ABSTRACT
The late Neogene megatooth shark, Otodus megalodon (Lamniformes: Otodontidae), is mostly known for its gigantic teeth and vertebrae. Re-examination of the rock matrix surrounding a previously described associated tooth set of O. megalodon from the upper Miocene of Japan resulted in the observation of numerous fragments of tessellated calcified cartilage and placoid scales. The morphology of each tessera and the arrangement of overall tessellated calcified cartilage are practically identical to those of extant chondrichthyans. Many placoid scales possess pronounced, rather broadly-spaced keels. A quantitative relationship between interkeel distances of keeled scales and reported cruising speeds across extant pelagic lamniforms and carcharhiniforms suggests that O. megalodon with a representative interkeel distance of ca. 100 µm was not a fast swimmer. We propose that O. megalodon was generally a slow cruising shark with occasional burst swimming for prey capture, where much of its metabolic heat through regional endothermy was possibly used to facilitate the digestion of large pieces of ingested meat as well as absorbing and processing nutrients. If so, the relative importance of the functional roles of regional endothermy possibly shifted from maintaining high cruising speeds to visceral food processing through the evolution towards gigantism in otodontids.
Introduction
The so-called megatooth shark, Otodus megalodon, is an iconic fossil shark belonging to the order Lamniformes (family Otodontidae) and is known from the late Neogene marine fossil record nearly worldwide (e.g. Cappetta Citation2012; Pimiento and Balk Citation2015; Boessenecker et al. Citation2019; Perez et al. Citation2019, Citation2021; Shimada et al. Citation2022). Although some vertebral remains are known (e.g. Bendix-Almgreen Citation1983; Uyeno and Sakamoto Citation1984; Uyeno et al. Citation1989; Gottfried et al. Citation1996; Kent Citation2018), O. megalodon is known mostly from teeth. The species was formerly classified into various genera such as Carcharocles, Procarcharodon, Megaselachus, or Carcharodon. However, it is now commonly regarded as a species of Otodus (Otodontidae) considering the taxonomic priority as well as avoiding direct phylogenetic linkage to Carcharodon (Lamnidae) and Otodus non-monophyly (Ehret et al. Citation2012; Shimada et al. Citation2017). Otodus megalodon has been estimated to reach at least 15 m in total length (TL) and up to about 18‒20 m TL (e.g. Pimiento and Balk Citation2015; Razak and Kocsis Citation2018; Shimada Citation2019; Cooper et al. Citation2020; Perez et al. Citation2021), although populations in cooler regions (higher latitudes) appear to have produced larger individuals more frequently than those in warmer regions (Shimada et al. Citation2022; but see also Herraiz et al. Citation2020). Based on bite marks on bones of various marine mammals (e.g. Aguilera et al. Citation2008; Collareta et al. Citation2017; Godfrey et al. Citation2018, Citation2021) as well as geochemical composition of fossil teeth (Martin et al. Citation2015; Kast et al. Citation2022; McCormack et al. Citation2022), O. megalodon clearly occupied a high trophic position in its ecosystem.
Uyeno et al. (Citation1989) reported 73 associated teeth of Otodus megalodon from a single individual found in an upper Miocene marine deposit in northern Saitama Prefecture, central Japan ()). Uyeno et al. (Citation1989) also briefly noted their observation of numerous small fragments of what they considered to be pieces of calcified cartilage from the same O. megalodon individual. Subsequently, Nishimoto et al. (Citation1992) reported several placoid scales of O. megalodon from a lower Miocene marine deposit in Gifu Prefecture, Japan, that were allegedly associated with at least several teeth of the species. However, no specific description or discussions have been made on Uyeno et al.’s (Citation1989) potential non-vertebral calcified cartilage materials or Nishimoto et al.’s (Citation1992) report on placoid scales which had remained unnoticed or neglected in subsequent studies of O. megalodon.
Figure 1. Representative examples of associated teeth and tessellated calcified cartilage fragments from an individual of Otodus megalodon from the upper Miocene of northern Saitama Prefecture, Japan. (a), part of associated tooth set in rock matrix (archival photograph of SMNH: see Uyeno et al. Citation1989, plate 1a); (b), fragment of tessellated calcified cartilage in rock matrix (SMNH-VeF-377); (c), close-up view of tessellated calcified cartilage depicted in Figure 1(b) (SMNH-VeF-377); (d), three samples of disaggregated tesserae (SMNH-VeF-377: left and middle samples = exterior view; right sample = broken surface showing transverse cross-sectional view). Scale bar: (a) and (b) = 1 cm; (c) and (d) = 1 mm.
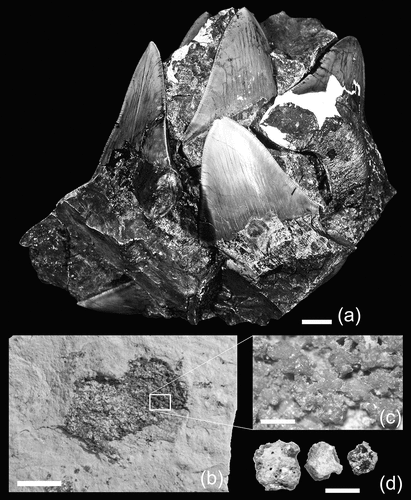
Recently, the rock matrix surrounding the tooth set of Otodus megalodon described by Uyeno et al. (Citation1989) was re-examined and confirmed that the specimen includes fragments of non-vertebral calcified cartilage. Moreover, further observation led to the discovery of numerous placoid scales (= dermal denticles) of the shark. In this present paper, we describe the morphology of the non-vertebral calcified cartilage and placoid scales in detail and also review the placoid scales described by Nishimoto et al. (Citation1992). We also discuss the biological significance of those anatomical components, especially the placoid scales as they offer new insights into the swimming ability of this fossil shark with profound ecological and evolutionary implications.
Geologic setting
The tooth set of Otodus megalodon described by Uyeno et al. (Citation1989) was discovered in 1986 from the Aketo stratigraphic section near Aketo Weir, Fukaya City (formerly Kawamoto Town) in northern Saitama Prefecture, central Japan. The Aketo section exposes a ~570-m-thick Miocene sedimentary rock sequence along the Arakawa River and shows regressive facies from the marine Tsuchishio Formation, which yielded the O. megalodon specimen, to the deltaic Yagii Formation (Homma Citation1986, Citation1987; Suto et al. Citation2003). According to Suto et al. (Citation2003), the Tsuchishio Formation at the Aketo section is at least 270 m thick and consists of diatomaceous siltstone, sandy siltstone, and alternating fine-grained sandstone and sandy siltstone with intercalation of tuff beds. The tooth set of O. megalodon occurred in and around a large concretion within the grey siltstone of the basal part of the section, of which the specific horizon is located at ~1 m above the ‘T-3 Tuff bed’ within the Tsuchishio Formation (Uyeno et al. Citation1989). On the basis of diatom biostratigraphy, the geologic age of the Tsuchishio Formation at the Aketo section is placed at the uppermost part of the North Pacific Diatom Zone NPD 5C of Yanagisawa and Akiba (Citation1998), specifically between the diatom horizons D55.8 and D56 (Suto et al. Citation2003). These diatom horizons are dated 10.2 and 10.0 Ma, respectively (Yanagisawa and Ando Citation2020). Therefore, the Tsuchishio Formation at the Aketo section is lower upper Miocene, specifically middle Tortonian, in age.
The lower part of the Tsuchishio Formation at the Aketo section is largely devoid of macrofossils. In fact, besides the Otodus megalodon specimen reported by Uyeno et al. (Citation1989), the only other fossil reported from the lower part of the formation is a pleuronectid (righteye flounder) bony fish, Saitamapsetta nomurai (Sakamoto and Uyeno Citation1992). The upper part of the formation, on the other hand, is fossiliferous, although all reported fossils are represented by invertebrates. Molluscs including Anadara, Macoma, and Mya, a majid decapod, and benthic foraminifers have been reported from the upper part of the formation, indicating a temperate shallow marine environment (Takeda and Fujiyama Citation1984; Hiki Research Group Citation1989; Akimoto and Tanaka Citation2004; Kurihara Citation2010).
Materials and methods
Uyeno et al. (Citation1989) described 73 teeth, including multiple teeth still encased in five blocks of rock. However, only eight actual fossil teeth (SMNH-VeF38, 39, 40, 41, 42, 43, 44, and 376) and 57 teeth in the form of replicas (collectively as SMNH-Om1) are formally curated at the Saitama Museum of Natural History (SMNH) in Nagatoro, Saitama Prefecture, Japan. The rest of the actual 65 teeth regrettably remain in the private collection of the discoverer of the associated tooth set. Whereas Otodus megalodon may have reached up to about 20 m TL especially in cooler waters (e.g. Perez et al. Citation2021; Shimada et al. Citation2022), this specific O. megalodon individual was moderately large. The crown height (CH) of the tallest upper anterior tooth measures 98.9 mm (Uyeno et al. Citation1989, table 2). Based on Shimada’s (Citation2019) linear equation showing the quantitative relationship between the CH of upper anterior teeth and total length (TL) derived from the extant white shark (Carcharodon carcharias) (i.e. TL = 11.788·CH + 2.143), the individual is conservatively (see Perez et al. Citation2021) estimated to have measured 11.7 m TL, corresponding to an estimated possible age of about 60 years old at the time of its death (see Shimada et al. Citation2021, figure 2(a)).
