Abstract
Background
To explore the risk factors of proteinuria in Omicron variant patients and to construct and verify the risk predictive model.
Methods
1091 Omicron patients who were hospitalized from August 2022 to November 2022 at Tianjin First Central Hospital were defined as the derivation cohort. 306 Omicron patients who were hospitalized from January 2022 to March 2022 at the same hospital were defined as the validation cohort. The risk factors of proteinuria in derivation cohort were screened by univariate and multivariate logistic regression analysis, and proteinuria predicting scoring system was constructed and the receiver operating characteristic(ROC)curve was drawn to test the prediction ability. The proteinuria risk model was externally validated in validation cohort.
Results
7 factors including comorbidities, blood urea nitrogen (BUN), serum sodium (Na), uric acid (UA), C reactive protein (CRP) and vaccine dosages were included to construct a risk predictive model. The score ranged from −5 to 16. The area under the ROC curve(AUC) of the model was 0.8326(95% CI 0.7816 to 0.8835, p < 0.0001). Similarly to that observed in derivation cohort, the AUC is 0.833(95% CI 0.7808 to 0.9002, p < 0.0001), which verified good prediction ability and diagnostic accuracy in validation cohort.
Conclusions
The risk model of proteinuria after Omicron infection had better assessing efficiency which could provide reference for clinical prediction of the risk of proteinuria in Omicron patients.
Keywords:
Background
The COVID-19 pandemic has affected millions of people in the past three years. The pathogenesis is complicated with infectious, inflammatory, and thrombotic mechanisms involved [Citation1]. The clinical manifestation of COVID-19 varies with a remarkable kidney tropism [Citation2,Citation3]. Existing studies have shown that SARS-CoV-2 virus may specifically attack the kidney, causing acute kidney injury (AKI), hematuria and proteinuria [Citation4,Citation5].
Since first detected in South Africa in November 2021, Omicron, the novel coronavirus variant, had displaced Delta and rapidly spread as the predominant variant around the world. Although disease severity appears to be lower, a rapid increase of transmission has led to a relatively substantial volume of hospitalizations [Citation6]. Our team have found that among patients without a CKD history, about 14.2% of Omicron variant patients exhibited proteinuria [Citation7], which was much higher than the natural prevalence of CKD (10.8%) in the general population of China [Citation8]. Another report showed that proteinuria was shown in up to 44% of COVID-19 patients [Citation9]. So far, we have recognized that proteinuria was an independent predictor of the progression of COVID-19 and may reflect early direct or indirect renal damage [Citation10,Citation11]. Despite all this, proper prognostic models of patients admitted to a hospital who have developed proteinuria is lack.
Identification of important comorbidities and laboratory predictors among routinely performed tests may contribute to better discover of progressive renal injury. Thus, using the clinical data easily obtained in practical work, we aim to explore the risk factors of proteinuria in Omicron variant patients, and to construct and verify the risk model which can provide objective and quantitative basis for accurate warning and early diagnosis of proteinuria.
Methods
Study population
The present observational derivation-validation study was performed in two non-overlapping cohorts. All participants were diagnosed with SARS‐CoV‐2 Omicron variant infection and then admitted to hospital for further treatment. Nucleic acid was extracted from respiratory samples using commercial kits (Zybio). The WHO protocol was used to identify two target genes, the open reading frame of 1ab (ORF1ab) and the nucleocapsid protein (N), using reverse transcription‐polymerase chain reaction (RT‐PCR) to confirm Omicron variant [Citation12]. Enrolled patients were required to have at least one urinalysis. Patients with positive urine protein for the first time needed to repeat the urine test at least once, and those who were still positive were confirmed as proteinuria. Patients aged <13 years old, foreign nationality, or with CKD history were excluded. CKD is defined as abnormalities of kidney structure or function, present for >3 months, with implications for health. Markers of kidney damage include one or more: albuminuria (AER ≥30 mg/g (≥3 mg/mmol)), urine sediment abnormalities, electrolyte and other abnormalities due to tubular disorders, abnormalities detected by histology, structural abnormalities detected by imaging, history of kidney transplantation. Function abnormalities mean that GFR <60 mL/min per 1.73 m2 (GFR categories G3a-G5). During hospitalization, patients continued to take regular medication to treat underlying diseases such as hypertension or diabetes or cardiovascular diseases.
