of 400×. An unbiased counting frame[Citation22] was placed on the reference and the look-up sections on the screen of the PC, to perform the counting according to the dissector counting method. The bottom and the left-hand edges of the counting frame were considered to be the forbidden (exclusion) lines together with the extension lines. Other boundaries of the frame that were the top-right edges were considered to be inclusion lines, and any particle that hit these lines or was located inside the frame was counted as a dissector particle (see and ). The size of the unbiased counting frame was adjusted to a count of approximately 600 glomeruli from each sample.
The dimension of the counting frame on the PC screen was 20 × 20 cm, and the real dimension of this counting frame (1 cm2) was estimated by the following formula:
Glomeruli seen in the reference section but not in the look-up section were counted.[Citation23] The mean numerical density of glomeruli [Nv(glomeruli)] per cm3 was estimated using the following formula:where Q glomeruli are the total number of glomeruli counted in the reference section, t is the mean section thickness (5 μm), and A is the area of the unbiased counting frame.
Thus, the total number of glomeruli (TN(glomeruli)) in the whole rat kidney was estimated by the following equation:where Nv(glomeruli) is the numerical density of glomeruli per cm3; TN(glomeruli), the total number of glomeruli in the whole kidney, was calculated by using Nv(glomeruli) and the kidney volume results estimated by the Cavalieri method. Finally, histopathological examinations were carried out on images of the same sections.
Statistical Analysis
Differences in volumetric data, numerical density, total number, and glomerular height between the two groups were tested using the independent samples t-test (two-tailed, with a significance limit of p = 0.05 in this test). All statistical calculations were performed using SPSS 13.0 software for Windows.
RESULTS
Stereological Results
All stereological results of the study are summarized in the following .
Kidney Volume
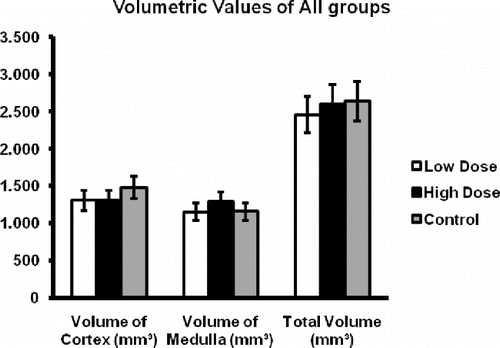
Kidney tissue volumes were estimated by the Cavalieri principle on a series of H-E stained sections. The estimates of the volumes of the kidney of the control and haloperidol-treated rats are given in . The kidney volumes in all groups were 2.637, 2.6052, and 2.453, respectively. Statistical analysis by independent samples t-test revealed a significant difference between these estimates of successive groups (p < 0.01).
Volumes of Cortex and Medulla
The volumes of the cortex and medulla of kidney tissue were additionally estimated by the Cavalieri principle on a series of H-E stained sections. The estimates of the volumes of the cortex and medulla of the control rats and those of the low-dose and high-dose haloperidol-administered rats are given in . The volumes of the cortex and medulla were 1480 mm3 and 1157 mm3, respectively, for the rats in the control group. By low-dose haloperidol application, the volume of the kidney cortex and medulla was estimated at 1306 mm3 and 1209 mm3, respectively. In the high-dose haloperidol group, the volumes of the kidney cortex and medulla were estimated at 1305 cm3 and 1150 mm3, respectively. Statistical analysis by independent samples t-test revealed a significant difference between these estimates of successive groups (p < 0.05).
Mean Numerical Density of Glomeruli (Nv(Glomeruli))
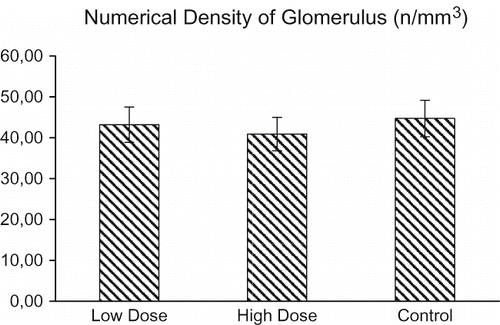
The mean numerical density of glomeruli for all groups is shown in . There were approximately 44.7 glomeruli /mm3 in the kidneys of the control rats. However, the numerical density of glomeruli in the kidneys decreased significantly after low-dose treatment compared to the numerical density of glomeruli in the kidneys after high-dose treatment. There were approximately 43.2 glomeruli /mm3 and 40.9 glomeruli /mm3 in the kidneys of the low-dose- and high-dose-administered rats. Specifically, the mean glomerulus density in the high-dose group decreased by 8.6% relative to the control group (p > 0.05).