Table 1. Representative interkeel distance (IKD) of Otodus megalodon and two other extinct lamniforms compared to reported representative (‘modal’ or average) IKD of ‘non-fin’ (= head or body) placoid scales, along with reported cruising speed (CS), in non-embryonic pelagic lamniform and carcharhiniform sharks measuring 100+ cm TL with keeled placoid scales (excluding Mitsukurina owstoni, Alopias superciliosus, Triaenodon obesus, and Galeocerdo cuvier that have thorn-like and/or sparsely distributed (= less dense) placoid scales on their body, but Megachasma pelagios is included because it has rather densely spaced scales with well-defined keels: see Reif Citation1985b; Frumkin and Shimada Citation2020). Note that, although the TL of each individual for which IKD was taken is given, which does not necessarily correspond to the TL of the individual for which CS was measured, it is assumed here that IKD is independent of TL based on Raschi and Elsom’s (Citation1986) study. Additional legends: ‘L’ for Lamniformes and ‘C’ for Carcharhiniformes under ‘Order’; brackets ([]), tentative CS estimation based on regression equation in (see text for detail); asterisk (*), average value.
Table 2. Reported cruising speeds of extant pelagic lamniform and carcharhiniform sharks represented in in terms of ‘body length/second’ (BL/s) (based on Cooper et al. Citation2022, data S1, which also provide original references; each BL/s is based on the largest individual for each species listed in the data set; excludes Alopias vulpinus), along with interkeel distance (IKD) from (see text for treatment of species with multiple samples).
Materials described in this present study were recovered from rock pieces (ca. 1,800 cm3 in total volume) that immediately surrounded the associated tooth set and that were retained by SMNH. Original inspection of rock surfaces resulted in the observation of numerous fragmentary tessellated calcified cartilage sheets and scattered individual tesserae (for terminology, see Dean and Summers Citation2006) as well as a few placoid scales. Whereas some rock samples were highly indurated, other pieces were sufficiently soft to be able to break down into sediments by lightly tapping with a hammer. Approximately 300 cm3 of disaggregated sediments, each measuring generally <4 mm in maximum dimension were produced, rinsed with tap water, dried, and examined under a dissecting microscope, where a pair of forceps was used to extract most of the fossil materials described in this study. Additional placoid scales were also collected through surface examination of small rock pieces mostly measuring 4–15 mm in maximum dimension that amassed to about 900 cm3. The described tessellated calcified cartilage pieces and all collected placoid scales are collectively curated as SMNH-VeF-377 and SMNH-VeF-378, respectively, at SMNH.
The placoid scales described by Nishimoto et al. (Citation1992) are reviewed and briefly discussed based on the original description. However, we determined that they should not be heavily relied upon for further biological considerations for the following two primary reasons. First, the nature of the placoid scales’ association with a tooth set of Otodus megalodon is not described in Nishimoto et al.’s (Citation1992) paper. This situation means that any possible accidental inclusion of placoid scales from one or more other contemporaneous sharks cannot be ruled out. Second, where no referable catalogue number was provided for those specimens, Nishimoto et al. (Citation1992) illustrated a total of 10 placoid scales to demonstrate a total of five morphological scale types. However, they reported neither the total number of scales collected nor the total number of samples representing each of the five morphological types, making meaningful biological inferences difficult.
Results
The examined rock and sediment samples contained many small fragments of tessellated calcified cartilage as well as placoid scales. Whereas only select examples of disaggregated tesserae were sampled because most calcified cartilage pieces were fragmentary, 589 placoid scales were collected. The placoid scales measure 0.3–0.8 mm in maximum dimension, and each consists of an enameloid crown with a constricted base and a dentine root with a centrally located nutritive pore on the basal face. The collected placoid scales can be classified morphologically into three broad categories, and they are arbitrarily assigned to Types ‘I’, ‘II’, and ‘III’, where Types II and III each consisting of two subcategories are arbitrarily assigned to ‘a’ and ‘b’ for the purpose of this study. All placoid scales were found individually disarticulated except for two ‘Type II-a’ scales remaining articulated. Of the nearly 600 placoid scales collected, the morphotypes are distributed as 12% Type I (n = 71), 17% Type II-a (n = 100), 25% Type II-b (n = 147), 31% Type III-a (n = 183), and 15% Type III-b (n = 88). Along with the nature of the tessellated calcified cartilage, each scale type is described below.
Tessellated calcified cartilage
One of the largest sheets of tessellated calcified cartilage with discernable broken edges measures about 3 cm × 2 cm ()). However, the vast majority of tessellated calcified cartilage pieces preserved in the rock matrix are even more fragmentary. Their broken surfaces in indurated rocks appear smooth and vitreous and offer very little anatomical information. Each larger fragment of calcified cartilage that offers more anatomical detail, particularly those in softer rocks, consists of a layer of tessellated calcified cartilage. Each tessera typically measures 0.8–1.5 mm and is polygonal, commonly hexagonal (some snowflake-shaped: e.g. centre-most tessera in )) but some pentagonal (e.g. right-most sample in )), where its five or six extremities or sides meet with extremities or sides of adjacent tesserae to form a tessellated calcified cartilage sheet ()). Well-preserved tesserae may exhibit minute, irregularly scattered pores on the exterior surface (e.g. left-most example in )). The exact thickness of each tessellated calcified cartilage sheet is difficult to measure due to the way it is generally preserved and because most disaggregated tesserae are broken, but it appears to be only about 1.0–1.5 mm.
‘Type I’ placoid scales
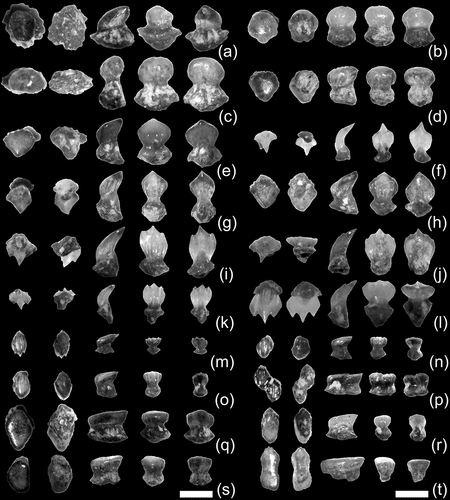
This scale type ()–(d)) is characterised by a bulbous crown and massive root, where the root is about equal in size to, or slightly larger than, the crown. The Type I scales range from 0.5 to 0.8 mm in maximum dimension, which includes the largest placoid scales among the samples collected (e.g. )). The bulbous crown may have a posteriorly directed blunt point or rim making a teardrop shape in apical view () and (d)) or may form a spherical to laterally ovoid shape () and (c)). Some of the Type I scales exhibit a few weak ridges (= keels) and grooves on the anterior face of the crown (e.g. )), that are somewhat similar to Type III-b scales (see below), but they are classified into Type I for the presence of rounded apical surface with a poor demarcation to the lateral sides, making the bulbous appearance. The junction between the crown and root is relatively horizontal. The basal root surface exhibiting a nutritive pore faces basally and is gently convex, where the periphery of the root may be expanded entirely (e.g. ) and (b)) or only laterally (e.g. )), or may be simply rounded (e.g. )).
Figure 2. Representative examples of placoid scales (SMNH-VeF-378) from an individual of Otodus megalodon from the upper Miocene of northern Saitama Prefecture, Japan. (a)–(d), ‘Type I’ scales; (e)–(h), ‘Type II-a’ scales; (i)–(l), ‘Type II-b’ scales; (m)–(p), ‘Type III-a’ scales; (q)–(t), ‘Type II-b’ scales (see text for scale types). Orientations (from left to right): apical (anterior to top), basal (anterior to top), profile (anterior to left), anterior, and posterior views. Scale bar = 0.5 mm.
‘Type II’ placoid scales
This scale type ()–(l)) is characterised by an apicobasally extended crown with one or more sharp apices and a rather gracile root with a lateral expansion near its base, where the root is about equal in size to, or slightly smaller than, the crown. The Type II scales range from 0.4 to 0.7 mm in maximum dimension (e.g. )). The crown, which is laterally expanded and relatively thin anteroposteriorly, is gently curved apicoposteriorly with a convex apicoanterior (= exterior) face. The crown with a single apex (monocuspidate) is here referred to as Type II-a ()–(h)), whereas that with three, but some with five, apices (multicuspidate) is referred to as Type II-b ()–(l)). The exterior face of the crown is largely smooth in Type II-a, although three to six weak, round keels (= ridges bounding grooves in between) may be present near the base of the crown that directly faces anteriorly. On the other hand, such keels may be significantly pronounced and may continue posteriorly to the terminal end of the multiple apices of the crown in Type II-b, although keels may become less pronounced posteriorly. Interkeel distances (IKD) on the apical surface in Type II-b scales range from ca. 75 to 130 µm (n = 147), with the modal IKD of around 100 µm. The junction between the crown and root in Type II strongly slants apicoposteriorly, rather than horizontally observed in Types I and III. The basal face of the root slants apicoanteriorly, where both sides typically spread laterally, forming a laterally elongate diamond shape in basal view.
