Abstract
Brain tumors (BTs) are a common pediatric malignancy. Improved treatment has resulted in higher survival rates. There is, however, increasing concern about adverse effects of the disease and its treatment, including effects on social competence (i.e. effective social functioning in everyday life). The aim of this study is to examine multiple levels of social competence (i.e. social skills and social adjustment) in newly diagnosed pediatric BT patients. Thirty newly diagnosed BT patients aged 5–12 years were assessed shortly after diagnosis with a neuropsychological test battery focusing on social competence, including tests for IQ, social skills (i.e. social-affective and executive functioning) and social adjustment (rated by parents and teachers). Their performance was compared to 95 healthy controls who completed the same assessment. Patients and healthy controls were largely comparable with regard to demographic and environmental factors and did not differ on measures of IQ, social skills and social adjustment. Furthermore, age was found to have a positive significant effect on social skills independent of group. Shortly after diagnosis, pediatric BT patients did not perform different from healthy controls on IQ and measures of social skills and social adjustment. This is an encouraging finding. However, because of potentially neurotoxic adjuvant therapy and the ongoing development of social skills, longitudinal follow-up studies are needed to investigate long-term outcome regarding social competence in BT survivors.
Introduction
With the increased survival of pediatric brain tumor (BT) patients, there is a growing attention for the late effects of the disease and its treatment. Studies in pediatric BT survivors show impairment in social competence (i.e. effective social functioning in everyday life) which puts patients at risk of developing psychological problems. Social competence is an important predictor of quality of life and also of (later) academic, vocational and romantic functioning. Currently, it is unclear how, when and at which level(s) of social competence these deficits arise in BT survivors.
The current study is the first to investigate multiple levels of social competence (i.e. social skills and social adjustment) in newly diagnosed BT patients prospectively. Patients between the ages of five to twelve were assessed with tests for intelligence, social-affective and executive functions (i.e. social skills) and social adjustment and compared to healthy controls. No differences were found between BT patients and healthy controls regarding social skills and social adjustment. Age had a significant positive effect on social skills which supports the ongoing development of social competence throughout childhood.
It is comforting and of clinical and scientific relevance for pediatric BT patients, their family and clinicians to know that social competence, despite its complexity, appears not to be significantly affected in the early stage of the disease. However, adjuvant treatment, particularly cranial radiation therapy, may contribute to later poor social skills and adjustment. Furthermore, brain damage related to the tumor and/or surgery may only result in obvious deficits many years later. A follow-up study of these patients has, therefore, been planned in order to study social competence in brain tumor survivors several years after diagnosis.
Introduction
Brain tumors (BTs) are the second most common pediatric malignancy, accounting for approximately 20–25% of pediatric cancers. Average long-term (i.e. 5 year) survival is around 60%.Citation1 Medical treatment includes surgery, cranial or craniospinal radiation (CRT) and/or chemotherapy.Citation2,Citation3 With improved survival, increasing concern has emerged about adverse late effects of the disease and its treatment. Deleterious late effects include neurological, endocrine and (neuro)psychological impairment with subsequent negative effects on school career, employment and quality of life.Citation3 Furthermore, psychosocial functioning and in particular social competence (i.e. effective social functioning in everyday life) appears to be affected in BT survivors and can increase the risk of developing psychological problems.Citation4 Currently, it is unclear how, when and at which level(s) of social competence these deficits arise in pediatric BT patients.
