Abstract
The authors aimed to investigate the efficacy of epoetin-alpha on hemoglobin levels and red cell transfusion requirement in children with both hematologic malignancy (HM, n = 27) and solid tumors (ST, n = 14). Epoetin-alpha was given (150 U/kg or 250 U/kg, thrice weekly) for 12 weeks. Epoetin alpha significantly increased the hemoglobin levels at the 2nd and 3rd months of therapy (p <. 05). At the 3rd month, the patients required less red cell transfusion. At the dose of 150 U/kg, only three patients with HM, but none of the ST patients, required red cell. However, none required red cell transfusion after 2nd month on epoetin alpha 250 U/kg. Epoetin-alpha administration increases hemoglobin levels and decreases red cell transfusion requirement in children with malignancy.
Keywords:
Anemia is a common problem for the patients with malignancy. In this population chronic anemia due to primary disease is aggrevated by chemo-therapy Citation[1]. Most of the patients with malignancy become seriously anemic and they require transfusion during treatment Citation[2, 3]. However, transfusion complications in acute and chronic period may be hazardous as shown in adult patients with cancer Citation[2]. Many studies on adult patients with malignancy have shown that correction of anemia with recombinant human erythropoietin is related with increased hemoglobin (Hb) levels and increased quality of life Citation[4–7]. There are a few studies that have investigated the role of erythropoietin in children with malignancy Citation[8–13]. Nevertheless, most of the published studies enrolled patients predominantly in solid tumors. There are two studies that enrolled the patients in both leukemias and solid tumors, but in limited patient numbers. Therefore, we aimed to investigate the efficacy of epoetin-alpha on hemoglobin levels and red cell transfusion requirement in children with both hematologic malignancy and solid tumors that were followed-up at our institution in a controlled, nonrandomized study design.
MATERIALS AND METHODS
Study Design
This trial was a single-institutional study of safety and efficacy of epoetin alpha. The study protocol was approved by the local ethical committee. A written parental consent was also obtained.
Patients
This study enrolled patients in hematological malignancy (HM) and solid tumor (ST) that have Hb levels below 10 g/dL as determined at least twice. Exclusion criteria were clinical symptoms of anemia, hypertension, gastrointestinal bleeding, deficiencies of iron, folate, or vitamin B12.
The study group included 41 patients (17 males, 24 females) aged 5 months–17 years. The HM group aged 1–16 years and consisted of 27 patients (17 males, 10 females). There were 9 acute lymphoblastic leukemias, 6 acute myelogenous leukemias, 2 non-Hodgkin's lymphomas. The ST group aged 5 months–17 years and included 14 patients (8 males, 6 females). There were 8 osteogenic sarcomas, 3 Ewing family tumors, 1 neuroblastoma, 1 medulloblastoma, 1 rhabdomyosarcoma in this group.
The patients with leukemia and NHL were treated with BFM protocols (ALL-BFM-95, AML-BFM-1998, and NHL-BFM-95). Other therapy protocols were Mayo Clinic Pilot II Study for osteogenic sarcoma, CCG 7881 Study for Ewing's sarcoma, modified GPO-NB-90 for neuroblastoma, POG 9031 for medulloblastoma, and SIOP-MMT 89 for rhabdomyosarcoma.
The age-matched (2–17 years) control group included 30 patients (16 males and 14 females). There were 19 patients with HM (11 acute lymphoblastic leukemias, 2 acute myelogenous leukemias, 6 non-Hodgkin's lymphomas) and 11 patients with ST (6 osteogenic sarcomas, 2 CNS tumors, 2 neuroblastomas, 1 Ewing family tumor). The patients of the control group were treated with similar chemotherapy protocols according to each malignancy type.
During the study period thromboembolic events, convulsion, hypertension, organ dysfunction and death were defined as serious adverse events.
Methods
The patients with HM or ST, were randomized to receive epoetin-alpha 150 U/kg (thrice weekly, SC) or 250 U/kg (thrice weekly, SC) for 12 weeks. Thirteen patients with HM and 8 patients with ST received epoetin-alpha 150 U/kg and 13 patients with HM and 7 patients with ST received epoetin-alpha 250 U/kg.
The patients that have ferritin levels below 400 μg/dL were also given iron supplementation (peroral or intravenously).
All patients continued to receive chemotherapy and/or radiotherapy during study period. In both groups patients received transfusion if Hb levels decrease below 7 g/dL.
A routine evaluation performed at the beginning of the study that consisted of anthropometric and blood pressure measurements, physical examination, a complete blood cell count including reticulocyte count, and blood chemistry. Complete blood count was repeated weekly. Other evaluations were repeated twice a month.
