Abstract
This systematic review aimed to statistically profile the medication burden and associated influencing factors, and outcomes in patients with dialysis-dependent chronic kidney disease (DD-CKD). Studies of medication burden in patients with DD-CKD in the last 10 years from 1 January 2013 to 31 March 2024 were searched from PubMed, Embase, and Cochrane databases. Newcastle-Ottawa Scale (NOS) or Agency for Healthcare Research and Quality (AHRQ) methodology checklist was used to evaluate quality and bias. Data extraction and combining from multiple groups of number (n), mean, and standard deviation (SD) were performed using R programming language (version4.3.1; R Core Team, Vienna, Austria). A total of 10 studies were included, and the results showed a higher drug burden in patients with DD-CKD. The combined pill burden was 14.57 ± 7.56 per day in hemodialysis (HD) patients and 14.63 ± 6.32 in peritoneal dialysis (PD) patients. The combined number of medications was 9.74 ± 3.37 in HD and 8 ± 3 in PD. Four studies described the various drug classes and their proportions, in general, antihypertensives and phosphate binders were the most commonly used drugs. Five studies mentioned factors associated with medication burden. A total of five studies mentioned medication burden-related outcomes, with one study finding that medication-related burden was associated with increased treatment burden, three studies finding that poor medication adherence was associated with medication burden, and another study finding that medication complexity was not associated with self-reported medication adherence. Limitations: meta-analysis was not possible due to the heterogeneity of studies.
Introduction
Chronic kidney disease (CKD) is a global public health problem, affecting 10% of the world’s population [Citation1].
Patients with CKD require renal replacement therapy when faced with progressive loss of renal function to renal failure. Dialysis has been the dominant treatment modality for advanced CKD in patients who are not suitable or unlikely to receive renal transplantation. In general, patients with advanced CKD who receive renal replacement therapy in the form of dialysis or renal transplantation are expected to have a longer survival time compared to conservative treatment.
Dialysis-dependent chronic kidney disease (DD-CKD) patients often suffer from a large number of comorbidities, such as hypertension, diabetes, cardiovascular disease (CVD), CKD-related bone and mineral disorders, anemia, and so on, which result in the need for multiple medications to alleviate symptoms associated with CKD and to slow down the progression of comorbidities [Citation2]. Therefore, the total medication burden in dialysis patients can be high. In fact, observational studies have reported that the median number and types of prescription drugs per day can be as high as 19 and 12, respectively, more than for any other chronic disease [Citation3,Citation4]. Polypharmacy renders them vulnerable to medication-related problems, including adverse drug reactions (ADRs) [Citation5]. Poor adherence to complex multimodal therapies contributes to increased morbidity and mortality in these patients [Citation6].
Over decades, there has been an increasing effort to conceptualize the treatment-related burden of chronic diseases [Citation7]. There are various forms of treatment for chronic diseases, one of the most common forms of treatment is the use of medication. In recent years, there has been an increasing attention about medication burden in patients with DD-CKD, and there is a need for a comprehensive review and synthesis of existing studies.
The purpose of this systematic review is to profile the medication burden and associated influencing factors, and outcomes in patients with DD-CKD in recent years.
Methods
Data source and search strategy
The review was prospectively registered in PROSPERO (42023476767). We searched all English articles in PubMed, Embase, and Cochrane according to the PRISMA checklist (see Table S1). We used the keywords ‘chronic kidney disease’, ‘medication regimen complexity’, ‘medication burden’, ‘pill burden’, ‘Dialysis’, and so on, full search terms are described in . The search strategy was developed in collaboration with experienced librarians and adapted to each database. In addition to the electronic search, we also conducted a manual search for the references in the included articles.
Table 1. Search terms used in the PUBMED, EMBASE, and COCHRANE databases.
Study selection
Articles should meet any of the following criteria to be included: (1) every study assessing the medication burden in patients with DD-CKD. (2) The type of literature is clinical research. (3) Ten years of literature in English from 1 January 2013 to 31 March 2024. Exclusion criteria were: (1) review, meta-analysis, expert opinion, animal experiment, case report, letter, and experimental protocol. (2) Literature with no access to valid data. (3) Repeatedly published literature, literature with incomplete information, and no access to full text.
Quality assessment
The quality of cohort studies was evaluated using the Newcastle-Ottawa Scale (NOS), which assesses the quality of each study through three dimensions: selection of study participants (0–4 points), comparability (0–2 points), and exposure/outcome of interest (0–3 points). The maximum score for studies that met all entries on these three dimensions was 9 [Citation8]. Cross-sectional studies were assessed using the Agency for Healthcare Research and Quality (AHRQ) methodology checklist [Citation9,Citation10]. The AHRQ checklist consists of 11 entries, each of which is adjudicated using yes, no, unclear, and not applicable. In this study, the qualities of articles were categorized into four levels, namely ‘excellent’ (≥10 responses as ‘yes’), ‘good’ (7–9 responses as ‘yes’), ‘weak’ (4–6 responses as ‘yes’), and ‘poor’ (1–3 ‘yes’ answers) [Citation11].