The derivation cohort comprised 1205 inpatients from August 2022 to November 2022 at Tianjin First Central Hospital. The excluded participants included 4 cases of foreign infection, 6 cases of CKD history and 109 cases of age <13 years old. Finally, 1091 inpatients were enrolled in the study. The validation cohort comprised 430 inpatients from January 2022 to March 2022 at the same hospital. The excluded participants included 8 cases of CKD history, 12 cases without urinalysis results and 104 cases of age <13 years old. Finally, 306 inpatients were enrolled in the study.
The study was approved by Haihe Laboratory of Cell Ecosystem (Ethics approval document HHL2022005‐EC‐1) and was conducted following the principle of the Helsinki Declaration.
Data collection
Data available in hospital documentation was collected including the demographic parameters, clinical features, and laboratory measurement indexes. Demographic and clinical information contained age, gender, body mass index (BMI), comorbidities such as hypertension, diabetes, cardiovascular and cerebrovascular diseases (CVD), COVID-19 clinical classification and vaccination. Laboratory data contained hemoglobin (HGB), white blood cell (WBC), platelets (PLT), alanine transaminase (ALT), Lactate Dehydrogenase (LDH), blood urea nitrogen (BUN), creatinine (Cr), sodium (Na), potassium (K), calcium (Ca), uric acid (UA), fasting blood glucose (FBG), C reactive protein (CRP), interleukin 6 (IL-6) and urinalysis. Proteinuria was defined as the presence of 1+ protein or greater in urinalysis after ruling out urinary tract infection. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation.
All laboratory results were obtained on-admission, defined as first 48h from hospital arrival. According to Chinese recommendations for diagnosis and treatment of novel coronavirus (SARS-CoV-2) infection (trial 8th version), we classified all inpatients as asymptomatic, mild, ordinary, or severe patients with COVID-19 [Citation13]. The vaccine type and dosage for each patient was recorded.
Statistical analysis
Normally distributed continuous variables were presented as mean ± standard deviation, non-normally distributed continuous variables as medians and interquartile ranges, and categorical variables as frequencies and percentages. We divided the participants into proteinuria group and no proteinuria group. Independent Sample T or Mann–Whitney U‐test was used to compare continuous variables between groups. If appropriate, the categorical variables were compared using the chi‐squared test or Fisher’s exact test. Univariate and multivariate Logistic regression analysis were applied to screen the indicators with risk value. The efficiency was evaluated by the area under curve (AUC) of the receiver operating characteristic (ROC) curve, and it was considered that AUC >0.7 was the risk model with better assessing efficiency in endpoint events.
A weighted score was created to assess the occurrence, in the derivation cohort, of proteinuria in Omicron variant patients based on the demographic, clinical features, and laboratory parameters in hospital. Firstly, to establish a scoring system, all continuous variables were converted into categorical variables. Youden’s index was calculated according to the ROC curve of proteinuria. The value corresponding to the largest Youden’s index was used as the cutoff of categorical variables, for example, the cutoff of HGB was >15.1 g/L. Secondly, univariate and multivariate logistic regression analysis were applied to screen the clinical variables associated with the risk of proteinuria in the derivation cohort. Variables with univariate p value <0.1 were entered into multivariate logistic regression model and those with p value <0.05 were selected into the final scoring model. Thirdly, we assigned scores to these meaningful variables of the scoring model. The corresponding point assignment for independent variables were calculated by using the β regression coefficient values. We divided the β coefficient of each variable in the final multivariable model by one-half of the smallest coefficient and rounded to the nearest integer as the assignment point. In this study, Na >141.4 mmol/L is the smallest β regression coefficient. By this mean, we assigned the weighted point for proteinuria to each variable, for example, the score value of Diabetes was 5. In view of the protective effect of 2 and 3 dosage of vaccine against proteinuria compared with no vaccination, negative values were given. Fourthly, we added up all the weighted points of variables to obtain the final total risk score of each patient. Therefore, we generated a scoring system for assessing proteinuria in Omicron patients. Lastly, the ROC curves were drawn to test the assessing ability and the diagnostic accuracy of the proteinuria risk scoring model in the derivation and validation cohorts separately.
All statistical analyses were conducted using SPSS version 22 (IBM) and STATA version 14 (StataCorp LP). A p value <0.05 was considered statistically significant.
