Abstract
Successful first diagnostic lumbar puncture (LP) is crucial because intrathecal chemotherapy has not yet protected the central nervous system against cancer cells. If blood contaminates the cerebrospinal fluid (CSF) with blasts, they may enter the central neural system and compromise the patient’s health. We retrospectively determined the incidence of traumatic lumbar punctures (TLP) in 2,507 LPs of 250 pediatric hemato-oncology patients aged from one to 18 years, including both diagnostic and intrathecal treatment procedures, and 2,617 LPs of 1,525 other age-matched pediatric patients. We used ≥10 erythrocytes/µL in the CSF sample as the criterion of TLP. TLPs were less frequent in hemato-oncology patients than in other patients (31.6% vs. 48.5%, p < 0.0001). The incidence of TLP was significantly lower in the first diagnostic LP than in subsequent intrathecal treatment LPs (20.5% vs. 31.6%, p = 0.0046). According to logistic regression analysis, the odds of TLP was 1.6-fold if the LP procedure was not performed in the hemato-oncology department. The odds of the patient’s next LP being traumatic were threefold if the previous first LP was traumatic. A week or less time between the first and next LP tripled the odds of TLP as well. The patient’s age category was not significantly associated with the incidence of TLP. Given the risks of TLP, hemato-oncology patients’ first diagnostic LP should include administration of chemotherapy, as generally recommended, and be performed under general anesthesia or deep sedation by an experienced physician to optimize not only the success of the first LP procedure but also following procedures.
Introduction
Lumbar puncture (LP) is an essential clinical procedure in children with acute lymphoblastic leukemia (ALL), first for diagnostics and then for the follow-up and administration of intrathecal chemotherapy according to the patient’s specified treatment protocol. The success of the first diagnostic LP is crucial because the chemotherapy has not yet protected the central nervous system against cancer cells. If blood leaks into the spinal canal during the first diagnostic puncture and contaminates the cerebrospinal fluid (CSF) with blasts, the cancer cells may enter the central neural system and compromise the patient’s health. Therefore, the current guidelines for pediatric leukemia recommend administrating chemotherapy along with the first diagnostic LP.Citation1
A high number of blasts in the CSF sample of the diagnostic LP is associated with poorer event-free survival of pediatric patients with ALL and relapse of the disease.Citation2–7 Further, increased amounts of white blood cells in the CSF sample may trigger more intensive intrathecal therapy and lead to extra strain on the patient.Citation3 Thus, avoidance of bleeding into the subarachnoid space is of paramount clinical importance in children with hematologic malignancies. However, it is yet debatable whether the CSF samples drawn from the diagnostic LP are less blood-tinged than those from the subsequent intrathecal treatment punctures.Citation7,Citation8
Traumatic LPs (TLP) causing blood leakage into CSF are common in clinical practice.Citation9–12 Many factors, including the use of sedation and sedation level, the use of needle guidance, anatomic puncture level, patient anatomy and age, physician’s expertise, clinical specialty, and setting all affect the incidence of traumatic punctures.Citation7,Citation9–11,Citation13–19 In pediatric hemato-oncology, the criterion used for the definition of TLP is set to 10 erythrocytes/µL,Citation2–7 but it can vary a lot between studies,Citation9,Citation10,Citation13–19 up to 10,000 erythrocytes/µL.Citation14,Citation18 This large variation in the used criteria is likely to confound the interpretation of results from different studies.
In this retrospective study of hospital register data, we evaluated the incidence of TLP both in the first diagnostic LP procedures and subsequent intrathecal treatment procedures in pediatric hemato-oncology patients. So far, this specific topic is only scarcely studied with contrasting findings.Citation7,Citation8 Shaikh et al. reported similar 19.7% and 17.8% incidences of TLP in the first diagnostic LP and subsequent treatment LPs, respectively.Citation7 In contrast to the comparable 18.7% incidence of TLP in the first LP, Howard et al. observed a much higher 32.1% incidence of TLP in the subsequent LPs.Citation8 Besides the above research question, we compared the incidence of TLP in pediatric hemato-oncology patients to that in other pediatric patients who had undergone LP for other indications than hemato-oncology. We also examined factors that may account for the incidence of TLP after the first LP in both pediatric populations. In addition, we propose a graphical means to determine the incidence of TLP for any given criterion from the distribution of erythrocyte counts in the CSF samples.