‘Type III’ placoid scales
This scale type ()–(t)) is characterised by a massive crown with a flat apical face that is well-demarcated with the lateral sides and a massive root that is about equal in size to the crown. The Type III scales range from 0.3 to 0.7 mm in maximum dimension, which includes the smallest placoid scales among the samples collected (e.g. )). The ‘flat-topped’ crown is typically anteroposteriorly elongated and outlines an oval to rounded diamond shape in apical view. The vast majority of Type III scales have a single blunt posterior end, although a few exhibit a three-pronged posterior rim. The crown exhibiting typically three, but as many as five, keels, is here referred to as Type III-a ()–(p)), whereas that with no keels on the apical face is referred to as Type III-b ()–(t)) although many Type III-b scales exhibit rounded keels on the anterior face. IKDs on the apical surface in Type III-a scales range from ca. 75 to 85 µm (n = 183), with the modal IKD of about 80 µm. The junction between the crown and root is relatively horizontal. The basal root surface exhibiting a nutritive pore is flat or gently convex and typically forms an anteroposteriorly directed diamond shape.
Among the Type III scales are two notable samples. The sample belonging to Type III-a depicted in ) consists of two articulated placoid scales. They exemplify the fact that the anteroposteriorly directed diamond-shaped crown and root of each scale allow the articulation of each scale to adjacent scales to occur diagonally, rather than side by side or immediately anteroposteriorly. The sample referable to as Type III-b depicted in ) consists of a single placoid scale that is exceptionally elongated anteroposteriorly. The middle portion of the complete crown and damaged root is slightly compressed laterally, revealing that it may be two placoid scales abnormally developed in a conjoined manner.
Discussion
Taphonomic interpretation
In this study, we described the recovery of numerous tessellated calcified cartilage remains and placoid scales belonging to the same individual of Otodus megalodon originally described by Uyeno et al. (Citation1989) on the basis of 73 associated teeth. Besides their abundance and proximity to the associated teeth, these tessellated calcified cartilage remains and placoid scales are interpreted to unequivocally belong to the same individual shark because, where vertebrate fossils are generally uncommon in the stratigraphic unit (see Geologic setting above), no remains of other sharks or vertebrates were found in the examined rock matrix or sediments.
Although Otodus megalodon is known to have possessed well-calcified vertebral centra that represent areolar calcification (see Dean and Summers Citation2006), this present study confirms that calcification of cartilage occurred also in other parts of its skeleton in the form of tessellated calcified cartilage. Cartilage associated with vertebrae, or broadly ‘vertebral cartilage’, may include tessellated calcified cartilage sheathing uncalcified cartilage making up the body of neural and haemal arches (Dean and Summers Citation2006). However, the fact that the specimen of O. megalodon does not preserve any vertebrae but is instead chiefly represented by its associated teeth (Uyeno et al. Citation1989) suggests the likelihood that the tessellated calcified cartilage described in this paper came from skeletal elements of the head, such as the neurocranium, splanchnocranium (including jaw cartilage), or possibly pectoral girdles or fins (also see further discussion below on potential types of placoid scales represented in the specimen). In addition, the fact that tessellated calcified cartilage is represented by fragmentary pieces, along with the fact that the associated tooth set represents a taphonomically induced ‘disarticulated tooth set’ (sensu Shimada Citation2006), indicates that the carcase of O. megalodon did not experience rapid burial but rather laid on a relatively quiet ocean floor for some period of time leading to its incomplete decay before it became buried. The abundance of minute, mostly disarticulated placoid scales found, that would otherwise have drifted away before burial if the water current was strong (e.g. see Schäfer Citation1972, p. 56), also supports this taphonomic interpretation.
Physical aspects of tesselated calcified cartilage
Tesserae have been present in chondrichthyans for over 400 million years to stiffen their skeleton (Maisey Citation2013; Maisey et al. Citation2021), and several layers of tessellated calcified cartilage may be present at mechanically demanding parts of the skeleton, such as the corners of the jaws in large species and durophagous forms (Dingerkus et al. Citation1991; Summers Citation2000; Dean et al. Citation2006). The Otodus megalodon individual examined here was moderately large for the species (conservative estimate of 11.7 m; see Materials and Methods above), and because it possessed large, serrated teeth that would have required the jaws to be mechanically demanding for cutting function, it is plausible that at least parts of its jaws could have had multiple layers of tessellated calcified cartilage. However, we did not observe any multi-layered tessellated calcified cartilage, although the lack of such observation may be due to the fragmentary nature of most tessellated calcified cartilage.
It is noteworthy that the morphology of each tessera (e.g. predominantly hexagonal) and the arrangement of tesserae as a tessellated calcified cartilage sheet in Otodus megalodon are practically identical to those of extant chondrichthyans (see Seidel et al. Citation2021, and references therein), further exemplifying remarkable morphological conservatism of tessellated calcified cartilage in chondrichthyans. More significantly, whereas the vast majority of tesserae in extant taxa examined previously are based on small (<2 m) chondrichthyans (see Seidel et al. Citation2021, and references therein), the fact that the size range of tesserae observed in the estimated 11.7-m-TL individual of O. megalodon is comparable to that of extant chondrichthyans suggests that larger body size does not necessarily produce larger tesserae. This observation in turn suggests that skeletal elements sheathed by tesserae developed through biomineralization along the margins of existing tesserae to form new tesserae ontogenetically (Dean et al. Citation2009; Seidel et al. Citation2021) also occurred in O. megalodon that achieved exceptional gigantism (see Shimada et al. Citation2020).
Physical aspects of placoid scales
The placoid scales of Otodus megalodon depicted in capture the overall morphological variation observed in 589 samples collected, where we recognise three major morphological types, and two subtypes for two of the three major types. Similarly, Nishimoto et al. (Citation1992) reported a total of five morphological types, where some of their scales resemble forms reported herein whereas others do not. For example, Nishimoto et al.’s (Citation1992) ‘Types I and II’ scales are equivalent to the morphological range seen in our Type II-a scales. One of Nishimoto et al.’s (Citation1992) three examples of their ‘Type III’ (their plate 34, figures 5a, b) closely resemble Type III-b in our study, but the other two (their plate 34, figures 1a, b, 6a, b) are equivalent to our Type I. The more intriguing aspect of Nishimoto et al.’s (Citation1992) report is the fact that the scales they referred to ‘Types IV and V’ characterised by a large number (7 or 10, respectively) of exceptionally prominent keels with average IKDs of 50 and 35 µm, respectively, are not represented in our samples at all. It is possible that Nishimoto et al.’s (Citation1992) Types IV and V may represent placoid scales from different regions of the body not represented by our samples (see below for further discussion of scales on different body regions). However, we note that Nishimoto et al.’s (Citation1992) Types IV and V scales are more similar to carcharhinid scales than to lamniform scales (e.g. see Reif Citation1985b; Motta et al. Citation2012; Frumkin and Shimada Citation2020; Paig-Tran et al. Citation2022). Carcharhinid (e.g. Carcharhinus spp.) teeth, among many other elasmobranch fossils, are common in the specific stratigraphic horizon (Yamanouchi Member) within the Akeyo Formation of the Mizunami Group where Nishimoto et al.’s (Citation1992) placoid scale samples were collected (e.g. see Itoigawa et al. Citation1985). In fact, a relatively high concentration of multiple types of isolated placoid scales likely from different elasmobranch taxa is not necessarily unusual even in a small amount of sediment in the fossil record (e.g. Gorman et al. Citation2014; Nelms et al. Citation2014; Sibert and Rubin Citation2021). Therefore, it is quite possible that Nishimoto et al.’s (Citation1992) Types IV and V scales, which occurred with only eight teeth putatively coming from a single O. megalodon individual, may have been derived from one or more different shark species. In contrast, the placoid scales of O. megalodon from the Tsuchishio Formation described here are interpreted to have come exclusively from one shark individual, particularly because of the co-occurrence with an exceptionally high concentration of associated teeth and calcified cartilage fragments () and because vertebrate fossils, including shark teeth, are uncommon throughout the stratigraphic formation (see Geological setting above). The different types and subtypes represented in the placoid scale samples reported here most certainly reflect scales from different parts of the body. The interpretation that they all come from a single shark is also supported by the fact that those types and subtypes are represented rather in a continuum in size and morphology.
It is well documented that a wide range of variations in dermal denticle morphology typically exists within an individual shark (e.g. Reif Citation1985a, Citation1985b; Ankhelyi et al. Citation2018; Feichtinger et al. Citation2021; Naylor et al. Citation2021). Otodus megalodon is a lamniform shark, and significant intraindividual variation in placoid scale morphology also exists in extant lamniforms (e.g. Reif Citation1985b; Motta et al. Citation2012; Frumkin and Shimada Citation2020) that consist of eight families, 10 genera, and 15 species (e.g. Stone and Shimada Citation2019; Ebert et al. Citation2021). The most detailed survey of intraindividual variations in lamniforms that has so far been conducted is on the extant shortfin mako, Isurus oxyrinchus (Lamnidae) (Motta et al. Citation2012). Otodus megalodon is not a lamnid shark, but the vast majority of morphological scale types observed in I. oxyrinchus are also represented in the placoid scales of O. megalodon reported here. For example, Type I scales are similar to placoid scales of I. oxyrinchus found along the ventral side of the body between the pelvic and anal fins (scale sampling site ‘A3’ of Motta et al. Citation2012). Type II-a scales are similar to placoid scales of I. oxyrinchus found primarily in the head (scale sampling site ‘H2’ of Motta et al. Citation2012), and Type II-b scales resemble most scales of I. oxyrinchus distributed on the trunk between the head and the level of the pelvic fins as well as the posterior margin of the pectoral fins (scale sampling sites ‘B1’–B6’, ‘A2’, and ‘P3’ of Motta et al. Citation2012). Type III-a and Type III-b scales are reminiscent of placoid scales of I. oxyrinchus located on the dorsal face and anterior edge of the pectoral fins, respectively (respectively, scale sampling sites ‘P2’ and ‘P1’ of Motta et al. Citation2012). However, decisive examples of the morphology of placoid scales observed towards and on the caudal fin of I. oxyrinchus, which are characterised by exceptionally elongate posterior prongs of the crown, are not present in the collected scale samples of O. megalodon. Whether the distribution of scale morphologies observed in different parts of the body in I. oxyrinchus was the same in O. megalodon cannot be ascertained from the present fossil record. However, because the rock samples that yielded the placoid scales of O. megalodon contained its tooth set (= mouth or head region), the observed range of scale morphology of O. megalodon does not contradict the fact that it corresponds to the types of scales found primarily on the anterior half of the body in I. oxyrinchus. In fact, 88% of the total scale samples collected (Types II-a, II-b, III-a, and III-b combined; see above for the percentage of each scale type) are identified to be placoid scales from the anterior half of the body, about half of which (46%: Types III-a and III-b combined) are scales reminiscent to those on the pectoral fins of I. oxyrinchus.