Data extraction and analysis
After identifying the title, two independent reviewers (XL and XJ) analyzed the study by screening the title and abstract, and if neither researcher was sure whether the abstract met the inclusion criteria or was inconsistent, the full text was retrieved and reviewed. Relevant articles were selected for full-text review, and after full-text review, the two reviewers extracted, tabulated, and analyzed the data from the full-text articles that met the inclusion criteria separately and then cross-checked them. Disagreements between the two reviewers were resolved by a third author (PC) reviewing the problematic articles. For articles in which the original text was missing and did not report the data of the primary or secondary outcomes measures, journal editors or authors have been contacted via email.
Data extraction and combining from multiple groups of number (n), mean, and standard deviation (SD) were performed using R programming language (version4.3.1; R Core Team, Vienna, Austria) [Citation12,Citation13].
Results
Search results
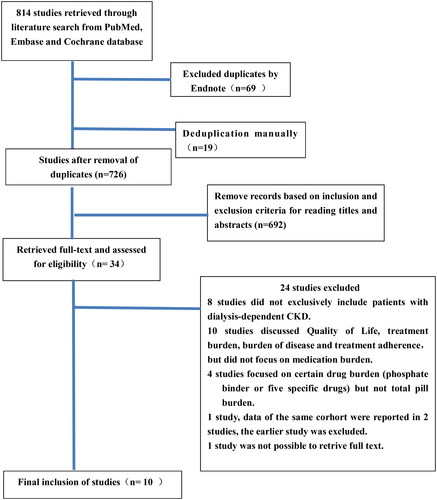
Initially, 814 articles were searched, and 745 articles were obtained after deduplicated by Endnote software. There were 726 articles left after deduplicated manually. Based on the inclusion and exclusion criteria, 716 articles were excluded by reading the titles, abstracts, full texts, and relevant references. Ten articles were finally included, which were in line with the themes, study types, research indicators, and inclusion populations of the literature. The final flowchart of the selection process of the articles is shown in .
Characteristics of selected studies
A total of 10 studies were included [Citation14–23]. shows a detailed overview of the 10 studies. Six were cross-sectional studies [Citation14–16,Citation19,Citation21,Citation22], one was a prospective cohort study [Citation17], two were retrospective cohort studies [Citation20,Citation23], and one was an intervention cohort study [Citation18]. One study was published in 2014 [Citation21], one in 2017 [Citation22], two in 2018 [Citation14,Citation15], one in 2019 [Citation17], four in 2021 [Citation18–20,Citation23], and one most recently in 2023 [Citation16]. One was from the Middle East [Citation16], four from Asia [Citation14,Citation15,Citation18,Citation22], three from Europe [Citation17,Citation21,Citation23], one from North America [Citation20], and one from Australia [Citation19]. The smallest study included 150 patients [Citation18], and the largest study included 27,573 patients [Citation23]. Four studies included only hemodialysis (HD) patients [Citation14,Citation18,Citation20,Citation22], and the remaining studies included patients with various dialysis types of CKD (PD and/or HD). One study included elderly patients with CKD ≥ 65 years of age [Citation17].
Table 2. Characteristics of included studies.
Quality assessment
Four cohort studies had the NOS of 7–9. The AHRQ methodology checklist results of six cross-sectional studies were ‘good’ in four studies and ‘weak’ in two studies. shows the detailed quality assessment results for the characteristics of these 10 studies.
Table 3. Literature quality assessment.
Study outcomes
Current status of medication burden epidemiology
The methods used to assess medication burden in the included articles included pill burden/drug burden in seven articles [Citation14–16,Citation18,Citation19,Citation21,Citation22], drug classes/number of medications in five articles [Citation15,Citation16,Citation18,Citation20,Citation23], and the medication regimen complexity index (MRCI) in one article [Citation17].
Pill burden is defined as the total number of pills taken by the patients in a unit of time (per day/week), including as-needed and over-the-counter medications [Citation17]; oral medication includes pills, capsules, powders, and liquid formulations; each dose is considered to be one pill [Citation14]. The number of medications (the number of drug classes) is defined as the number of different medications for a patient, including over-the-counter medications and as-needed medications [Citation17]. The use of five or more medications per day is often considered polypharmacy [Citation24].
MRCI is a tool for assessing medication complexity and consists of 65 items in three parts: A (dosage forms), B (dosing frequency), and C (additional direction), with 32, 23, and 10 items. The minimum MRCI score for someone on medicines is 1.5, which represents a single tablet or capsule taken once a day as needed; there is no established maximum as the score increases with the increase in medication complexity [Citation25].