The patients in both groups served as their own controls. Their Hb levels and transfusion requirements during 3 months before starting epoetin-alpha were also used to compare with the parameters recorded during 3 months after epoetin alpha treatment started.
Statistics
Student t test and Wilcoxon rank sign test (SPSS Software, USA) were used to test differences in means and the chi square test was used to test differences in proportions. Numerical values are expressed as mean ± SD.
RESULTS
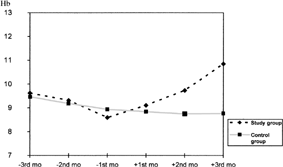
gives mean Hb concentrations of the study group and of the control group by months before and after epoetin-alpha treatment. Monthly comparisons of Hb were done using by two consecutive months. Hb levels of whole treatment group increased monthly after epoetin alpha started (, ).
Mean Hb Changes by Months in the Study and the Control Groups
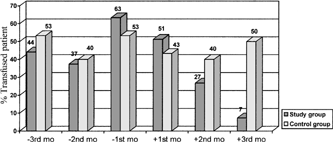
The mean Hb levels of either HM or ST groups were found to have significantly higher than those of the control group at the 2nd and 3rd months of epoetin- alpha treatment (). Similarly at the 3rd month of treatment, the patients have been found to require less red cell transfusion ().
Mean Hemoglobin Levels (g/dL) of the Study and the Control Groups. First 3-Month Period Served as Their Own Controls
Red Cell Transfusion Requirement by Months in the Study and the Control Groups (Unit Per Patient)
As to erythropoietin dose, none of the 20 patients at the dose of 250 U/kg required red cell transfusion beyond 2nd month of epoetin-alpha treatment. However, at the dose of 150 U/kg, only 3 out of 13 patients with HM, but none of the ST patients, required red cell transfusion after 2nd month.
clearly shows the decline in red cell transfusion requirement of the study group after erythropoietin started. Considering the total study period, it has been shown that the patients on epoetin alpha required less red cell transfusion as compared to their previous 3 months (85.3% vs 58.5%; p =. 01). But the control group did not show any decrease in terms of red cell transfusion requirement between their last 3-month period and first 3-month period (p >. 05).
Whether Hb level is taken above 8 g/dL to start epoetin-alpha for the patients with HM, these patients likely respond to treatment more and require less red cell as compared to those that have below 8 g/dL (p =. 02 for Hb increase and p =. 03 for red cell decline). Clearly the patients that have Hb level below 8 g/dL seem to respond better to epoetin-alpha at the dose of 250 U/kg rather than 150 U/kg ().
Mean Hb Concentrations of the Patients that Received Epoetin-Alpha at the Dose of 150 U/kg According to Initial Hb Concentrations of the Treatment
Mean Hb Changes by Months During Epoetin-Alpha Treatment at the Dose of 250 U/kg According to Initial Hb Levels of the Treatment
During the study period we did not record any serious adverse effect. However most of the patients experienced local pain and skin irritation at the injection site.
DISCUSSION
High cure rates for childhood cancers raise the questions of transfusion-associated diseases and quality of life in the patients on cancer therapy and in the long-term survivors. The more children are transfused by blood products, the more likely they become infected by the viruses in the developed and, particularly, in the developing countries. Therefore, use of erythropoeitin appears to be an option for treatment of anemia in children who undergo concomitant chemotherapy Citation[14].
There are only a few studies that have investigated the role of epoetin-alpha in children with cancer, however, no precise data about optimal dose and duration of erythropoietin are available in the current literature Citation[8–13]. In a phase I–II study done by Beck and Beck Citation[10], short term use of epoetin-alpha 100 U/kg for 14 days resulted no response to treatment in 18 children with different types of cancer. In a small study group including 5 leukemias and 10 solid tumors, Bolonaki et al. Citation[8] administered epoetin-alpha 150 U/kg thrice weekly for 8 weeks and found that 46% of the patients responded to this dose, but the rest required more erythropoietin doses such as 250 to 400 U/kg. In that trial it has been shown that red cell transfusion requirement decreased to 66% from 100%. Similarly, Porter et al. Citation[9] reported reduction of red cell and platelet transfusion requirements in patients with sarcoma by the concomitant administration of erythropoietin (150–300 U/kg) and iron for 16 weeks. Leon et al. Citation[13] administered epoetin-alpha 150 U/kg 5 times a week for 3 months and achieved 72% response rate in 25 children with different solid tumors. Varan et al. Citation[11] also recommended to use epoetin-alpha (without iron supplement) 150 U/kg thrice weekly for 2 months in patients with solid tumors. In our study 41 patients with either hematologic malignancy (21 patients) or solid tumor (14 patients) responded to epoetin-alpha administration, since their Hb levels increased significantly by the 2nd and 3rd months and red cell transfusion requirement decreased by the 3rd month of therapy. We did not find any difference among the patients on cisplatinum or non-cisplatinum chemotherapy, as most of the patients with solid tumor have been receiving cisplatinum-based chemotherapy.