Materials and methods
Data description
Using the hospital registers, we retrospectively searched for the erythrocyte count data collected from LPs of pediatric patients between 1 January 2010, to 31 May 2017, in Tampere and Turku University Hospitals, Finland. These two hospitals provide tertiary care for a population of about 1.8 million people, including 360,000 children.
The erythrocyte count data from pediatric hemato-oncology LP procedures were identified from the hospital department codes (hemato-oncology population), while the data from other pediatric departments served as the reference data (reference population). The LP procedures in the hemato-oncology population were always performed in respective departments. The erythrocyte count was routinely determined from the second or third vial of the CSF sample in the hospital laboratories with standard cytometric methods. Besides the erythrocyte count, the collected data contained the date of the procedure, hospital department code, and patient’s age at the time of the procedure. For this study, the patients’ age range was confined to 1–18 years to reduce potential bias due to different age distributions in the study populations. Among the pediatric hemato-oncology patients, patients younger than one year were rare (∼5%), whereas more than half (∼55%) of the reference patients were this young. In total, the collected data comprised 2,507 LP procedures of 250 pediatric hemato-oncology patients and 2,617 LP procedures of 1,525 other pediatric patients.
The regional Ethics Committee of the Expert Responsibility area of Tampere University Hospital approved the study. Because of being a register-based study, all patient data were pseudonymized before the analysis and no informed consent from individual patients or their parents was needed.
Data analysis
We used mean, median, standard deviation (SD), interquartile range (IQR) and range as descriptive data, as appropriate for the variable of interest. For the erythrocyte counts, we used the cumulative distribution curve as the main descriptive statistics. This curve illustrates the respective proportions of CSF samples for any value of the erythrocyte count. For the chosen erythrocyte count-based criterion of TLP, the incidence of TLP equals the vertical distance from the curve up to the 100% level. We used ≥10 erythrocytes/µL in the CSF sample as the criterion of TLP, which is the standard in hemato-oncology.Citation2–7 In addition, we used ≥400 erythrocytes/µL as another criterion of bloody TLP for comparing the patient populations. The ≥400 erythrocytes/µL criterion denotes approximately the visual threshold of blood in the CSF sample,Citation9 and permits further comparison to the literature.
We evaluated the influence of the first diagnostic LP of pediatric hemato-oncology patients on the incidence of TLP by comparing the distributions of erythrocyte counts between the first diagnostic LPs and subsequent intrathecal treatment LPs. Be it noted that intrathecal chemotherapy was also administered along with the first diagnostic LP. For this comparison, we required erythrocyte count data from the first diagnostic LP and at least one intrathecal treatment LP of each patient. The influence of the patient population on the incidence of TLP was assessed by comparing the distributions of erythrocyte counts between the hemato-oncology LP and reference LP procedures using the two above-described criteria of TLP. The Chi-squared test was used for these comparisons.
We used logistic regression analysis to reveal statistically significant factors that account independently for the incidence of TLP, defined as a dichotomous variable by the criterion of ≥10 erythrocytes/µL. First, the incidence of TLP in the patient’s first LP was analyzed using the patient population (hemato-oncology vs. reference) and age category (1 - < 3 years, 3 - < 5 years, 5 - < 10 years, 10 − 18 years) as independent variables. Second, the incidence of TLP in the patient’s next LP after the previous first procedure was analyzed using the patient population, whether the first procedure was TLP and whether the next LP procedure was repeated within the following time categories (0 − 7 days, 8 − 28 days, and 29 − 365 days after the first procedure), and the age category as independent variables. Estimated odds ratios (OR) are reported with 95% confidence intervals (95% CI).
