Abstract
Objectives
The aim of this study was to determine the strength of the association between frailty and adverse outcomes in patients undergoing maintenance hemodialysis.
Design
A systematic review and meta-analysis.
Setting and participants
Patients aged ≥18 years who were undergoing maintenance hemodialysis.
Methods
PubMed, Web of Science, Embase, the Cochrane Library, Scopus, the China Knowledge Resource Integrated Database, the Wanfang Database and the Weipu Database were searched from inception until 11 April 2024. The reviewers independently selected the studies, extracted the data and evaluated the quality of the studies. Stata 15.1 software was used to perform the meta-analysis.
Results
A total of 36 articles were included in this study, including 56,867 patients. The primary outcome events in this study were mortality, hospitalization, and vascular access events. The secondary outcomes were depression, cognitive impairment, falls, fracture, sleep disturbances, and quality of life. This study suggested that frailty was associated with mortality in patients undergoing maintenance hemodialysis [hazard ratio (HR), 1.97; 95% CI, 1.62–2.40]. Frailty increased the risk of mortality in patients [odds ratio (OR), 2.33; 95% CI, 1.47–3.68]. In addition, we found that frailty was significantly associated with hospitalization in patients undergoing maintenance hemodialysis (OR, 2.47; 95% CI, 1.52–4.03). Patients who were undergoing maintenance hemodialysis and who were frail had a greater risk of hospitalization [RR, 1.47; 95% CI, 1.05–2.08] and emergency visits (RR, 2.28; 95% CI, 1.78–2.92). The results of this study also suggested that frailty was associated with a greater risk of vascular access events (HR, 1.72; 95% CI, 1.50–1.97). Finally, frailty increased the risk of depression (OR, 4.31; 95% CI, 1.83–10.18), falls and fractures, and reduced quality of life.
Conclusions
The findings of this study suggested that frailty was an important predictor of adverse outcomes in patients undergoing maintenance hemodialysis. In the future, medical staff should regularly evaluate signs of weakness, formulate individual diagnosis and treatment plans, adjust dialysis plans according to the patient’s condition, and reduce the occurrence of adverse events.
Registration
The study protocol was registered on PROSPERO (https://www.crd.york.ac.uk/PROSPERO/, number: CRD42023486239).
Introduction
Maintenance hemodialysis (MHD) is an important renal replacement therapy for patients with end-stage renal disease. According to a 2019 research survey [Citation1], approximately 11,797 patients received dialysis in China, 90.96% of whom had undergone MHD. Due to long-term dialysis treatment, patients on MHD lose large amounts of protein and bound amino acids [Citation2]. When these losses are combined with prolonged periods of sitting or lying down, it leads to impaired muscle synthesis and decreased physical fitness, which can easily result in frailty [Citation3]. Frailty is a medical syndrome characterized by a decline in physiological reserves or multiple-system dysfunction, leading to increased vulnerability and decreased stress resistance in the body [Citation4]. Many studies [Citation5–7] have explored associations between frailty and adverse outcomes, including mortality, hospitalization, disability and falls. Adverse outcomes related to frailty can have a negative impact on patients undergoing MHD.
There is a greater incidence of frailty (42.27%) in patients on MHD [Citation8]. Another meta-analysis showed that the prevalence of MHD patients was 46% [Citation9]. Frailty is an important risk factor for bone loss in MHD patients, which increases the risk of fractures or falls in MHD patients and extends hospital stays [Citation10,Citation11]. In addition, frailty is associated with mortality in patients undergoing MHD, reduces patients’ self-care ability, and significantly affects their quality of life [Citation12,Citation13]. Therefore, it is necessary to investigate the relationship between frailty and adverse outcomes in patients undergoing maintenance hemodialysis. Through a systematic review and meta-analysis, we summarized the associations between frailty and adverse outcomes in patients undergoing maintenance hemodialysis, aiming to provide a reference for clinical application.
Methods
Design
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Citation14]. The PRISMA checklist is shown in Supplementary Table 1. This study has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42023486239).
Search strategy
The researchers systematically searched PubMed, Web of Science, Embase, the Cochrane Library, Scopus, the China Knowledge Resource Integrated Database (CNKI), the Wanfang Database and the Weipu Database (VIP) from inception until 11 April 2024. In this study, subject terms and free terms were used for the search. The search terms included the following: frailty, frail or frailness, frailty syndrome, debility, frail, prefrail, renal dialysis, hemodialysis, extracorporeal, extracorporeal dialysis, intradialytic, etc. The search strategy is shown in Supplementary Table 2.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) patients undergoing MHD; (2) people aged 18 years or older; (3) cohort studies, longitudinal studies or cross-sectional studies; (4) reports of hazard ratios (HRs), odds ratios (ORs), or risk ratios (RRs) with 95% CIs; and (5) a diagnosis of frailty that was not limited but was clearly defined. The Fried Phenotype (FP) assessment included weight loss, slowness, exhaustion, low physical activity, and weakness, in which ≥3 items indicated frailty, 1–2 items indicated prefrailty, and 0 items indicated robustness [Citation15].