Seven studies referred to pill burden [Citation14–16,Citation18,Citation19,Citation21,Citation22]. Only three of these included the pill burden for HD patients [Citation14,Citation18,Citation22]. Iwashita et al. reported that oral tablets per day in HD patients was 16.4 ± 8.34 on dialysis days and 16.3 ± 8.55 on non-dialysis days, which were not statistically different from each other. Regarding the frequency of oral medication, most of the patients took oral medication 3 (31% and 33%), 4 (24% and 22%), and 5 (31% and 30%) times per day [Citation14]. George et al. reported that the mean number of pills was 17.3 ± 6.6 (pills/day) [Citation18] and Gupta et al. reported that the total number of pills in HD patients was 10.5 ± 3.2 (pills/day) [Citation22]. Four of these included the pill burden for patients with various dialysis types of CKD (PD and/or HD) [Citation15,Citation16,Citation19,Citation21], Cirevalli et al. reported that pill burden per day was 10 ± 3 for HD and 13 ± 5 for PD patients [Citation15]. Parker et al. reported mean pill burden (pills/day), 16 ± 7 in MHD (in-center HD), 11 ± 7 in HHD (home HD), 16 ± 7 in peritoneal dialysis (PD) [Citation21]. Al-Mansouri et al. described that the median (IQR) of drug burden was 122 (61) pills per week in HD patients, and 109 (33) medications per week in pre-dialysis patients [Citation16]. Murali et al. showed a median of 21 pills per day for ESKD dialysis patients and 9 pills per day for CKD patients [Citation19]. The pill burden of HD patients was presented as mean ± SD in five studies [Citation14,Citation15,Citation18,Citation21,Citation22], the combined pill burden was 14.57 ± 7.56 per day. The combined pill burden of PD patients from two studies was 14.63 ± 6.32 per day [Citation15,Citation21].
Five studies referred to the number of medications (the number of drug classes) [Citation15,Citation16,Citation18,Citation20,Citation23]. Two of these just included the number of medications for HD patients [Citation18,Citation20]. George et al. reported that the mean number of medications was 10.8 ± 3.2 in HD patients [Citation18]. Paik et al. described the number of drug classes and showed a gradual decrease in HD patients with CKD from 2013 to 2017, which were 7.4 ± 3.8, 7.0 ± 3.7, 7.0 ± 3.7, 7.0 ± 3.6, and 6.8 ± 3.6, respectively. The number of patients taking multiple medications (≥5 medications) concurrently was also decreased (77.5% in 2013 and 73.8% in 2017) [Citation20]. Three of these included the number of medications for patients with various dialysis types of CKD (PD and/or HD) [Citation15,Citation16,Citation23], Cirevalli et al. reported that drug classes per day was 7 ± 2 for HD and 8 ± 3 for PD patients [Citation15], Al-Mansouri et al. described the number of medications for HD patients was 12 (5) oral medications and 3 (2) parenteral medications [Citation16]. Van Oosten et al. defined PP at three levels: concurrent use of five medications (PP), 10 medications (excessive PP (EPP)), and 15 medications (hyper PP (HPP)). The median number of dispensed medications in dialysis patients was 12; the prevalence of PP, EPP, and HPP was 93.4, 69.3, and 31.5% for all medications, and 70.0%, 15.1%, and 1.2% for chronic medications [Citation23]. The number of medications (the number of drug classes) of HD patients was presented as mean ± SD in two studies [Citation15,Citation18], the combined number of medications was 9.74 ± 3.37. The number of medications for PD patients from one study was 8 ± 3 [Citation15].
Only one study related to MRCI [Citation17], Parker et al. assessed MRCI and concluded it to be 24.7 ± 7.9 in HD, and 23.3 ± 6.4 in PD patients.
As for specific medication types and their proportion, two studies described specific medication types in HD patients [Citation14,Citation20]; one study involved HD and pre-dialysis patients [Citation16]; another study involved all dialysis patients [Citation15]. In general, antihypertensives and phosphate-binding agents were more commonly used.
Paik et al. found that the overall medication burden for HD ESRD patients decreased slightly from 2013 to 2017, prescribing of harmful medications (including non-benzodiazepine sleeping pills, benzodiazepines, and opioids) also decreased during this period [Citation20].
Medication burden-related factors
A total of five studies mentioned factors related to medication burden [Citation15,Citation17,Citation20,Citation21,Citation23].