Total Number of Glomeruli
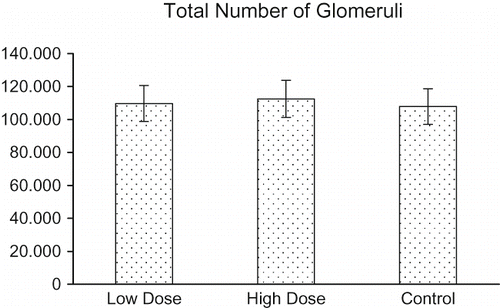
shows the total number of glomeruli in the kidneys of the control and haloperidol-treated rats. The control rats had 112,536 glomeruli in the kidney. By increasing the haloperidol dose, the total number of glomeruli in the kidney decreased to 109,649 in the low-dose group and 107,853 in the high-dose group. Statistical analysis performed by independent samples t-test revealed that the total number of glomeruli in the kidney did not change significantly from the beginning to the end of the treatment (p > 0.05). This decrease, however, was dose-dependent.
Mean Height of Glomerulus
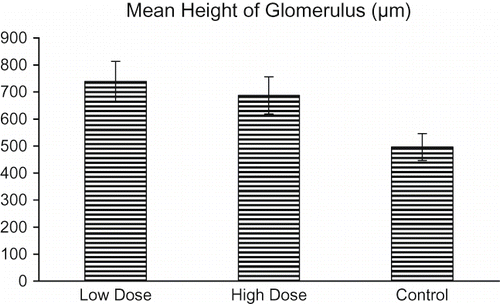
The mean heights of glomeruli for the control, low-, and high-dose groups are shown in . The mean heights were 496.25, 739.25, and 687.5 in all groups, respectively. Specifically, the mean glomerular height in the low-dose group increased by 48.9% relative to the control group (p < 0.05).
Histological Results
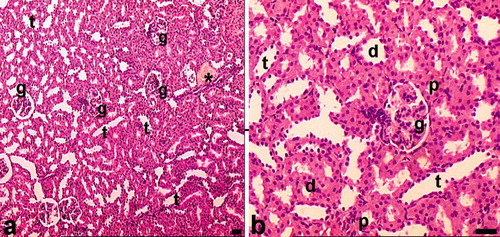
The renal cortex of the control rats is composed of glomeruli, blood vessels, tubules, and interstitium (see ). When evaluating these renal specimens by light microscopy on hematoxylin-eosin stained sections, the overall cellularity and symmetry of the glomerulus were detected (see and ). Renal tubules (long and winding neck) formed as the proximal tubule, the loop of Henle, and the distal tubule. All kinds of tubules were formed as continued cells. Among the tubular structures, very little interstitium was present in the cortex. Light microscopic findings of kidney sections from haloperidol-treated rats are summarized as follows.
Figure 6. Light microscopy of kidneys in the control group. In this figure, glomeruli and tubuli with healthy appearance are seen. Abbreviations: g = glomerulus, d = distal tubules, p = proximal tubules, t = collecting tubules. *Blood vessel. Dye: Hematoxylin-eosin; Magnification bars: 50 μm.
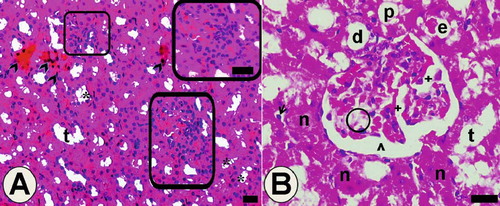
In the low-dose group, irregularity of the tubular area and focal necrotic areas was seen conspicuously (see ). Cells that have pyknotic nuclei and eosinophilic cytoplasm were detected in some areas. Deductions in the tubule wall were caused by the loss of tubule cells. Hydropic vacuolization in tubule cells, eosinophilic accumulations, and desquamated cell in some tubule lumens were observed (see and ). In addition, some pathological changes occurred in glomeruli, such as dilatation of glomerular capillaries and Bowman space, thickening in the basal membrane of intraglomerular capillary, and mesangial cell damage.