The FRAIL scale consists of five components (fatigue, resistance, ambulation, illness, and loss of weight), with results categorized as robust (0 items), prefrail (1–2 items), and frail (≥3 items) [Citation16]. The Frailty Index (FI) is based on the deficit accumulation model, where the types and number of deficits selected may vary depending on the study (≥30). It categorizes individuals into robust (<0.12), prefrail (0.12–0.25), and frail (>0.25) states [Citation17,Citation18]. The Edmonton Frailty Scale (EFS) consists of 9 dimensions, namely, cognition (1 item), health status (2 items), independent living ability (1 item), social support (1 item), medication (2 items), nutritional status (1 item), emotion (1 item), incontinence (1 item) and activity ability (1 item), with a total score ranging from 0 to 17. The participants were divided into light, moderate and severe frailty groups. The Hospital Frailty Risk Score (HFRS) uses a pooled score calculated from 109 ICD-10 codes associated with frailty to classify patients as having a low (<5), medium (5–15), or high (>15) risk of frailty [Citation19]. When the same cohort was used in multiple studies, the study with the most complete or largest amount of participant data was selected.
The exclusion criteria were as follows: (1) patients who underwent peritoneal dialysis or kidney transplantation; (2) low-quality literature; (3) studies with incomplete data; and (4) non-English language literature.
Study selection and data extraction
Two researchers (MC and LPN) independently searched and selected studies and then extracted data for cross-examination (κ = 0.8). If a disagreement arose during the selection process, a third party (HYG) was consulted to resolve the issue. After reading the article title and abstract, studies that clearly did not meet the acceptance criteria were excluded. The following data were extracted: study author, year of publication, country, age, study design, prevalence of frailty, sample size, proportion of women, frailty criteria, follow-up time, study effect measure, outcome indicators, and quality grade score.
Quality assessment
We used the New Castle Ottawa Scale (NOS) [Citation20] to assess the quality of the included longitudinal and cohort studies. The NOS assesses the risk of bias by evaluating the quality of each study on three dimensions: selection, comparability, and outcome. The total possible score was 9 points. A score of 7–9 was considered high-quality research, 4–6 was considered moderate-quality research, and less than 4 was considered low-quality research. The cross-sectional studies included were evaluated using the Agency for Healthcare Research and Quality (AHRQ) [Citation21]. The AHRQ included an 11-item checklist. A score of 8–11 was considered high-quality research, 4–7 was considered moderate-quality research, and less than 4 was considered low-quality research. The quality of the literature was independently assessed by two people (MC and HCL), and in cases of disagreement, a third party (QL) was consulted.
Statistical analysis
The primary outcomes of this study were mortality, hospitalization and vascular access events. Other data were treated as secondary outcome indicators. The clinical outcome of frailty was estimated using the HR, OR or RR along with the 95% CI. Stata 15.1 was used for statistical analysis and graph generation. The statistical heterogeneity between studies was examined with Cochran’s Q and I2 tests. We defined heterogeneity >50% as a statistically significant difference. When statistically significant heterogeneity was found between the included studies, a random effects model was used to determine the effect size; otherwise, a fixed effects model was used. Subgroup analysis was used to detect heterogeneity. Sensitivity analysis was performed by excluding individual studies. Funnel plots were constructed, and Begg’s test and Egger’s test were used to test publication bias. When publication bias was identified, the trim-and-fill method was used.
Results
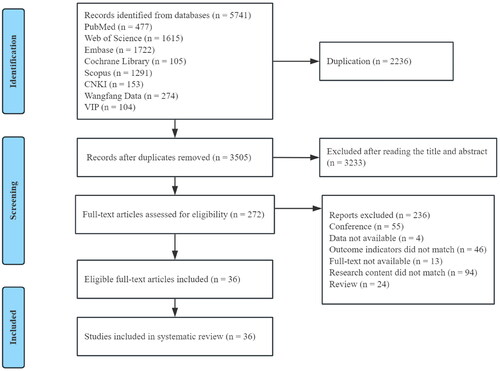
In this study, a total of 5741 studies were retrieved. After removing 2236 duplicates, 3505 studies were reviewed for eligibility. We selected 272 articles after reading the title and abstract. After reading the full texts of the 272 studies, 236 were excluded, and 36 studies were ultimately included. The flow chart of the research screening process is shown in .
Study characteristics and quality assessment
A total of 36 studies were included in this meta-analysis, including 56,867 participants. The study characteristics are presented in . The proportion of women ranged from 30.7 to 57.3%. Studies were conducted in Asia [Citation12,Citation22–35], America [Citation11,Citation36–47], Oceania [Citation48,Citation49] and Europe [Citation13,Citation50–54]. A total of five frailty assessment tools were used in this study, including FP [Citation11,Citation12,Citation22–28,Citation30–33, Citation35–46,Citation49–53], FI [Citation13], FRAIL [Citation29,Citation34,Citation47], EFS [Citation54], and HFRS [Citation48]. In addition, this study included 27 cohort studies [Citation11,Citation12,Citation24–28,Citation31–36,Citation38,Citation40–46,Citation48–53], three longitudinal studies [Citation13,Citation47,Citation54], and six cross-sectional studies [Citation22,Citation23,Citation29,Citation30,Citation37,Citation39] with follow-up periods ranging from three months to five years. The quality of each study was evaluated according to the assessment criteria of the NOS and the AHRQ. Twenty-three studies were considered high quality, and 13 were considered moderate quality. The quality assessment results are shown in Supplementary Tables 3 and 4.
Table 1. Characteristics and quality assessment of the included studies.
Table 3. Subgroup analysis of the association between frailty and hospitalization in patients undergoing maintenance hemodialysis.