Five studies addressed the correlation between age and medication burden, three were statistically significant [Citation15,Citation20,Citation23], and two were not [Citation17,Citation21]. One study showed that mean pills per day among PD patients was significantly higher in patients ≥60 years than in 18–59 years (p = .002). Drug classes among HD patients showed significantly higher in patients aged ≥ 60 years than in those aged 18–59 years (p = .013) [Citation15]. Paik et al. found that the mean number of medications was greater in patients aged <65 years compared to those aged ≥ 65 years. Additionally, they observed a decrease in the mean number of medications over time in both age groups. Specifically, for patients aged <65 years, the number decreased from 7.7 medications in 2013 to 7.2 medications in 2017, while for patients aged ≥ 65 years, it decreased from 7.0 medications in 2013 to 6.4 medications in 2017 [Citation20]. However, a study showed that patients aged 75 had a lower risk of EPP (OR 0.74 (95% CI 0.59–0.91)) compared with patients aged 20–64 years [Citation23].
Two studies mentioned relationships between gender and medication burden and suggested significant correlations [Citation17,Citation20]. Paik et al. stated that when examining prescribing patterns by gender, the average number of medications was higher in female patients compared to males. Additionally, they observed a decrease in the average number of medications over time in both groups. Specifically, for female patients, the number decreased from 7.9 medications in 2013 to 7.2 medications in 2017, while for male patients, it decreased from 6.9 medications in 2013 to 6.4 medications in 2017 [Citation20]. Parker et al. showed that females (unstandardized beta coefficient: 2.44, 95% CI: −0.07 to 4.81, p = .044) were a significant determinant of MRCI in a multivariate linear regression analysis [Citation17].
Two studies reported that dialysis type was a drug burden-related factor. Cirevalli et al. mentioned dialysis modalities and showed that both drug classes per day and mean pills per day were significantly higher in the PD population than in HD [Citation15]. Parker et al. found that the only significant factor influencing pill burden was modality setting (dialysis modalities) [Citation21].
Paik et al. mentioned race [Citation20] and found variability. When medication prescribing patterns were examined for race, the average number of medications was higher for white patients than for black and Asian patients. The number of medications per patient during each year for White patients decreased from 7.5 in 2013 to 7.0 in 2017; for Black patients decreased from 7.2 to 6.6; and for Asian patients decreased from 7.0 to 6.5 [Citation20].
Three studies discussed the relationship between comorbidities and medication burden, two with significance and one without [Citation17,Citation21,Citation23]. Parker et al. assessed comorbidities using the Charlson Comorbidity Index (CCI) [Citation17]. This index consists of 19 weighted comorbidities that are added together to give a total score [Citation26]. CCI were significant determinants of the MRCI (CCI 4 or 5 vs. 2 or 3, unstandardized beta coefficient: 2.56, 95% CI: 0.27–4.85, p = .029). Van Oosten et al. found the most pronounced risk factors for EPP in dialysis patients were diabetes (OR 3.69, 95% CI: 3.08–4.43) and vascular disease (OR 2.08, 95% CI: 1.72–2.51) [Citation23]. Nevertheless, Parker et al. concluded that the Liu comorbidity score was not associated with an increase in pill burden [Citation21].
Other relevant factors included LIS status (low-income subsidy status), where the average number of medications was higher in patients receiving LIS compared to those not receiving LIS [Citation20]; use of phosphate binders was associated with more complex medication regimens [Citation17].
Interventions targeting the medication burden
Implementation of a proactive deprescription program could go a long way toward reducing polypharmacy. Deprescribing can be defined as ‘the systematic process of identifying and discontinuing drugs in instances in which existing or potential harms outweigh existing or potential benefits within the context of an individual patient’s care goals, current level of functioning, life expectancy, values, and preference’ [Citation27]. George et al. examined the mean number of drug classes and pill burden per patient 12 weeks before and after the implementation of a proactive deprescription program [Citation18], and after deprescription, the number of drug classes was significantly reduced from 11 (IQR 8–13.25) to 8 (IQR 6–9) (p < .001), and the number of pills was significantly reduced from 16 (IQR 12.75–21.25) to 11 (IQR 8–14.25) (p < .001). The pill burden derived using the Living with Medication Questionnaire-Visual Analogue Scale (LMQ-VAS) score was also significantly reduced from 7 (IQR 5–8) to 4 (IQR 3–5) (p < .001).
Medication burden-related outcomes
A total of five studies mentioned medication burden-related outcomes [Citation16–19,Citation22]. One study mentioned that drug-related burden in HD patients was associated with increased treatment burden. Patients with a higher weekly drug burden (mean 117–239 drugs per week) perceived a significantly higher treatment burden compared to patients with a lower drug burden [Citation16]. Three studies found that medication adherence was associated with medication burden, Murali et al. found that ESKD patients had a higher medication burden and significantly poorer medication non-adherence compared to CKD patients [Citation19]. The study by George et al. showed a significant negative correlation between the number of medications before discontinuation and medication adherence (Modified Medication Adherence Rating Scale (MARS)) (R = 0.200 and p < .014) [Citation18]. Gupta et al. concluded that high pill burden has an adverse impact on medication adherence [Citation22].