Among the Type III scales are two articulated placoid scales ()). The scales, that are interpreted to have come from a pectoral fin (see above), demonstrate that scales densely covered the surface of the paired fins, consistent with scales on the pectoral fins of many extant pelagic lamniform and carcharhiniform taxa (e.g. see Reif Citation1985b). In the contrary, whether scales belonging to Types I and II were densely spaced cannot be ascertained. However, the presence of parallel keels in Type II scales does not preclude the possibility that they were also densely organised based on scales of the head and body of many extant pelagic lamniform and carcharhiniform taxa (e.g. Reif Citation1985b), unlike widely spaced, thorn-like scales with non-parallel keels seen in body scales of Mitsukurina owstoni and Alopias superciliosus (Frumkin and Shimada Citation2020). It should be added that the collected Type III scales include a specimen presumably consisting of two scales that abnormally developed in a conjoined manner ()). Whereas new scales develop at places where either old scales are shed or the growth of the skin creates new spaces (Reif Citation1982), cases of a fusion of two scales are known to occur in extant sharks (Reif Citation1985b), suggesting that the same developmental mechanism of placoid scales that operates in extant sharks was present in O. megalodon.
The placoid scales of most extant lamniform sharks are generally smaller (crown widths of ca. 0.1–0.3 mm: Motta et al. Citation2012; Frumkin and Shimada Citation2020) than the collected samples of placoid scales of Otodus megalodon (crown widths of ca. 0.3–0.7 mm: ). Rather, the scale size range of O. megalodon is comparable to that of representative extant pelagic carcharhiniform sharks (e.g. Prionace, Carcharhinus, and Isogomphodon: Raschi and Elsom Citation1986; Motta et al. Citation2012; Naylor et al. Citation2021; Paig-Tran et al. Citation2022, figure 3.13) as well as Carcharias taurus (Lamniformes: Carchariidae; 0.3–0.6 mm based on Raschi and Elsom Citation1986), many of which exhibit the morphology of Types II and III. Furthermore, the scale size range of O. megalodon, along with the observed morphological range in Types II and III, is also comparable to that of many Late Cretaceous pelagic lamniforms, such as Cretoxyrhina (Cretoxyrhinidae) and Cretodus (Pseudoscapanorhynchidae) that reached at least 5 m TL as well as Squalicorax spp. (Anacoracidae) that were generally <3 m TL (Shimada Citation1997; Shimada and Cicimurri Citation2005; Shimada and Everhart Citation2019; Amalfitano et al. Citation2022). There are also many isolated placoid scales in the Late Cretaceous fossil record where Cretoxyrhina and Squalicorax are common (e.g. Gorman et al. Citation2014; Nelms et al. Citation2014; Guzzo and Shimada Citation2018) that are not only similar to the placoid scales of O. megalodon size-wise but also morphology-wise. These observations suggest that there is considerable conservation of scale morphology as well as scale size among many pelagic lamniform sharks at least since the Late Cretaceous. More significantly, although most extant lamniforms for which placoid scales were previously surveyed were from individuals <2.5 m TL (e.g. Reif Citation1985b; Motta et al. Citation2012; Frumkin and Shimada Citation2020), the fact that the general size of placoid scales represented by the vast majority of studied extant pelagic lamniforms and carcharhiniforms as well as extinct lamniform taxa such as Cretoxyrhina, Cretodus, and Squalicorax is similar to the overall scale size of much larger O. megalodon at least demonstrates that the exceptional gigantism seen in O. megalodon (Shimada et al. Citation2020) did not necessarily yield exceptionally large placoid scales. Rather, more new placoid scales of similar small size were added as the fossil shark grew through ontogeny.
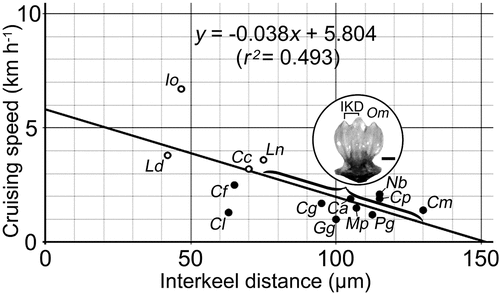
Figure 3. Linear regression showing the relationship between interkeel distances (IKD) of placoid scales and reported cruising speeds across 14 extant lamniform and carcharhiniform species with keeled placoid scales (see and text; excludes Alopias spp. and Sphyrna spp. with highly derived body forms; solid circle plots indicate ectothermic taxa, and open circle plots indicate regionally endothermic taxa). The bracket along the regression line shows the total range of IKDs (75–130 µm) observed in Type II-b placoid scales of Otodus megalodon reported here, and the inset shows a representative example in anteroapical view (one of SMNH-VeF-378; scale bar = 100 µm). Species abbreviations: Ca, Carcharhinus amblyrhynchos; Cc, Carcharodon carcharias; Cf, Carcharhinus falciformis; Cg, Carcharhinus galapagensis; Cl, Carcharhinus limbatus; Cm, Carcharhinus melanopterus; Cp, Carcharhinus plumbeus; Gg, Galeorhinus galeus; Io, Isurus oxyrinchus; Ld, Lamna ditropis; Ln, Lamna nasus; Mp, Megachasma pelagios; Nb, Negaprion brevirostris; Om, Otodus megalodon; Pg, Prionace glauca.
Cruising speed of Otodus megalodon based on placoid scales
Whereas placoid scales serve to accommodate sensory and bioluminescent organs, they themselves also have various functional roles, such as abrasion resistance, biological (including against parasites) and mechanical protection, antifouling, hydrodynamic drag reduction, and increased thrust (e.g. Reif Citation1978, Citation1985a, Citation1985c; Bechert et al. Citation1985; Raschi and Musick Citation1986; Raschi and Tabit Citation1992; Carman et al. Citation2006; Schumacher et al. Citation2007; McKenzie et al. Citation2014). In particular, their hydrodynamic drag reduction function has received considerable attention. This is because the morphology of placoid scales, particularly in reference to the presence or absence of keels on the crown as well as the distance between keels if present, has been found to reflect the manoeuverability of each shark in the water (e.g. Reif and Dinkelacker, Citation1982; Frumkin and Shimada Citation2020; Popp et al. Citation2020). For example, scales with a simple narrow thorn-like crown and a multi-keeled broad crown are considered to reflect slow swimming and fast swimming, respectively (Reif Citation1982, Citation1985a; Frumkin and Shimada Citation2020). Drag reduction, especially in keeled scales, is said to occur by reducing crossflow turbulence near the crown surface resulting in the reduction of shear stress, as well as by controlling flow separation around the body to reduce pressure drag (e.g. Bechert et al. Citation2000; Lang et al. Citation2011; Du Clos et al. Citation2018; Paig-Tran et al. Citation2022).
Cooper et al. (Citation2022) reconstructed a hypothetical 3D model of Otodus megalodon by considering that the species likely resembled the extant white shark, Carcharodon carcharias, because O. megalodon was inferred to have had similar ecological and thermophysiological attributes to C. carcharias, including likely being regionally endothermic (Ferrón Citation2017). Based on their 3D reconstruction that requires testing for its accuracy, Cooper et al. (Citation2022) concluded that O. megalodon was a fast swimmer (see also Jacoby et al. Citation2016; Ferrón Citation2017) and was capable of long-distance travel. However, their conclusion is logically circular because their study already assumed that the regionally endothermic extant C. carcharias capable of fast and long-distance swimming is the model organism in the first place. Therefore, independent evidence is required to test whether O. megalodon was a fast swimmer capable of long-distance travel. In this regard, one notable finding of this present study is the abundance of scales with parallel keels in O. megalodon without any sharply pointed ‘thorn-like’ dermal denticles seen in Mitsukurina owstoni or the trunk portion of Alopias superciliosus that characterise slow-cruising sharks (see Frumkin and Shimada Citation2020). Therefore, an attempt was made to infer the swimming speed of O. megalodon on the basis of its placoid scales. Although O. megalodon achieved exceptional gigantism (Shimada et al. Citation2020), such comparisons are found to be worthwhile because, despite the fact that the crown size of placoid scales may increase ontogenetically, the spacing of the parallel keels (= IKD) on the crown surface is known to remain practically constant throughout the life of each shark with keeled scales (Raschi and Elsom Citation1986), meaning that IKD can be assumed independent of body size.