Primary outcomes
Mortality
Meta-analysis of studies using OR
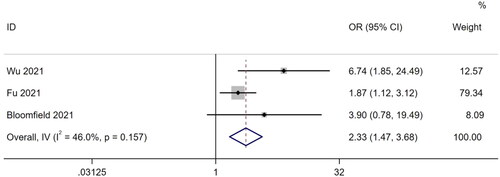
A total of three studies [Citation30,Citation32,Citation49] reported ORs of mortality. There was little heterogeneity among the studies (I2 = 46%, p = .157), so a fixed-effects model was used for the meta-analysis. The results showed that frailty increased the mortality of patients undergoing MHD by 2.33 times (OR, 2.33; 95% CI, 1.47–3.68; p < .001). The results are shown in .
Meta-analysis of studies using HR
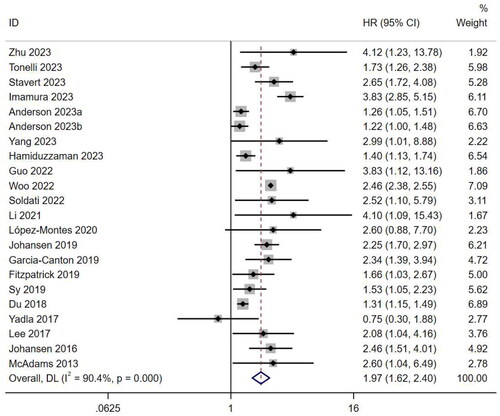
Twenty-two studies [Citation11–13,Citation22,Citation24,Citation25,Citation27,Citation31,Citation33,Citation35,Citation36,Citation38,Citation40–43,Citation46,Citation48,Citation50–52,Citation54] reported the HR as a risk measure of mortality. There was high heterogeneity among the studies (I2 = 90.4%, p < .001), so a random effects model was used for analysis. Frailty was closely associated with mortality in patients undergoing MHD (HR, 1.97; 95% CI, 1.62–2.40; p < .001) ().
Hospitalization
Meta-analysis of studies using OR and HR
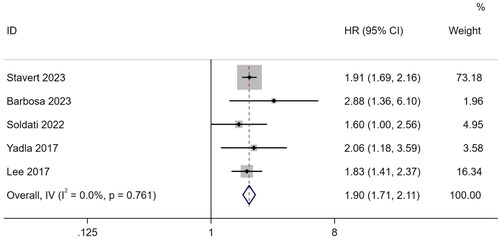
Three studies [Citation25,Citation30,Citation49] reported ORs for hospitalization. The results showed that frailty significantly increased the risk of hospitalization in patients undergoing MHD (OR, 2.47; 95% CI, 1.52–4.03; I2 = 24.7%; p < .001). Five studies provided HR as a risk measure for hospitalization [Citation12,Citation13,Citation35,Citation47,Citation48]. Based on the heterogeneity test (I2 = 0.0%, p = .761), a fixed-effects model was used for analysis. The results showed that the incidence of hospitalization in frail patient undergoing MHD was 1.90 times greater than that in nonfrail patients (HR, 1.90; 95% CI, 1.71–2.11; p < .001) ().
Meta-analysis of studies using RR
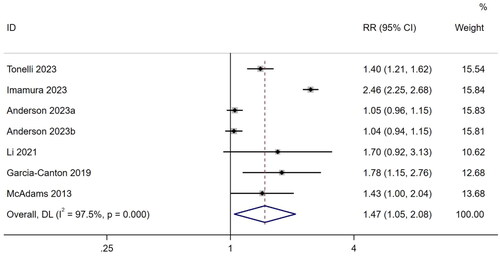
Seven studies [Citation11,Citation24,Citation31,Citation46,Citation50,Citation51,Citation54] reported the RR as a risk measure for hospitalization. A random-effects model was used for the meta-analysis based on heterogeneity (I2 = 97.5%, p < .001). The results showed that frailty increased hospitalization risk by 1.47 times in patients undergoing MHD (RR, 1.47; 95% CI, 1.05–2.08; p = .027). The results are shown in . In addition, three studies [Citation22,Citation31,Citation54] reported RRs for emergency visits. Combined rates of emergency visits were significantly greater in patients with frailty (RR, 2.28; 95% CI, 1.7–2.92; I2 = 7.3%, p < .001) (Supplementary Figure 1).
Vascular access events
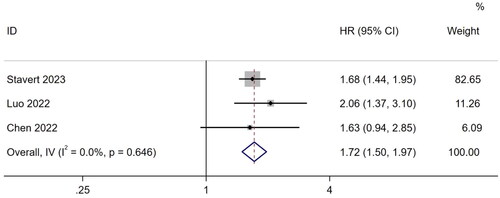
Three studies [Citation26,Citation28,Citation48] described the association between frailty and vascular access events. A meta-analysis using a fixed-effects model showed that frail patients undergoing MHD were more likely to experience vascular access events (HR, 1.72; 95% CI, 1.50–1.97; I2 = 0.0%; p < .001) ().
Secondary outcomes
Depression
Four studies [Citation29,Citation30,Citation37,Citation40] described the association between frailty and depression in patients undergoing MHD. The studies suggested that frailty significantly increased the risk of depression in patients on MHD (OR, 4.31; 95% CI, 1.83–10.18; I2 = 54.9%; p = .001) (Supplementary Figure 2).
