Abstract
Objectives: This study aimed to identify psychological predictors of oral hypoglycaemic agent (OHA) adherence and to compare adherence rates between a novel and well-known instrument, i.e. the Probabilistic Medication Adherence Scale (ProMAS) and Medication Adherence Report Scale (MARS-5).
Design and main outcome measures: A longitudinal study design was applied with surveys at baseline and 6-month follow-up. At baseline, OHA adherence using the ProMAS and MARS-5, socio-cognitive determinants and demographics were assessed. At follow-up, the ProMAS was applied as outcome measure, on which socio-cognitive determinants and demographics were regressed using linear regression analysis.
Results: The baseline and follow-up sample included 304 and 231 participants, respectively. When applying cut-off points of ≥15 for the ProMAS and ≥23 for the MARS-5, 47.4 and 89.5% adhered to their OHAs. Consistent predictors of better adherence comprised a low education, lower severity perceptions, and higher self-efficacy and intention. After correcting for baseline adherence, a low education and higher self-efficacy remained significant adherence predictors.
Conclusions: Compared to the MARS-5, ProMAS data was less skewed, similar to objectively collected data, and yielded insights in a broader spectrum of (non)-adherence behaviours. Results stress the need for adherence improving interventions which particularly should target higher educated patients and patients with low self-efficacy, low intention and high severity perceptions.
Introduction
The long-term beneficial effects of intensive glycemic control in type 2 diabetes patients on disease progression, cardiovascular risks and early mortality are well-known (Holman, Paul, Bethel, Matthews, & Neil, Citation2008). Initial treatment strategies comprise healthy lifestyle recommendations (Krass, Schieback, & Dhippayom, Citation2015), i.e. improving dietary patterns, regular physical activity, smoking cessation, moderate alcohol consumption, and a healthy Body Mass Index (BMI) (King, Mainous, Carnemolla, & Everett, Citation2009). Only 3.5% of the 40–75-year-old patients achieves adherence to all these recommendations which often makes the initiation of pharmaceutical interventions inevitable (King, Mainous, Carnemolla, & Everett, Citation2009). Depending on the necessity of treatment intensity, oral hypoglycaemic agents (OHAs) are usually considered primary pharmaceutical strategies. As insulin secretory capacities of the pancreas progressively decrease, eventually many patients will require more than one drug, whether or not combined with insulin therapy.
Despite the pivotal role of OHAs in pursuing glycaemic control, adherence rates are suboptimal (Krass et al., Citation2015). Suboptimal adherence not only attenuates beneficial treatment outcomes (Holman et al., Citation2008), but is also associated with reduced quality of life, increased healthcare costs and hospitalisations, and early mortality (Asche, LaFleur, & Conner, Citation2011). Adherence rates range widely across studies between 38.5 and 93.1% which is probably largely attributable to the variety of objective and subjective measurement instruments used (Krass et al., Citation2015). Subjective measurement instruments, i.e. self-report questionnaires, are most often employed, given their low costs, usability, potential to test large sample sizes, and the wide range of adherence behaviours that can be assessed (Clifford, Perez-Nieves, Skalicky, Reaney, & Coyne, Citation2014; Kleppe, Lacroix, Ham, & Midden, Citation2015; Nguyen, La Caze, & Cottrell, Citation2014). The Medication Adherence Report Scale (MARS-5) (Horne, Hankins, & Jenkins, Citation2001) and the Morisky Medication adherence Scale (MMAS) are frequently used self-report instruments that have been developed for adherence research involving chronic illnesses (Clifford et al., Citation2014; Horne & Weinman, Citation2002). These validated questionnaires have demonstrated sufficient internal reliability (Horne et al., Citation2001), but faced criticism regarding their often highly skewed adherence distributions and limited range of adherence behaviours assessed (Lehmann et al., Citation2014; MacLaughlin et al., Citation2005). Therefore, new instruments are needed that assess adherence behaviour more accurately.
The Probabilistic Medication Adherence Scale (ProMAS) (Kleppe et al., Citation2015) has recently been developed in response to flaws in existing self-report instruments and could potentially solve these measurement issues. The ProMAS assesses eighteen adherence behaviours with varying item difficulty, selected from extensive literature review and patient interviews (Kleppe et al., Citation2015). Hitherto, research showed that the ProMAS, when compared with the MARS-5, yields less skewed adherence distributions, shows a better match with data collected by objective methods (Sabaté, Citation2003), and provides in depth insights in which adherence behaviours are improvable (Kleppe et al., Citation2015). However, additional research is needed to confirm findings, particularly to assess the applicability of the ProMAS for specific disease states such as type 2 diabetes.