lists the reported ‘representative’ (‘modal’ or average depending on studies) IKDs of some extinct lamniforms with keeled placoid scales compared with those of non-embryonic, extant lamniform and carcharhiniform taxa considered as ‘fast pelagic hunting sharks’ or ‘large near-shore hunters’ (sensu Reif Citation1985b) with densely spaced, keeled scales located primarily on their trunk region. One exception is Megachasma pelagios which is regarded as a sluggish planktivorous shark for comparison because it does have placoid scales with densely spaced, well-developed keels (Frumkin and Shimada Citation2020). The IKD of about 100 µm for O. megalodon is the modal IKD observed in Type II-b scales with the assumption that they possibly represent scales from the trunk region excluding fins (see above). Type III-a scales that have well-defined keels with narrower IKDs (ca. 80 µm: see above) are not included because they are considered to represent placoid scales from the pectoral fins (see above), but it is noteworthy that both IKD values (100 µm for Types II-b and 80 µm for Type III-a) falls within the range of the IKD of extant taxa listed in . Likewise, the IKD of 45 µm for Cretoxyrhina mantelli as well as that of 135 µm for Cretodus houghtonorum fall within the total recorded IKD range. It should be added that Amalfitano et al. (Citation2022) illustrated three keeled placoid scales of Cretodus crassidens, but the species is not included in because of the lack of their apical view which prevented adequate examination of their IKDs. Additionally, Shimada and Cicimurri (Citation2005) reported some scales of a Late Cretaceous anacoracid lamniform, Squalicorax falcatus, and noted that the species has keeled placoid scales with an average IKD of 30 µm. However, besides the fact that such a small IKD is not recorded (), the re-examination of the images of the placoid scales (Shimada and Cicimurri Citation2005, fig. 10) suggests that the keels in S. falcatus are somewhat irregular and non-parallel and do not reach the crown apex, so the species is also not included in .
also lists reported cruising speeds of respective extant taxa, where known, in order to examine whether the cruising speed (CS) is indeed related to IKD of placoid scales. Our primary sources of CS data are those compiled by Watanabe et al. (Citation2015) and Harding et al. (Citation2021) whose studies depended on the accuracy of CSs of diverse fishes, where additional sources were used for taxa not reported in these two studies. We should note that Cooper et al. (Citation2022, data S1) presented an extensive compilation of swimming speed data, but they are not used here because their data set consists of a mixture of different speed extrapolation methods and examination durations, that may not necessarily capture the representative CS of each species (see further discussion below). Reported swimming speeds of sharks include values using different distance and/or time units as well as different types of speed measurements, such as ‘burst speed’, ‘maximum speed’, and ‘body lengths per second’. For the purpose of this study, we used published CSs (distance per unit time: sensu Watanabe et al. Citation2015, which were considered to be broadly equivalent to the ‘routine swimming speeds’ of Lauder and Di Santo Citation2015) to capture the ordinary swimming state of each shark species, and unit conversions were made where necessary to standardise our data in terms of ‘km per hour’ (km h−1). contains ‘IKD-CS’ pairs of a total of 18 extant species, comprising seven lamniforms and 11 carcharhiniforms, where for the purpose of this paper, we used the average IKD for species with more than one sample listed in the table (i.e. IKD of 47.75 µm for Alopias vulpinus, 46.7 µm for Isurus oxyrinchus, 70 µm for Carcharodon carcharias, 112.5 µm for Prionace glauca, 115 µm for Carcharhinus plumbeus, and 45 µm for Sphyrna zygaena).
Based on the IKD-CS pairs of 18 extant lamniform and carcharhiniform species, we generated two regression lines relating the IKD (x) with their respective CS (y). One regression line was based on all 18 species combined, which gave an equation of y = −0.014x + 3.298 with a very low statistical significance (p = 0.221) and coefficient of determination (r2 = 0.092). The 18 species include two genera that are highly derived morphologically, Alopias (thresher sharks) with an exceptionally elongated caudal fin and Sphyrna (hammerhead sharks) with a peculiar laterally expanded head. Although the exact body form of Otodus megalodon is not known (Sternes et al. Citation2022), one may consider a typical shark body plan (e.g. Gottfried et al. Citation1996; Cooper et al. Citation2020, Citation2022; Sternes and Shimada Citation2020; Sternes et al. Citation2022) to be more parsimonious for the body form of O. megalodon than considering it having an atypical body design like Alopias and Sphyrna with different hydrodynamics of the body that may affect its swimming speed. Therefore, we experimented with the second regression analysis by examining the IKD-CS pairs of only 14 species in without Alopias spp. and Sphyrna spp. The resulting regression line () has an equation of y = −0.038x + 5.804 with a drastically improved, albeit still weak, statistical significance (p = 0.005) and coefficient of determination (r2 = 0.493). This indicates that the IKD-CS relationship of Alopias, Sphyrna, or both may be fundamentally different from that of other large pelagic sharks examined or additional or more accurate data are needed. Regardless, if the second regression equation is used at face value despite its relatively weak coefficient of determination, the estimated CS of O. megalodon is calculated to be 2.0 km h−1, whereas that of Cretoxyrhina mantelli and Cretodus houghtonorum 4.1 and 0.7 km h−1, respectively (). These results would mean that Cretoxyrhina mantelli was a fast swimmer, Cretodus houghtonorum a sluggish swimmer, and O. megalodon an ‘intermediate’ swimmer, even among the CS values of the 14 extant species included in the analysis are considered. In fact, the general position of a rather tight cluster of several plots observed at 100 µm near the x-axis in indicates that the CS range of 1–2 km h−1 for O. megalodon appears reasonable.
One major observation that can be gleaned from is that the lowest limit of the IKD range of Otodus megalodon (75 µm) marks the highest limit of the IKD range observed among the extant lamnids that are regionally endothermic (Ferrón Citation2017). It should be noted that the linear regression equation exclusively of the lamnids (; n = 4) is y = −0.054x + 7.494 with a very weak statistical significance (p = 0.442) and coefficient of determination (r2 = 0.312), and that exclusively of non-Alopias and non-Sphyrna extant ectothermic taxa (; n = 10), y = −0.005x + 2.112, has practically no correlation (r2 = 0.005) with even weaker statistical significance (p = 0.543). However, it is also noteworthy that, if the IKD of 100 µm is experimentally applied to the equation derived only from the regionally endothermic lamnid taxa, the estimated CS is 2.1 km h−1, which is still about the same as the estimate (2.0 km h−1) derived from all 14 extant taxa (). Whether the quantitative relationship between the IKDs and CSs in is linear, or even real (especially in the ectothermic taxa), needs further investigation. However, if it is considered at face value and if O. megalodon with the IKDs ranging up to as much as 130 µm was indeed regional endothermic, our study suggests that O. megalodon would represent an exception to the general rule that endothermy promotes fast swimming in sharks (Harding et al. Citation2021; see further discussion below).
Otodus megalodon has been regarded as a fast swimmer relative to other sharks with its previous cruising speed estimates ranging from 4.8 to 5.1 km h−1 (Jacoby et al. Citation2016; Ferrón Citation2017; Cooper et al. Citation2022). Even though the most recent study on this topic conducted by Cooper et al. (Citation2022) examined the CS of O. megalodon primarily in terms of ‘body length/second’ (BL/s), however, our observation does not support their interpretation that ‘O. megalodon was able to cruise faster than all living species’ (p. 2; note that their study examined 28 extant shark species that included fast swimmers). We should note that we conducted an additional linear regression analysis to examine the relationship between IKD (as ‘x’) and reported CSs of extant pelagic lamniform and carcharhiniform sharks represented in in terms of ‘body length/second’ (BL/s; as ‘y’) based on Cooper et al.’s (Citation2022, data S1) data (). It resulted in an equation y = −0.0009x + 0.406 with an exceptionally low BL/s predictability from IKD (p = 0.523; r2 = 0.053). Even if we forcefully calculate the BL/s value for O. megalodon with the IKD of 100 µm using the equation, the resulting 0.316 BL/s is not the greatest value compared to BL/s values in , suggesting that Cooper et al.’s (Citation2022) assertion, like Ferrón (Citation2017), that O. megalodon was an exceptionally fast swimming shark cannot be supported. Furthermore, it should be noted that the IKD of 100 µm based on the Type II-b placoid scales was used in our analysis, but even if the modal IKD of 80 µm based on the Type III-a scales is used, the estimated CS would be 2.8 km h−1, which is still slower than any of the examined extant lamnids, including Carcharodon carcharias that is the slowest among them ().