As to erythropoetin dose, only 3 patients with hematologic malignancy required red cell transfusion at the dose of 150 U/kg, but none of the patients at the dose of 250 U/kg required transfusion after 2nd month of therapy. However, the patients with solid tumors did not require red cell transfusion at any dose of epoetin-alpha after 2nd month of treatment. It is clear that patients with solid tumors should not be given higher doses of eryhtropoetin other than 150 U/kg.
For all patients, despite red cell requirement of three-month period was 85% before epoetin-alpha treatment, it significantly decreased to 58% after erythropoietin started. This was in accordance to a previous finding Citation[8]. However, the control group displayed similar transfusion requirement trend during the second 3-month period of cancer treatment as it was observed during the first 3 months. This means that Hb increase and red cell transfusion decrease of the patients on epoetin-alpha is likely related to epoetin-alpha itself, but not to the dose intensity of the chemotherapeutic agents between the first and the last 3-month periods.
In conclusion, based on our results, epoetin-alpha administration three times weekly was effective and safe in children with hematologic malignancies or solid tumors. Epoetin-alpha administration results in Hb increases and patients need less red cell transfusion during cancer treatment. Further studies should address the optimum dose of epoetin-alpha for malignancy type in large randomized trials.
REFERENCES
- Skillings J R, Sridhar F G, Wong C, Paddock L. The frequency of red cell transfusion for anemia in patients receiving chemotherapy: a retrospective cohort study. Am J Clin Oncol.. 1993; 16: 22–25. [PUBMED], [INFOTRIEVE]
- Mohandas K, Aledort L. Transfusion requirements, risks, and costs for patients with malignancy. Transfusion. 1995; 35: 427–430. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Ferencz T, Csaki C S, Koos R, et al, Transfusion requirements following chemotherapy in pediatric solid tumors. Erythropoiesis. 1994; 5: 115–118
- Henry D H, Abels R I. Recombinant human erythropoietin in the treatment of cancer and chemotherapy-induced anemia: results of double blind and open label follow-up. Sem Oncol.. 1994; 21: 21–28
- Leitgeb C, Pecherstorfer M, Fritz E, Ludwing H. Quality of life in chronic anemia of cancer during treatment with recombinant human erythropoietin. Cancer. 1994; 73: 2535–2542. [PUBMED], [INFOTRIEVE]
- Ludwig H, Leitgeb C, Fritz E, et al, Erythropoietin treatment of chronic anemia of cancer. Eur J Cancer. 1993; 29 A(supp 12)8–12
- Littlewood T J, Bajetta E, Nortier J W, et al, Effects of epoetin alpha on hematologic parameters and quality of life in cancer patients receiving non-platinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol.. 2001; 19: 2865–2874. [PUBMED], [INFOTRIEVE]
- Bolonaki I, Stiakaki E, Lydaki E. Treatment with recombinant human erythropoietin in children with malignancies. Pediatr Hematol Oncol.. 1996; 13: 111–121. [PUBMED], [INFOTRIEVE]
- Porter J C, Leahey A, Polise K, et al, Recombinant human erythropoietin reduces the need for erythrocyte and platelet transfusions in pediatric patients with sarcoma: a randomized, double blind placebo-controlled trial. J Pediatr. 1996; 129: 656–660. [PUBMED], [INFOTRIEVE]
- Beck M N, Beck D. Recombinant erythropoietin in acute chemotherapy induced anemia of children with cancer. Med Ped Oncol.. 1995; 25: 17–21
- Varan A, Büyükpamukçu M, Kutluk T, Akyüz C. Recombinant human erythropoietin treatment for chemotheraphy related anemia in children. Pediatrics. 1999; 103: E16, [CROSSREF], [PUBMED], [INFOTRIEVE]
- Tamary H, Danon Y L. Recombinant human erythropoietin in children with cancer. Pediatr Hem Oncol. 1996; 13: 305–308
- Leon P, Jimenez M, Barona P, Sierrasesumaga L. Recombinant human erythropoietin for the treatment of anemia in children with solid malignant tumors. Med Ped Oncol.. 1998; 30: 110–116
- Macmillan M L, Freedman M H. Recombinant human erythropoietin in children with cancer. J Ped Hem Oncol.. 1998; 20: 187–189