In contrast, one study found that medication complexity was not associated with self-reported medication adherence, as assessed by the eight-item Morisky Medication Adherence Scale (MMAS-8), in elderly CKD patients [Citation17].
Discussion
This systematic review primarily highlights the current status of drug burden and examines factors associated with drug burden and outcomes in DD-CKD patients.
The results showed a higher drug burden in patients with DD-CKD. The pill burden of HD patients was presented as mean ± SD in five studies. The combined pill burden was 14.57 ± 7.56 per day. The combined pill burden of PD patients from two studies was 14.63 ± 6.32 per day. The number of medications (the number of drug classes) of HD patients was presented as mean ± SD in two studies. The combined number of medications was 9.74 ± 3.37. The number of medications of PD patients from one study was 8 ± 3. Only one study related to MRCI concluded as 24.7 ± 7.9 in HD, and 23.3 ± 6.4 in PD patients. According to the World Health Organization, the top 3 chronic diseases of the causes of death in 2019 were ischemic heart disease (IHD), stroke, and chronic obstructive pulmonary disease (COPD) [Citation28]. The results of this systematic review showed that the medication burden in patients with DD-CKD is comparable to that of patients with IHD and COPD. The MRCI of coronary artery disease patients was 17.0 (IQR 10.5) [Citation29]; the mean number of medications of acute coronary syndrome patients during the peri-hospitalization period was prescribed 8.6 medications on admission, 11.4 medications on discharge, and 11.1 medications after discharge (within 90 days after discharge). In COPD patients, the number of prescription medications was 6 (range 2–14) [Citation30], total MRCI score was 24 (18.5, 31) [Citation31].
Factors associated with medication burden as aggregated from the five papers include age, dialysis modality, gender, race, LIS status, CCI, etc. Similar findings have been reported in other chronic disease studies, such as age and comorbidities. Dialysis patients not only have a large number of comorbidities, but also specific complications (e.g., anemia, hyperkalemia, and osteodystrophy) that require specific medications (e.g., anti-anemic agents, potassium-binding agents, phosphate-binding agents, etc.) to treat them [Citation32]. Multiple comorbidities and complications usually contribute to particularly high levels of combination medication [Citation33]. Factors associated with high medication regimen complexity in patients with IHD with statistically significant differences were heart failure, hypertension, diabetes mellitus, COPD, and combined five or more diseases [Citation29,Citation34,Citation35]. Relevant studies showed no significant correlation between MRCI and diabetes and hypertension in COPD patients [Citation36], combined cardiovascular, gastrointestinal, or metabolic disease alone results in a higher total MRCI score [Citation31].
Potential interventions on these associated factors may help to reduce pill burden and produce positive outcomes. Age, sex, dialysis modality, and so on are uncontrollable risk factors; we can focus on interventions to controllable risk factors. Drug burden in DD-CKD patients is composed mainly of antihypertensive and phosphorus-lowering drugs, suggesting a clinical focus. Mitigation of the high drug burden associated with hypertension can be achieved by enhancing blood pressure control through lifestyle modification and dialysis treatment. Weight loss, reduction of dietary sodium, moderate alcohol consumption, and physical activity are the best-proven interventions for the primary prevention of hypertension [Citation37]. More frequent HD reduces blood pressure more consistently and requires less antihypertensive medication to achieve the same blood pressure control as the traditional three-times-weekly regimen [Citation38]. Another way to control fluid volume in dialysis patients is to set an appropriate dry weight (DW) [Citation39]. The combined use of non-pharmacological therapies, in particular, dietary sodium restriction, dialysate sodium adjustment, and the use of antihypertensive medications (preferably cardioprotective drugs) may be the best option to optimize blood pressure control [Citation40].
Phosphate binders are one of the most commonly used medications in dialysis patients with renal failure. The use of phosphate-binding agents may cause gastrointestinal side effects such as vomiting, constipation, and diarrhea, increasing the drug burden. Commonly used calcium-based binders include calcium carbonate, calcium acetate, sevelamer, lanthanum carbonate, iron hydroxyl oxidized sucrose, and iron citrate [Citation41]. Some research demonstrated that SO (sucroferric oxyhydroxide) monotherapy was associated with a reduction in pill burden, better adherence, and improvements in phosphorus control among HD and PD patients [Citation42,Citation43]. Other options for phosphorus reduction include limiting dietary phosphate intake (while ensuring adequate protein intake) and increasing the frequency or duration of dialysis (for patients requiring renal replacement therapy) [Citation44].