Statistical analyses were done with IBM SPSS statistics software (Version 27.0). A p-value less than 0.05 was considered statistically significant.
Results
At the time of their first LP, the mean age (SD) of pediatric hemato-oncology patients was 6.7 (4.5) years and that of the reference pediatric patients 9.6 (5.5) years. Respective age ranges were from 1.0 to 17.6 years or from 1.0 to 18 years. The median time (IQR) between the patient’s first and next procedure was 7 (7 − 11) days in the hemato-ongology population and 7 (2 − 31) days in the reference population.
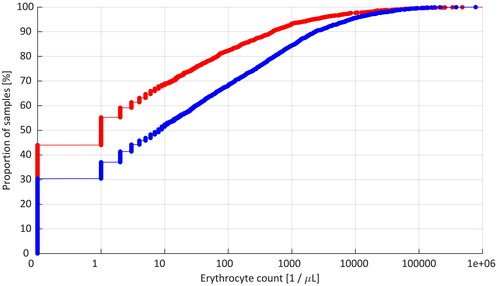
Cumulative distribution curves of the erythrocyte count data in all CSF samples from hemato-oncology and reference LP procedures are shown in . Of the hemato-oncology LPs, 44.0% of the CSF samples were erythrocyte-free, whereas 30.4% of the samples were erythrocyte-free in the reference LPs (p < 0.0001). Using the criterion of ≥10 erythrocytes/µL for TLP, the respective incidences were 31.6% and 48.5% (p < 0.0001). For the criterion ≥400 erythrocytes/µL, the incidences of TLP were 11.3% and 22.2%, respectively (p < 0.0001).
Figure 1. Cumulative distribution curves of the erythrocyte count data in LPs of pediatric hemato-oncology patients (red line) and reference populations (blue line).
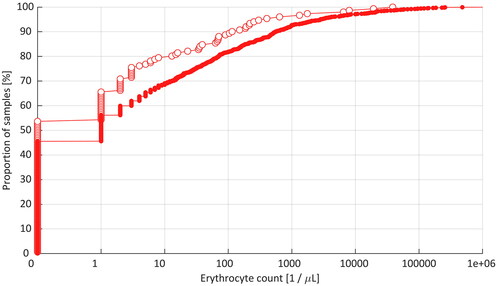
In the first diagnostic LP, the incidence of TLP was 20.5% and 31.6% in the subsequent intrathecal treatment LPs according to the criterion of ≥10 erythrocytes/µL, differing statistically significantly from each other (p = 0.0046). Cumulative distribution curves of the erythrocyte count data in the CSF samples in the first and subsequent LP procedures are shown in .
Figure 2. Cumulative distribution curves of the erythrocyte count data in the first diagnostic LPs (open circles) and subsequent intrathecal treatment LPs (solid circles) in pediatric hemato-oncology patients.