As OHA adherence can vary markedly between individuals, not only new instruments that measure adherence more accurately are needed, but preferably these instruments should also identify factors which predict adherence. This in order to develop effective adherence improving interventions. Reviews conclude that adherence is a complex process in which various domains of adherence determinants interact, i.e. social and economic factors, healthcare system factors, therapy-related factors, condition-related factors, and patient-related factors (Sabaté, Citation2003). Although all domains may provide relevant intervention targets to improve adherence, most of these domains are difficult to change and might only influence adherence indirectly through perceptions of the patient (Sabaté, Citation2003). Hence, patient-related factors, including socio-cognitive determinants such as a person’s awareness, motivation, intention and self-regulation (Vries, Citation2017), seem to be the most viable domain to intervene in. Nonetheless, only few studies have applied socio-cognitive theoretical models to explain OHA adherence behaviour (Chao, Nau, Aikens, & Taylor, Citation2005; Jannuzzi, Rodrigues, Cornelio, Sao-Joao, & Gallani, Citation2014) and only one was quantitative in nature. This cross-sectional study showed negative associations of perceived general barriers and perceived side-effect barriers, and a positive association of self-efficacy with medication adherence as measured with the MMAS (Chao et al., Citation2005). To the best of our knowledge, studies have not yet applied longitudinal designs, allowing the identification of factors with predictive value for OHA adherence, and have not yet applied promising adherence assessing instruments such as the ProMAS. Therefore, the aim of the current study was twofold.
The first aim was to compare OHA adherence between the ProMAS and MARS-5. The second aim was to identify socio-cognitive predictors of OHA adherence using the ProMAS as outcome.
Materials and methods
A longitudinal study design was applied in type 2 diabetes patients with surveys at baseline and 6-month follow-up. The study was approved by the Medical Ethical Committees of the Maastricht University Medical Centre (15-4-181) and Zuyderland Hospital (15-N-209).
Participants and procedure
The current study is part of a larger project which also aims to identify socio-cognitive predictors of adherence to insulin therapy. Two hospitals with outpatient clinics and 55 general practices in the southern part of the Netherlands were approached to aid in recruiting participants. All hospitals and six practices indicated their willingness to participate. Patients were eligible if diagnosed with type 2 diabetes, aged 40–70 years old, and used at least one type of blood glucose lowering medication, i.e. either OHAs and/or insulin therapy. Patients not able to speak, understand or write the Dutch language were excluded. A total of 1674 potential participants were invited by the research team to participate via postal mail by an information letter (personalised with a signature from their own physician), an informed consent form and the baseline questionnaire. In case participants completed and returned the baseline questionnaire, they were sent the follow-up questionnaire 6 months afterwards. For the purpose of this study, we aimed to retain 182 participants at follow-up to ensure enough study power, taking into account an α of 0.05 and a study power of 0.8 (Faul, Erdfelder, Lang, & Buchner, Citation2007).
Questionnaires
The baseline questionnaire consisted of 130 questions and the follow-up questionnaire consisted of 78 questions on adherence to OHAs, socio-cognitive determinants, and demographics.
Adherence to OHAs
Adherence to OHAs was assessed by the ProMAS (Kleppe et al., Citation2015) and MARS-5 (Horne & Weinman, Citation2002). The ProMAS was assessed at baseline and follow-up, the MARS-5 at baseline. Both instruments assess adherence without a time frame over which adherence is assessed. This implies that adherence is assessed from medication initiation onwards which in most diabetes patients can add up to several decades. This might not only lead to a questionable relevance of results, but also to results which are subject to recall-bias due to a not recent enough recall time-frame (Clifford et al., Citation2014). Moreover, in the Netherlands, most patients visit their physician quarterly for a diabetes check-up in which guidelines recommend that therapy adherence should be one of the main discussion topics (Federatie, Citation2017). Based on these arguments, adding a 3-month time-frame over which adherence behaviours were assessed to each item of the ProMAS and MARS-5, was considered tenable, e.g. ‘it has happened at least once in the last three months that I forgot to take (one of) my OHAs’. The 18 items of the ProMAS can be scored on a binary answering scale (1 = yes, true or 0 = no, not true) and assess various adherence behaviours, e.g. forgetting medication, taking less or more medication, and changing medication dosages. A higher sum score represents better adherence (range 0–18). The outcome can be applied either as continuous or dichotomised measure in which a score of ≥15 is considered high adherence (Kleppe et al., Citation2015).
The MARS-5 consists of five items which can be scored on a five-point scale (1 = always – 5 = never) and assesses adherence behaviours, e.g. forgetting, stopping, and skipping medication (Horne & Weinman, Citation2002). Its sum score ranges from 5 to 25, with higher scores representing better adherence, and is most often dichotomised to distinguish between non-adherence and adherence. Although no golden standard exists, a cut-off point at ≥23 is widely applied and considered high adherence (Koster, Philbert, Winters, & Bouvy, Citation2015).