For interventions targeting the medication burden, only one study of reducing drug burden in patients with DD-CKD by proactively canceling prescription plans has shown better results. In addition, a study about the development and implementation of a deprescribing tool for specific medications in the HD unit indicated that five medication classes were selected, at the end of the study, 57% of patients were taking fewer medications than at baseline, and no adverse events were observed [Citation45]. Deprescribing is an important measure in the management of polypharmacy and inappropriate use of medication, and the more widely recognized method of deprescribing in the industry is the ‘five-step-approach’ [Citation27]. Influenced by many factors, such as prescription, patients, and society, deprescribing still faces serious challenges, and there is still a lack of unified streamlining methodology and guidelines, and the evaluation of the clinical effect of deprescribing is also a difficult point. The use of multi-targeted, fixed-combination products, such as atorvastatin/amlodipine and aspirin/pravastatin, reduces drug burden in patients with CVD [Citation46] and COPD [Citation31]. Home medication management is an important part of polypharmacy management, which mainly focuses on medication therapy management (MTM) services provided to patients by family pharmacists and family physicians. The service objective of medication management is to identify and resolve problems associated with the patient’s medication process, such as promoting rational drug use [Citation47]. Under the medication management model, the active participation of family members in health education can bring into play the family support role of family members to help patients use medication rationally and change their bad living habits. All those above measures will help to reduce the drug burden in the DD-CKD patients.
This review also noted that excessive medication burden can lead to increased treatment burden, poorer medication adherence, and reduced quality of life [Citation16,Citation18,Citation19,Citation22]. However, one study showed that medication complexity was not associated with self-reported medication adherence [Citation17], it may be caused by the use of different medication adherence scales to rate medication adherence, and different demographic characteristics of the enrolled patients. Non-adherence to medication was also present in patients with IHD and stroke [Citation29,Citation48], but not in COPD patients; Federman et al. found no statistically significant association between the complexity of treatment regimen and medication adherence in COPD patients [Citation36]. One study showed that the complexity of medication regimens in elderly CKD patients was not significantly associated with 30-day readmissions; however, it was associated with significantly shorter 12-month readmissions [Citation49]. In patients with a primary diagnosis of heart failure, there was a significant association between MRCI and 30-day readmission, which was defined as readmission within 30 days of discharge [Citation50]. However, no studies have been reported on whether drug burden is associated with primary endpoint events such as death and readmission in DD-CKD patients, which is a direction for future research.
Strengths of this study: (1) as far as we know, this is the first review of medication burden in DD-CKD; (2) the search was conducted according to a rigorous methodology; (3) showed the present complete understanding of serious medication burden in DD-CKD, and suggested calling for studies on key clinical outcomes and interventions targeting medication burden. Limitations of this study: (1) only English language studies were included in this study and the comprehensiveness of the findings was limited. (2) Heterogeneity between studies was high, with differences in sample size, variables, and group characteristics. (3) No randomized controlled trials (RCTs) studies were included, weakening the strength of the evidence. (4) In the end, there were no rigorous clinical outcomes or long-term outcomes.
Conclusions
Drug burden is a significant problem in patients with DD-CKD, which is associated with multiple factors and adverse outcomes, exploring interventions is future study directions.
Author contributions
XL, PC, DX, and XJ contributed to the conceptualization and design of the study. XL, PC, and XJ contributed to the literature systematic search and validation of study selection. XL, PC, YL, and DX analyzed and interpreted the data. XJ and PC drafted the first version of the article with early revision by XJ and DX. All authors critically revised the article and approved the final version for publication. DX and XJ had full access to all data in the study and had the ultimate responsibility for the decisions submitted for publication and the modifications required. All authors have read and agreed to the published version of the manuscript.
Ethical approval
Not applicable.
Consent form
Not applicable.
Supplemental Material
Download MS Word (33.4 KB)Acknowledgements
We would like to thank the staff at the Center for Big Data Research in Health and Medicine, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, for their valuable contribution.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Additional information
Funding
References
- KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024;105(4):684–701. doi:10.1016/j.kint.2023.10.018.
- Schmidt IM, Hübner S, Nadal J, et al. Patterns of medication use and the burden of polypharmacy in patients with chronic kidney disease: the German Chronic Kidney Disease Study. Clin Kidney J. 2019;12(5):663–672. doi:10.1093/ckj/sfz046.
- Chiu YW, Teitelbaum I, Misra M, et al. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089–1096. doi:10.2215/cjn.00290109.
- Manley HJ, Garvin CG, Drayer DK, et al. Medication prescribing patterns in ambulatory haemodialysis patients: comparisons of USRDS to a large not-for-profit dialysis provider. Nephrol Dial Transplant. 2004;19(7):1842–1848. doi:10.1093/ndt/gfh280.
- Cardone KE, Bacchus S, Assimon MM, et al. Medication-related problems in CKD. Adv Chronic Kidney Dis. 2010;17(5):404–412. doi:10.1053/j.ackd.2010.06.004.
- Schmid H, Hartmann B, Schiffl H. Adherence to prescribed oral medication in adult patients undergoing chronic hemodialysis: a critical review of the literature. Eur J Med Res. 2009;14(5):185–190. doi:10.1186/2047-783x-14-5-185.