We must note that our estimated CS of 2.0 km h−1 for Otodus megalodon should be regarded as provisional because the validity of the assumption of constant spacing of the parallel keels on the scale surface through ontogeny based on Raschi and Elsom’s (Citation1986) remains uncertain for O. megalodon. In addition, the question remains as to whether the scale samples of O. megalodon we used for the analysis actually represent those from the head and trunk regions. More fundamentally, besides the fact that our calculated CS for O. megalodon is based on a linear equation with the rather weak p- and r2-values (see above), we must also point out that the CS and IKD data listed in should be viewed only as first approximations for each shark species for at least two major factors. First, it is possible that the reported CS value of each extant species () may not be accurate because determining the exact CSs of sharks is exceptionally difficult (e.g. Lauder and Di Santo Citation2015; Kai and Fujinami Citation2020). For example, although we relied largely on the CS data used by Watanabe et al. (Citation2015) and Harding et al. (Citation2021) (), Cooper et al.’s (Citation2022, data S1) compilation of swimming speed data reveals a rather wide range of reported swimming speeds for each species with multiple samples. The sources of speed variations may come from the fact that reported speeds are based on individuals of different sizes or ontogenetic stages using different tracking approaches (e.g. two-dimensional vs. three-dimensional tracking), and because speed data are also influenced by the duration of tracked time of each sample as well as water currents and intraspecific behavioural differences (Kai and Fujinami Citation2020). Second, the IKD values used for our analysis () were based on multiple studies with somewhat variable scale sampling sites, even though our study limited measurements from the placoid scales assumed to have come from the head and trunk regions. We also note that some studies reported only one general IKD value for a species, whereas some other studies gave a range of IKDs or a ‘modal’ or average IKD measured either from a single shark or multiple sharks at the same, or a similar but not necessarily identical, scale sampling site, making comparisons challenging. Therefore, the choices and treatment of data as well as comparative and analytical methods in this study—and hence the estimated CS for the fossil species—should be considered exploratory or tentative.
Nevertheless, our tentative CS estimate of 2.0 km h−1 for Otodus megalodon derived from presently available empirical data at least provides a point of reference. For instance, although we used the most representative IKD value of 100 µm in our analysis, the IKDs of the Type II-b scales range from 75 to 130 µm (see above). Therefore, the CS of O. megalodon could have been slightly faster (3.0 km h−1) or slower (0.9 km h−1) if the regression equation in is used at face value, although it is worth pointing out that the faster estimated CS of 3.0 km h−1 is still smaller than any of the reported CSs of any extant lamnids in . In addition, even though Ferrón (Citation2017) inferred it to be about 37.2 km h−1, the true possible ‘burst swimming’ speed of O. megalodon cannot be ascertained from the fossil record; however, it is reasonable to assert that it must have been much faster than 2.0 km h−1. Furthermore, we compared O. megalodon with sharks that have densely spaced, keeled placoid scales that are considered optimal for surface drag reduction for swimming (Reif and Dinkelacker Citation1982; Reif Citation1985b), but if placoid scales on the body of O. megalodon were actually sparsely distributed in life, one could argue that the CS of O. megalodon must have been slower than 2.0 km h−1.
Although we interpreted that Nishimoto et al.’s (Citation1992) Type IV or V scales may not belong to Otodus megalodon, if they indeed belong to O. megalodon and their respective IKDs of 75 and 60 µm are used to estimate the CS based on the linear equation in , they would be 3.0 and 3.5 km h−1, which are close to, or comparable with, CSs of non-Isurus lamnids. However, besides the similarity in morphology, placoid scales of similar sizes (about 0.4 mm in maximum dimension) and IKDs also occur in other sharks, such as Carcharhinus spp., particularly those on their pectoral fins (Reif Citation1985b; Raschi and Elsom Citation1986). An additional noteworthy aspect of Nishimoto et al.’s (Citation1992) putative placoid scales of O. megalodon is that their other scale types (their Types I–III) have keels confined to the anterior crown margin without extending to the posterior edge, unlike many scales identified here as Type II-b inferred to have come from the body. Even so, it is equally noteworthy that not all Type II-b scales had keels fully extended to the posterior margin, which may indicate that fully-keeled placoid scales were possibly not the dominant scale form in O. megalodon. This situation, in turn, further supports the interpretation that O. megalodon was not necessarily a fast-swimming shark as previous studies (e.g. Jacoby et al. Citation2016; Ferrón Citation2017; Cooper et al. Citation2022) contended.
Lifestyle and roles of regional endothermy and gigantism
The thermophysiology of Otodus megalodon, together with its smaller otodontid ancestor Cretalamna (up to 3.5 m TL: Shimada et al. Citation2020), has been inferred to be regionally endothermic which is considered as a possible evolutionary driver for its gigantism (Ferrón Citation2017; Ferrón et al. Citation2017). Harding et al. (Citation2021) subsequently revealed that endothermic fishes evolved to increase their swimming speed, where regional endothermy is said to allow for greater muscle power generation compared to ectothermic fishes (Dickson and Graham Citation2004). New empirical geochemical evidence suggests that O. megalodon was indeed endothermic sensu lato (Griffiths et al. Citation2023). It is therefore reasonable to assert that it had large quantities of red muscles that would have allowed sustained swimming (e.g. see Bernal et al. Citation2012).
Where some sharks have shown to have higher energetic costs at slower swimming speeds (Gruber and Dickson Citation1997; Lowe Citation2001; Bernal et al. Citation2012), larger sharks are said to have higher optimal swimming speeds presumably simply because of their increased stride length compared to smaller sharks (Videler and Nolet Citation1990). In fact, the ‘total cost of transport’ that entails the overall impact of swimming and energetic costs (Schmidt-Nielsen Citation1972, Citation1984) must have been very low for gigantic individuals of Otodus megalodon compared to that of smaller sharks (see Parsons Citation1990). These observations do not seem to agree with the tentatively estimated, slower-than-expected CS of 2.0 km h−1 for O. megalodon. This is especially true considering the fact that the recorded CSs of the two largest extant sharks that are ectothermic and comparable to O. megalodon size-wise (Pimiento et al. Citation2019), the whale shark (Rhincodon typus) and basking shark (Cetorhinus maximus), are as much as ca. 3.1 and 3.9 km h−1, respectively (Sims Citation2000; Gleiss et al. Citation2011), even though they are typically characterised as slow-moving sharks (e.g. Compagno Citation2002). Although their exact placoid scale sampling sites are uncertain, published illustrations show that, while placoid scales of C. maximus are thorn-like (Bigelow and Schroeder Citation1948, fig. 23E, Castro Citation2010, fig. 62e) reminiscent of the scales of Mitsukurina owstoni (Frumkin and Shimada Citation2020, fig. 3D), placoid scales of R. typus are fine (200–250 µm) and keeled with IKDs ranging 75–100 µm, but their keels are exceptionally pronounced compared to those of O. megalodon and reach to the scales’ posterior edge (Bigelow and Schroeder Citation1948, fig. 30E, F; Castro Citation2010, fig. 52e). Their ability to achieve considerably high CSs, however, can be explained by their planktivory where larger spatial coverages are needed for filter-feeding. This seemingly perplexing discrepancy for the gigantic O. megalodon inferred to be endothermic, however, does have a possible alternative explanation—notably, by the fact that, besides generating greater muscle power, regional endothermy is said to also facilitate digestion compared to ectothermic fishes (Dickson and Graham Citation2004).
Visceral countercurrent heat exchangers occur in endothermic fishes and retain an elevated metabolic rate from food processing, such as digestion, absorption, and protein synthesis (Dickson and Graham Citation2004). In fact, the warmest visceral organ in extant lamnids is the spiral valve intestine (Carey et al. Citation1981, Citation1985; Bernal et al. Citation2001) and is said to be the likely main site of heat production for ‘visceral endothermy’ (Dickson and Graham Citation2004). Furthermore, the large lipid-rich liver that is associated with the suprahepatic rete is warm in lamnid sharks (Carey and Teal Citation1969; Carey et al. Citation1985; Bernal et al. Citation2001). In addition to speeding rates of digestion and enhancing nutrient absorption and processing (Stevens and McLeese Citation1984; Carey et al. Citation1985), a warm viscera contributes to the further elevation of the body core temperature that additionally helps at least some endothermic fishes to be able to exploit cool waters (Dickson and Graham Citation2004). Whereas the fossil record suggests that gigantic individuals of O. megalodon were more common in cooler waters (Shimada et al. Citation2022), regardless, the need of being able to process food and absorb nutrients efficiently for O. megalodon must have been critical given that a large individual (e.g. 15 + m TL) could have fed on prey size of 3–6 m in few bites (Cooper et al. Citation2022).
On the basis of geochemical evidence, Otodus megalodon is inferred to have occupied as high as, or higher than, the tropic position represented by the extant Carcharodon carcharias (Martin et al. Citation2015; McCormack et al. Citation2022; Kast et al. Citation2022). Bite marks on bones of extinct cetacean and pinniped in the fossil record suggest that O. megalodon (or possibly its ancestral chronospecies, O. chubutensis) had a diet of marine mammals (Purdy et al. Citation2001; Aguilera et al. Citation2008; Collareta et al. Citation2017; Godfrey et al. Citation2018, Citation2021). Whether those bite marks represent predation or scavenging by the shark generally cannot be ascertained, but because carrion is available only episodically as a pulsed resource in many ecosystems (Nowlin et al. Citation2008), it is reasonable to assert that O. megalodon frequently hunted for prey because exclusive reliance on unpredictably available carcases was likely not sustainable for the gigantic O. megalodon.
The Tsuchishio Formation in northern Saitama Prefecture is rather non-fossiliferous (see Geological Setting above), but marine mammal fossils are known from other Tortonian marine strata in the vicinity, such as mysticete cetacean and odobenid pinniped remains from Tochigi Prefecture (e.g. Kimura et al. Citation2014; Kawano et al. Citation2022). Our present study suggests that Otodus megalodon was not necessarily a fast swimmer, but this does not preclude its ability to engage in occasional burst swimming for prey capture. For example, even a large, ectothermic, planktivore like Cetorhinus maximus is capable of breaching the water nearly vertically at a maximum recorded exit speed of 5.6 m s−1 (Rudd et al. Citation2021; equivalent to 20.2 km h−1 vs. aforementioned cruising speed of 3.9 km h−1 for the species). Therefore, we contend that O. megalodon was generally a slow cruising shark with occasional burst swimming to hunt for prey aided by its great stride length from being large, where much of its metabolic heat through regional endothermy was used to facilitate digesting large pieces of ingested meat and absorbing and processing nutrients through its digestive tract.