Socio-cognitive determinants
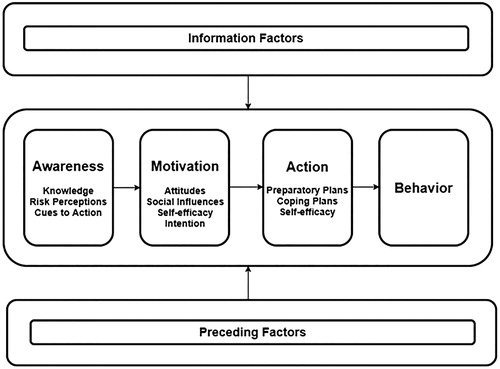
Socio-cognitive determinants were derived from the I-Change Model (ICM) (Vries, Citation2017), which is a theoretical framework integrating various well-known socio-cognitive theories (Ajzen, Citation1991; Janz & Becker, Citation1984; Prochaska & DiClemente, Citation1983). The ICM () has been applied frequently to map salient beliefs of health behaviour (change), including medication adherence (Voncken-Brewster et al., Citation2014). The model assumes that behaviour can partly be explained by socio-cognitive determinants and that behaviour change is a phased process. The ICM distinguishes between three phases; an awareness, motivation and action phase, influenced by information and preceding factors. Awareness determinants are believed to have a distal indirect influence on behaviour and include a person’s knowledge, risk perceptions, i.e. how susceptible someone feels to get a certain illness and how severe this illness is valued, and salient cues to action, i.e. prompts which trigger engagement in health behaviour. Subsequently, these awareness determinants influence distinct motivational determinants such as attitudes towards performing health behaviour (pros and cons), perceptions of social influences (support, modelling and norms), and self-efficacy, i.e. a person’s perception of their own competence to successfully execute a health behaviour in difficult situations. A person’s intention to change health behaviour is influenced by these motivational determinants. However, a high intention towards behaviour change does not consistently warrant successful behaviour change (Sniehotta, Scholz, & Schwarzer, Citation2005). Action phase determinants facilitate this process by the formation of preparatory plans and coping plans, which are assumed to increase the likelihood of successful translation of expressed intentions into the pursued behaviour. Distinct determinants were assessed through several salient beliefs, derived from earlier research (Vluggen, Hoving, Schaper, & de Vries, Citation2018), and combined into scales.
Knowledge was assessed by ten items, e.g. shaking and sweating are signs of a too high blood glucose level, which could be scored on a binary answering scale (1 = true or 0 = false). An overall sum score was calculated ranging from 0 to 10, with higher scores representing more knowledge.
Risk perceptions were assessed with eight items each for perceived susceptibility (α = 0.83), e.g. susceptibility of developing visual conditions in the future (1 = very unlikely–5 = very likely) and perceived severity (α = 0.88), e.g. severity of having a heart attack in the future is (1 = not serious at all–5 = very serious).
Cues to action (α = 0.94) were measured by eleven items, e.g. when I read information about OHAs, I am prompted to use my OHAs as prescribed (1 = totally disagree–5 = totally agree).
Attitudes were measured using six items each for perceived pros (α = 0.87), e.g. when I use my OHAs as prescribed, I (1 = do not feel healthier–4 = feel much healthier), and perceived cons (α = 0.70), e.g. when I use my OHAs as prescribed, I (1 = suffer a lot of side effects–4 = do not suffer side effects).
Perceived social influences were assessed with eleven items including support (4 items, α = 0.80), e.g. my partner supports me to use my OHAs as prescribed, modelling (3 items, α = 0.78), e.g. my family members use their medicines as prescribed, and norms (4 items, α = 0.82), e.g. my physician thinks I should use my OHAs as prescribed (1 = totally disagree–5 = totally agree).
Self-efficacy was measured with eleven items, representing eleven salient out of routine situation in which self-efficacy to be adherent might be reduced (α = 0.89), e.g. how difficult or easy is it for you to use your OHAs as prescribed when being on vacation (1 = very difficult–5 = very easy).
Intention was assessed with two items (α = 0.90), i.e. I plan/I want to use my OHAs as prescribed (1 = totally disagree–5 = totally agree).
Preparatory plans (α = 0.77), e.g. I plan to put my OHAs in a fixed place, and coping plans (α = 0.98), e.g. I have a plan to use my OHAs in a difficult situation such as being on vacation, were assessed with seven and eleven items respectively (1 = totally disagree–5 = totally agree).
Demographics and dossier data
Demographic characteristics included gender (1 = male, 2 = female), age, education level (1 = low, 2 = medium, 3 = high), relationship status (1 = alone, 2 = together with partner), nationality, length, weight, consultation content and a description of prescribed medication. From the participants’ electronic patient dossier data were extracted on systolic and diastolic blood pressure, as well as the laboratory values of HbA1c-level, creatinine clearance, and LDL-cholesterol. Body Mass Index (BMI) was calculated as weight/length2.