Social competence is defined as a multi-level construct consisting of three interrelated factors ().Citation5 The first factor, social skills, refers to the child’s cognitive functions that are relevant for competent social functioning: social-affective and executive functions. Social skills influence the efficiency of the child’s social interaction or performance (factor 2) in daily life (e.g. being prosocial, aggressive, withdrawn). The extent to which the child’s performance is developmentally appropriate (perceived by self and others) constitutes social adjustment (factor 3). Schulte and Barrera concluded in their review that BT survivors experience persisting social adjustment problems.Citation4 Deficits in social-affective functions (i.e. the ability to understand and interact with other people) and executive functions (i.e. cognitive functions needed for efficient and goal-directed behavior) have also been shown in BT patients after treatment.Citation6–12 The few pretreatment studies on social adjustment or (parent rated) executive functioning reported group scores within the normal range.Citation13–15 So far, none of the studies examined social-affective and executive functioning (using neuropsychological tests) as well as social adjustment in newly diagnosed BT patients before the start of adjuvant therapy. Social-affective and executive functions are subserved by a complex network of brain structures including (pre)frontal and temporal areas, the anterior cingulate cortex, the amygdala, anterior insula and the cerebellum.Citation6,Citation16–18 This network, which is still developing throughout childhood and adolescence, may be affected by brain damage caused by the BT itself, surgical procedures and/or CRT. This makes age an essential variable in determining social consequences of brain damage.Citation19 So far, the factor consistently found to be negatively related to development of social skills and social adjustment in pediatric BT patients is time since diagnosis.Citation4,Citation9 Other possible risk factors include a history of CRT and a younger age at diagnosis.Citation7,Citation9,Citation20 Furthermore, factors like premorbid functioning, intellectual ability, social context and family situation (e.g. socio-economic status (SES), number of parents in the home) as well as the psychological impact of having a serious (chronic) illness are also of importance.Citation5,Citation20–23
Figure 1. Simplified model of social competence. An adapted version of Yeates’ integrative, heuristic model of social competence in children with brain disorders.Citation5
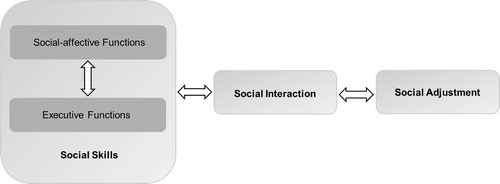
The aim of the current study is to examine multiple levels of social competence (i.e. social skills and social adjustment) in newly diagnosed pediatric BT patients compared to healthy controls.
Materials and methods
Participants
BT patients were recruited between 2011 and 2014 through four hospitals in the Netherlands and Belgium: University Medical Center Groningen (UMCG), Radboud University Medical Center Nijmegen, VU University Medical Center Amsterdam and University Hospitals Leuven (UZL). Patients, parents and teachers were asked for participation when the child was newly diagnosed, aged between 5 and 12 with a stable medical condition (e.g. no poor prognosis and with a life expectancy of >1 year). Exclusion criteria were autistic spectrum disorder (ASD), history of brain disease or neurological condition that affected normal development, IQ below 70 or severe sensory handicaps and/or behavioral problems hampering reliable neuropsychological assessment.
In total, 35 patients were included and assessed. Five children were excluded after assessment because of genetic disorders (n = 2), ASD (n = 1), abnormal development due to prematurity (n = 1) or assessment after receiving CRT (n = 1). Consequently, 30 patients were included (50% girls) for further analysis. All children underwent neurosurgical intervention (i.e. resection or biopsy). Fifteen children were assessed before brain surgery, 14 children were assessed after surgery but before receiving adjuvant therapy. In one case, only questionnaires were administered. In case of hydrocephalus and/or heightened intracranial pressure, assessments were scheduled after drain placement or third ventriculostomy (n = 5). All children had Dutch as a first language. Mean age at diagnosis was 8 years and 4 months and mean age at assessment was 8 years and 5 months. On average, patients that were seen before surgery were 11 months older at time of assessment than patients assessed after surgery.
During the same period, healthy Dutch and Flemish children were randomly selected per class from four primary schools. Flemish children were also recruited through personal contacts of the researchers. To create a control group with an equal representation of different age groups and an average IQ within 1 SD of the population mean, a pre-selection of children was made based on age and school performance (i.e. focusing on the lower scoring half of the class) at two additional Dutch primary schools. After the pre-selection, a random selection was made. Inclusion and exclusion criteria for healthy controls were similar to those of patients. In total, 108 children were included and assessed. Four children were removed from the sample after assessment due to IQ below 70 (n = 1), rheumatoid arthritis (n = 1) or ASD (n = 2). Another nine children were randomly removed because one of their siblings also participated in this study. The final sample consisted of 95 children (55% girls) with a mean age at assessment of 8 years and 9 months.
This study was approved by the UMCG Medical Ethical Committee and the UZL Ethical Board. All parents, teachers and children aged 12 years and older gave their informed consent before study inclusion.
Measures
Social-affective functions
The Facial Affect Recognition (FAR) subtest of the NEPSY-II-NL was administered in all children to assess the ability to discriminate between facial expressions. The NEPSY-II-NL Theory of Mind (ToM) subtest was used in all children to examine the ability to understand beliefs, intentions, deception, emotion, imagination and pretending (i.e. basic ToM). The reliability of both subtests is adequate. The validity of these subtests seems acceptable, however this judgement is based on a minimal amount of information presented in the manual.Citation24 In children aged 8 years and over, complex ToM was assessed with a Dutch translation of a short form of the Strange Stories Test (SST).Citation25,Citation26 Because the SST is an experimental test, no research on reliability and validity has been conducted. However, the SST is sensitive to mentalizing deficits in children with high functioning ASD and shows a moderate intercorrelation with classic false belief tasks.Citation25 The total correct scores for all tests were used for analysis.
