ABSTRACT
Cisplatin and carboplatin are effective antineoplastic agents. They are also considered to be potentially highly ototoxic. To date, no long-term follow-up data from well-documented cohorts with substantial numbers of childhood cancer survivors (CCS) with platinum-related hearing loss are available. Therefore, in this study, we studied the reversibility of ototoxicity from discontinuation of treatment onwards in a national cohort of platinum-treated survivors with hearing loss at the end of cancer treatment. Of the 168 CCS with follow-up audiograms, we longitudinally evaluated the course of hearing function in 61 CCS who showed hearing impairment at discontinuation of treatment according to the Münster criteria (>20 dB at ≥4–8 kHz). Survivors were treated with platinum (median total cumulative dose cisplatin: 480 mg/m2 and median total cumulative dose carboplatin: 2520 mg/m2). Median follow-up time was 5.5 years (range: 1.0–28.8 years). The results showed that none of these survivors revealed improvement of hearing function even till 28.8 years after discontinuation of treatment (grade <2b during long-term follow-up). An increase in hearing loss with two or three Münster degrees was observed in five of 61 survivors after 1.6–19.6 years. Overall, this indicates that ototoxicity after platinum treatment may be irreversible and that longitudinal clinical audiological monitoring and care is required in long-term survivors of childhood cancer on a large scale.
Introduction
As survival rates of children with platinum-treated solid tumors have substantially improved, the understanding of both short- and long-term side effects of platinum treatment becomes increasingly important. Ototoxicity has been reported as a severe potential side effect of platinum agents Citation[1–3]. Platinum-induced ototoxicity is characterized by high-frequency sensorineural hearing loss, and newer audiologic methods such as extended high-frequency audiometry show that hearing loss can already occur at 12 kHz. This can subsequently progress during treatment toward hearing loss at lower frequencies involved in speech (0.5–6 kHz), of which 1–2 kHz frequencies are most important for speech. Consequently, ototoxicity may lead to a delay in speech, language, and social-emotional development in children, and subsequently it contributes to learning and developmental difficulties Citation[4, 5].
To date, only scarce data are available on the longitudinal development of hearing impairment from discontinuation of treatment toward later in life. Ototoxicity most often appears during therapy. Nine studies included follow-up data on the course of hearing function after platinum treatment in childhood cancer survivors (). However, these studies were relatively small and did not report the influence of environmental factors such as the coexistence of middle ear problems or head trauma. Other reports comprised small series and case-reports of childhood cancer survivors with ototoxicity Citation[6–9]. Studies including larger number of patients with hearing loss that investigate the reversibility of hearing loss during long-term follow-up are lacking.
Figure 1. Flow chart of childhood cancer survivors with hearing impairment at discontinuation of treatment.
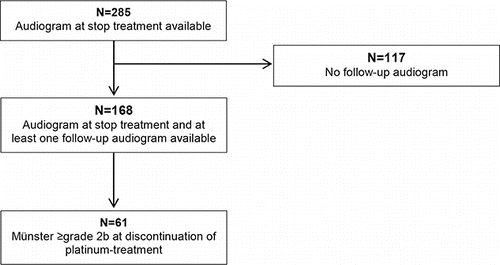
Figure 2. Childhood cancer survivors with hearing impairment at discontinuation of treatment (Münster ≥2b). Survivors with hearing impairment that persisted with equal severity (A). Survivors with aggravating hearing impairment over time (B). Survivors with >2 grade loss over time are depicted in red and indicated by an asterisk. Hearing did not recover in any of the survivors.
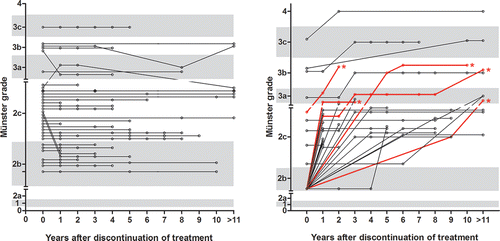
Table 1. Studies focusing on the course of hearing impairment after discontinuation of platinum treatment.