Falls
A total of six studies [Citation30,Citation31,Citation34,Citation35,Citation45,Citation53] described the relationship between frailty and falls in patients undergoing MHD. Of these, two studies [Citation30,Citation34] reported ORs for falls, with a combined value of 1.88 (95% CI, 0.72–4.94; p = .198). The results showed no significant associations. Three studies reported RRs for falls [Citation31,Citation45,Citation53]; the pooled incidence rate ratio was 2.42 (95% CI, 1.39–4.21; p = .002). This suggested that frailty in patients undergoing MHD was associated with falls. Another study [Citation36] showed that frail patients were 2.1 times more likely to fall than nonfrail patients (HR, 2.1; 95% CI, 1.21–3.92).
Fracture
Two studies [Citation39,Citation44] reported on the relationship between frailty and fractures. Jafari et al. [Citation39] showed no correlation between frailty and fracture in patients undergoing MHD (OR, 1.14; 95% CI, 0.26–5.94; p = .82). Another study [Citation44] showed that frail patients were 1.6 times more likely to experience falls and fractures than nonfrail patients were (HR, 1.60; 95% CI, 1.16–2.20; p<.01). Due to the limited number of included studies, more studies can be conducted in the future.
Cognitive impairment
One study [Citation51] described associations between frailty and cognitive impairment in patients undergoing MHD. The results showed no significant correlation between frailty and cognitive impairment in patients on MHD (HR, 1.19; 95% CI, 0.96–1.48; p=.11).
Sleep disturbances
A cross-sectional study [Citation23] of 360 patients undergoing MHD in Japan showed that frailty was associated with sleep disturbances (OR, 1.12; 95% CI, 1.05–1.20; p<.01).
Quality of life
One study [Citation30] described the relationship between frailty and quality of life. The results showed that frailty was negatively associated with quality of life in patients undergoing MHD (OR, 0.955; 95% CI, 0.925–0.987; p<.01).
Subgroup analysis
Mortality
A subgroup analysis was performed for studies that reported HR values of mortality. Subgroup analyses were based on frailty assessment tools, region, study design, sample size, follow-up time and female sex ratio. The results were as follows. Based on the frailty assessment tools, the FP group had the lowest mortality (HR, 1.91; 95% CI, 1.54–2.36; p < .001). According to the region, the European group had a lower mortality than the other groups (HR, 1.50; 95% CI, 1.16–1.93; p = .002). According to the study design, the cohort study group (HR, 1.91; 95% CI, 1.55–2.35; p < .001) had lower mortality than did the longitudinal study group (HR, 2.39; 95% CI, 1.54–3.72; p < .001) and cross-sectional study group (HR, 4.12; 95% CI, 1.23–13.78). In terms of sample size, the mortality in the ≥500 group (HR, 2.00; 95% CI, 1.62–2.47; p < .001) was significantly greater than that in the <500 group (HR, 1.94; 95% CI, 1.49–2.52; p < .001). Based on the follow-up time, mortality was greater in the ≥2 years group (HR, 1.99; 95% CI, 1.56–2.55; p < .001). According to the female ratio, the mortality of the ≥50% group (HR, 4.11; 95% CI, 1.68–10.03; p = .002) was lower than that of the <50% group (HR, 1.92; 95% CI, 1.57–2.34; p < .001). The results are shown in .
Table 2. Subgroup analysis of the association between frailty and mortality in patients undergoing maintenance hemodialysis.
Hospitalization
We performed a subgroup analysis of studies that provided hospitalization RR values. There were three subgroups: frailty assessment tools, region, and follow-up time. When the FP test was used to assess frailty, there was no significant association between frail patients undergoing MHD and hospitalization (RR, 1.43; 95% CI, 0.99–2.08; p = .057). In terms of region, there was a significant difference between frailty and hospitalization in the American group (RR, 1.40; 95% CI, 1.22–1.61; p < .001) and the Asian group (RR, 2.33; 95% CI, 1.79–3.02; p < .001), but there was no significant difference between frailty and hospitalization in the European group (RR, 1.09; 95% CI, 0.96–1.24; p = .202). According to the follow-up time, there was no association between frailty and hospitalization in the <2 years group (RR, 1.12; 95% CI, 0.97–1.29; p = .114) ().
Sensitivity analysis
We conducted a sensitivity analysis of 22 studies reporting mortality HRs. The random deletion of references in the study did not affect the results of the study, indicating that the calculation results of the random effects were stable (Supplementary Figure 3).
Publication bias
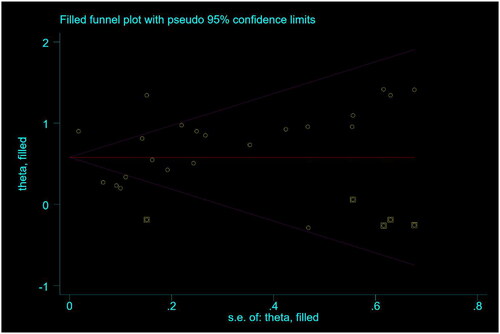
We constructed a funnel plot (Supplementary Figure 4) and performed Egger’s test (p = .151) and Begg’s test (p = .009) for 22 studies reporting mortality HRs. We found a slight bias; therefore, we performed a trim-and-fill test. The results showed no significant differences before (HR, 1.97; 95% CI, 1.62–2.40; p < .001) or after adjustment (HR, 4.59; 95% CI, 3.59–6.13; p < .001) (). Therefore, a large publication bias effect was excluded.
Discussion
A total of 36 studies were included in this study to explore the relationship between frailty and adverse outcomes in patients undergoing MHD to facilitate the development of clinical interventions. The results suggested that frailty increased the incidence of adverse outcome events in patients on MHD, including primary outcome events such as mortality, hospitalization, and vascular access events and secondary outcome events such as depression, cognitive impairment, falls, fracture, sleep disturbances, and reduced quality of life. In addition, subgroup analyses of the outcome indicators mortality (HR) and hospitalization (RR) were performed in this study.