Results
Characteristics of derivation and validation cohorts
showed the demographic and clinical characteristics in derivation and validation cohorts. The average age was 38.49 ± 16.10 and 45.93 ± 16.14 years, respectively. The average BMI was 24.39 ± 3.96 and 25.25 ± 4.70 in two cohorts. 53.4% of patients were males in derivation cohort and 40.8% in validation cohort. It suggested that the patients in the validation cohort were older which can explain the more comorbidities. Meanwhile, BMI was higher, and the proportion of women was lower in the validation cohort. There was a significant difference between two groups in vaccination. The vaccination rate was higher in derivation cohort than in validation cohort (97.3% vs 92.5%), as well as the three dosages vaccination rate (52.7% vs 48.4%). The main types of vaccinations were all Sinovac vaccine (38.3% vs 48.1%) in two cohorts with statistical difference in proportion. In terms of clinical classification, there were also significant differences between two groups. Asymptomatic type was common (89.2%) in derivation cohort, while ordinary type was common (69.8%) in validation cohort. In addition, there were differences in inflammatory indicators levels between the two cohorts. CRP was higher in validation cohort while IL-6 was higher in derivation cohort. There were no differences between other laboratory indicators.
Table 1. Demographic and clinical characteristics in derivation cohort and validation cohort.
In terms of renal involvement, no patients in both cohorts met the diagnostic criteria for AKI. The incidence of proteinuria in derivation and validation cohorts were 4.59% and 19.93%, respectively. We further compared the clinical data between proteinuria group and no proteinuria group, taking derivative cohort as an example. It is worth noting that serum BUN and eGFR were within the normal range in two groups, however, patients in proteinuria group had higher BUN, lower eGFR. Additionally, patients in proteinuria group were older, more men, higher BMI, more complications, higher UA, higher CRP, higher proportion of unvaccinated patients, and lower rate of three dosage vaccination. The above results were consistent in validation cohort.
Model derivation
Firstly, all continuous variables were converted into categorical variables and cutoff values were shown as follows: age >60.5 years, BMI >27.2 Kg/m2, HGB >15.1 g/L, WBC >5.4 × 109/L, BUN >4.62 mmol/L, Cr >73.2 μmol/L, Na >141.4 mmol/L, Ca <2.23 mmol/L, UA >395.9 mmol/L, FBS >5.4 mmol/L, CRP >9.83 mg/L and IL-6 >10.1 pg/ml. Secondly, to find the risk factors associated with proteinuria in the derivation cohort, we conducted the logistic regression analysis. Age, sex, BMI, hypertension, diabetes, CVD, HGB, WBC, BUN, Cr, Na, Ca, UA, FBS, CRP, IL-6 and vaccine dosage showed p value <0.1 in the univariate logistic regression. Furtherly, Diabetes, CVD, BUN, Cr, Na, UA, CRP, and vaccine dosage were confirmed as the independent risk factors for proteinuria in the multivariate model (). Because of the collinearity of BUN and Cr, we retain the BUN with a larger OR and a total of 7 parameters were included in the final scoring model.
Table 2. Logistic regression analysis of variables assessing proteinuria in derivation cohort.
Construction of the risk score
showed the point assignment for assessing variables in the derivation cohort. The score value of Diabetes was 5, CVD was 4, BUN >4.6 mmol/L was 3, Na >141.4 mmol/L was 2, UA >395.9 mmol/L was 3, CRP >9.8 mg/L was 4, unvaccinated and one dose of vaccination were 0, two doses of vaccination was −4 and three doses of vaccination was −5. According to the generated scoring system, we obtained the final proteinuria risk score of each patient in the derivation cohort. The score ranged from − 5 to 16. The higher the score, the higher the risk of proteinuria after Omicron infection. The ROC curve was drawn with the score as a parameter to assess the occurrence of proteinuria and AUC is 0.8326(95% CI 0.7816 to 0.8835, p < 0.0001)which meant good predictive ability ().
Figure 1. ROC curve of the risk model for the derivation and validation cohort.
Area under the ROC curve to determine the assessing ability of the model in two cohorts, respectively, representing the sensitivity on the ordinate axis and specificity in the abscissa. Blue represents ROC curve of the Derivation cohort. Red represents ROC curve of the Validation cohort.