In the current study, we prospectively evaluated the course of serious hearing impairment at the time of completion of treatment after long-term follow-up in platinum-treated pediatric CCS based on a national cohort.
Materials and methods
This study aims to describe the course of hearing function in long-term survivors of childhood cancer that revealed ototoxicity at discontinuation of treatment. The assessment of determinants of ototoxicity after platinum treatment is subject of another study by the Dutch Childhood Oncology Group (DCOG)—Long-term Effects after Childhood Cancer (LATER) group Citation[10].
We performed a cross-sectional, multicenter study in long-term survivors of childhood cancer who received platinum agents but not cranial irradiation, diagnosed between April 1980 and September 2012. The study was approved by the local medical ethical committee of the Erasmus Medical Center, Rotterdam, the Netherlands (MEC-2015-269, EMC). Treatment details, as well as patient characteristics, were retrieved from databases and medical records of all seven pediatric oncology centers in the Netherlands as part of the DCOG LATER study. We excluded survivors with known pre-existing persistent hearing impairment at start therapy, as well as those who had received cranial or local irradiation to the ear/neck region. We included platinum-treated patients with clinical relevant hearing loss at discontinuation of platinum treatment, as defined by Münster grade ≥2b (>20 dB at ≥4–8 kHz) Citation[11].
Pure tone audiometry (PTA) was used to evaluate hearing function in children over 5 years of age. PTA is performed at six different frequencies (0.25, 0.5, 1, 2, 4, and 8 kHz) in both ears. Conditioned play audiometry was used for children from approximately age 2 years onward, and visual reinforcement audiometry was used for children aged between 6 months and 2 years. The co-existence of middle ear problems was excluded by further audiological measures and tests. Patients with unreliable tests were also excluded. All audiograms were interpreted by a well-trained investigator (EC). For quality control, a random sample of 10% of the audiograms was examined by a second, independent, international experienced audiologist (AaZ-D). Hearing impairment was evaluated using Münster grading system () Citation[11]. The Münster's grading system was used as it is considered as providing the highest sensitivity, specificity, and positive and negative predictive value compared to other ototoxicity classification systems Citation[12, 13]. In addition, the International Society of Pediatric Oncology (SIOP) Boston grading system was used as a secondary, independent validation Citation[11, 14]. The SIOP Boston scale is considered a useful scale for studies of platinum-induced ototoxicity Citation[11, 14].
Table 2. Münster and SIOP Boston classification for platinum-induced ototoxicity.
Significant improvement in hearing function was defined as a return to <Münster 2b at follow-up. Aggravation of hearing impairment was defined as >1 (Münster) scale increase. Audiograms performed within 1 year after discontinuation of platinum treatment were used as baseline audiograms. Audiograms performed more than 1 year after discontinuation of platinum treatment were used as follow-up audiograms.
Results
The study population consists of CCS treated at the Erasmus Medical Center, Rotterdam, the Netherlands and a cohort of CCS treated between 1963 and 2002 as part of the DCOG LATER study. The included cohort consists of CCS treated with platinum who were not cranial irradiated and with an audiogram <1 year after discontinuation of platinum treatment (n = 285). In 117 of these 285 survivors, no follow-up audiograms were performed (). The remaining 168 survivors were included in the analysis with one or more audiograms after discontinuation of treatment (). The survivors with follow-up audiograms (n = 168) were not different with respect to age at diagnosis, gender, and diagnosis than survivors without follow-up audiograms (n = 117). In contrast, survivors with audiograms received a slightly higher median total cumulative dose cisplatin (480 mg/m2 vs. 408 mg/m2, p = 0.044) and were more often treated with cisplatin than carboplatin (p = 0.031). Of the 168 survivors with follow-up audiograms, 61 (36.3%) had hearing impairment at discontinuation of treatment (Münster grade ≥2b). CCS were diagnosed with sarcoma (59%), neuroblastoma (18%), hepatoblastoma (9.8%), germ cell tumor (8.2%), low-grade glioma (3.3%), and nephroblastoma (1.6%). Median age at diagnosis was 9.4 years (range: 0.1–17.2 years). Follow-up time ranged between 1.1 and 27.2 years after completion of treatment, with a median of 5.9 years. Forty-six CCS had been treated with cisplatin only at a median cumulative dose of 480 mg/m2 (range: 180–900 mg/m2). Two CCS had been treated with carboplatin only at a cumulative dose of 1288 and 3230 mg/m2. The remaining thirteen CCS had been treated with both platinum agents. The median cumulative dose of cisplatin received was 400 mg/m2 (range: 300–570 mg/m2), and the median cumulative dose of carboplatin received was 1700 mg/m2 (range: 992–3938 mg/m2 ().