Our study suggested that frailty in patients undergoing MHD was associated with mortality. HR and OR values of combined mortality showed that frail patients on MHD have a greater risk of mortality. This finding was consistent with the findings of Lee et al. [Citation9], who showed that frailty significantly increased the risk of mortality in patients undergoing MHD (HR, 2.02; 95% CI, 1.65–2.48). Another study [Citation55] also showed that frailty was associated with mortality in patients undergoing MHD (OR, 1.34; 95% CI, 1.04–1.72; p = .002). In addition, this study showed that frailty was associated with hospitalization in patients on MHD. Combining the HR, OR, and RR values of hospitalization, the results suggested that frailty increased the risk of hospitalization. A recent cohort study [Citation25] showed that frailty was an independent predictor of hospitalization in patients undergoing MHD (OR, 2.276; 95% CI, 1.034–5.010; p = .041). Another study [Citation12] revealed that frailty was associated with composite outcomes (all-cause mortality or cardiovascular hospitalization) in patients undergoing MHD (HR, 1.63; 95% CI, 1.01–2.65; p = .047). Our study suggested that frailty was associated with vascular access events in patients on MHD. Chao et al. [Citation56] reported that patients with frailty had a 2.6-fold greater risk of vascular access disturbances than nonfrail patients. Another study [Citation57] showed that frail patients undergoing MHD often exhibited vascular endothelial dysfunction due to chronic inflammation and oxidative stress, which is one of the causes of vascular access dysfunction. The most common cause of vascular access dysfunction is arteriovenous anastomosis stenosis [Citation58]. Oxidative stress markers, such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), induce progressive intimal proliferation and extracellular matrix deposition, leading to increased inflammation and adhesion and ultimately result in plaque or thrombus formation and vascular access dysfunction [Citation58–60]. In addition, there is evidence that oxidative stress and chronic inflammation are common pathological mechanisms of frailty and depression, and the two are closely related [Citation61,Citation62]. This finding was consistent with our study, which suggested that frailty increased the risk of depression in patients. Anderson et al. [Citation63] also showed that frailty was related to depression in patients undergoing MHD. Another study [Citation64] showed that frailty and depression can lead to disability, cognitive dysfunction, and even death. Therefore, it is necessary to perform frailty screening for patients undergoing MHD to reduce the occurrence of adverse events.
In this study, the prevalence of frailty ranged from 12.6 to 82%, which was associated with the use of different frailty assessment tools. At present, there is no uniform standard for frailty assessment tools. Our subgroup analysis revealed a relatively low risk of mortality in the FP group. This may be because FP is easy to apply and does not consider psychological and social factors. The FP assessment is one of the most widely used frailty assessment scales [Citation65]. A scoping review of 164 studies on frailty assessment tool use in dialysis and chronic kidney disease patients showed that the most commonly used frailty assessment tool was the FP [Citation66]. The FP assessment is easy to perform and has high flexibility, which helps to identify prefrailty and predict adverse outcomes in patients. However, the FP did not significantly differ between frail and hospitalized patients undergoing MHD, which we speculate may be due to the limited number of included studies.
Our subgroup analysis revealed that although the European region was not associated with hospitalization in patients undergoing MHD, mortality was significantly reduced. We hypothesized this was related to differences in eating habits. The European diet was favored over the Mediterranean diet [Citation67]. The Mediterranean diet is a healthy plant-based diet that improves inflammation, oxidative stress, and insulin resistance, which helps to delay frailty [Citation68,Citation69]. Another study [Citation70] revealed that following a Mediterranean diet can reduce the incidence of chronic kidney disease, improve kidney function, and reduce hemodialysis rates and mortality. In terms of sex, our subgroup analysis results indicated that the mortality rate was lower when a smaller proportion of patients were females (<50%). Another study showed that estrogen in women can delay the process of apoptosis and fibrosis and has a protective effect on the kidneys, which helps to slow frailty [Citation71]. Moreover, testosterone in men is thought to increase oxidative stress and activate the renin-angiotensin system, damaging kidney function and increasing dialysis rates [Citation72]. However, a large cohort study showed that women who received dialysis had a greater risk of mortality than men [Citation73]. This contradicted our results, and we speculated that the reason was related to the sample size included in the subgroup analysis. Further exploration of the relationship between sex and mortality should be conducted in the future. In addition, heterogeneity in mortality was associated with study design, sample size, and follow-up when analyzed using a random effects model.
Limitations of this study include the following: (a) the use of five frailty assessment tools in the literature may have had an impact on heterogeneity; (b) one of the cross-sectional studies that provided mortality OR values had a wide confidence interval, which may have led to an overestimation of the results; (c) the age span of the population included in this study was large, which may have influenced the results; (d) due to the limited number of included studies, this study only carried out sensitivity analysis on mortality as an outcome indicator; and (e) three types of research were included in this study (cross sectional studies, cohort studies, and longitudinal studies) and the study time ranged from three months to five years, which may have affected the stability of the results.
Conclusion
In this study, the adverse outcomes of frailty in patients undergoing MHD included mortality, hospitalization, vascular access events, depression, cognitive impairment, falls, fracture, sleep disturbances, and reduced quality of life. The results of this study suggested that frailty increases the incidence of adverse outcomes in patients undergoing MHD. Therefore, early identification of frailty in patients undergoing MHD is essential to help prevent adverse outcome events.