Statistical analyses
Analyses were performed using SPSS 24.0, applying a significance level of 0.05. Missing data was imputed applying principles of Downey and colleague (Downey & King, Citation1998). Participants missing over 20% of the total data or participants missing data on outcome measure(s) were excluded from the analysis. Descriptive statistics and frequencies were applied to describe sample characteristics. A logistic regression was performed regarding demographic variables, i.e. gender, age, education level and relationship status, to identify potential selective attrition between baseline and follow-up. To answer the first aim, descriptive statistics and frequencies were applied to map both overall adherence scores of the baseline ProMAS and MARS-5 as well as scores on individual questionnaire items. Overall adherence scores were compared by applying dichotomised outcome measures. Subsequently, bivariate analyses between demographics, socio-cognitive determinants, and follow-up adherence (ProMAS) were performed using Pearson’s correlations. Additionally, a correlational analysis was performed between the continuous baseline outcome of the ProMAS and MARS-5. To answer the second aim, multiple linear regression analysis was performed by regressing demographics, socio-cognitive determinants and the baseline ProMAS sum score on the continuous ProMAS follow-up adherence sum score. The selection of variables to include in the multiple regression analysis was based on the assumption of the theoretical framework that was applied in the study (Heinze, Wallisch, & Dunkler, Citation2018). The model assumes that socio-cognitive variables (e.g. knowledge and self-efficacy), patient background variables (e.g. age, gender, and education level), and clinical outcomes (e.g. HbA1c level) may either directly or indirectly influence adherence behaviour (Vries, Citation2017). Independent predictor variables were entered hierarchically in the analysis according to the assumptions of the ICM: (1) demographics and dossier data, (2) awareness determinants, (3) motivational determinants and intention, (4) action determinants, and (5) baseline behaviour. Baseline behaviour was added in the last regression model to examine its impact on socio-cognitive predictors entered in previous models.
Results
Participants
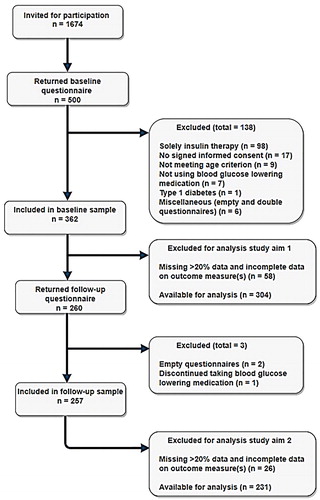
The flow of participants throughout the study is shown . Five hundred participants returned the baseline questionnaire (29.9% response rate). Of the 362 participants included in the baseline sample, 260 returned the follow-up questionnaire (72% response rate). After exclusion of participants missing over 20% of the data or with missing data on the outcome measure(s) (Downey & King, Citation1998), data of 304 participants remained for the analysis of the first study aim, and data of 231 participants for the second study aim. As described in , two third of population was male, the mean age was 60.8 years and the majority of participants lived together with a partner. Education level was fairly equally distributed. Participants had a relative long average disease duration of over 12 years. The majority of participants was obese with inadequate blood glucose control as reflected by the elevated mean HbA1c-level, while the mean LDL-cholesterol was well within target range for many participants (Federatie, Citation2017). Two thirds used one class of OHA medication; nearly 90% used metformin. Near half of the participants applied insulin therapy alongside their OHAs. The attrition analysis revealed that participants with a low education level were more likely to drop-out during follow-up (OR = 1.85, p = 0.02).
Table 1. Sample characteristics.
Overall ProMAS and MARS-5 adherence scores
The average baseline adherence score was 13.6 (range 2–18, SD = 3.6) for the ProMAS and 24.1 (range 16–25, SD = 1.4) for the MARS-5. With a cut-off point of ≥15, 47.7% of the respondents adhered to their OHAs regarding the ProMAS, while 89.5% of the respondents achieved OHA adherence regarding the MARS-5 when using a cut-off point of ≥23.
Adherence assessment scores
Scoring percentages to items assessed in the ProMAS and MARS-5 are shown in . Regarding the ProMAS, items two and six were reported as non-adherent by more than half of the participants, and both comprise adherence behaviours concerning medication timing. Items 1, 5 and 10 were reported as non-adherent by over 40% of the participants, and comprise adherence behaviours such as forgetting medication, being positive about having taken all the required medication, and having taken medication at a different moment than prescribed (e.g. with breakfast or in the evening). Items which comprised adherence behaviours such as (temporarily) stopping medication, changing medication dosages, filling prescriptions, initiating medication taking, and taking more medication than prescribed, were reported as non-adherent by less than 5% of participants.
Table 2. Baseline scoring percentages per item (ProMAS and MARS-5).
In the MARS-5 questionnaire, slightly more than 50% of the participants reported to have never forgotten to take their OHAs, while nearly all others indicated that this occurred rarely or sometimes. Over 95% of the participants indicated to have never altered the dose of their OHAs, stopped taking their OHAs, or decided to skip one of their OHAs intakes.
Bivariate analyses
Correlations of demographics and socio-cognitions with follow-up adherence
As shown in , older participants, as well as participants perceiving more pros, having a higher self-efficacy and higher intention to adhere, showed higher levels of adherence. Moreover, participants who made a higher number of preparatory plans and coping plans for difficult situations, showed better adherence. Significant negative correlations with adherence indicated that participants with a high education level had lower adherence scores compared to low educated participants, and that participants with a lower HbA1c-level and lower risk perceptions showed better adherence. Participants perceiving more cues to action and more cons significantly showed lower levels of adherence.