Table 3. Characteristics of the survivors.
Among the 61 CCS with Munster grade ≥2b hearing impairment, Münster score remained unaltered over time in 32 survivors after a median time of 5.1 years (range 1.1–21.3 years; and Supplementary Table S1). A subset of 24 CCS showed one Münster grade increase after a median time of 3.5 years (range: 1.1–21.3 years). After a median time of 2.1 years (range 1.6–9.9 years), three CCS showed an increase of two Münster grades and after a median time of 12.4 years (range 5.2–19.6), two survivors showed an increase of three Münster grades (, Supplementary Table S1). There are no significant risk factors for developing none vs. ≥2 grades of hearing impairment over time. In none of the survivors, Münster score improved over time.
Using the SIOP Boston scale, 53/168 (32%) of the CCS had hearing impairment at discontinuation of treatment. Among these 53 survivors with SIOP Boston ≥grade 2 hearing impairment, SIOP Boston score remained unaltered over time in 47 survivors after a median time of 9 years (range 1.1–21.3 years; Supplementary Table S1). A subset of 5 CCS showed one SIOP Boston scale increase after a median time of 3.8 years (range: 1.6–24.7 years) and one CCS showed two SIOP Boston scales increase after 1.1 years. Similar to the Münster criteria, in none of the survivors, SIOP Boston scale improved over time.
Discussion
The purpose of this study was to reveal the course of hearing loss during long-term follow-up in a cohort of CCS with hearing impairment at time of discontinuation of platinum treatment. Although the risk of platinum-related hearing loss is common knowledge, so far long-term follow-up data on the (ir)reversibility of hearing impairment are scarce and are based on limited numbers and incomplete data. In the current study, we showed that none of the survivors of childhood cancer with clinical relevant hearing impairment according to both Münster and SIOP Boston scale at discontinuation of treatment demonstrated recovery of hearing function to better ranges (Münster grade <2b or SIOP Boston grade <2) during follow-up. This indicates that hearing impairment is irreversible on the long term with its attendant consequences for speech development, social-emotional development, and learning development.
This finding is in agreement with those of previous literature describing the pathophysiology of platinum-induced ototoxicity in mice, Citation[15, 16] which shows that cisplatin causes DNA lesions by forming intrastrand and interstrand crosslinks, activating several signal transduction pathways. In vitro, this results in the activation of apoptosis Citation[17]. Furthermore, cisplatin induces reactive oxygen species formation, leading to apoptosis of the hair cells Citation[15, 18]. The consequently degenerated cochlear inner and outer hair cells, once damaged, are suggested not to have the potential to regenerate.
In five of the 61 survivors, we found serious aggravation of hearing loss (two or three degrees of severity) after 1.6–19.6 years. These survivors with ≥2 grade hearing loss over time were all women, whereas in the total cohort, women only represent 48%. They were diagnosed between 9 and 12.7 years of age with neuroblastoma (n = 1), osteosarcoma (n = 2), and germ cell tumor (n = 2).