Geolocation information
China
Supplemental Material
Download Zip (336.4 KB)Acknowledgement
The authors wish to thank Mei He, from Mianyang Central Hospital, and Liping Ning, Haoyue Gan, Hangcheng Liu and Qin Liu from North Sichuan Medical College, for providing data support for this study. There is no funding.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zhang L, Zhao MH, Zuo L, et al. China kidney disease network (CK-NET) 2015 annual data report. Kidney Int Suppl. 2019;9(1):1–13. doi: 10.1016/j.kisu.2018.11.001.
- Fang W, Fuxu W, Hongwei Z. Progress of clinical research on protein energy consumption in maintenance hemodialysis patients. Chinese Blood Purif. 2019;18(02):127–130. doi: 10.3969/j.issn.1671-4091.2019.02.013.
- Wei Q, Zhaohua ZOU, Zihan Y, et al. Construction of risk prediction model for patients with maintenance hemodialysis with frailty and pre-frailty. Nurs Res. 2024;38(02):233–239. doi: 10.12102/j.issn.1009-6493.2024.02.007.
- Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433–441. doi: 10.2147/CIA.S45300.
- Lu YW, Chang CC, Chou RH, et al. Sex differences in the frailty phenotype and mortality in the I-Lan longitudinal aging study cohort. BMC Geriatr. 2024;24(1):182. doi: 10.1186/s12877-024-04785-w.
- Xu J, Xu W, Qiu Y, et al. Association of prefrailty and frailty with all-cause mortality, acute exacerbation, and hospitalization in patients with chronic obstructive pulmonary disease: a meta-analysis. J Am Med Dir Assoc. 2023;24(7):937–944.e933. doi: 10.1016/j.jamda.2023.03.032.
- Sawa R, Doi T, Tsutsumimoto K, et al. Overlapping status of frailty and fear of falling: an elevated risk of incident disability in community-dwelling older adults. Aging Clin Exp Res. 2023;35(9):1937–1944. doi: 10.1007/s40520-023-02474-z.
- Dandan W, Kanfei Y, Xuanxue Z. Comparison of performance of three machine learning algorithms for predicting fateful risk in maintenance hemodialysis patients. Nurs Res. 2024;38(01):8–16. doi: 10.12102/j.issn.1009-6493.2024.01.002.
- Lee HJ, Son YJ. Prevalence and associated factors of frailty and mortality in patients with end-stage renal disease undergoing hemodialysis: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(7):3471. doi: 10.3390/ijerph18073471.
- Yoneki K, Kitagawa J, Hoshi K, et al. Association between frailty and bone loss in patients undergoing maintenance hemodialysis. J Bone Miner Metab. 2019;37(1):81–89. doi: 10.1007/s00774-017-0898-4.
- Tonelli M, Wiebe N, Gill JS, et al. Frailty and clinical outcomes in patients treated with hemodialysis: a prospective cohort study. Kidney Med. 2023;5(8):100684. doi: 10.1016/j.xkme.2023.100684.
- Lee SW, Lee A, Yu MY, et al. Is frailty a modifiable risk factor of future adverse outcomes in elderly patients with incident end-stage renal disease? J Korean Med Sci. 2017;32(11):1800–1806. doi: 10.3346/jkms.2017.32.11.1800.
- Soldati A, Poggi MM, Azzolino D, et al. Frailty index and adverse outcomes in older patients in haemodialysis. Arch Gerontol Geriatr. 2022;101:104673. doi: 10.1016/j.archger.2022.104673.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4.
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16(7):601–608. doi: 10.1007/s12603-012-0084-2.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. doi: 10.1093/gerona/62.7.722.
- Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775–1782. doi: 10.1016/S0140-6736(18)30668-8.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z.
- Ma LL, Wang YY, Yang ZH, et al. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7(1):7. doi: 10.1186/s40779-020-00238-8.
- Shulin W. Longitudinal study of frailty and short-term adverse outcomes in elderly patients with maintenance hemodialysis [dissertation]. Shandong University of Chinese Medicine; 2021. doi: 10.27282/d.cnki.gsdzu.2021.000313.
- Yoshikoshi S, Yamamoto S, Suzuki Y, et al. Association between physical frailty and sleep disturbances among patients on hemodialysis: a cross-sectional study. Nephron. 2024;148(3):152–159. doi: 10.1159/000533418.
- Imamura K, Yamamoto S, Suzuki Y, et al. Prevalence, overlap, and prognostic impact of multiple frailty domains in older patients on hemodialysis. Arch Gerontol Geriatr. 2023;114:105082. doi: 10.1016/j.archger.2023.105082.
- Jafari M, Anwar S, Kour K, et al. T scores, FRAX, frailty phenotype, falls, and its relationship to fractures in patients on maintenance hemodialysis. Can J Kidney Health Dis. 2021;8:20543581211041184. doi: 10.1177/20543581211041184.
- Yang Y, Yang H, Diao Z, et al. Frailty and adverse outcomes after SARS-CoV-2 infection in elderly patients on maintenance hemodialysis: a cohort study. Clin Interv Aging. 2023;18:1937–1948. doi: 10.2147/CIA.S429226.
- Guojuan Z, Jianyun X, Lili S, et al. Influence of frailty on prognosis of elderly hemodialysis patients. International Journal of Urology. 2023;43(5):874–877. doi: 10.3760/cma.j.cn431460-20220321-00223.