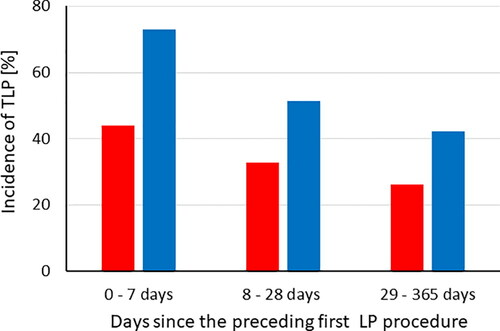
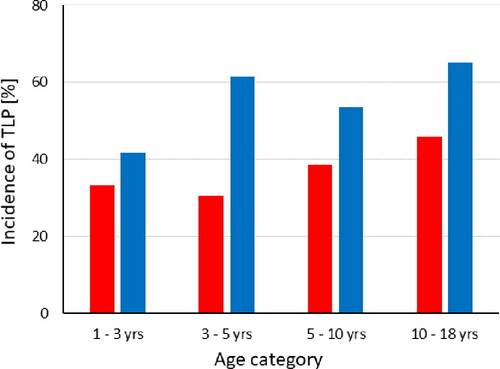
According to the logistic regression analysis, the odds of TLP in the patient’s first LP were 1.6 times higher [OR 1.58 (95% CI 1.11 − 2.26), p = 0.011), if the procedure was performed in other pediatric departments than hemato-oncology. The age category was not associated with the incidence of TLP (p = 0.96). In the next LP after the first procedure, the odds of TLP were similarly higher [OR 1.68 (95% CI 0.98 − 2.88), p = 0.057) in the reference patients compared to hemato-oncology patients, but if the first LP procedure was traumatic the odds of TLP tripled [OR 2.97 (95% CI 1.76 − 5.02), p < 0.0001) in the next procedure, and further, if the LP procedure was repeated within one week the odds of TLP tripled [OR 3.06 (95% CI 1.60 − 5.88), p = 0.001) compared to the time category longer than four weeks. If the procedure was repeated within four weeks, the odds of TLP were 1.5-fold and non-significant (p = 0.25). The age group was not associated with the incidence of TLP (p = 0.24) but the analysis suggested doubled odds of TLP among the oldest children [OR 2.09 (95% CI 0.88 − 5.00), p = 0.096). For illustrative purpose, the incidences of TLP in hemato-oncology and reference patients broken down by the time category since the patient’s previous first LP procedure and the age category are shown in and , respectively.
Discussion
Existing scientific evidence on whether the first diagnostic LP in pediatric hemato-oncology patients is less likely traumatic is yet scarce and contradictory.Citation7,Citation8 Our study adds to this knowledge and gives support to this largely intuitive notion. Collating the data of almost 1,400 first diagnostic LPs and over 11,000 subsequent treatment LPs from the present and two earlier studies,Citation7,Citation8 the incidence of TLP is 19.1% in the first LP and 25.7% in the subsequent LPs. These aggregate figures imply that one out of five diagnostic LPs and one out of four intrathecal treatment LPs of these patients are traumatic with the standard criterion of ≥10 erythrocytes/µL. For comparison, half of the pediatric reference LP procedures are traumatic as per this criterion. Considering the patient’s next LPs after the first procedure, a traumatic previous LP – not primarily the patient population – contributed to whether the next LP is traumatic as evinced by threefold odds of TLP. In earlier studies of pediatric hemato-oncology patients,Citation7.Citation8 previous TLP has been associated with 1.4–1.6 times higher odds. These differences may be partly explained by different statistical adjustments and the fact that we compared the patient’s first LP to their next LP in a setting that comprised not only pediatric hemato-oncology patients but also age-matched reference patients. Also, in line with earlier findings in pediatric hematology,Citation7,Citation8 a short time (from a day up to a week) between successive LP procedures has been linked to at least tripled odds of TLP. Whereas the age category did not significantly account for the incidence of TLP, the observed trend suggested doubled odds of TLP among those older than 10 years. This finding is in line with Shaikh et al.,Citation7 who found twofold odds of TLP among same-aged patients but not with the data presented by Howard et al.Citation8 indicating no clear trend of higher incidence of TLP in adolescents. The potential interaction of patient’s age and other yet unspecified factors with the incidence of TLP calls for further investigations.
