Abstract
The metabolic capacity of the intestinal microflora and its influence on the human body are not fully understood. One area of interest is the metabolism of drugs used to treat disease. To gain a better understanding, major genera present in the intestine were tested for their ability to metabolize phenobarbital, a pharmaceutical drug used to treat epilepsy. We tested Bifidobacterium, Bacteroides, Enterococcus, Eubacterium, Clostridium, and Staphylococcus. Growth analysis at different concentrations of phenobarbital and gas chromatography-mass spectrometry analysis were used to determine the response and metabolism of phenobarbital. Our growth curve results demonstrated that the lag time for some bacteria was affected in the presence of high concentrations of phenobarbital. The generation time was also affected based on the concentration and specific bacterial type. Finally, it was shown that Bifidobacteirum adolescentis and Bifidobacterium bifidum were unique in metabolizing phenobarbital (reductively cleaved) into the compound alpha-ethyl-benezeneacetamide, which was an unexpected metabolite. The data demonstrate for the first time the ability of Bifidobacterium to metabolize phenobarbital into the potentially toxic metabolite alpha-ethyl-benezeneacetamide. The consequences of the generated metabolite may affect the surrounding intestinal bacterial population or the effectiveness of the drug in treating epilepsy.
Introduction
The metabolic capacity of intestinal microorganisms is enormous due to the diverse population of bacteria found there. Our understanding of these organisms’ participation in the metabolism of different xenobiotics is incomplete Citation[1–3]. Among this population are found the genera Bacteroides, Clostridium, Eubacterium, Enterococcus, and Bifidobacterium.
One area of interest related to the metabolism of xenobiotics is the mechanisms associated with degradation of therapeutic drugs and the impact that this may have on the effectiveness of the parent compound. For example, phenobarbital is used to treat epilepsy but unfortunately the enzyme systems associated with the metabolism of phenobarbital are not fully understood. Current evidence suggests that the metabolism of phenobarbital occurs in the liver as a result of the CYP 2C cytochrome P450 Citation[4]. The product generated is either the para-hydroxyphenobarbital and/or phenobarbital N-glucoside Citation[5]. The extent to which phenobarbital is converted to these metabolic products is not known, as the total recovery of the parent compound and its metabolites has not been reported. Therefore, other systems in the human body may be involved in the metabolism of phenobarbital. The purpose of this study was to determine if major intestinal strains are involved in the anaerobic metabolism of phenobarbital.
The broad importance of the study will provide information regarding the possible effect of intestinal microflora metabolism on the physiology of the host. In addition, as probiotic therapy becomes more popular, it is important that we understand the different metabolic systems that are found in resident microflora. Finally, identifying and characterizing human intestinal strains that are involved in the metabolism of xenobiotics is important as they may affect human health.
Materials and methods
Bacterial strains
Bacteria were selected on the basis of isolates determined to be present in human fecal samples Citation[6]. All cultures were obtained directly from the American Type Culture Collection (ATCC; Rockville, MD, USA): Bifidobacterium adolescentis ATCC 15703, Bifidobacterium bifidum ATCC 15696, Bacteroides fragilis ATCC 25285, Enterococcus faecalis ATCC 27274, Eubacterium aerofaciens ATCC 25986, Clostridium perfringens ATCC 19574, and Staphylococcus aureus ATCC 13150.
Chemicals
Phenobarbital (Sigma, St Louis, MO, USA; molecular weight, 232.24, C12H12N2O3 CAS registry 50-06.6). The gas chromatography-mass spectrometry data for B indicate the metabolite to be alpha-ethyl-benzeneacetamide (molecular weight, 163.22, C10H13NO, CAS registry 90-26-6).