- Luo CM, Hsieh MY, Cheng CH, et al. Association of frailty with thrombosis of hemodialysis vascular access: a prospective Taiwanese cohort study. Am J Kidney Dis. 2022;80(3):353–363.e351. doi: 10.1053/j.ajkd.2021.12.017.
- Guo Y, Tian R, Ye P, et al. Frailty in older patients undergoing hemodialysis and its association with all-cause mortality: a prospective cohort study. Clin Interv Aging. 2022;17:265–275. doi: 10.2147/CIA.S357582.
- Chen CH, Hsieh YL, Chuang SY, et al. The impact of frailty on the outcomes of hemodialysis vascular access. Acta Cardiol Sin. 2022;38(1):29–38. doi: 10.6515/ACS.202201_38(1).20210711A.
- Chi CY, Lee SY, Chao CT, et al. Frailty as an independent risk factor for depression in patients with end-stage renal disease: a cross-sectional study. Front Med. 2022;9:799544. doi: 10.3389/fmed.2022.799544.
- Li Y, Zhang D, Ma Q, et al. The impact of frailty on prognosis in elderly hemodialysis patients: a prospective cohort study. Clin Interv Aging. 2021;16:1659–1667. doi: 10.2147/CIA.S329665.
- Fu W, Zhang A, Ma L, et al. Severity of frailty as a significant predictor of mortality for hemodialysis patients: a prospective study in China. Int J Med Sci. 2021;18(14):3309–3317. doi: 10.7150/ijms.51569.
- Du LY, Hu X, Wang XH, et al. Frailty in 380 patients with maintenance hemodialysis and its influence on prognosis. J Nurs. 2018;25(20):37–41. doi: 10.16460/j.issn1008-9969.2018.20.037.
- Lin Z, Zhenghong W, Yi W, et al. Incidence of frailty in maintenance hemodialysis patients and its effect on falls. Modern Clin Care. 2017;16(11):9–13. doi: 10.3969/j.issn.1671-8283.2017.11.003.
- Yadla M, John JP, Mummadi M. A study of clinical assessment of frailty in patients on maintenance hemodialysis supported by cashless government scheme. Saudi J Kidney Dis Transpl. 2017;28(1):15–22. doi: 10.4103/1319-2442.198102.
- Hamiduzzaman A, Wu R, Murray V, et al. Comparing the Fried frailty phenotype versus the Veterans Affairs frailty index among dialysis dependent patients. Hemodial Int. 2023;27(4):444–453. doi: 10.1111/hdi.13101.
- Santos D, Ferreira LGS, Pallone JM, et al. Association between frailty and depression among hemodialysis patients: a cross-sectional study. Sao Paulo Med J. 2022;140(3):406–411. doi: 10.1590/1516-3180.2021.0556.R1.14092021.
- Woo K, Gascue L, Norris K, et al. Patient frailty and functional use of hemodialysis vascular access: a retrospective study of the US renal data system. Am J Kidney Dis. 2022;80(1):30–45. doi: 10.1053/j.ajkd.2021.10.011.
- Sy J, McCulloch CE, Johansen KL. Depressive symptoms, frailty, and mortality among dialysis patients. Hemodial Int. 2019;23(2):239–246. doi: 10.1111/hdi.12747.
- Johansen KL, Delgado C, Kaysen GA, et al. Frailty among patients receiving hemodialysis: evolution of components and associations with mortality. J Gerontol A Biol Sci Med Sci. 2019;74(3):380–386. doi: 10.1093/gerona/gly206.
- Fitzpatrick J, Sozio SM, Jaar BG, et al. Frailty, body composition and the risk of mortality in incident hemodialysis patients: the predictors of arrhythmic and cardiovascular risk in end stage renal disease study. Nephrol Dial Transplant. 2019;34(2):346–354. doi: 10.1093/ndt/gfy124.
- Johansen KL, Dalrymple LS, Glidden D, et al. Association of performance-based and self-reported function-based definitions of frailty with mortality among patients receiving hemodialysis. Clin J Am Soc Nephrol. 2016;11(4):626–632. doi: 10.2215/CJN.03710415.
- Delgado C, Shieh S, Grimes B, et al. Association of self-reported frailty with falls and fractures among patients new to dialysis. Am J Nephrol. 2015;42(2):134–140. doi: 10.1159/000439000.
- McAdams-DeMarco MA, Suresh S, Law A, et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol. 2013;14(1):224. doi: 10.1186/1471-2369-14-224.
- McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896–901. doi: 10.1111/jgs.12266.
- Barbosa EMS, Pereira AG, Mori V, et al. Comparison between FRAIL Scale and Clinical Frailty Scale in predicting hospitalization in hemodialysis patients. J Nephrol. 2023;36(3):687–693. doi: 10.1007/s40620-022-01532-5.
- Stavert B, Monaro S, Naganathan V, et al. Frailty predicts increased risk of reintervention in the 2 years after arteriovenous fistula creation. J Vasc Access. 2023;24(6):1428–1437. doi: 10.1177/11297298221088756.
- Bloomfield K, Wu Z, Chan L, et al. Frailty prevalence in Aotearoa New Zealand haemodialysis patients and its association with hospitalisations. N Z Med J. 2021;134(1546):95–108.
- Anderson BM, Qasim M, Correa G, et al. Self-reported health change in haemodialysis recipients modulates the effect of frailty upon mortality and hospital admissions: outcomes from a large prospective UK cohort. Nephrol Dial Transplant. 2023;38(5):1297–1308. doi: 10.1093/ndt/gfac287.