Table 3. Pearson’s correlations between demographics, socio-cognitions and adherence.
Correlations of outcome measures
A moderate to large correlation was observed between the ProMAS and the MARS-5 (r = 0.67; p = 0.00), representing a substantial positive association between both measures (Hinkle, Wiersma, & Jurs, Citation2003).
Predictors of OHA adherence
Results of the regression analysis are displayed in . Results of model one demonstrates that higher age and a low education level predicted better adherence. In model two, participants with a high score on cues to action and severity perceptions were less adherent. Participants with a low education maintained showing better adherence. In model three, education level and severity perceptions remained significant, while cues to action became insignificant. Living together, a high self-efficacy and intention were other predictors of better adherence. In the fourth model, the significance of relationship status disappeared, while the other factors remained significant. In the fifth model, the significance of severity perceptions and intention disappeared, and a low education level, high self-efficacy and baseline adherence predicted better adherence at follow-up. The fifth model explained 48% of the total variance in OHA adherence.
Table 4. Predictors of OHA adherence at 6-month follow-up.
Discussion
Our first aim was to compare OHA adherence between the ProMAS and MARS-5. Overall adherence differed markedly between these questionnaires, with a difference around 40% in adherence percentages, which is in line with earlier publications (Kleppe et al., Citation2015; Krass et al., Citation2015).
Patients are generally considered to be adherent if the adherence percentage to prescribed medication matches or exceeds 80% (Osterberg & Blaschke, Citation2005). This implies a submaximal, but acceptable adherence percentage, which leaves room for improvements in patients’ adherence. Applying this acceptable principle, the ProMAS adherence percentage of 47.7% virtually matches the adherence percentage as collected by objective methods, in contrast to the adherence percentage of 89.5 as observed in the MARS-5 (Sabaté, Citation2003). As shown in our study and previous application of the ProMAS, when dichotomized, the percentage of patients being adherent is about 50%. These data match data gathered from objective measures, assessing medication adherence of chronic ill patients, or more specific patients applying OHAs such as those assessed by our study. Although we did not use an objective measure ourselves, data on OHA adherence through objective measures is widely available. These data also show that around 50% of the patients adhered to their OHAs (Hansen, Farley, Droege, & Maciejewski, Citation2010; Hertz, Unger, & Lustik, Citation2005; Kreyenbuhl et al., Citation2010).
In addition, the ProMAS covers a broader spectrum of adherence behaviours compared to the MARS-5. For instance, additionally to forgetfulness which has been reported earlier as the most common reason for non-adherence (Guenette et al., Citation2015), the ProMAS also identified incorrect medication timing as a major issue in non-adherence. By contrast, the skewed adherence distribution of the MARS-5 only slightly discriminates between adherent and non-adherent patients. Therefore, current results confirm earlier work in which the ProMAS and MARS-5 are compared and underline the promising character of this novel adherence instrument to assess OHA adherence in type 2 diabetes patients (Kleppe et al., Citation2015).
We observed a moderate to large correlation (r=.67) between the ProMAS and MARS-5. This implies that there is a fairly substantial linear coherence between the measures. However, by squaring the correlation, which results in an R2 value, a more interpretable inference can be made about their cohesion. This results in an R2 of .45, which represents a shared variability of 45% by the ProMAS and MARS-5 score. To put this value into perspective, still 55% of the variability is left to be accounted for by other variables. This shows that the shared variance between the ProMAS and MARS-5 is rather moderate.
Our second study aim was to identify socio-cognitive predictors of OHA adherence using the ProMAS as outcome. In the fourth multivariate model, a low education level, lower severity perceptions, and higher self-efficacy and intention predicted better OHA adherence. A low education level and higher self-efficacy remained significant predictors even after adjusting for baseline behaviour.
While DiMatteo (DiMatteo, Citation2004) and Kirkman and colleagues (Kirkman et al., Citation2015) associated higher levels of education with medication adherence, a recent study by Chew and colleagues (Chew, Hassan, & Sherina, Citation2015) indicated that higher education was associated with poor diabetes medication adherence. Another review by Jin and colleagues (Jin, Sklar, Min Sen Oh, & Li, Citation2008) reported inconsistent findings. Contradictory findings might be due to variations in study designs and populations, outcome measures applied and analysis methods (Chew et al., Citation2015; Kirkman et al., Citation2015; Mann, Ponieman, Leventhal, & Halm, Citation2009). However, a potential explanation for the identification of a low education level as predictor of OHA adherence in the current study, is that patients with a low education might believe more in their physicians’ treatment suggestions (Jin, Sklar, Min Sen Oh, & Li, Citation2008). As a result, lower educated patients might be more prompted to follow physicians’ prescriptions compared to higher educated patients.