Culture media and growth curves
The reference strains were initially cultured in anaerobic chopped meat carbohydrate medium (Morgan Hill, CA) for 48 h. To create a less nutrient-rich environment, the cultures were transferred aseptically to anaerobic TYG medium (10 g tryptone, 5 g yeast extract, 2 g dextrose per liter) and incubated for 24 h at 37°C Citation[7]. Following incubation, 100 µl of cells were subcultured to 5 ml TYG anaerobic tubes containing different concentrations of phenobarbital (final concentrations 0 mM, 5 mM, 10 mM, 20 mM, and 40 mM). The different solutions of phenobarbital were prepared by dissolving it in sterile water. A 0.45 µ syringe filter was used to add phenobarbital to the sterile TYG anaerobic medium. The experiments were performed in triplicate for all strains tested under anaerobic conditions. The growth was determined by measuring the optical density (OD) at 600 nm using a Spec-20 (Spectronic 20-D, Rochester, NY, USA). Time points were recorded between 0 and 72 h. After incubation, the samples were stored at −80°C. The OD data were compiled and graphed using Microsoft Excel Chart Wizard. The generation time for each culture was determined based on the log phase. To determine major protein induction, the protein profiles for each sample were analyzed by SDS-PAGE using a methanol-chloroform extraction procedure Citation[8].
Gas chromatograph-mass spectrometry (GC-MS)
The samples, which were stored −80°C, were also analyzed using GC-MS. To each sample (0.5 ml) in a borosilicate tube, 0.125 ml of 1 M K2HPO4 was added, followed by 3.25 ml of ether/chloroform (2:1) solution. This solution was vortex mixed for 1 min and then centrifuged at 1200 g for 2 min. The organic phase (top layer) was retained and the solvent was removed under a stream of N2. The sample was then dissolved with 0.1 ml of ethyl acetate and appropriate dilutions were made. Separation of the sample components by GC-MS (HP5890, HP5989B) was obtained using a DB5 (30 m) column with a 0.25 µ film thickness of the inner coating of the column. The phenobarbital gave an intense signal thereby standard electron impact ionization was used. The data were analyzed using a digital integrator.
Results
Growth analysis
shows the lag times for the bacterial strains tested under different concentrations of phenobarbital. There was an extended lag time for B. adolescentis and B. bifidum that began at a 20 mM concentration of phenobarbital. The lag time of the remaining test bacteria was not affected at any of the concentrations. However, E. aerofaciens did not grow in the presence of phenobarbital. shows the generation time for the test bacteria. The generation time of C. perfringens was greatly shortened for phenobarbital concentrations of 5–40 mM, suggesting that phenobarbital may serve as a carbon source. B. adolescentis showed a slight improvement in generation time at 5 mM but growth slowed dramatically at 40 nm phenobarbital. B. bifidum maintained a similar generation time to that of the control. The remaining test bacteria showed a general slowing of growth as the concentration of phenobarbital was increased. Increased generation time indicates some inhibitory effects of phenobarbital on bacterial growth. The SDS-PAGE gel showed no major induction of proteins based on all the concentrations tested (data not shown). All cultures were grown up to 72 h and prepared for GC-MS analysis.
Table I. Cell growth in the presence of phenobarbital and the generation of alpha-ethyl-benzeneacetamide.
GC-MS
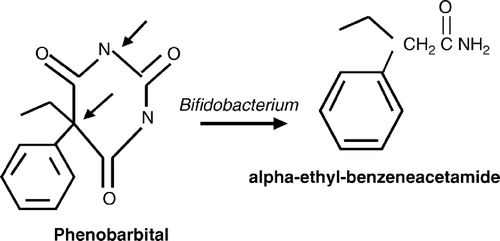
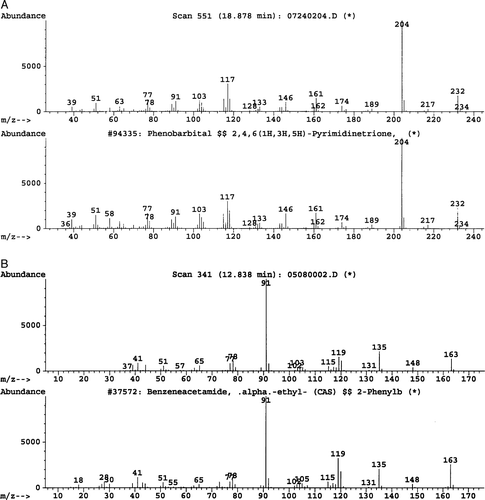
B. adolescentis and B. bifidum were the only bacteria that produced a major metabolite with all concentrations of phenobarbital (). A is a representation of an ion chromatogram showing the profile for nondegraded phenobarbital, while B shows degradation of 20 mM phenobarbital into alpha-ethyl-benezeneacetamide by B. adolescentis. Retention times at 18.49 min and 12.84 min were observed. The 20 mM phenobarbital degradation control (no bacteria present) showed only one major peak at 18.89 min (A). The mass spectrometry data identified the two major peaks as phenobarbital (18.49 min) and alpha-ethyl-benezeneacetamide (12.84 min). The molecular spectrum of the peaks revealed that the molecular masses were at m/z 232 and m/z 163 (). Similar results were observed for B. bifidum. The remaining test bacteria that grew in the presence of phenobarbital did not generate alpha-ethyl-benezeneacetamide, as no major products were identified. The 0 mM phenobarbital blank (bacteria present) and sterile medium (no bacteria and no phenobarbital present) showed no major peaks that corresponded to either phenobarbital or alpha-ethyl-benezeneacetamide. Therefore, the data suggest that the metabolism of phenobarbital into alpha-ethyl-benezeneacetamide was due to Bifidobacterium. In addition, the data showed that phenobarbital underwent reductive cleavage, as the terminal nitrogen obtained a hydrogen atom. It appears that the Bifidobacterium spp. cleaved phenobarbital at two positions, releasing the CONCO molecule ().