Figure 7. Light microscopy of kidney in the low-dose haloperidol-given group. Arrow heads: cells of tubules that have pyknotic nuclei and eosinophilic cytoplasm. *Hydropic vacuolation in tubule cells. Abbreviations: t = tubular lumen, boxed areas subjected to focal necrosis; d = distal tubule surrounded with vacuolated cells; n = necrotic tubules and necrotic tubule cells; arrow = desquamated cell in the lumen; e = eosinophilic accumulation in proximal tubule lumen and vacuolated tubule cells; ˆ = dilated Bowman space. Circled area = space that occurred after mesangial cells damage; + = dilated glomerular capillaries. Also in B, + indicates thickening in the basal membrane of the intraglomerular capillary. The inset shows boxed areas of A at high magnification. Dye: Hematoxylin-eosin; Magnification bars: 40 μm.
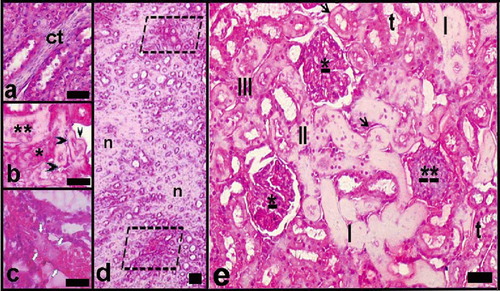
In the high-dose group, necrotic areas were detected (see and ). Increase of connective tissue among necrotic tubules (see ), membrane thickening, and hyaline deposits at the tubular basal membrane (see and ); varying degrees of necrosis in both distal and proximal tubules (see and ); hyaline accumulation in tubule's lumen (see , , and ); and venous thrombus (see ) were seen. Glomerular mesangial cell proliferation and focal necrosis were also observed (see and ).
Figure 8. Light microscopy of kidney in the high-dose haloperidol-given group. Abbreviations: ct = enlarged connective tissue among the necrotic tubules. **Necrotic distal tubule. *Necrotic proximal tubule. Black arrow head = tubular basal membrane thickening and hyaline deposits; white arrows = venous thrombus and hyaline deposit-filled necrotic tubules. d indicates general view of renal medulla; n = necrotic areas, limited areas, blood vessels, and damaged tubules. e indicates a general view of the renal cortex. *Mesangial cell proliferation and focal necrosis in glomeruli; **Damaged glomerulus. t = tubules with hydropic degeneration; III, II, I = necrotic tubules at increasing degrees (the most in III). Arrows = tubular basal membrane thickening and hyaline deposits. Dye: Hematoxylin-eosin; Magnification bars: 50 μm.
DISCUSSION
In the present study, stereological methods were used to investigate the mean numerical density and total number of glomeruli and volume of the whole kidney, cortex only, medulla only, and their fraction to the whole kidney of the rats from all groups.[Citation21,Citation22] Stereological quantifications and histopathological examinations were performed on the light microscopic sections. The results of both approaches confirmed each other. All findings of this study are discussed as follows.
Studies performed by quantitative methods, which give a numerical density or any morphometric data, contribute to obtaining architectural information on the functionality of that structure. Previously, many papers have been reported that used histological methods to estimate the parameters of glomerulus and other structures in rat kidneys. These studies investigated stereological parameters such as estimation of glomerular number and volume, volume of tubules, and volume of renal cortical interstitium.[Citation24,Citation25] However, none of these studies mentioned the effects of neuroleptics on the kidney. In our study, many structures of the kidney were stereologically analyzed and detailed with histological examinations.
According to our stereological results, the total number of glomeruli and numerical density of glomerulus and in the haloperidol-treated groups was not changed by increasing the dose in comparison to the control group; this means haloperidol did not cause glomerular atrophy at any important level. Mean height of glomerulus significantly increased, especially in the low-dose groups. This increase may have occurred due to dilatation in the capillary of the glomerulus resulting in renal failure. Thus, it can be inferred that the increase of glomerular height led to a volumetric enlargement in the glomeruli. Both the cortical volume and total volume of kidneys was significantly changed. In the haloperidol-treated groups, volumetric fractions of the cortex to the whole kidney of the rats were significantly decreased by increasing the dose. Moreover, volumetric fractions of the medulla to the whole kidney of the rats were increased in parallel by the given dose at a significant level. This increase in volumetric fraction of the medulla may have occurred due to hyaline accumulation of tubules, vascular occlusion, and enlarged interstitium. Thus, in this situation, the volumetric fraction of the cortex seems to have relatively decreased.