Unexpectedly, severity perceptions were significantly inversely related to medication adherence in both bivariate and multivariate analyses; current socio-cognitive models assume that higher levels of perceived threat should result in higher levels of medication adherence (Janz & Becker, Citation1984; Vries, Citation2017). One potential explanation could be that our analyses were confounded by depression, which was not measured in our study. Depression was in earlier studies associated with higher severity perceptions (Chao et al., Citation2005) and with lower self-efficacy and medication adherence (Krass et al., Citation2015). According to the Protection Motivation Theory, it is assumed that adverse actions will be preserved if there is a high threat perception but low perceived self-efficacy (Rogers, Citation1975). Clearly further research is needed to explore the inverse relation of severity perceptions and medication adherence.
Self-efficacy has been reported extensively in earlier studies as a factor associated with adherence to diabetes medication (Chao et al., Citation2005; Guenette et al., Citation2015; Vluggen et al., Citation2018). Particularly, lower self-efficacy to adhere to medication tends to occur in out-of-routine situations, e.g. being on vacation, out for dinner, busy or ill (Guenette et al., Citation2015; Vervloet et al., Citation2013; Vluggen et al., Citation2018). Associating medication taking with everyday activities has been found to aid in OHA adherence, while a change in daily routine would make adherence more difficult (Farmer, Kinmonth, & Sutton, Citation2006; Guenette et al., Citation2015). Achieving medication adherence in routinely situations, might increase self-efficacy beliefs through mastery expectations. On the contrary, it might be more difficult to achieve medication adherence in out-of-routine situations because no association can be made with everyday activities, given the irregularity of the situation (Bandura, Citation1977). Repeated success, i.e. adherence to medication in situations when routine is disrupted, might increase self-efficacy and in turn medication adherence and should in that regard be facilitated by linking adherence to daily activities (Bandura, Citation1977; Guenette et al., Citation2015).
Strengths and limitations
This study is subject to some strengths and limitations. To our knowledge, this is the first study that applied a longitudinal study design in assessing socio-cognitive predictors of OHA adherence and to use the ProMAS to assess adherence. Moreover, this study added a time-frame of 3 months over which adherence is assessed, which might have decreased recall-bias and provided a more accurate adherence assessment. Limitations include the social desirability issues of self-report adherence measures (Lehmann et al., Citation2014). Despite the considerable advantages of assessing adherence through self-report instruments, a combination of subjective and objective measures is preferred (Lehmann et al., Citation2014) and should, when feasible, be included in future studies assessing adherence. By including both self-report and objective measures, one is able to make mutual performance comparisons. An advantage of applying both measures simultaneously in one study is that results will be obtained from the exact same study population contrary to comparing results across studies and study populations.
Our approach to predicting OHA adherence assumed that adherence is a rational process, partly explained by socio-cognitions. Although a large part of the variance in adherence behaviour was explained by these socio-cognitions, a substantial contribution to non-adherence behaviour was made by forgetfulness, which is considered unintentional, i.e. non-rational behaviour (Wroe, Citation2002). Hence, future studies should examine a combination of rational processes and processes underlying unintentional non-adherence, to contribute to a potential holistic understanding of OHA adherence.
Conclusion and recommendations
Results primarily indicate that OHA adherence is suboptimal, independent of measurement instrument and cut-off point applied. Our results confirm earlier research on the wide variety of OHA adherence percentages, which largely depends on methodology and cut-off points applied. Consensus on the best method to assess adherence is lacking and no golden standard exists in dichotomizing adherence scores which may hamper interpretability of divergent results. However, when applying sub-maximal cut-off points, which are generally acceptable, the ProMAS yielded results similar to objectively collected data. Moreover, when compared to the MARS-5, the ProMAS yielded less skewed data towards adherence and insights in a broader spectrum of (non)-adherence behaviours. Hence, the ProMAS seems better equipped to deal with flaws present in existing self-report instruments. Adding a relevant time-frame might decrease recall bias and provide more accurate estimates of adherence.
Suboptimal adherence stresses the need for effective adherence improving interventions. Decreasing forgetfulness and medication taking timing issues might increase adherence rates. Interventions should particularly target higher-educated patients and patients with low self-efficacy in out-of-routine situations as these characteristics predict lower adherence levels. Result regarding severity perceptions requires further inspection to examine potential explanations suggested in this article.
Acknowledgements
The funding source had no involvement in preparing or conducting the research or research article. The study was designed and guaranteed by all authors. S.V. wrote the manuscript and researched data. C.H. reviewed/edited the manuscript and assisted in the analysis. N.S. and H.V reviewed/edited the manuscript.
Disclosure statement
None declared.