Executive functioning
The Digit Span Backward subtest of the Wechsler Intelligence Scales for Children (WISC-III-NL) or the Numbers Backward subtest from the Children’s Memory Scale was administered from 6 years onward to assess working memory (WM). Reliability of both subtests is acceptable.Citation27,Citation28 The Trail Making Test (TMT) intermediate form was administered from 8 years onward and the time needed to complete part B was used as a measure of cognitive flexibility.Citation29 Reliability of part B seems acceptable, the test is sensitive to brain damage in children and associated with vocational outcome in adulthood after childhood traumatic brain injury.Citation30 Furthermore, planning and problem solving was assessed by the total correct score of the Zoo Map Part 1 of the Behavioral Assessment of the Dysexecutive Syndrome for Children which was administered from 8 years onward. The Zoo Map part 1 has adequate reliability. The validity of this test seems questionable based on the little data that is presented in the manual.Citation31 Parents and teachers completed the Behavior Rating Inventory of Executive Function (BRIEF) which assessed executive functions. Reliability of this questionnaire is adequate and validity acceptable.Citation32 The total scores of Behavioral Regulation and Metacognition were used for analysis.
Social adjustment
Social adjustment was assessed by the Child Behavior Checklist (CBCL) and the Teacher Report Form (TRF) 6–18. The CBCL Social Competence score and the CBCL and TRF Social Problem subscales were used for analysis. Both questionnaires have adequate reliability and validity. Reliability for the CBCL and TRF Social Problem subscales is adequate, but low for the CBCL Social Competence scale. The latter is explained by the fact that this scale is not aimed at measuring one characteristic like the Social Problem subscales but measures several skills using different question formats within one domain.Citation33
Intellectual abilities
Intellectual ability was measured by the WISC-III-NL Similarities, Vocabulary (verbal IQ; VIQ), Block Design and Object Assembly (performance IQ) subtests in children aged 6 years and over. In 5-year-olds and in two 6-year-old children for whom the WISC appeared too difficult (both assessed after surgery), equivalent subtests of the Wechsler Preschool and Primary Scale of Intelligence˗III-NL were used. Both intelligence tests have adequate reliability and validity and the reliability of the individual subtests is acceptable.Citation34 In seven patients (five assessed before and two assessed after surgery) and one control, a proxy of VIQ was used, based on one verbal subtest. Due to an error in the administration of Object Assembly, only Block Design was included in the analyses as a measure of visuospatial ability.
Sample characteristics
Parents provided information on their occupation, education, family structure (e.g. living with one or two parents, number of siblings, birth order). Furthermore, parents were asked about the presence of developmental disorders in their child by means of a single open-ended question. Based on parental occupation and education, a SES score was calculated and used for analysis.Citation35 For BT patients, information about diagnosis and treatment was collected from medical records. Medical information and data on family structure were used descriptively.
Procedure
Neuropsychological assessments in Dutch BT patients were conducted by TBK and Flemish patients were assessed by JL. Intelligence tests and questionnaires in Flemish patients were administered as part of standard hospital protocol by a colleague neuropsychologist of JL. Data in Dutch patients was mostly gathered during one session, in Flemish patients it was gathered over two sessions. In BT patients, the administration of tests was not conducted in a fixed order because it was often combined with a standard clinical assessment. In all cases test administration did not exceed 1.5 hours, contained frequent breaks and was adjusted to the patient in such a way that the child was still motivated and able to complete as many tests as possible. Parents received the questionnaires immediately after the introduction of the study. However, because of the nature of the disease and timely surgical intervention, not all parents were able to complete the questionnaires before the surgery. All parents were specifically instructed to complete the questionnaires based on their observations of their child prior to brain surgery. Parents completed the questionnaires before, during or after the assessment (BRIEF: 34.58 ± 72.68; CBCL: 26.57 ± 75.15; range: −1 to 291 days relative to neuropsychological assessment). Teachers completed questionnaires after consent of parents and/or patient was given to approach them (BRIEF: 103.44 ± 111.82; TRF: 92.55 ± 121.78; range: −35 to 287 days relative to assessment).