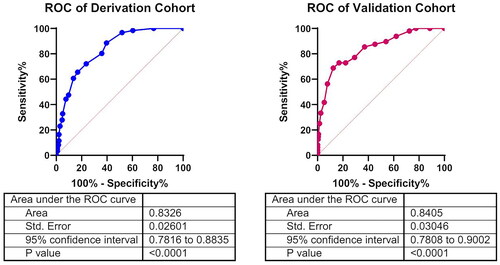
Table 3. The point assignment for assessing variables in the derivation cohort.
External validation of risk score
Taking the same methods, we obtained the final proteinuria risk score of each patient in the validation cohort and ROC curve was plotted. Similarly to that observed in the derivation cohort, the AUC is 0.833(95% CI 0.7808 to 0.9002, p < 0.0001), which verified good assessing ability in the validation cohort ().
Performance of the risk score in the derivation and validation cohorts
The models’ assessing characteristics in terms of sensitivity and specificity for the derivation and validation cohort were shown in . According to different scores, the sensitivity and specificity of proteinuria risk score were listed.
Table 4. Diagnostic accuracy of the assessing score for after omicron proteinuria in derivation cohort and validation cohort.
Discussion
Kidney involvement secondary to Omicron infection is common [Citation14]. Proteinuria can occur alone or in combination with AKI [Citation11,Citation15]. Up to present, the infection of Omicron COVID-19 has been like common respiratory viruses. However, patients with proteinuria have a significantly higher risk of mortality [Citation16]. Early detection of proteinuria is a meaningful study. Hence, we aimed to develop and validate a weighted score model to assess the occurrence of proteinuria in Omicron patients. To this end, we retrospectively analyzed a single-center derivation cohort to get the “Omicron related proteinuria risk model” including 7 parameters, covering four major aspects: comorbidities, biochemical indicators, inflammatory indicators, and vaccine dosage. Then, we verified the result in an independent validation cohort. The scoring model derived in our study showed a good assessing ability verified in both derivation and validation cohorts.
COVID-19 severity seems to correlate with previous conditions such as hypertension and diabetes mellitus (DM) [Citation17]. DM is one of the most common comorbidities in COVID-19 patients and related to poor outcomes [Citation18,Citation19]. Patients with DM have a higher incidence of proteinuria and higher level of CRP on admission [Citation20]. The susceptibility of severe disease in DM patients may be attributed to several mechanisms including elevated viral replication [Citation21], suppressed immune response [Citation22] induced by hyperglycemia and having more comorbidities. In the present study, combined DM and CVD were risk factors for proteinuria in Omicron patients. However, we could not identify that the proteinuria in our case series were signs of Omicron infection as early renal damage caused by DM or CVD may be ignored before admission, although CKD participants were excluded in advance.
Serum UA, BUN, and creatinine are the blood biochemistry indexes directly reflecting kidney function. A study revealed the incidence of elevated creatinine and BUN in hospitalized COVID-19 patients was 15.5% and 14.1%, respectively [Citation23]. Another study revealed that hematuria, proteinuria, elevated baseline serum creatinine and BUN and decrease baseline eGFR were independent risk factors for disease progression after adjusted confounders. Meanwhile, the dynamic trajectories of UA were significantly related to disease progression [Citation24]. In our study, although no AKI occurred in derivation cohort, BUN and eGFR in the proteinuria group were significantly different from those in no proteinuria group. Elevated serum BUN and creatinine were independent risk factors of proteinuria in Omicron patients.
Elevated serum UA often leads to endothelial injury [Citation25], which essentially through inflammatory reaction and oxidative stress [Citation26]. Jia et al. found that blood UA even just exceeding physiological value will lead to inflammatory reaction [Citation27]. It is universally acknowledged that endothelial damage exists with COVID-19 infection and may cause albuminuria [Citation28–30]. From this point of view, when COVID19 encounters higher serum UA level, the incidence of proteinuria will greatly increase.
The relationship between elevated serum sodium and proteinuria has not been reported. We speculate that the increase of blood sodium means insufficient intake and reduced effective blood volume, resulting in renal hypo-perfusion and renal injury secondary to hemodynamic changes, and therefore the occurrence of proteinuria.