- Anderson BM, Qasim M, Correa G, et al. Cognitive impairment, frailty, and adverse outcomes among prevalent hemodialysis recipients: results from a large prospective cohort study in the United Kingdom. Kidney Med. 2023;5(4):100613. doi: 10.1016/j.xkme.2023.100613.
- López-Montes A, Martínez-Villaescusa M, Pérez-Rodríguez A, et al. Frailty, physical function and affective status in elderly patients on hemodialysis. Arch Gerontol Geriatr. 2020;87:103976. doi: 10.1016/j.archger.2019.103976.
- Zanotto T, Mercer TH, van der Linden ML, et al. The relative importance of frailty, physical and cardiovascular function as exercise-modifiable predictors of falls in haemodialysis patients: a prospective cohort study. BMC Nephrol. 2020;21(1):99. doi: 10.1186/s12882-020-01759-z.
- Garcia-Canton C, Rodenas A, Lopez-Aperador C, et al. Frailty in hemodialysis and prediction of poor short-term outcome: mortality, hospitalization and visits to hospital emergency services. Ren Fail. 2019;41(1):567–575. doi: 10.1080/0886022X.2019.1628061.
- Oki R, Hamasaki Y, Tsuji S, et al. Clinical frailty assessment might be associated with mortality in incident dialysis patients. Sci Rep. 2022;12(1):17651. doi: 10.1038/s41598-022-22483-8.
- Chao CT, Chiang CK, Huang JW, et al. Self-reported frailty among end-stage renal disease patients: a potential predictor of dialysis access outcomes. Nephrology (Carlton). 2017;22(4):333–334. doi: 10.1111/nep.12961.
- Mansur HN, Lovisi JC, Colugnati FA, et al. Association of frailty with endothelial dysfunction and its possible impact on negative outcomes in Brazilian predialysis patients with chronic kidney disease. BMC Nephrol. 2015;16(1):157. doi: 10.1186/s12882-015-0150-1.
- Chan JS, Hsiao PJ, Chiang WF, et al. The role of oxidative stress markers in predicting acute thrombotic occlusion of haemodialysis vascular access and progressive stenotic dysfunction demanding angioplasty. Antioxidants. 2021;10(4):569. doi: 10.3390/antiox10040569.
- Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45.
- Shiu YT, Rotmans JI, Geelhoed WJ, et al. Arteriovenous conduits for hemodialysis: how to better modulate the pathophysiological vascular response to optimize vascular access durability. Am J Physiol Renal Physiol. 2019;316(5):F794–f806. doi: 10.1152/ajprenal.00440.2018.
- Deng MG, Liu F, Liang Y, et al. Association between frailty and depression: a bidirectional Mendelian randomization study. Sci Adv. 2023;9(38):eadi3902. doi: 10.1126/sciadv.adi3902.
- Majd M, Saunders EFH, Engeland CG. Inflammation and the dimensions of depression: a review. Front Neuroendocrinol. 2020;56:100800. doi: 10.1016/j.yfrne.2019.100800.
- Anderson BM, Qasim M, Correa G, et al. Depression is associated with frailty and lower quality of life in haemodialysis recipients, but not with mortality or hospitalization. Clin Kidney J. 2023;16(2):342–354. doi: 10.1093/ckj/sfac241.
- Potter GG, McQuoid DR, Whitson HE, et al. Physical frailty in late-life depression is associated with deficits in speed-dependent executive functions. Int J Geriatr Psychiatry. 2016;31(5):466–474. doi: 10.1002/gps.4351.
- Panza F, Lozupone M, Solfrizzi V, et al. Different cognitive frailty models and health- and cognitive-related outcomes in older age: from epidemiology to prevention. J Alzheimers Dis. 2018;62(3):993–1012. doi: 10.3233/JAD-170963.
- Kennard AL, Rainsford S, Glasgow NJ, et al. Use of frailty assessment instruments in nephrology populations: a scoping review. BMC Geriatr. 2023;23(1):449. doi: 10.1186/s12877-023-04101-y.
- Shannon OM, Ashor AW, Scialo F, et al. Mediterranean diet and the hallmarks of ageing. Eur J Clin Nutr. 2021;75(8):1176–1192. doi: 10.1038/s41430-020-00841-x.
- Joshi S, Kalantar-Zadeh K, Chauveau P, et al. Risks and benefits of different dietary patterns in CKD. Am J Kidney Dis. 2023;81(3):352–360. doi: 10.1053/j.ajkd.2022.08.013.
- Wu PY, Chen KM, Tsai WC. The Mediterranean dietary pattern and inflammation in older adults: a systematic review and meta-analysis. Adv Nutr. 2021;12(2):363–373. doi: 10.1093/advances/nmaa116.
- Hansrivijit P, Oli S, Khanal R, et al. Mediterranean diet and the risk of chronic kidney disease: A systematic review and meta-analysis. Nephrology. 2020;25(12):913–918. doi: 10.1111/nep.13778.
- Valdivielso JM, Jacobs-Cachá C, Soler MJ. Sex hormones and their influence on chronic kidney disease. Curr Opin Nephrol Hypertens. 2019;28(1):1–9. doi: 10.1097/MNH.0000000000000463.
- Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396(10250):565–582. doi: 10.1016/S0140-6736(20)31561-0.
- Vinson AJ, Zhang X, Dahhou M, et al. A multinational cohort study uncovered sex differences in excess mortality after kidney transplant. Kidney Int. 2023;103(6):1131–1143. doi: 10.1016/j.kint.2023.01.022.