Test administration in healthy controls took approximately 1 (5–7 year olds) to 1.5 hours (≥ 8 years). Master Psychology students of the University of Groningen and Catholic University of Leuven were thoroughly trained by TBK or JL and tests were administered at the child’s primary school or home in one or two sessions in the following order: TMT, WISC-III-NL Object Assembly, Digit Span Backward or CMS Numbers Backward, NEPSY-II-NL ToM, NEPSY-II-NL FAR, WISC-III-NL Similarities and Block Design, SST, Zoo Map part 1 and WISC-III-NL Vocabulary. Parents (BRIEF: 34.58 ± 72.68; CBCL: 12.37 ± 28.33; range: −7 to 164 days relative to assessment) and teachers (BRIEF: 23.51 ± 24.86; TRF: 25.74 ± 26.66; range: −9 to 90 days relative to assessment) of healthy controls completed the questionnaires around the time of the assessment. Parents of all included patients and healthy controls received a short written report of the assessment.
Statistical analysis
Missing data analyses were conducted to explore whether the reason of missing data in patients might induce a selection bias. Missing data were categorized per test as either due to disease related factors, time constraints or errors in administration. Demographic and environmental characteristics of healthy controls and BT patients were compared using a Mann Whitney U-test for the variable age and chi-square tests for categorical variables. Categorical variables that did not meet assumptions of a chi-square test, were described qualitatively only.
To gain insight in performance of BT patients assessed before or after surgery and to determine if patients assessed before or after surgery could be treated as one group, age corrected scores were calculated for tests of which published Dutch normative data were available (i.e. IQ, FAR, basic ToM and WM). The reason for presenting normative scores instead of means and standard deviations is that the latter would not be very informative due to age differences between the two BT patients groups. Because of the small sample sizes of the groups of BT patients assessed before or after surgery, data was only analyzed qualitatively. Normative scores were allocated to the following categories: above average (z > 1), average (-1≥ z ≤ 1), below average (-1> z ≤-2) or impaired (z <-2). No Dutch normative data was available for the TMT and SST. For these tests the raw scores were inspected qualitatively. Furthermore, because parents and teachers were asked to rate how the child was functioning prior to surgery on the BRIEF, CBCL and TRF, comparison of questionnaire data of BT patients who were assessed before or after surgery was not considered relevant. If large differences between patients who were assessed before or after surgery were observed for a particular variable, no further analyses were conducted.
Multiple linear regression analyses or derivatives of regression analyses (i.e. ANOVA or t-test) were performed to test for differences between patients and healthy controls while controlling for any relevant demographic variables. VIQ, visuospatial ability, social skills or social adjustment were used as dependent variables. Raw scores were used for all analyses with the exception of VIQ which is a composite score that automatically includes a correction for age. Independent variables were group (patients, healthy controls) and either age (months), gender and/or SES (medium, high). Effects of age, gender, SES and relevant interactions were tested separately and were only included in the final model if they had a significant effect on the dependent variable (i.e. VIQ, visuospatial ability, basic and complex ToM, WM, cognitive flexibility, CBCL Social Competence). If only categorical predictors were included in the model, an ANOVA or t-test was performed. Effect sizes (Cohen’s d, adjusted R2, partial η2) and 95% confidence intervals of means and regression coefficients were calculated.Citation36 If assumptions for the regression analysis were not met, we checked for the presence of outliers and performed the analyses with and without these outliers. If needed, log transformations of the dependent variables were conducted. In case of not normally distributed variables, healthy controls and BT patients were compared with a Mann Whitney U-test (i.e. Behavioral Regulation and Metacognition parents and teacher, CBCL and TRF Social Problems). Effect sizes (ƞη2), medians, interquartile ranges and boxplots were given.Citation37
Results
Missing data
On average and across variables, approximately 80% of missing data of BT patients was missing due to disease related factors; 20% was missing because of errors in administration and time constraints during testing. For the planning and problem solving task, more than half of the data were missing because of medical reasons (e.g. fatigue, diminished attention, blindness) and sample size was very small (n = 7). Therefore, outcomes were considered unrepresentative for the BT population and these data were not reported. Regarding questionnaires, the primary reason for missing data was non-response which was higher in teachers (range = 47%–58%) than parents (range = 8%–13%).
Sample characteristics
Age at assessment did not differ significantly between groups and neither did the distribution of gender and of medium and high SES across groups. A minor difference was observed between patients and healthy controls concerning birth order, but this difference was not significant. All other variables (i.e. developmental disorders, number of siblings and family composition) violated the assumptions of a chi-square test. However, qualitative inspection revealed no apparent differences between groups (). Clinical characteristics of BT patients are presented in .
Table 1. Demographic and environmental characteristics of brain tumor patients (N = 30) and healthy controls (N = 95).
Table 2. Clinical characteristics of brain tumor patients (N = 30).