In acute severe COVID-19, patients present with lung inflammation and AKI, accompanied by an exaggerated cytokine response [Citation31]. Cytokine storm may cause kidney immune inflammatory damage and endothelial dysfunction [Citation32]. Mortality due to COVID-19 has been proved to be correlated with laboratory markers of inflammation, such as CRP [Citation33]. Rozanovic believed that inflammatory markers were especially worthy of considering as further prognostic markers [Citation34]. However, even in COVID19 patients with mild symptoms, the inflammatory index are usually increased which may aggravates the immune inflammatory reaction, and then lead to proteinuria. Research showed that in asymptomatic/mild type patients, the IL-6 and CRP concentrations were significantly higher compared with the reference interval [Citation35]. Our previous study also found that the first batch of Omicron patients in Tianjin had elevated serum CRP with mild clinical symptoms [Citation7]. This study is no exception. In the derivative cohort composed of asymptomatic and mild symptoms, CRP is still higher than the normal value. Meanwhile, CRP in the proteinuria group was significantly higher than that in the non- proteinuria group. CRP >9.8 mg/L was included into proteinuria risk score model.
DM, CVD, elevated serum UA and inflammation, seemingly unrelated disease states, actually affect, correlate and aggravate each other, ultimately, through the damage of the vascular endothelium, inducing the occurrence of proteinuria. Omicron infection magnifies the interaction of the disease states, thereby increases the risk of proteinuria.
There is a significant and meaningful result in the current study, that is, the dosage of vaccine reception is a protective factor for proteinuria. The more inoculants, the stronger preventive effect. In fact, it has been confirmed that the protection of the vaccine is reflected in improving the prognosis of the overall course of disease. A recent study in the United States reported that the risks of COVID-19 were consistently the highest for unvaccinated persons. COVID-19 vaccine had a protective effect on SARS CoV-2 infection and COVID-19 associated hospitalization, and most protective among fully vaccinated persons or those with a booster [Citation36]. A study on 1090 participants in Shanghai, China discovered that vaccination of over 2 doses could shorten viral shedding time and provided protection of illness progression [Citation37] which was consistent with several previous studies [Citation38,Citation39]. Evidence from Malaysia suggested that the full vaccination had been highly effective in preventing COVID-19 infections, ICU admissions, and death [Citation40]. Even for older adults, the use of booster doses of vaccine was also supported [Citation41]. Compared with patients during the period of Delta predominance, Omicron-period patients had less severe illness, largely driven by an increased proportion who were fully vaccinated [Citation42]. From the perspective of mechanism, booster vaccination provided higher antibody titers and T cell response, consequently, sustained protection against severe outcomes following infection with the Omicron variant [Citation41,Citation43]. However, the effect of vaccine on proteinuria has not been reported. Our data support for the first time that enough dosage of vaccine can effectively reduce the onset of proteinuria. This is a reminder to us that COVID-19 vaccination, particularly a booster dose, continues to be critical in mitigating the health care burden of the Omicron variant.
The advantage of this study is that there are two groups of Omicron cohorts in different periods, and the sample size of the derived queue is large, and the verification method is external verification, which greatly increases the effectiveness of the risk model. Our study had some limitations. First, the population of the study was homogenous; all patients were Chinese. Thus, extrapolation of the results on other populations may be biased. Second, the generalization of the obtained results may be biased due to the retrospective single-center design. The presented findings should be confirmed in prospective multicenter trials. Third, our study was a retrospective study that utilized existing data including the urine test results (semi quantitative urine protein detection) of Omicron patients who underwent routine examinations on admission. Because it was not a prospective study designed by nephrologists and the patients were not in a nephrology ward, there were no ACR/PCR results. So, the confirmation of proteinuria was based on semi quantitative detection rather than ACR/PCR results.
Conclusions
In this study, a risk model of proteinuria after Omicron infection was constructed using simple and easily accessible clinical data. 7 parameters included suggested that clinicians should focus on diabetes, CVD, serum BUN, UA, Na, CRP, and vaccination dosage when facing Omicron patients. The risk model has better assessing efficiency, which is helpful for early and accurate warning, recognition, diagnosis, and intervention of kidney involvement. Vaccination is a protective factor. It is necessary to support the public to be vaccinated on time to minimize the kidney injury of Omicron.
Acknowledgments
We thank everyone involved in medical care and epidemic prevention for their tireless efforts during the pandemic. We thank everyone at Tianjin First Central Hospital.
Disclosure statement
The authors declare no potential conflict of interest. The funding sponsor did not take part in the design of the study and collection, analysis, and interpretation of data, and in writing the manuscript.
Additional information
Funding
References
- Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27(1):28–33. doi: 10.1038/s41591-020-01202-8.