The phylogenetic origin of the genus Otodus stems from an otodontid ancestor, Cretalamna (Shimada et al. Citation2017, and references therein), which is inferred to have already been regionally endothermic (Ferrón Citation2017). However, Cretalamna likely reached only up to about 3.5 m TL (Shimada et al. Citation2020), which is comparable to most of the extant lamnid species (i.e. Lamna spp. and Isurus spp.) in size, and thus it is possible that regional endothermy in the otodontid clade first evolved to increase the swimming speed that is said to be the key driver for the evolution of regional endothermy in extant lamnids (e.g. Harding et al. Citation2021). The Otodus lineage is represented by evolving chronospecies, where although the exact number of species is debatable, the following five species are typically recognised in literature: O. obliquus from the early Palaeocene to the early Eocene; O. auriculatus in the mid–late Eocene; O. angustidens in the early Oligocene; O. chubutensis in the early to mid-Miocene; and O. megalodon in the mid-Miocene–early Pliocene (Pimiento and Balk Citation2015; Trif et al. Citation2016; Perez et al. Citation2019; Shimada et al. Citation2020). Through the evolution of the clade, the body size is interpreted to have increased gradually from O. obliquus that possibly reached up to about 8 m TL to the terminal species, O. megalodon, which likely reached up to at least 15 m TL (Shimada Citation2019; Perez et al. Citation2021), with the total lengths of other Otodus species (i.e. O. auriculatus, O. angustidens, and O. chubutensis) ranging between 8 and 15 m TL (Shimada et al. Citation2020). However, geochemical evidence suggests that the exceptionally high trophic position was already achieved early in the Otodus clade by O. auriculatus in the mid–late Eocene (Kast et al. Citation2022). If our interpretations about O. megalodon’s estimated CS and the use of endothermically derived metabolic heat primarily for facilitating visceral food processing are true, it is conceivable that the relative importance of the functional roles of regional endothermy could have switched from maintaining high CSs to the visceral food processing as a consequence of the evolution of exceptional gigantism through the otodontid clade as the general sizes of ingested food increased.
Conclusions
Because energetics is tightly linked to the fitness of organisms (Gleiss et al. Citation2022), it is not an overstatement that the structure and dynamics of ecosystems are a direct consequence of organisms’ metabolism (Brown et al. Citation2004). Therefore, understanding the biology of gigantic organisms like Otodus megalodon and its phylogenetic relatives is meaningful because they must have played significant roles in the evolution of their ecosystems. This present study has offered new insight into such issues by examining associated tessellated calcified cartilage and placoid scales from an individual O. megalodon estimated to be 11.7 m TL collected from the lower upper Miocene (middle Tortonian) Tsuchishio Formation in central Japan. The morphology of each predominantly hexagonal tessera and the arrangement of overall tessellated calcified cartilage in O. megalodon are practically identical to those of the extant chondrichthyans, suggesting that the same mechanism for the ontogenetic development of cartilage operated in O. megalodon. The collected placoid scales can be classified into three broad categories, where their overall sizes are comparable to many extant pelagic lamniforms and carcharhiniform sharks, indicating that the exceptional gigantism seen in O. megalodon did not necessarily produce exceptionally large placoid scales. Many of the scales possess pronounced parallel keels on the crown surface with a representative IKD of about 100 µm. Our study demonstrates that, albeit somewhat weak, there is a quantitative relationship between IKDs of keeled placoid scales and reported CS values among extant pelagic lamniform and carcharhiniforms, and it suggests that O. megalodon was not a fast-swimming shark. Specifically, our inferred cruising speed for O. megalodon is approximately 2.0 km h−1 with a range of 0.9–3.0 km h−1. This result is in contrast with previous studies (Jacoby et al. Citation2016; Ferrón Citation2017; Cooper et al. Citation2022) that suggested that O. megalodon was a fast-swimming (4.8–5.1 km h−1) shark. Whereas O. megalodon fed on marine mammals based on the fossil record, our interpretation is that O. megalodon was generally a relatively slow cruising shark with occasional burst swimming for prey capture; however, much of its metabolic heat through regional endothermy was possibly used to promote digestion of large pieces of meat it ingested as well as absorbing and processing nutrients. If our interpretations are correct, then, the relative importance of the functional roles of regional endothermy possibly shifted from maintaining high CSs in the early part of the otodontid history marked by ~3 m TL Cretalamna to visceral food processing through the evolution towards gigantism leading to 15+ m TL O. megalodon that must have swallowed large pieces of food.
Acknowledgments
The first author (KS) takes this opportunity to thank all authors of Uyeno et al. (Citation1989) (T. Uyeno, O, Sakamoto, and H. Sekine) as well as those who were involved in the excavation of the original tooth set material (I. Okabe, S. Shinozaki, and T. Naito as noted by Uyeno et al. Citation1989), for their mentorship and friendship during his teenage years. Besides the second author (YY) who arranged KS’s examination of the rock samples for the purpose of this study, KS is also delighted to coauthor this work with the third (YK) and fourth (YT) authors, who were his ‘fossil hunting buddies’ in the mid-1980s. The serendipitous nature of this study is particularly notable as KS vividly recalls a personal phone call he received from H. Sekine in 1986 starting with “Guess what I found?” [in Japanese] soon after Sekine first discovered the original Otodus megalodon tooth set described by Uyeno et al. (Citation1989). Logistical support provided by the Department of Environmental Science and Studies and the Department of Biological Sciences at DePaul University, Chicago, Illinois, USA, is also appreciated. We also thank J. A. Cooper and V. J. Perez for their review which greatly improved the quality of this paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aguilera OA, García L, Cozzuol MA. 2008. Giant-toothed white sharks and cetacean trophic interaction from the Pliocene Caribbean Paraguaná Formation. Paläont Z. 82:204–208. doi:10.1007/BF02988410
- Akimoto K, Tanaka M. 2004. Sublittoral benthic foraminiferal assemblages from the Tsuchishio Formation (upper Miocene), northeastern margin of the Kanto Mountains, central Japan. Asso Geol Collabor Japan Monogr. 52. 75–79. in Japanese with English abstract
- Amalfitano J, Dalla Vecchia F, Carnevale G, Fornaciari E, Roghi G, Giusberti L. 2022. Morphology and paleobiology of the Late Cretaceous large-sized shark Cretodus crassidens (Dixon, 1850) (Neoselachii; Lamniformes). J Paleontol. 96:1166–1188. doi:10.1017/jpa.2022.23
- Ankhelyi MV, Wainwright DK, Lauder GV. 2018. Diversity of dermal denticle structure in sharks: skin surface roughness and three‐dimensional morphology. J Morphol. 279:1‒23. doi:10.1002/jmor.20836
- Bechert DW, Bruse M, Hage W, Meyer R. 2000. Fluid mechanics of biological surfaces and their technological application. Naturwissenschaften. 87:157–171. doi:10.1007/s001140050696
- Bechert DW, Hoppe G, Reif W-E. 1985. On the drag reduction of the shark skin. Am Inst Aeronau Astronau. 85-0546:1–18. doi:10.2514/6.1985-546
- Bendix-Almgreen SE. 1983. Carcharodon megalodon from the Upper Miocene of Denmark, with comments on elasmobranch tooth enameloid: coronoïn. Bulletin of the Geological Society of Denmark. 32:1–32. doi:10.37570/bgsd-1983-32-01
- Bernal D, Carlson JK, Goldman KJ, Lowe CG. 2012. Energetics, metabolism, and endothermy in sharks and rays. In: Carrier JC, Musick JA, Heithaus MC, editors. Biology of sharks and their relatives, 2 ed. Boca Raton (Florida): CRC Press; p. 211‒237.
- Bernal D, Dickson KA, Shadwick RE, Graham JB. 2001. Review: analysis of the evolutionary convergence for high performance swimming in lamnid sharks and tunas. Comp Biochem Physiol. 129(2–3):695–726. doi:10.1016/S1095-6433(01)00333-6
- Bigelow HB, Schroeder WC. 1948. Fishes of the western North Atlantic, Part I: lancelets, Cyclostomes, Sharks. In: Tee-Van J, Breder CM, Hildebrand SF, Parr AE, Schroeder WC, editors. Fishes of the western North Atlantic. Part 1. New Haven (Connecticut): Sears Foundation for Marine Research. Yale University, New Haven; p. 59–576.
- Boessenecker RW, Ehret DJ, Long DJ, Churchill M, Martin E, Boessenecker SJ. 2019. The Early Pliocene extinction of the mega-toothed shark Otodus megalodon: a view from the eastern North Pacific. PeerJ. 7:e6088. doi:10.7717/peerj.6088
- Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. 2004. Toward a metabolic theory of ecology. Ecology. 85:1771–1789. doi:10.1890/03-9000
- Cappetta H. 2012. Chondrichthyes. Mesozoic and Cenozoic Elasmobranchii: teeth. In: Schultze H-P, editor. Handbook of paleoichthyology, volume 3E. Munich: Verlag Dr. Friedrich Pfeil; p. 1‒512.