Figure 1. Representation of an ion chromatogram showing the degradation of phenobarbital into benzeneacetamide. (A) 20 mM phenobarbital only. (B) B. adolescentis and 20 mM phenobarbital.
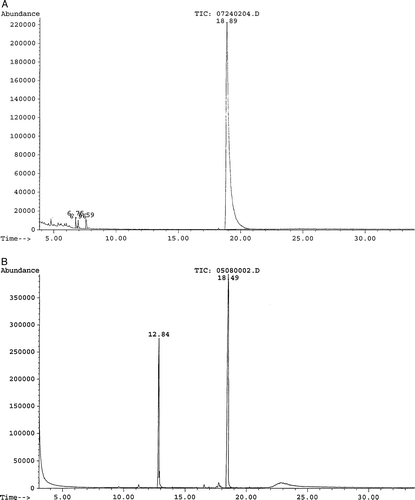
Figure 2. Mass spectra of phenobarbital and alpha-ethyl-benezeneacetamide. (A) Phenobarbital: top frame shows the experimental B. adolescentis fragmentation pattern, and the bottom frame shows the database fragmentation pattern. (B) Alpha-ethyl-benezeneacetamide: top frame shows the experimental B. adolescentis fragmentation pattern, and the bottom frame shows the database fragmentation pattern.
Discussion
Intestinal flora display a very diverse array of enzyme reactions, and as a result there is interest in understanding the role these enzyme reactions have in human health Citation[9–11]. Unfortunately, our understanding of these reactions remains poor. One area of interest is the metabolism of drugs used to treat disease. Metabolites can alter the expression of enzymes such as cytochrome P450 at the gastrointestinal tract or the liver, as the effects of intestinal flora on the expression of liver cytochrome P450 have been documented Citation[12]. The altered expression can affect the detoxification and metabolite activation processes, which can impact human health. Therefore, an understanding as to how specific intestinal microbes metabolize xenobiotics is important.
The objective of the present study was to determine if phenobarbital, a potent inducer of cytochrome P450 and a drug used to treat epilepsy, was metabolized by different intestinal bacteria. Our results demonstrated an effect on growth for both B. adolescentis and B. bifidum as an extended lag phase at the higher phenobarbital concencentration (>20 mM) was observed. The generation time for B. adolescentis increased threefold at 40 mM, whereas the generation time for B. bifidum remain relatively steady for all concentrations, which suggests that B. adolescentis is more sensitive to higher concentrations of phenobarbital. Interestingly, C. perfringens had an approximately twofold reduction in the lag phase for all phenobarbital concentrations. In addition, C. perfringens improved its generation time by an average of fourfold for all phenobarbital concentrations, which suggests that phenobarbital may serve as a carbon source.
Unexpectedly, when major metabolites were identified, only two Bifidobacterium species were able to metabolize phenobarbital into alpha-ethyl-benezeneacetamide, which is different from published phenobarbital studies Citation[13], Citation[14]. Furthermore, the improved growth of C. perfringens did not produce alpha-ethyl-benezeneacetamide, supporting the possibility that phenobarbital may be completely mineralized by this organism. Finally, B. adolescentis was one of several intestinal species that displayed a P450-like protein, hence the reductive activity observed may be related to cytochrome P450 Citation[8].