- Motavalli R, Abdelbasset WK, Rahman HS, et al. The lethal internal face of the coronaviruses: kidney tropism of the SARS, MERS, and COVID19 viruses. IUBMB Life. 2021;73(8):1005–1015. doi: 10.1002/iub.2516.
- Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400.
- Joseph A, Zafrani L, Mabrouki A, et al. Acute kidney injury in patients with SARS-CoV-2 infection. Ann Intensive Care. 2020;10(1):117. doi: 10.1186/s13613-020-00734-z.
- Martinez-Rojas MA, Vega-Vega O, Bobadilla NA. Is the kidney a target of SARS-CoV-2? Am J Physiol Renal Physiol. 2020;318(6):F1454–F1462. doi: 10.1152/ajprenal.00160.2020.
- Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods—United States, December 2020–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146–152. doi: 10.15585/mmwr.mm7104e4.
- Teng LB, Chang WX. The investigation of kidney involvement in 430 hospitalized patients with omicron COVID-19 in Tianjin, China. Blood Purif. 2023;52(5):437–445. doi: 10.1159/000528734.
- Zhang LX, Long JY, Jiang WS, et al. Trends in chronic kidney disease in China. N Engl J Med. 2016;375(9):905–906. doi: 10.1056/NEJMc1602469.
- Cheng YC, Luo R, Wang K, et al. kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005.
- Perico L, Benigni A, Casiraghi F, et al. Immunity, endothelial injury, and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol. 2021;17(1):46–64. doi: 10.1038/s41581-020-00357-4.
- Pei GC, Zhang ZG, Peng J, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31(6):1157–1165. doi: 10.1681/ASN.2020030276.
- World Health Organization. Laboratory testing strategy recommendations for COVID‐19: interim guidance; 2020. World Health Organization. https://apps.who.int/iris/handle/10665/331509.
- National Health Commission of the People’s Republic of China. Chinese recommendations for diagnosis and treatment of novel coronavirus (SARSCoV2) infection (Trial 8th version). Chin J Clin Infect Dis. 2020;13(5):321–328.
- Zheng XZ, Yang HY, Li XL, et al. Prevalence of kidney injury and associations with critical illness and death in patients with COVID-19. Clin J Am Soc Nephrol. 2020;15(11):1549–1556. doi: 10.2215/CJN.04780420.
- Portolés J, Marques M, López-Sánchez P, et al. chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrol Dial Transplant. 2020;35(8):1353–1361. doi: 10.1093/ndt/gfaa189.
- Nlandu Y, Mafuta D, Sakaji J, et al. Predictors of mortality in COVID-19 patients at Kinshasa Medical Center and a survival analysis: a retrospective cohort study. BMC Infect Dis. 2021;21(1):1272. doi: 10.1186/s12879-021-06984-x.
- Kania M, Mazur K, Terlecki M, et al. Characteristics, mortality, and clinical outcomes of hospitalized patients with COVID-19 and diabetes: a reference single-center cohort study from Poland. Int J Endocrinol. 2023;2023:8700302–8700311. doi: 10.1155/2023/8700302.
- Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020.
- Wang DW, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585.
- Guo T, Shen QX, Ouyang XL, et al. Clinical findings in diabetes mellitus patients with COVID-19. J Diabetes Res. 2021;2021:7830136–7830137. doi: 10.1155/2021/7830136.
- Hill MA, Mantzoros C, Sowers JR. Commentary: COVID-19 in patients with diabetes. Metabolism. 2020;107:154217. doi: 10.1016/j.metabol.2020.154217.
- Seminog OO, Goldacre MJ. Risk of pneumonia and pneumococcal disease in people hospitalized with diabetes mellitus: English record-linkage studies. Diabet Med. 2013;30(12):1412–1419. doi: 10.1111/dme.12260.
- Yang XB, Yu Y, Xu JQ, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5.
- Xia TT, Zhang WJ, Xu Y, et al. Early kidney injury predicts disease progression in patients with COVID-19: a cohort study. BMC Infect Dis. 2021;21(1):1012. doi: 10.1186/s12879-021-06576-9.
- Yin W, Zhou QL, Ouyang SX, et al. Uric acid regulates NLRP3/IL-1β signaling pathway and further induces vascular endothelial cells injury in early CKD through ROS activation and K + efflux. BMC Nephrol. 2019;20(1):319. doi: 10.1186/s12882-019-1506-8.