Qualitative exploration of data of BT patients assessed before or after surgery
While no marked group differences were observed for VIQ, visuospatial ability, basic ToM, SST and WM, BT patients assessed after surgery seemed to obtain impaired FAR scores more often than patients assessed before surgery and healthy controls (). Also, patients assessed after surgery performed relatively worse for their age on cognitive flexibility than those assessed prior to surgery and healthy controls. Therefore, no further statistical analyses were conducted for FAR and cognitive flexibility performance.
Table 3. Age corrected scores based on published Dutch normative data of the brain tumor patients that were assessed before (N = 15) or after surgery (N = 14) for tumor removal and healthy controls (N = 95).
Quantitative comparison of BT patients and healthy controls
Performance of patients and healthy controls on measures of IQ, social skills and social adjustment can be found in .
Table 4. Outcomes on measures of verbal IQ, social-affective functions, executive functions and social adjustment for brain tumor patients (N = 30) and healthy controls (N = 95).
Intelligence
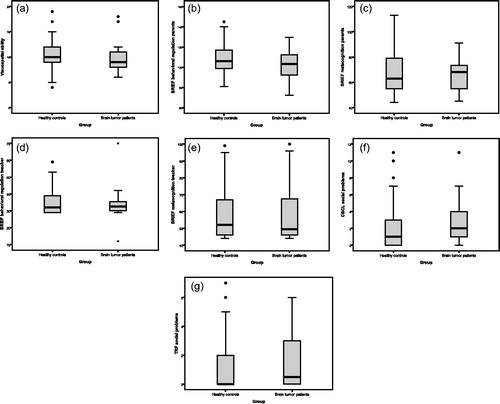
A log transformation normalized VIQ data and a two-way ANOVA was performed. The interaction effect was not significant (F(1,105) = 0.01, p = .92, partial η2 < .01). The model with only the main effects showed a significant result for SES (F(1,106) = 6.44, p = .01, partial η2 = .06) while group (F(1,106) = 1.01, p = .32, partial η2 = .01) did not contribute significantly. Patients and healthy controls did not differ regarding visuospatial ability (U = 701.00, z = −1.30, p = .19, ƞη2 = .02, ).
Figure 2. Boxplots of performances of brain tumor patients (N = 30) and healthy controls (N = 95) on visuospatial ability, executive functions and social adjustment.
Note. BRIEF = Behavior Rating Inventory of Executive Function; CBCL = Child Behavior Checklist; TRF = Teacher Report Form; A) N = 19 brain tumor (BT) patients and N = 91 healthy controls B) N = 27 BT patients and N = 87 healthy controls; C) N = 27 BT patients and N = 87 healthy controls; D) N = 16 BT patients and N = 79 healthy controls; E) N = 16 BT patients and N = 79 healthy controls; F) N = 22 BT patients and N = 72 healthy controls; G) N = 10 BT patients and N = 70 healthy controls.
Social-affective and executive functions
Results indicated that age (β = .71, p < .001, B = 0.11 [0.09;0.13]) predicted 52% of variance in basic ToM scores (F(3,109) = 41.71, p < .001). Group (β = .01, p = .92, B = 0.07 [−1.29;1.44]) and gender (β = −.11, p = .10, B = −0.87 [−1.93;0.18]) did not contribute significantly to the model. Complex ToM performance was significantly influenced by age (β = .41, p < .001, B = 0.04 [0.02;0.07]) and gender (β = −.27, p = .02, B = −.95 [−1.73;−0.17]) but not by group (β = .02, p = .84, B = 0.11 [−0.96;1.19]). The overall model fit was 18% (F(3,66) = 6.18, p = .001). Age (β = .38, p < .001, B = 0.03 [0.01;0.04]) significantly explained WM scores, while group (β = −.01, p = .94, B = −0.03 [−0.72;0.67]) did not significantly add to the model (adjusted R2 = 12%, F(2,98) = 8.03, p = .001). Healthy controls and patients did not differ significantly on parent and teacher rated Behavioral Regulation (parent: U = 1247.00, z = 0.48, p = .63, ƞη2 < .01; teacher: U = 640.00, z = 0.08, p = .94, η2 < .01) and Metacognition (parent: U = 1135.50, z = −0.26, p = .80, η2 < .01; teacher: U = 643.50, z = 0.12, p = .91, η2 < .01, to ).
Social adjustment
Regarding CBCL Social Competence, age was a significant predictor (β = .23, p = .03, B = 0.02 [0.01;0.03]) but group was not (β = −.10, p = .34, B = −0.42 [−1.28;0.45]). The total model explained 4% of variance (F(2,91) = 3.07, p = .05). No significant group differences were found for the TRF (U = 376.00, z = 0.42, p = .67, η2< .01) and CBCL Social Problem scales (U = 890.50, z = 0.12, p = .37, η2 < .01, and ).