Seitz and Gill reported that neuroleptic malignant syndrome (NMS) is a potentially fatal adverse event associated with the use of antipsychotics. The authors also stated that NMS developed in cases involving men with agitated delirium who received relatively high doses of parenteral haloperidol.[Citation26] Likewise, early reports listed neuroleptic malignant syndrome and acute myoglobinuric renal failure developing in patients after they had received a large dose of haloperidol. In this research, in patients subjected to haloperidol treatment, rhabdomyolysis was demonstrated by biochemical methods as well as by a muscle biopsy. Sanai et al. reported that acute renal failure induced from rhabdomyolysis developed in patients who had previously received haloperidol.[Citation27]
Since the recent development of stereological quantification methods, a large amount of numerical data has been readily obtained.[Citation28,Citation29] In the histological step of our study, dilatation of glomerular capillaries and large blood vessels was seen. In addition to tubular deformations, hyaline accumulation, necrosis, and vacuolar degenerations supported our stereological data. In addition, the presented quantitative findings showing that haloperidol is associated with many alterations in rat kidneys are the first in the literature.
In conclusion, the results of the present study suggest that there is a significant relationship between haloperidol treatment and structural changes in kidneys such as tubular deformations, prominent dilatation of the renal vessels and tubules, enlarged glomeruli, and glomerular basal membrane thickening. Thus, these structural abnormalities may cause corruption of renal morphometry. The findings of this study clarify that the stereological and histopathological reasons underlying the dose-dependent renal injury are associated with the chronic administration of haloperidol. There is more research to be done; we suggest that there may be an advantage of using low doses of antipsychotics with chronic administration to decrease their side effects on the kidney.
DECLARATION OF INTEREST
None of the authors has a commercial interest, financial interest, and/or other relationship with manufacturers of pharmaceuticals, laboratory supplies, and/or medical devices or with Commercial providers of medically related services.
This research was conducted in the Laboratory of Pharmacology at Ataturk University, School of Medicine, 25240 Erzurum, Turkey, and the Laboratory of Histology and Embryology, School of Medicine, at Ataturk University, 25240 Erzurum, Turkey.
REFERENCES
- Borc JJ, Larson DB, Lyons JS, Beardsley RS. Expenditures for psychotropic medications in the United States. Am J Psychiatry. 1991; 148: 644–647
- Baldessarini RJ, Frankenburg FR. Clozapine: A novel antipsychotic agent. N Engl J Med. 1991; 324: 746–754
- Levenson JL. Neuroleptic malignant syndrome. Am J Psychiatry. 1985; 142: 1137–1145
- Sovner R, Dimascio A, Killam F. Psychopharmacology: A generation of progress. Extrapyrmidal syndromes and the other neurological side effects of psychotropic drugs, MA Lipton, F Killam. Raven Press, , New York 1978; 1021–1032, In:(eds.)