- Cai W, Duan XM, Liu Y, et al. Uric acid induces endothelial dysfunction by activating the HMGB1/RAGE signaling pathway. Biomed Res Int. 2017;2017:4391920–4391911. doi: 10.1155/2017/4391920.
- Jia SD, Wang YG, Li HF, et al. Oxidative stress and endothelial dysfunction at different serum uric acid levels. Zhonghua Nei Ke Za Zhi. 2008;47(8):638–641.
- Pelle MC, Zaffina I, Lucà S, et al. Endothelial dysfunction in COVID-19: potential mechanisms and possible therapeutic options. Life (Basel). 2022;12(10):1605–1628. doi: 10.3390/life12101605.
- Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41(32):3038–3044. doi: 10.1093/eurheartj/ehaa623.
- Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3.
- Melhorn J, Alamoudi A, A Mentzer J, et al. Persistence of inflammatory and vascular mediators 5 months after hospitalization with COVID-19 infection. Front Med (Lausanne). 2023;10:1056506. doi: 10.3389/fmed.2023.1056506.
- Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14(5):315–328. doi: 10.1038/nri3665.
- Park C, Tavakoli-Tabasi S, Sharafkhaneh A, et al. Inflammatory biomarkers differ among hospitalized veterans infected with alpha, delta, and omicron SARS-CoV-2 variants. Int J Environ Res Public Health. 2023;20(4):2987. doi: 10.3390/ijerph20042987.
- Rozanovic M, Domokos K, Márovics G, et al. Can we predict critical care mortality with non-conventional inflammatory markers in SARS-CoV-2 infected patients? Clin Hemorheol Microcirc. 2023;84(1):71–82. doi: 10.3233/CH-231697.
- Trofin F, Nastase EV, Vâță A, et al. The immune, inflammatory and hematological response in COVID-19 patients, according to the severity of the disease. Microorganisms. 2023;11(2):319. doi: 10.3390/microorganisms11020319.
- Danza P, Koo TH, Haddix M, et al. SARS-CoV-2 infection and hospitalization among adults aged ≥18 years, by vaccination status, before and during SARS-CoV-2 B.1.1.529 (Omicron) variant predominance—Los Angeles County, California, November 7, 2021–January 8, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(5):177–181. doi: 10.15585/mmwr.mm7105e1.
- Chen XH, Wang HY, Ai JW, et al. Identification of CKD, bedridden history and cancer as higher-risk comorbidities and their impact on prognosis of hospitalized Omicron patients: a multi-center cohort study. Emerg Microbes Infect. 2022;11(1):2501–2509. doi: 10.1080/22221751.2022.2122581.
- Bernal JL, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;13(373):n1088. doi: 10.1136/bmj.n1088.
- Kaabi NA, Oulhaj A, Hosani AFI, et al. The incidence of COVID-19 infection following emergency use authorization of BBIBP-CORV inactivated vaccine in frontline workers in the United Arab Emirates. Sci Rep. 2022;12(1):490. doi: 10.1038/s41598-021-04244-1.
- Hamdan NEA, Fahrni ML, Lazzarino AI. COVID-19 vaccination prioritization strategies in Malaysia: A retrospective analysis of early evidence. Vaccines (Basel). 2023;11(1):48–62. doi: 10.3390/vaccines11010048.
- Fong CHY, Zhang XJ, Chen LL, et al. Effect of vaccine booster, vaccine type, and hybrid immunity on humoral and cellular immunity against SARS-CoV-2 ancestral strain and Omicron variant sublineages BA.2 and BA.5 among older adults with comorbidities: a cross sectional study. EBioMedicine. 2023;88:104446. doi: 10.1016/j.ebiom.2023.104446.
- Modes ME, Directo MP, Melgar M, et al. Clinical characteristics and outcomes among adults hospitalized with laboratory-confirmed SARS-CoV-2 infection during periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant predominance—One Hospital, California, July 15–September 23, 2021, and December 21, 2021–January 27, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(6):217–223. doi: 10.15585/mmwr.mm7106e2.
- Stirrup O, Shrotri M, Adams NL, et al. Clinical effectiveness of SARS-CoV-2 booster vaccine against omicron infection in residents and staff of long-term care facilities: a prospective cohort study (VIVALDI). Open Forum Infect Dis. 2023;10(1):ofac694. doi: 10.1093/ofid/ofac694.