Discussion
Newly diagnosed BT patients and healthy controls aged 5 to 12 years did not differ in performance on IQ and measures of social skills and social adjustment. This is in line with previous pretreatment studies in newly diagnosed BT patients which reported scores within the normal range for measures of social adjustment or (parent rated) executive functioning.Citation13–15 We extended these findings by showing no differences between patients and healthy controls on standardized neuropsychological tests for social˗affective and executive functioning. However, inspection of (normative) scores showed that BT patients assessed after surgery seemed to be impaired on FAR performance more often and performed relatively worse for their age on a cognitive flexibility task than patients assessed before surgery and healthy controls. We should be careful in attributing these differences to negative effects of surgery because 25% of the healthy controls also showed impaired FAR performance and no normative data was available for the cognitive flexibility task. Additionally, more severely ill patients were more likely to be assessed after surgery, effects of surgery are not always negative (e.g. relieve of intracranial pressure) and secondary (temporary) effects of illness and treatment like tiredness, pain or effects of general anesthetics might also have played a role.
It is encouraging that there were no significant differences between patients and healthy controls on measures of social skills and adjustment shortly after diagnosis and that only relatively few patients showed clinically impaired social skill performance. However, we cannot simply assume that the development of social competence in patients will follow a normal trajectory. Besides adverse effects of the tumor and/or surgery, late effects of CRT have also been suggested as a possible risk factor for diminished social skills and adjustment.Citation7,Citation9,Citation20 Second, also patients who have not received CRT may later develop (more) problems that were not present around the time of diagnosis.Citation2 This phenomenon is known as growing into deficit and may pose a risk to the development of social competence in BT survivors.Citation38 The age effects that were found in the current sample confirm the ongoing development of social-affective and executive functions in middle childhood.Citation16–18 Because of this continued development, the full impact of brain damage on social competence in BT survivors will not be clear until development is completed. These concerns are supported by retrospective studies in BT survivors that indicated significant deficits in social˗affective and executive functioning and social adjustment several years after treatment.Citation4,Citation7–10
Study limitations and implications
Our study comes with certain limitations (i.e. relatively small sample size, heterogeneity of the sample regarding age, diagnosis and presentation of the disease as well as differences in timing of assessment) which are part of performing a prospective study in severely ill children. Because of their illness, there were more missing data for BT patients than for healthy controls. This might have resulted in an overrepresentation of scores of patients that were least affected by disease or surgery, with possibly less affected cognitive and behavioral functioning, because they were most likely to complete the entire assessment. Furthermore, assessments in BT patients were not as standardized as in healthy controls, since they had to be adjusted to the individual needs and disabilities of patients. Although not all tests show adequate reliability and validity and adjustments to standard assessment were made, we are confident that we have selected the best test battery possible from the tests that were available at the start of this study. Furthermore, we have no reason to believe that the selected tests and questionnaires were inadequate in addressing social competence problems experienced by pediatric BT patients. The complexities of daily (social) life are obviously not fully captured by administering neuropsychological tests in a standardized setting. Most tests of social-affective functioning are rather structured and mostly involve children’s responses on hypothetical social situations.Citation39 Nevertheless, neuropsychological studies are relevant for understanding and treating social competence problems in BT patients.Citation40 Future studies could, however, further focus on the development of more ecologically valid measures of social-affective functioning in children. In addition, they could also include standardized observation during assessment to study social interaction and include patient reports or peer ratings to assess social adjustment in more detail.Citation41 Within the present study, several environmental factors were assessed that could be of influence on social competence. These factors focused on the family context (e.g. SES, family structure). It would be interesting to also address the social context (e.g. social support, peer acceptance) and more psychological family factors (e.g. family functioning, parental coping) in future research.Citation5,Citation39
Conclusion
The results of the current study are an important starting point in studying social competence after treatment of a BT in childhood because this is the first study that looks into multiple levels of social competence in newly diagnosed BT patients. The results show that, on average, performance of patients did not differ from healthy controls on measures of IQ, social skills and social adjustment. It is comforting and of clinical and scientific relevance for patients, their families and clinicians to know that social competence, despite its complexity, appears not to be significantly affected in the early stage of the disease. However, only the combination of the current findings with the follow-up of this sample will enable us to make more specific recommendations regarding clinical management of these problems. Adjuvant treatment, particularly CRT, may contribute to later poor social skills and adjustment. Furthermore, brain damage related to the tumor and/or surgery may only result in obvious deficits many years later. Therefore, more prospective longitudinal studies with larger samples of patients would contribute to the understanding of long-term development of social competence in pediatric BT survivors.