In our study, no detailed information about noise exposure, or use of ototoxic medication after discontinuation of treatment, could be retrieved because of the cross-sectional design of the study. In future studies, prospective collection of such confounding factors may be of value in the identification of survivors for early monitoring of hearing impairment in the future. Timing of audiometric testing was not equal among survivors, and follow-up audiograms varied between CCS, from 1.6 to 21.3 years after the end of treatment. On one hand, survivors with a late follow-up audiogram could have progression of hearing loss already early after the first follow-up audiogram. On the other hand, survivors with brief follow-up could have progression of hearing loss at a later time point that was missed.
We are the first to report the irreversibility of hearing loss after platinum treatment in a well-documented substantial cohort of platinum-treated survivors of childhood cancer that were noncranial irradiated. Our study confirms the results of a previous smaller study in 13 CCS, which showed that hearing loss is indefinite Citation[19]. To date, only a few studies have issued the course of platinum-induced hearing loss during follow-up Citation[6–8, 20–22]. These studies included limited number of patients, Citation[6–8, 21] included cranial irradiated patients, Citation[8, 20, 21] and did only cover one or two platinum-treated malignancy types Citation[20–22]. Nitz et al. studied children with osteosarcoma and soft-tissue sarcoma and identified 55/112 with hearing loss (<4 kHz and ≥4 kHz). Of these, 51 showed no recovery of function and four showed improvement of hearing function over time. However, it was not described if hearing function improved to normal hearing ranges Citation[20]. Qaddoumi et al. Citation[21] suggested that in 2/12 retinoblastoma patients treated with carboplatin, hearing impairment (Brock grade 1 and grade 4) resolved, but it was unsure up to what level. Stohr et al. Citation[22] described a frequency of 20/42 osteosarcoma patients with hearing impairment after discontinuation of cisplatin treatment. After a median follow-up time of 5.5 years, 18 patients had permanent hearing loss. Only 2/20 showed hearing improvement to <grade 2 (self-designed scoring system). Confounding factors with regard to infection and co-medication were not taken into account in any report at time of assessment of hearing function. An unpublished study by Dutton et al. Citation[23] reported 14 patients without recovery of hearing function at 2.5–10 years after treatment. Although some of these studies do not seem to be in line with our results, improvement of hearing function to normal values has never been well documented after initial hearing loss in the range of Münster grade ≥2b or equal levels of hearing loss using other classification methods (e.g. Brock or Chang) Citation[20–22].
We conclude from our study in noncranial irradiated pediatric patients that the risk of permanent platinum-related hearing loss is substantial when this is confirmed at the end of cancer treatment. Although this hearing loss is constant in most cases, 30 survivors even showed aggravation (≥1 Münster grade). Therefore, clinical surveillance is necessary before more significant frequencies for speech perception are affected. On an international level (International Guideline Harmonization Group (IGHG)), in collaboration with the survivors group of PanCare, clinical surveillance guidelines for long-term follow-up of hearing loss in childhood cancer survivors are now being harmonized. This will aid in the awareness of health-care providers of this cancer-related permanent high-impact treatment-related health risk and will guide optimum care for survivors.
Compliance with ethical standards
This study has been submitted by the medical ethical committee and was approved as a non-WMO study (MEC-2015-269) because only data were retrieved from databases and medical records.
Conflicts of interest
The authors declare no conflicts of interest.
Supplementary_Table_1.docx
Download MS Word (22.8 KB)Funding
E.C. and S.F.M.P. are supported by the PanCareLIFE project that has received funding from the European Union's Seventh Framework Programme for research, technological development, and demonstration under grant agreement no 602030. A.C.H.d.V. is supported by the Paediatric Oncology Centre Society for Research (KOCR), Rotterdam, the Netherlands.
References
- Grewal S, Merchant T, Reymond R, McInerney M, Hodge C, Shearer P. Auditory late effects of childhood cancer therapy: a report from the Children's Oncology Group. Pediatrics. 2010;125(4):e938–950.