- Mohammed MA, Moles RJ, Chen TF. Medication-related burden and patients’ lived experience with medicine: a systematic review and metasynthesis of qualitative studies. BMJ Open. 2016;6(2):e010035. doi:10.1136/bmjopen-2015-010035.
- Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14(1):45. doi:10.1186/1471-2288-14-45.
- AHRQ Methods for Effective Health Care. Methods guide for effectiveness and comparative effectiveness reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008.
- Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. doi:10.1111/jebm.12141.
- Rodrigues H, Cobucci R, Oliveira A, et al. Burnout syndrome among medical residents: a systematic review and meta-analysis. PLOS One. 2018;13(11):e0206840. doi:10.1371/journal.pone.0206840.
- Altman D, Machin D, Bryant T, et al. Statistics with confidence. 2nd ed. London: BMJ Books; 2000. p. 28–31.
- Higgins JPT, Li T, Jj D. Chapter 6: choosing effect measures and computing estimates of effect. In: Cochrane handbook for systematic reviews of interventions. Cochrane; 2023. Available from: https://training.cochrane.org/handbook/current/chapter-06#section-6-5-2
- Iwashita Y, Ohya M, Kunimoto S, et al. A survey of drug burden in patients undergoing maintenance hemodialysis in Japan. Intern Med. 2018;57(20):2937–2944. doi:10.2169/internalmedicine.0108-17.
- Cirevalli P, Nimmanapalli HD, Parlapalli LK, et al. Pill burden, drug class distribution and financial burden for buying medicines in different modalities of chronic kidney disease patients: cross-sectional study. Int J Pharm Pharm Sci. 2018;10(6):165–170. doi:10.22159/ijpps.2018v10i6.26186.
- Al-Mansouri A, Hamad AI, Al-Ali FS, et al. Pill-burden and its association with treatment burden among patients with advanced stages of chronic kidney disease. Saudi Pharm J. 2023;31(5):678–686. doi:10.1016/j.jsps.2023.03.008.
- Parker K, Bull-Engelstad I, Aasebø W, et al. Medication regimen complexity and medication adherence in elderly patients with chronic kidney disease. Hemodial Int. 2019;23(3):333–342. doi:10.1111/hdi.12739.
- George JS, Joseph R, Thomas ETA, et al. Active deprescribing program in chronic kidney disease patients undergoing haemodialysis. Nephrology. 2021;26(11):890–897. doi:10.1111/nep.13936.
- Murali K, Mullan J, Roodenrys S, et al. Medication non-adherence in patients with kidney disease: importance of depression, health literacy and pill burden. Nephrology. 2021;26(Suppl. 2):24. doi:10.1111/nep.13930.
- Paik JM, Zhuo M, York C, et al. Medication burden and prescribing patterns in patients on hemodialysis in the USA, 2013–2017. Am J Nephrol. 2021;52(12):919–928. doi:10.1159/000520028.
- Parker K, Nikam M, Jayanti A, et al. Medication burden in CKD-5D: impact of dialysis modality and setting. Clin Kidney J. 2014;7(6):557–561. doi:10.1093/ckj/sfu091.
- Gupta V, Jasani R, Kumar R, et al. Pill burden and PILL adherence in dialysis patients. Indian J Nephrol. 2017;27:S93.
- Van Oosten MJM, Logtenberg SJJ, Hemmelder MH, et al. Polypharmacy and medication use in patients with chronic kidney disease with and without kidney replacement therapy compared to matched controls. Clin Kidney J. 2021;14(12):2497–2523. doi:10.1093/ckj/sfab120.
- Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65(9):989–995. doi:10.1016/j.jclinepi.2012.02.018.
- Ghimire S, Peterson GM, Castelino RL, et al. Medication regimen complexity and adherence in haemodialysis patients: an exploratory study. Am J Nephrol. 2016;43(5):318–324. doi:10.1159/000446450.
- Beddhu S, Bruns FJ, Saul M, et al. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med. 2000;108(8):609–613. doi:10.1016/s0002-9343(00)00371-5.
- Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827–834. doi:10.1001/jamainternmed.2015.0324.
- World Health Organization. The top 10 causes of death; 2020 [cited 2023 Oct 10]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- Tinoco MS, Groia-Veloso RCS, Santos J, et al. Medication regimen complexity of coronary artery disease patients. Einstein. 2021;19:eAO5565. doi:10.31744/einstein_journal/2021AO5565.
- Noteboom B, Jenkins S, Maiorana A, et al. Comorbidities and medication burden in patients with chronic obstructive pulmonary disease attending pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2014;34(1):75–79. doi:10.1097/hcr.0000000000000036.
- Negewo NA, Gibson PG, Wark PA, et al. Treatment burden, clinical outcomes, and comorbidities in COPD: an examination of the utility of medication regimen complexity index in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2929–2942. doi:10.2147/copd.S136256.