Declaration of interest
The authors report no conflict of interest.
Acknowledgements
We would like to thank the members of the pediatric neuro-oncology team of the University of Groningen for their cooperation in this study. In particular, we are grateful to Drs. J.M. Fock for all her help in recruiting newly diagnosed patients.
Additional information
Funding
References
- Gatta G, Botta L, Rossi S, et al. Childhood cancer survival in Europe 1999–2007: Results of EUROCARE-5-a population-based study. Lancet Oncol. 2014;15(1):35–47. doi:10.1016/S1470-2045(13)70548-5.
- Aarsen FK, Paquier PF, Reddingius RE, et al. Functional outcome after low-grade astrocytoma treatment in childhood. Cancer. 2006;106(2):396–402. doi:10.1002/cncr.21612.
- Lassaletta A, Bouffet E, Mabbott D, et al. Functional and neuropsychological late outcomes in posterior fossa tumors in children. Childs Nerv Syst. 2015;31(10):1877–1890. doi:10.1007/s00381-015-2829-9.
- Schulte F, Barrera M. Social competence in childhood brain tumor survivors: A comprehensive review. Support Care Cancer. 2010;18(12):1499–1513. doi:10.1007/s00520-010-0963-1.
- Yeates KO, Bigler ED, Dennis M, et al. Social outcomes in childhood brain disorder: A heuristic integration of social neuroscience and developmental psychology. Psychol Bull. 2007;133(3):535–556. doi:10.1037/0033-2909.133.3.535.
- Frith CD, Frith U. Social cognition in humans. Curr Biol. 2007;17(16):724–732.
- Bonner MJ, Hardy KK, Willard VW, et al. Social functioning and facial expression recognition in survivors of pediatric brain tumors. J Pediatr Psychol. 2008;33(10):1142–1152. doi:10.1093/jpepsy/jsn035.
- Hopyan T, Laughlin S, Dennis M. Emotions and their cognitive control in children with cerebellar tumors. J Int Neuropsychol Soc. 2010;16(6):1027–1038. doi:10.1017/S1355617710000974.
- Wolfe KR, Madan-Swain A, Kana RK. Executive dysfunction in pediatric posterior fossa tumor survivors: A systematic literature review of neurocognitive deficits and interventions. Dev Neuropsychol. 2012;37(2):153–175. doi:10.1080/87565641.2011.632462.
- Longaud-Vales A, Chevignard M, Dufour C, et al. Assessment of executive functioning in children and young adults treated for frontal lobe tumours using ecologically valid tests. Neuropsychol Rehabil. 2016;26(4):558–583. doi:10.1080/09602011.2015.1048253.
- Waber DP, Pomeroy SL, Chiverton AM, et al. Everyday cognitive function after craniopharyngioma in childhood. Pediatr Neurol. 2006;34(1):13–19. doi:10.1016/j.pediatrneurol.2005.06.002.
- Huizinga M, Dolan CV, van der Molen MW. Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia. 2006;44(11):2017–2036. doi:10.1016/j.neuropsychologia.2006.01.010.
- Brookshire B, Copeland DR, Moore BD, et al. Pretreatment neuropsychological status and associated factors in children with primary brain tumors. Neurosurgery. 1990;27(6):887–891. doi:10.1227/00006123-199012000-00005.
- Varela M, Liakopoulou M, Alexiou GA, et al. Presurgical neuropsychological and behavioral evaluation of children with posterior fossa tumors. J Neurosurg Pediatr. 2011;8(6):548–553. doi:10.3171/2011.8.PEDS11223.
- Thigpen JC, Pearson M, Robinson KE, et al. Presurgical assessment of cognitive function in pediatric brain tumor patients: Feasibility and initial findings. Neurooncol Pract. 2016;3(4):261–267. doi:10.1093/nop/npv066.
- Yuan P, Raz N. Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neurosci Biobehav Rev. 2014;42:180–192. doi:10.1016/j.neubiorev.2014.02.005.
- Blakemore SJ. Development of the social brain in adolescence. J R Soc Med. 2012;105(3):111–116. doi:10.1258/jrsm.2011.110221.
- Riva D, Giorgi C. The cerebellum contributes to higher functions during development: Evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123(5):1051–1061. doi:10.1093/brain/123.5.1051.