- Vilner BJ, Bowen WD. Sigma receptor-active neuroleptics are cytotoxic to C6 glioma cells in culture. Eur J Pharmacol. 1993; 244: 199–201
- Vilner BJ, De Costa BR, Bowen WD. Cytotoxic effects of sigma ligands: Sigma receptor-mediated alterations in cellular morphology and viability. J Neurosci. 1995; 15: 117–134
- Behl C, Lezoulac'h F, Widmann M, Rupprecht R, Holsboer F. Oxidative stress-resistant cells are protected against haloperidol toxicity. Brain Res. 1996; 717: 193–195
- Halici Z, Dursun H, Keles ON, et al. Effect of chronic treatment of haloperidol on the rat liver: A stereological and histopathological study. Naunyn Schmiedebergs Arch Pharmacol. 2009; 379: 253–261
- Gepdiremen A, Aydin N, Halici Z, Sahin O, Unal B, Aydin MD, Bakuridze K. Chronic treatment of haloperidol causes vasoconstriction on basilar arteries of rats, dose dependently. Pharmacol Res. 2004; 50: 569–574
- Sarsilmaz M, Kaplan S, Songur A, et al. Effects of postnatal formaldehyde exposure on pyramidal cell number, volume of cell layer in hippocampus and hemisphere in the rat: A stereological study. Brain Res. 2007; 1145: 157–167
- Razga Z, Nyengaard JR. Estimation of the number of angiotensin II AT1 receptors in rat kidney afferent and efferent arterioles. Anal Quant Cytol Histol. 2007; 29: 208–216
- Medeiros FJ, Aguila MB, Mandarim-de-Lacerda CA. Renal cortex remodeling in streptozotocin-induced diabetic spontaneously hypertensive rats treated with olive oil, palm oil and fish oil from Menhaden. Prostaglandins Leukot Essent Fatty Acids. 2006; 75: 357–365
- Mayhew TM. Second-order stereology and ultrastructural examination of the spatial arrangements of tissue compartments within glomeruli of normal and diabetic kidneys. J Microsc. 1999; 195: 87–95
- Bertram JF. Analyzing renal glomeruli with the new stereology. Int Rev Cytol. 1995; 161: 111–172
- Chang WH, Jaw SS, Tsay L. Chronic haloperidol treatment with low doses may enhance the increase of homovanillic acid in rat brain. Eur J Pharmacol. 1989; 62: 151–156
- Di Matteo V, Cacchio M, Di Giulio C, Di Giovanni G, Esposito E. Biochemical evidence that the atypical antipsychotic drugs clozapine and risperidone block 5-HT(2C) receptors in vivo. Pharmacol Biochem Behav. 2002; 71: 607–613
- Cruz-Orive LM, Weibel ER. Recent stereological methods for cell biology: A brief survey. Am J Physiol. 1990; 258: 148–156
- Gundersen HJ, Bagger P, Bendtsen TF, et al. The new stereological tools: Dissector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 1988; 96: 857–881
- Tunc AT, Turgut M, Aslan H, et al. Neonatal pinealectomy induces Purkinje cell loss in the cerebellum of the chick: A stereological study. Brain Res. 2006; 1067: 95–102
- Gundersen HJG, Jensen EB. The efficacy of systematic sampling in stereology and its prediction. J Microsc. 1987; 147: 229–263
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the dissector. J Microsc. 1984; 134: 127–136
- Unal B, Özbek E, Aydin MD, et al. Effect of haloperidol on the numerical density of neurons and nuclear height in the rat hippocampus: A stereological and histopathological study. Neurosci Res Commun. 2004; 34: 1–9
- Kaplan S, Gokyar A, Unal B, et al. A simple technique for localizing consecutive fields for dissector pairs in light microscopy: Application to neuron counting in rabbit spinal cord following spinal cord injury. J Neurosci Methods. 2005; 145: 277–284
- Cunha AR, Aguila MB, Mandarim-de-Lacerda CA. Effects of early postnatal hyperglycaemia on renal cortex maturity, endothelial nitric oxide synthase expression and nephron deficit in mice. Int J Exp Pathol. 2008; 89: 284–291
- Piecha G, Koleganova N, Gross ML, Geldyyev A, Adamczak M, Ritz E. Regression of glomerulosclerosis in subtotally nephrectomized rats: Effects of monotherapy with losartan, spironolactone, and their combination. Am J Physiol Renal Physiol. 2008; 295: 137–144
- Seitz DP, Gill SS. Neuroleptic malignant syndrome complicating antipsychotic treatment of delirium or agitation in medical and surgical patients: Case reports and a review of the literature. Psychosomatics. 2009; 50: 8–15
- Sanai T, Matsui R, Hirano T, et al. Successful treatment of six patients with neuroleptic malignant syndrome associated with myoglobulinemic acute renal failure. Ren Fail. 2006; 28: 51–55
- Sala MA, Valeri V, Matheus M, Marcos R, Monteiro RA, Rocha E. Stereological analysis of syncytiotrophoblast from human mature placenta. Arch Anat Microsc Morphol Exp. 1983; 72: 99–106
- Marcos R, Monteiro RA, Rocha E. Design-based stereological estimation of hepatocyte number, by combining the smooth optical fractionator and immunocytochemistry with anti-carcinoembryonic antigen polyclonal antibodies. Liver Int. 2006; 26: 116–124