The present overall 32% incidence of TLP compares well to earlier findings in pediatric patients with ALL: 12%Citation3, 18%,Citation7 19%,Citation5 21%,Citation2 29%,Citation8 36%,Citation4 and 38%.Citation6 However, the large variability in reported incidences between the studies of comparable target populations is striking. Ideally, the CSF sample should reflect the composition of CSF as it would be without the influence of the performed procedure and potential puncture-related trauma of tissues. In the present study, truly erythrocyte-free CSF samples were obtained from 43.9% of pediatric hemato-oncology patients and 30.4% of the reference pediatric patients. In practice, visual verification of erythrocyte counts as low as 10 erythrocytes/µL is not possible, whereas checking the sample quality and cell content requires cytometric analysis. For visual verification, more than 40x higher erythrocyte concentrations are necessary to observe whether the CSF sample is blood-tinged or not.Citation9 Visible blood in the CSF sample, classified as a bloody sample in hemato-oncology,Citation8 was evident in 11.3% of the present hemato-oncology CSF samples, while the incidence of bloody LP was twice that in the reference population. These figures compare well with the 11% incidence of bloody LP in patients with ALL,Citation8 40% incidence in pediatric patients comprising mostly infants,Citation14 32% incidence both in infants and children,Citation17 and 26% incidence in infants and 13% in children.Citation16
A non-traumatic patient’s first LP, irrespective of the patient population, is crucial in reducing TLPs in subsequent LPs as the present study showed. Also shown by the present study and the literature,Citation7,Citation8 a short time between successive LPs (up to two weeks) increases further the incidence of TLP. The incidence of TLP is linked to incidental tissue trauma caused by the spinal needle that leads to blood leakage from the damaged capillaries or venules into the spinal canal.Citation9 It is possible that a couple of days is not sufficient for complete healing of the puncture site, and the site remains vulnerable to blood leakage when the patient’s next LP procedure is performed. If so, this may partly explain the higher incidence of TLP in the following LP procedure. In the short term, a higher number of puncture attempts during a single LP procedure is associated with a doubled risk of TLP.Citation16 In addition, several procedural, patient, and physician-related factors contribute to the low incidence of TLP in pediatric hemato-oncology patients.Citation7,Citation8,Citation19 Patient movement during the procedure makes LP more challenging to perform, and the risk of TLP increases,Citation14 whereas sufficient sedation or anesthesia prevents patient movements and reduce the incidence of TLP.Citation14,Citation17 However, sedation or general anesthesia involves health risks that need to be weighed against received benefits case-by-case, which explains the variation in sedation practices between specialties and partly the variation and differences in the incidence of TLP. Pediatric hemato-oncology patients are routinely kept in deep sedation or general anesthesia during the LP procedure, while experienced physicians are preferred as providers of LP to improve the success rate and reduce the incidence of TLP.Citation7 However, deep sedation may not always be necessary as a recent study of pediatric patients with ALL found acceptable performance in intrathecal treatment LPs also without deep sedation.Citation20 In the present study, the low incidence of TLP at the first LP procedure pertains to the criticality of this procedure and a consequent focus on its overall success. This means that experienced pediatric hematologists primarily perform the patient’s first diagnostic LP and general anesthesia is employed as the standard practice in Finland. Given the promising recent results,Citation20 however, the utility of unsedated LPs in pediatric hemato-oncology patients warrants further studies.
The main strength of the present study is the large number (about 5,000 in total) of pediatric LP procedures performed in two Finnish university hospitals, one half of those being hemato-oncology LPs. We consider that the present dataset represented well age-matched pediatric patient populations undergoing LP procedures in different clinical settings, making the observed incidences of TLP robust and unbiased. Thus, with this large dataset, we could establish the actual distributions of erythrocyte counts in the CSF samples collected from pediatric hemato-oncology LPs, broken down into the diagnostic and intrathecal treatment procedures, and other pediatric LPs performed for other medical indications. From the cumulative distribution curves describing the distribution of erythrocyte counts in different patient populations, we could derive reliable estimates for the population-specific incidences of TLP.
The main limitation pertains to many potential factors that we could not consider because we had only a minimized, pseudonymized dataset collected retrospectively from the hospital registers at our disposal. Obviously, the sequence leading to TLP is multifactorial modulated by several intrinsic and extrinsic factors that we could not specifically include in statistical models. These factors include depth of sedation, use of topical analgesics, patient’s position during the procedure, patient’s obesity and anatomy, specific blood properties such as platelet count or coagulopathy, physician’s specialty and experience in performing LPs, use of image guidance, or the gauge and needle type used in LP.Citation7,Citation8,Citation12–17,Citation19 More detailed information on patient-, physician-, and procedure-related factors might have allowed us to reveal some new specific relationships that could have further explained potential etiologies of TLP in pediatric LPs.