- Ailion AS, Hortman K, King TZ. Childhood brain tumors: A systematic review of the structural neuroimaging literature. Neuropsychol Rev. 2017;27(3):220–244. doi:10.1007/s11065-017-9352-6.
- Barrera M, Atenafu EG, Schulte F, et al. Determinants of social competence in pediatric brain tumor survivors who participated in an intervention study. Support Care Cancer. 2017;25(9):2891–2898. doi:10.1007/s00520-017-3708-6.
- Hoskinson KR, Wolfe KR, Yeates KO, et al. Predicting changes in adaptive functioning and behavioral adjustment following treatment for a pediatric brain tumor: A report from the brain radiation investigative study consortium. Psychooncology. 2018;27(1):178–186. doi:10.1002/pon.4394.
- Carlson-Green B, Morris RD, Krawiecki N. Family and illness predictors of outcome in pediatric brain tumors. J Pediatr Psychol. 1995;20(6):769–784. doi:10.1093/jpepsy/20.6.769.
- Kullgren KA, Morris RD, Morris MK, et al. Risk factors associated with long-term social and behavioral problems among children with brain tumors. Journal of Psychosocial Oncology. 2003;21(1):73–87. doi:10.1300/J077v21n01_04.
- Zijlstra HP, Kingma A, Swaab H, et al. Nepsy-II-NL. Enschede: Ipskamp; 2010.
- White S, Hill E, Happé F, et al. Revisiting the strange stories: Revealing mentalizing impairments in autism. Child Dev. 2009;80(4):1097–1117. doi:10.1111/j.1467-8624.2009.01319.x.
- Happé FG. An advanced test of theory of mind: Understanding of story characters' thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J Autism Dev Disord. 1994;24(2):129–154. doi:10.1007/BF02172093.
- Kort W, Schittekatte M, Bosmans M, et al. Wechsler Intelligence Scales for Children Third edition-NL. Amsterdam: Pearson Assessment and Information; 2005.
- Cohen MJ. Children's Memory Scale: Manual. San Antonio, TX: The Psychological Corporation, Harcourt Brace & Co; 1997.
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8(3):271–276. doi:10.2466/pms.1958.8.3.271.
- Strauss E, Sherman EMS, Spreen O, Attention In: Strauss E, Sherman EMS, Spreen O. eds. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. 3rd ed. New York: Oxford University Press; 2006:546–677.
- Tjeenk-Kalff AC, Krabbendam L. Behavioural Assessment of the Dysexecutive Syndrome for Children: Nederlandse Handleiding. Amsterdam: Pearson Assessment and Information; 2006.
- Smidts D, Huizinga M. BRIEF Executieve Functies Gedragsvragenlijst: Handleiding. Amsterdam: Hogrefe Uitgevers; 2009.
- Verhulst FC, van der Ende J. Handleiding ASEBA: Vragenlijsten Voor Leeftijden 6 t/m 18 Jaar. Rotterdam: ASEBA Nederland; 2013.
- Hendriksen J, Hurks P. Wechsler Preschool and Primary Scale of Intelligence Third edition-NL. Amsterdam: Pearson Assessment and Information; 2009.
- International Labour Office. International Standard Classification of Occupation Volume 1: Structure, Group Definitions and Correspondence Tables. Geneva: International Labour Office; 2012.
- Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi:10.1037/0033-2909.112.1.155.
- Fritz CO, Morris PE, Richler JJ. Effect size estimates: Current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141(1):2–18. doi:10.1037/a0024338.
- Anderson V, Northam E, Hendy J, et al. Child neuropsychology: Dimensions of theory and practice In: Anderson V, Northam E, Hendy J, Wrennall J, eds. Developmental Neuropsychology: A Clinical Approach. Hove, England: Psychology Press; 2001:3–37.
- Hocking MC, McCurdy M, Turner E, et al. Social competence in pediatric brain tumor survivors: Application of a model from social neuroscience and developmental psychology. Pediatr Blood Cancer. 2015;62(3):375–384. doi:10.1002/pbc.25300.
- Chaytor N, Schmitter-Edgecombe M. The ecological validity of neuropsychological tests: A review of the literature on everyday cognitive skills. Neuropsychol Rev. 2003;13(4):181–197. doi:10.1023/B:NERV.0000009483.91468.fb.
- Kok TB, Post WJ, Tucha O, et al. Social competence in children with brain disorders: A meta-analytic review. Neuropsychol Rev. 2014;24(2):219–235.
- Kort W, van den Bosch KP, Lutje Spelberg HC, et al. Dyslexie Screening Test (DST-NL) Handleiding. London: Pearson Assessment; 2005.