- Laville SM, Metzger M, Stengel B, et al. Evaluation of the adequacy of drug prescriptions in patients with chronic kidney disease: results from the CKD-REIN cohort. Br J Clin Pharmacol. 2018;84(12):2811–2823. doi:10.1111/bcp.13738.
- Marienne J, Laville SM, Caillard P, et al. Evaluation of changes over time in the drug burden and medication regimen complexity in ESRD patients before and after renal transplantation. Kidney Int Rep. 2021;6(1):128–137. doi:10.1016/j.ekir.2020.10.011.
- Cobretti MR, Page RL2nd, Linnebur SA, et al. Medication regimen complexity in ambulatory older adults with heart failure. Clin Interv Aging. 2017;12:679–686. doi:10.2147/cia.S130832.
- Wright EA, Steinhubl SR, Jones JB, et al. Medication burden in patients with acute coronary syndromes. Am J Manage Care. 2017;23(4):e106–e112.
- Federman AD, O'Conor R, Wolf MS, et al. Associations of medication regimen complexity with COPD medication adherence and control. Int J Chron Obstruct Pulmon Dis. 2021;16:2385–2392. doi:10.2147/copd.S310630.
- He J, Whelton PK. Epidemiology and prevention of hypertension. Med Clin North Am. 1997;81(5):1077–1097. doi:10.1016/s0025-7125(05)70568-x.
- Zimmerman DL, Ruzicka M, Hebert P, et al. Short daily versus conventional hemodialysis for hypertensive patients: a randomized cross-over study. PLOS One. 2014;9(5):e97135. doi:10.1371/journal.pone.0097135.
- Levin NW, Kotanko P, Eckardt KU, et al. Blood pressure in chronic kidney disease stage 5D-report from a kidney disease: improving global outcomes controversies conference. Kidney Int. 2010;77(4):273–284. doi:10.1038/ki.2009.469.
- Bucharles SGE, Wallbach KKS, Moraes TP, et al. Hypertension in patients on dialysis: diagnosis, mechanisms, and management. J Bras Nefrol. 2019;41(3):400–411. doi:10.1590/2175-8239-jbn-2018-0155.
- Scialla JJ, Kendrick J, Uribarri J, et al. State-of-the-art management of hyperphosphatemia in patients with CKD: an NKF-KDOQI controversies perspective. Am J Kidney Dis. 2021;77(1):132–141. doi:10.1053/j.ajkd.2020.05.025.
- Gray K, Ficociello LH, Hunt AE, et al. Phosphate binder pill burden, adherence, and serum phosphorus control among hemodialysis patients converting to sucroferric oxyhydroxide. Int J Nephrol Renovasc Dis. 2019;12:1–8. doi:10.2147/IJNRD.S182747.
- Kalantar-Zadeh K, Parameswaran V, Ficociello LH, et al. Real-world scenario improvements in serum phosphorus levels and pill burden in peritoneal dialysis patients treated with sucroferric oxyhydroxide. Am J Nephrol. 2018;47(3):153–161. doi:10.1159/000487856.
- Kidney Disease: improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-Mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009;2009(113):S1–S130. doi:10.1038/ki.2009.188.
- McIntyre C, McQuillan R, Bell C, et al. Targeted deprescribing in an outpatient hemodialysis unit: a quality improvement study to decrease polypharmacy. Am J Kidney Dis. 2017;70(5):611–618. doi:10.1053/j.ajkd.2017.02.374.
- Sleight P, Pouleur H, Zannad F. Benefits, challenges, and registerability of the polypill. Eur Heart J. 2006;27(14):1651–1656. doi:10.1093/eurheartj/ehi841.
- Jerath A, Panckhurst J, Parotto M, et al. Safety and efficacy of volatile anesthetic agents compared with standard intravenous midazolam/propofol sedation in ventilated critical care patients: a meta-analysis and systematic review of prospective trials. Anesth Analg. 2017;124(4):1190–1199. doi:10.1213/ane.0000000000001634.
- Lehmann J, Riedl D, Sztankay M, et al. The attitude towards polypills questionnaire (APPQ): a phase I–III development and validation study in patients with cerebrovascular disease. Eur J Neurol. 2021;28(12):4039–4050. doi:10.1111/ene.15088.
- Tesfaye WH, Peterson GM, Castelino RL, et al. Medication regimen complexity and hospital readmission in older adults with chronic kidney disease. Ann Pharmacother. 2019;53(1):28–34. doi:10.1177/1060028018793419.
- Colavecchia AC, Putney DR, Johnson ML, et al. Discharge medication complexity and 30-day heart failure readmissions. Res Social Adm Pharm. 2017;13(4):857–863. doi:10.1016/j.sapharm.2016.10.002.