- Brock PR, Bellman SC, Yeomans EC, Pinkerton CR, Pritchard J. Cisplatin ototoxicity in children: a practical grading system. Med Pediatr Oncol. 1991;19(4):295–300.
- Coradini PP, Cigana L, Selistre SG, Rosito LS, Brunetto AL. Ototoxicity from cisplatin therapy in childhood cancer. J Pediatr Hematol Oncol. 2007;29(6):355–360.
- Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear. 1998;19(5):339–354.
- Davis JM, Elfenbein J, Schum R, Bentler RA. Effects of mild and moderate hearing impairments on language, educational, and psychosocial behavior of children. J Speech Hear Disord. 1986;51(1):53–62.
- Berg AL, Spitzer JB, Garvin JH, Jr. Ototoxic impact of cisplatin in pediatric oncology patients. Laryngoscope. 1999;109(11):1806–1814.
- Bertolini P, Lassalle M, Mercier G, et al. Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol. 2004;26(10):649–655.
- Einarsson EJ, Petersen H, Wiebe T, Fransson PA, Magnusson M, Moell C. Severe difficulties with word recognition in noise after platinum chemotherapy in childhood, and improvements with open-fitting hearing-aids. Int J Audiol. 2011;50(10):642–651.
- Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23(34):8588–8596.
- Clemens E, de Vries AC PS, am Zehnhoff-Dinnesen A, et al. Determinants of ototoxicity in 451 platinum-treated Dutch survivors of childhood cancer: a DCOG late-effects study. Eur J Cancerq. 2016;69:77–85.
- Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol. 2012;30(19):2408–2417.
- Lanvers-Kaminsky C, Krefeld B, Dinnesen AG, et al. Continuous or repeated prolonged cisplatin infusions in children: a prospective study on ototoxicity, platinum concentrations, and standard serum parameters. Pediatr Blood Cancer. 2006;47(2):183–193.
- Schmidt CM, Bartholomaus E, Deuster D, Heinecke A, Dinnesen AG. The “Muenster classification” of high frequency hearing loss following cisplatin chemotherapy. Hno. 2007;55(4):299–306.
- Bass JK, Huang J, Onar-Thomas A, et al. Concordance between the chang and the International Society of Pediatric Oncology (SIOP) ototoxicity grading scales in patients treated with cisplatin for medulloblastoma. Pediatr Blood Cancer. 2014;61(4):601–605.
- van Ruijven MW, de Groot JC, Klis SF, Smoorenburg GF. The cochlear targets of cisplatin: an electrophysiological and morphological time-sequence study. Hear Res. 2005;205(1–2):241–248.
- McAlpine D, Johnstone BM. The ototoxic mechanism of cisplatin. Hear Res. 1990;47(3):191–203.
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22(47):7265–7279.
- Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33(1):9–23.
- Al-Khatib T, Cohen N, Carret AS, Daniel S. Cisplatinum ototoxicity in children, long-term follow up. Int J Pediatr Otorhinolaryngol. 2010;74(8):913–919.
- Nitz A, Kontopantelis E, Bielack S, et al. Prospective evaluation of cisplatin- and carboplatin-mediated ototoxicity in paediatric and adult soft tissue and osteosarcoma patients. Oncol Lett. 2013;5(1):311–315.
- Qaddoumi I, Bass JK, Wu J, et al. Carboplatin-associated ototoxicity in children with retinoblastoma. J Clin Oncol. 2012;30(10):1034–1041.
- Stohr W, Langer T, Kremers A, et al. Cisplatin-induced ototoxicity in osteosarcoma patients: a report from the late effects surveillance system. Cancer Invest. 2005;23(3):201–207.
- Dutton SC NM, Billett AE, LaVally B, Scott RM, Sallan SE, and Tarbell NJ. Progressive ototoxicity after combined modality treatmen of medulloblastoma. 38th annual meeting of the American Society for Therapeutic Radiology and Oncology (ASTRO); 27–30 Oct 1996; Los Angeles, United States.