Irrespective of the pediatric patient population, plenty of room remains for improvement concerning the incidence of TLPs. Means that help to avoid TLP in the patient’s first LP procedure would be of utmost importance as it tripled the odds of TLP in the next LP. Since success at the first attempt of LP may halve the incidence of TLP,Citation16 primary focus should be on performing the LP procedure as successfully as possible. Various image guidance methods have been used in children with ALL, particularly in LPs of the most challenging patients,Citation7,Citation19 and the ultrasound-assisted procedure was recently found well-adopted by pediatric oncologists.Citation21 Indeed, image guidance provides well-established means even to halve the incidence of TLP in different patient populations,Citation7,Citation11,Citation15,Citation22,Citation23 but at the expense of some added complexity (equipment, facilities, and expertise) and costs to the hospital. Further research and clinical studies are called for to devise feasible means to reduce the incidence of TLP and verify their effectiveness in pediatric patients.
In conclusion, the incidence of TLP in the first diagnostic LP of pediatric hemato-oncology patients was about one-third lower than in subsequent intrathecal treatment LPs. Regarding the hemato-oncology LPs in general, approximately one out of three LPs were traumatic whereas almost half of the other pediatric LPs performed not for hemato-oncology were traumatic according to the standard criterion of TLP. Irrespective of the pediatric patient population, the odds of TLP in the next LP was threefold if the patient’s first LP was traumatic, and a week or less time between the successive LPs also tripled the odds. While the incidence of TLP in pediatric hemato-oncology patients is relatively low, there is still room for improving the success in LP to achieve better clinical outcomes. Given the evident risks associated with TLP, the first diagnostic LP of hemato-oncology patients should include administration of chemotherapy, as generally recommended, and be performed under general anesthesia or deep sedation by an experienced physician to optimize not only the success of the first LP procedure but also the subsequent LPs.
Conflicts of interest and source of funding
HS, JK, and SH hold shares and are employees of Injeq Oy. All other authors declare no conflicts of interest regarding this study. This study was funded by Injeq Oy, a Finnish startup company that manufactures bioimpedance spinal needle system for lumbar punctures.
References
- Brown P, Inaba H, Annesley C, et al. Pediatric acute lymphoblastic leukemia, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(1):81–112. doi:10.6004/jnccn.2020.0001.
- Gajjar A, Harrison PL, Sandlund JT, et al. Traumatic lumbar puncture at diagnosis adversely affects outcome in childhood acute lymphoblastic leukemia. Blood. 2000;96(10):3381–3384.
- Burger B, Zimmermann M, Mann G, et al. Diagnostic cerebrospinal fluid examination in children with acute lymphoblastic leukemia: significance of low leukocyte counts with blasts or traumatic puncture. JCO. 2003;21(2):184–188. doi:10.1200/JCO.2003.04.096.
- Rech A, de Carvalho GP, Meneses CF, et al. The influence of traumatic lumbar puncture and timing of intrathecal therapy on outcome of pediatric acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2005;22(6):483–488. doi:10.1080/08880010591002242.
- Te Loo MWM, Kamps WA, Van der Does-Van den Berg A, et al. Prognostic significance of blasts in the cerebrospinal fluid without pleiocytosis or a traumatic lumbar puncture in children with acute lymphoblastic leukemia: experience of the Dutch Childhood Oncology Group. JCO. 2006;24(15):2332–2336. doi:10.1200/JCO.2005.03.9727.
- Cancela C, Murao M, Viana M, et al. Incidence and risk factors for central nervous system relapse in children and adolescents with acute lymphoblastic leukemia. Rev Bras Hematol Hemoter. 2012;34(6):436–441. doi:10.5581/1516-8484.20120109.
- Shaikh F, Voicu L, Tole S, et al. The risk of traumatic lumbar punctures in children with acute lymphoblastic leukemia. Eur J Cancer. 2014;50(8):1482–1489. doi:10.1016/j.ejca.2014.02.021.