Additional information
Funding
References
- Ajzen, I. (1991). The theory of planned behavior. Organizational Behavior and Human Decision Processes, 50(2), 179–211. doi:10.1016/0749-5978(91)90020-T
- Asche, C., LaFleur, J., & Conner, C. (2011). A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clinical Therapeutics, 33(1), 74–109. doi:10.1016/j.clinthera.2011.01.019
- Bandura, A. (1977). Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review, 84(2), 191–215. doi:10.1037//0033-295x.84.2.191
- Chao, J., Nau, D. P., Aikens, J. E., & Taylor, S. D. (2005). The mediating role of health beliefs in the relationship between depressive symptoms and medication adherence in persons with diabetes. Research in Social and Administrative Pharmacy, 1(4), 508–525. doi:10.1016/j.sapharm.2005.09.002
- Chew, B. H., Hassan, N. H., & Sherina, M. S. (2015). Determinants of medication adherence among adults with type 2 diabetes mellitus in three Malaysian public health clinics: A cross-sectional study. Patient Preference and Adherence, 9, 639–648. doi:10.2147/Ppa.S81612
- Clifford, S., Perez-Nieves, M., Skalicky, A. M., Reaney, M., & Coyne, K. S. (2014). A systematic literature review of methodologies used to assess medication adherence in patients with diabetes. Current Medical Research and Opinion, 30(6), 1071–1085. doi:10.1185/03007995.2014.884491
- DiMatteo, M. R. (2004). Variations in patients’ adherence to medical recommendations: A quantitative review of 50 years of research. Medical Care, 42(3), 200–209. doi:10.1097/01.mlr.0000114908.90348.f9
- Downey, R. G., & King, C. (1998). Missing data in Likert ratings: A comparison of replacement methods. The Journal of General Psychology, 125(2), 175–191. doi:10.1080/00221309809595542
- Farmer, A., Kinmonth, A. L., & Sutton, S. (2006). Measuring beliefs about taking hypoglycaemic medication among people with type 2 diabetes. Diabetic Medicine, 23(3), 265–270. doi:10.1111/j.1464-5491.2005.01778.x
- Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191.
- Federatie, N. D. (2017). De NDF Zorgstandaard diabetes type 2 volwassenen. Retrieved from http://www.zorgstandaarddiabetes.nl/type-2/
- Guenette, L., Lauzier, S., Guillaumie, L., Giguere, G., Gregoire, J. P., & Moisan, J. (2015). Patients’ beliefs about adherence to oral antidiabetic treatment: A qualitative study. Patient Preference and Adherence, 9, 413–420. doi:10.2147/Ppa.S78628
- Hansen, R. A., Farley, J. F., Droege, M., & Maciejewski, M. L. (2010). A retrospective cohort study of economic outcomes and adherence to monotherapy with metformin, pioglitazone, or a sulfonylurea among patients with type 2 diabetes mellitus in the United States from 2003 to 2005. Clinical Therapeutics, 32(7), 1308–1319. doi:10.1016/j.clinthera.2010.07.011
- Heinze, G., Wallisch, C., & Dunkler, D. (2018). Variable selection - A review and recommendations for the practicing statistician. Biometrical Journal, 60(3), 431–449. doi:10.1002/bimj.201700067
- Hertz, R. P., Unger, A. N., & Lustik, M. B. (2005). Adherence with pharmacotherapy for type 2 diabetes: A retrospective cohort study of adults with employer-sponsored health insurance. Clinical Therapeutics, 27(7), 1064–1073. doi:10.1016/j.clinthera.2005.07.009
- Hinkle, D. E., Wiersma, W., & Jurs, S. G. (2003). Applied Statistics for the Behavioral Sciences (5th ed.). Boston: Houghton Mifflin.
- Holman, R. R., Paul, S. K., Bethel, M. A., Matthews, D. R., & Neil, H. A. (2008). 10-year follow-up of intensive glucose control in type 2 diabetes. New England Journal of Medicine, 359(15), 1577–1589. doi:10.1056/NEJMoa0806470
- Horne, R., Hankins, M., & Jenkins, R. (2001). The Satisfaction with Information about Medicines Scale (SIMS): A new measurement tool for audit and research. Quality and Safety in Health Care, 10(3), 135–140. doi:10.1136/qhc.0100135
- Horne, R., & Weinman, J. (2002). Self-regulation and self-management in asthma: Exploring the role of illness perceptions and treatment beliefs in explaining non-adherence to preventer medication. Psychology & Health, 17(1), 17–32. doi:10.1080/08870440290001502
- Jannuzzi, F. F., Rodrigues, R. C., Cornelio, M. E., Sao-Joao, T. M., & Gallani, M. C. (2014). Beliefs related to adherence to oral antidiabetic treatment according to the theory of planned behavior. Revista Latino-Americana de Enfermagem, 22(4), 529–537. doi:10.1590/0104-1169.3578.2448
- Janz, N. K., & Becker, M. H. (1984). The Health Belief Model: A decade later. Health Education Quarterly, 11(1), 1–47. doi:10.1177/109019818401100101
- Jin, J., Sklar, G. E., Min Sen Oh, V., & Li, S. C. (2008). Factors affecting therapeutic compliance: A review from the patient’s perspective. Therapeutics and Clinical Risk Management, 4(1), 269–286.