The experimental concentrations of phenobarbital used in this study have clinical significance. A 5 mM final concentration is comparable to the upper level dose of an average adult Citation[13], while the 20 mM concentration is analogous to a dose that would be lethal to the average human. Since the total recovery of all phenobarbital and the metabolites has not been determined based on canine and human studies Citation[13], Citation[14], the alpha-ethyl-benezeneacetamide produced in this study and the influence on resident microflora as well as the expression of hepatic enzymes such as cytochrome P450 need further investigation. In addition, because the metabolite contains benzene, a potential toxicant, the absorption and further biotransformation by gastrointestinal and/or liver enzymes may affect human health.
Our results demonstrate for the first time that the intestinal bacteria, B. adolescentis and B. bifidum, are able to metabolize phenobarbital to a potentially toxic substance, alpha-ethyl-benezeneacetamide. These unexpected results are in contrast to previous published results that showed canines and humans metabolizing phenobarbital into the metabolites para-hydroxyphenobarbital and/or phenobarbital N-glucoside, which were generated from hepatic cytochrome P450. Based on our findings, it appears that Bifidobacterium spp. may alter the effectiveness of phenobarbital. Thereby, the possible metabolism of drugs by intestinal microflora should be considered in evaluating drug metabolism. Additional studies are needed to determine the metabolic response of resident and probiotic strains, as they may have similar responses to xenobiotics.
The financial support of the National Institutes of Health (DK52269-01) is gratefully acknowledged. The authors also thank Ronald Keith for assistance with the anaerobic media preparation, and Dr Robert V. Miller for reviewing the manuscript.
References
- Macfarlane G, Gibson GR, Forberg CW, Cheng KJ, White BR, Cotta MA, . Biochemical activities and metabolic transformations. Gastrointestinal microbiology, RI Mackie, BA White, et al. Chapman & Hall, New York, NY 1997; 269–620
- Heavey PM, Rowland IR. Gastrointestinal cancer. Best Pract Res Gastroenterol 2004; 18: 323–36
- Goldin BR. Intestinal microflora: metabolism of drugs and carcinogens. Ann Med 1990; 22: 43–8
- Lewis D. The CYP2 family: models, mutants, and interactions. Xenobiotica 1998; 28: 617–61
- Meatherall R. GC/MS confirmation of barbiturates in blood and urine. J Forensic Sci 1997; 42: 1160–70
- Moore WEC, Holdeman LV. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol 1974; 27: 961–79
- Fredrickson JK, Kostandarithes HM, Plymale AE, Daly MJ. Reduction of F(III), Cr(VI), U(VI), and Tc(VII) by Deinococcus radiodurans R1. Appl Environ Microbiol 2000; 66: 2006–11
- John GH, Walls S, Keith R, Good-fox-Jones J, Tucker K, Abraham KJ. The presence of a cytochrome P450-like protein in the human intestinal microflora Eubacterium aerofaciens. Microb Ecol Hlth Dis 2001; 13: 3–8
- Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med 2005; 230: 155–70
- Simons AL, Renouf M, Hendrich S, Murphy PA. Human gut microbial degradation of flavonoids: structure-function relationships. J Agric Food Chem 2005; 53: 4258–63
- Sinha VR, Kumria R. Microbially triggered drug delivery to the colon. Eur J Pharm Sci 2003; 18: 3–18
- Nugon-Baudon L, Rabot S, Flinois J-P, Lory S, Beaune Ph. Effects of the bacterial status of rats on the changes in some liver cytochrome P450 (EC 1.14.14.1) apoprotein consequent to a glucosinolate-rich diet Br J Nutr 1998;80:231–4.
- May T, Jurgens U, Rambeck B, Schnabel R. Comparison between premortem and postmortem serum concentrations of phenobarbital, phenytoin, carbamazepine and its 10,11-epoxide metabolite in institutionalized patients with epilepsy. Epilepsy Res 1999; 33: 57–65
- Butler T. The metabolic hydroxylation of phenobarbital. J Pharmacol Exp Ther 1955; 116: 326–36