- Howard SC, Gajjar AJ, Cheng C, et al. Risk factors for traumatic and bloody lumbar puncture in children with acute lymphoblastic leukemia. JAMA. 2002;288(16):2001–2007. doi:10.1001/jama.288.16.2001.
- Shah KH, Richard KM, Nicholas S, et al. Incidence of traumatic lumbar puncture. Acad Emerg Med. 2003;10(2):151–154. doi:10.1197/aemj.10.2.151.
- Srinivasan L, Shah SS, Abbasi S, et al. Traumatic lumbar punctures in infants hospitalized in the neonatal intensive care unit. Pediatr Infect Dis J. 2013;32(10):1150–1152. doi:10.1097/INF.0b013e31829862b7.
- Muthusami P, Robinson AJ, Shroff MM. Ultrasound guidance for difficult lumbar puncture in children: pearls and pitfalls. Pediatr Radiol. 2017;47(7):822–830. doi:10.1007/s00247-017-3794-0.
- Nath S, Koziarz A, Badhiwala JH, et al. Atraumatic versus conventional lumbar puncture needles: a systematic review and meta-analysis. Lancet. 2018;391(10126):1197–1204. doi:10.1016/S0140-6736(17)32451-0.
- Baxter AL, Fisher RG, Burke BL, et al. Local anesthetic and stylet styles: factors associated with resident lumbar puncture success. Pediatrics. 2006;117(3):876–878. doi:10.1542/peds.2005-0519.
- Nigrovic LE, Kuppermann N, Neuman MI. Risk factors for traumatic or unsuccessful lumbar punctures in children. Ann Emerg Med. 2007;49(6):762–771. doi:10.1016/j.annemergmed.2006.10.018.
- Yu SD, Chen MY, Johnson AJ. Factors associated with traumatic fluoroscopy-guided lumbar punctures: a retrospective review. AJNR Am J Neuroradiol. 2009;30(3):512–515. doi:10.3174/ajnr.A1420.
- Glatstein MM, Zucker-Toledano M, Arik A, Scolnik D, Oren A, Reif S. Incidence of traumatic lumbar puncture: experience of a large, tertiary care pediatric hospital. Clin Pediatr (Phila). 2011;50(11):1005–1009. doi:10.1177/0009922811410309.
- Procter C, Buys H, Carrara H, Thomas J. Risk factors for unsuccessful lumbar puncture in children. S Afr Med J. 2016;106(12):1230–1235. doi:10.7196/SAMJ.2017.v106i12.10703.
- Lyons TW, Cruz AT, Freedman SB, Pediatric Emergency Medicine Clinical Research Network (PEM CRC) Herpes Simplex Virus Study Group, et al. Interpretation of cerebrospinal fluid white blood cell counts in young infants with a traumatic lumbar puncture. Ann Emerg Med. 2017;69(5):622–631. doi:10.1016/j.annemergmed.2016.10.008.
- Frett MJ, Meeks H, Morgan KJ, et al. Retrospective analysis of predisposing factors for difficult lumbar punctures requiring image guidance in pediatric oncology patients. Pediatr Hematol Oncol. 2021;38(5):420–433. doi:10.1080/08880018.2020.1856986.
- Waters TW, Dickens DS. Reducing sedated lumbar punctures in pediatric patients with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2021;68(11):e29272. doi:10.1002/pbc.29272.
- Shaikh F, Arzola C, Alexander S, et al. Feasibility of ultrasound-assisted lumbar punctures performed by pediatric oncologists at the point of care. Pediatr Blood Cancer. 2021;68(7):e29015. doi:10.1002/pbc.29015.
- Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(1):85–96. doi:10.1111/acem.13682.
- Olowoyeye A, Fadahunsi O, Okudo J, et al. Ultrasound imaging versus palpation method for diagnostic lumbar puncture in neonates and infants: a systematic review and meta-analysis. BMJ Paediatr Open. 2019;3(1):e000412. doi:10.1136/bmjpo-2018-000412.