- King, D. E., Mainous, A. G., 3rd, Carnemolla, M., & Everett, C. J. (2009). Adherence to healthy lifestyle habits in US adults, 1988-2006. The American Journal of Medicine, 122(6), 528–534. doi:10.1016/j.amjmed.2008.11.013
- Kirkman, M. S., Rowan-Martin, M. T., Levin, R., Fonseca, V. A., Schmittdiel, J. A., Herman, W. H., & Aubert, R. E. (2015). Determinants of adherence to diabetes medications: Findings from a large pharmacy claims database. Diabetes Care, 38(4), 604–609. doi:10.2337/dc14-2098
- Kleppe, M., Lacroix, J., Ham, J., & Midden, C. (2015). The development of the ProMAS: A Probabilistic Medication Adherence Scale. Patient Preference and Adherence, 9, 355–367. doi:10.2147/PPA.S76749
- Koster, E. S., Philbert, D., Winters, N. A., & Bouvy, M. L. (2015). Adolescents’ inhaled corticosteroid adherence: The importance of treatment perceptions and medication knowledge. Journal of Asthma, 52(4), 431–436. doi:10.3109/02770903.2014.979366
- Krass, I., Schieback, P., & Dhippayom, T. (2015). Adherence to diabetes medication: A systematic review. Diabetic Medicine, 32(6), 725–737. doi:10.1111/dme.12651
- Kreyenbuhl, J., Dixon, L. B., McCarthy, J. F., Soliman, S., Ignacio, R. V., & Valenstein, M. (2010). Does adherence to medications for type 2 diabetes differ between individuals with vs without schizophrenia? Schizophrenia Bulletin, 36(2), 428–435. doi:10.1093/schbul/sbn106
- Lehmann, A., Aslani, P., Ahmed, R., Celio, J., Gauchet, A., Bedouch, P., … Schneider, M. P. (2014). Assessing medication adherence: Options to consider. International Journal of Clinical Pharmacy, 36(1), 55–69. doi:10.1007/s11096-013-9865-x
- MacLaughlin, E. J., Raehl, C. L., Treadway, A. K., Sterling, T. L., Zoller, D. P., & Bond, C. A. (2005). Assessing medication adherence in the elderly: Which tools to use in clinical practice?. Drugs & Aging, 22(3), 231–255. doi:10.2165/00002512-200522030-00005
- Mann, D. M., Ponieman, D., Leventhal, H., & Halm, E. A. (2009). Predictors of adherence to diabetes medications: The role of disease and medication beliefs. Journal of Behavioral Medicine, 32(3), 278–284. doi:10.1007/s10865-009-9202-y
- Nguyen, T. M. U., La Caze, A., & Cottrell, N. (2014). What are validated self-report adherence scales really measuring?: A systematic review. British Journal of Clinical Pharmacology, 77(3), 427–445. doi:10.1111/bcp.12194
- Osterberg, L., & Blaschke, T. (2005). Adherence to medication. New England Journal of Medicine, 353(5), 487–497. doi:10.1056/NEJMra050100
- Prochaska, J. O., & DiClemente, C. C. (1983). Stages and processes of self-change of smoking: Toward an integrative model of change. Journal of Consulting and Clinical Psychology, 51(3), 390–395. doi:10.1037//0022-006X.51.3.390
- Rogers, R. W. (1975). A protection motivation theory of fear appeals and attitude change. The Journal of Psychology, 91(1), 93–114. doi:10.1080/00223980.1975.9915803
- Sabaté, E. (2003). Adherence to long-term therapies: Evidence for action. Geneva, Switzerland: World Health Organization.
- Sniehotta, F. F., Scholz, U., & Schwarzer, R. (2005). Bridging the intention-behaviour gap: Planning, self-efficacy, and action control in the adoption and maintenance of physical exercise. Psychology & Health, 20(2), 143–160. doi:10.1080/08870440512331317670
- Vervloet, M., Spreeuwenberg, P., Bouvy, M. L., Heerdink, E. R., de Bakker, D. H., & van Dijk, L. (2013). Lazy sunday afternoons: the negative impact of interruptions in patients’ daily routine on adherence to oral antidiabetic medication. A multilevel analysis of electronic monitoring data. European Journal of Clinical Pharmacology, 69(8), 1599–1606. doi:10.1007/s00228-013-1511-y
- Vluggen, S., Hoving, C., Schaper, N. C., & de Vries, H. (2018). Exploring beliefs on diabetes treatment adherence among Dutch type 2 diabetes patients and healthcare providers. Patient Education and Counseling, 101(1), 92–98. doi:10.1016/j.pec.2017.07.009
- Voncken-Brewster, V., Tange, H., Moser, A., Nagykaldi, Z., de Vries, H., & van der Weijden, T. (2014). Integrating a tailored e-health self-management application for chronic obstructive pulmonary disease patients into primary care: A pilot study. BMC Family Practice, 15(1), 4. doi:10.1186/1471-2296-15-4
- Vries, H. (2017). An integrated approach for understanding health behavior; The I-change model as an example. Psychology and Behavioral Science International Journal, 2(2), 1–6. doi:10.19080/PBSIJ.2017.02.555585
- Wroe, A. L. (2002). Intentional and unintentional nonadherence: a study of decision making. Journal of Behavioral Medicine, 25(4), 355–372. doi:10.1023/A:1015866415552