- Carey FG, Casey JG, Pratt HL, Urquhart D, McCosker JE. 1985. Temperature, heat production and heat exchange in lamnid sharks. Mem South Calif Acad Sci. 9:92–108.
- Carey FG, Teal JM. 1969. Mako and porbeagle: warm-bodied sharks. Comp Biochem Physiol. 28(1):199–204. doi:10.1016/0010-406X(69)91335-8
- Carey FG, Teal JM, Kanwisher JW. 1981. The visceral temperatures of mackerel sharks (Lamnidae). Physiol Zool. 54(3):334–344. doi:10.1086/physzool.54.3.30159948
- Carman ML, Estes TG, Feinberg AW, Schumacher JF, Wilkerson W, Wilson LH, Callow ME, Callow JA, Brennan AB. 2006. Engineered antifouling microtopographies – correlating wettability with cell attachment. Biofouling. 22(1):11–21. doi:10.1080/08927010500484854
- Cartamil D, Wegner NC, Aalbers S, Sepulveda CA, Baquero CA, Graham JB. 2010. Diel movement patterns and habitat preferences of the common thresher shark (Alopias vulpinus) in the Southern California Bight. Mar Fr Res. 61:596‒604. doi:10.1071/MF09153
- Castro JI. 2010. The sharks of North America. New York: Oxford University Press; 640 p.
- Collareta A, Lambert O, Landini W, Di Celma C, Malinverno E, Varas-Malca R, Urbina M, Bianucci G. 2017. Did the giant extinct shark Carcharocles megalodon target small prey? Bite marks on marine mammal remains from the late Miocene of Peru. Palaeogeography, Palaeoclimatology, Palaeoecology. 469:84–91. doi:10.1016/j.palaeo.2017.01.001
- Compagno LJV. 2002. Sharks of the world: an annotated and illustrated catalogue of shark species known to date. Volume 2: bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes). FAO Sp Cat Fish Purp. 1(2):1–269.
- Cooper JA, Hutchinson JR, Bernvi DC, Cliff G, Wilson RP, Dicken ML, Menzel J, Wroe S, Pirlo J, Pimiento C. 2022. The extinct shark Otodus megalodon was a transoceanic superpredator: inferences from 3D modeling. Sci Adv. 8(33):9424. doi:10.1126/sciadv.abm9424
- Cooper JA, Pimiento C, Ferrón HG, Benton MJ. 2020. Body dimensions of the extinct giant shark Otodus megalodon: a 2D reconstruction. Sci Rep. 10:14596. doi:10.1038/s41598-020-71387-y
- Dean MN, Huber DR, Nance HA. 2006. Functional morphology of jaw trabeculation in the lesser electric ray Narcine brasiliensis, with comments on the evolution of structural support in the Batoidea. J Morphol. 267:1137–1146. doi:10.1002/jmor.10302
- Dean MN, Mull CG, Gorb SN, Summers AP. 2009. Ontogeny of the tessellated skeleton: insight from the skeletal growth of the round stingray Urobatis halleri. J Anat. 215:227–239. doi:10.1111/j.1469-7580.2009.01116.x
- Dean MB, Summers AP. 2006. Mineralized cartilage in the skeleton of chondrichthyan fishes. Zoology. 109:164–168. doi:10.1016/j.zool.2006.03.002
- Dickson K, Graham JB. 2004. Evolution and consequences in endothermy in fshes. Physiol Biochem Zool. 77:998–1018. doi:10.1086/423743
- Diemer KM, Mann BQ, Hussey NE. 2011. Distribution and movement of scalloped hammerhead Sphryna lewini and smooth hammerhead Sphyrna zygaena sharks along the east coast of Southern Africa. Af J Mar Sci. 33:229–238. doi:10.2989/1814232X.2011.600291
- Dingerkus G, Séret B, Guilbert E. 1991. Multiple prismatic calcium phosphate layers in the jaws of present-day sharks (Chondrichthyes; Selachii). Experientia. 47:38–40. doi:10.1007/BF02041246
- Du Clos KT, Lang A, Devey S, Motta PJ, Habegger ML, Gemmell BJ. 2018. Passive bristling of mako shark scales in reversing flows. J Roy Soc Interface. 15:20180473. doi:10.1098/rsif.2018.0473
- Ebert DA, Dando M, Fowler S. 2021. Sharks of the world: a complete guide. Princeton (New Jersey): Princeton University Press; 607 p.
- Ehret DJ, MacFadden BJ, Jones DS, Devries TJ, Foster DA, Salas-Gismondi R. 2012. Origin of the white shark Carcharodon (Lamniformes: Lamnidae) based on recalibration of the Upper Neogene Pisco Formation of Peru. Palaeontology. 55:1139‒1153. doi:10.1111/j.1475-4983.2012.01201.x
- Feichtinger I, Adnet S, Cuny G, Guinot G, Kriwet J, Neubauer TA, Pollerspöck J, Shimada K, Straube N, Underwood C, et al. 2021. Comment on “An early Miocene extinction in pelagic sharks. Science”. 374(6573):0632. doi:10.1126/science.abk0632
- Ferrón H. 2017. Regional endothermy as a trigger for gigantism in some extinct macropredatory sharks. PLoS ONE. 12:e0185185. doi:10.1371/journal.pone.0185185
- Ferrón HG, Martínez-Perez C, Botella H. 2017. The evolution of gigantism in active marine predators. Hist Biol. 30:712–716. doi:10.1080/08912963.2017.1319829
- Filmalter J, Cowley P, Forget F, Dagorn L. 2015. Fine-scale 3-dimensional movement behaviour of silky sharks Carcharhinus falciformis associated with fish aggregating devices (FADs). Mar Ecol Prog Ser. 539:207–223. doi:10.3354/meps11514
- Frumkin JA, Shimada K. 2020. Integument-based inferences on the swimming ability and prey hunting strategy of the bigeye thresher shark, Alopias superciliosus (Lamniformes: Alopiidae). Zoomorphology. 139:213–229. doi:10.1007/s00435-020-00484-3
- Gleiss AC, Norman B, Wilson RP. 2011. Moved by that sinking feeling: variable diving geometry underlies movement strategies in whale sharks. Funct Ecol. 25:595–607. doi:10.1111/j.1365-2435.2010.01801.x
- Gleiss AC, Treberg JT, Byrnes EE, Lear KO. 2022. Physiological and applied energetics of elasmobranch fishes. In: Carrier JC, Simpfendorfer CA, Heithaus MR, Yopak KE, editors. Biology of sharks and their relatives, 3 ed. Boca Raton (Florida): CRC Press; p. 289‒321.
- Godfrey SJ, Ellwood M, Groff S, Verdin MS. 2018. Carcharocles-bitten odontocete caudal vertebrae from the coastal eastern United States. Acta Palaeont Polo. 63:463–468. doi:10.4202/app.00495.2018
- Godfrey SJ, Nance JR, Riker NL. 2021. Otodus-bitten sperm whale tooth from the Neogene of the Coastal Eastern United States. Acta Palaeont Polo. 66:599–603. doi:10.4202/app.00820.2020
- Gorman KL, Shimada K, Witzke BJ. 2014. Late Cretaceous marine fishes from the basal Greenhorn Limestone in western Iowa. Trans Kansas Acad Sci. 117:91–99. doi:10.1660/062.117.0114
- Gottfried MD, Compagno LJV, Bowman SC. 1996. Size and skeletal anatomy of the giant “megatooth” shark Carcharodon megalodon. In: Klimley AP, Ainley DG, editors. Great white sharks: the biology of Carcharodon carcharias. San Diego: Academic Press; p. 55–66.
- Griffiths LM, Eagle RA, Kim SL, Flores R, Becker MA, Maisch HM IV, Trayler RB, Chan RL, McCormack J, Akhtara AA, et al. 2023. Endothermic physiology of extinct megatooth sharks. Proc Nat Acad Sci. doi:10.1073/pnas.2218153120
- Gruber SJ, Dickson KA. 1997. Effects of endurance training in the leopard shark, Triakis semifasciata. Physiol Zool. 70(4):481–492. doi:10.1086/515851
- Guzzo F, Shimada K. 2018. A new fossil vertebrate locality of the Jetmore Chalk Member of the Upper Cretaceous Greenhorn Limestone in north-central Kansas. U.S.A. Trans Kansas Acad Sci 121:59–68. doi:10.1660/062.121.0206
- Harding L, Jackson A, Barnett A, Donohue I, Halsey L, Huveneers C, Meyer C, Papastamatiou Y, Semmens JM, Spencer E, et al. 2021. Endothermy makes fishes faster but does not expand their thermal niche. Funct Ecol. 35:1951–1959. doi:10.1111/1365-2435.13869
- Herraiz JL, Ribé J, Botella H, Martínez-Pérez C, Ferrón HG. 2020. Use of nursery areas by the extinct megatooth shark Otodus megalodon (Chondrichthyes: Lamniformes). Biol Lett. 16:20200746. doi:10.1098/rsbl.2020.0746
- Hiki Research Group. 1989. The Shiobara-type molluscan fauna from the Tsuchishio Formation (Miocene), northeastern margin of the Kanto Mountains, central Japan. Earth Sci. (Chikyu Kagaku). 43:58–61. doi:10.15080/agcjchikyukagaku.43.1_58
- Homma T. 1986. Miocene series along the middle part of the River Ara-kawa in the northeastern marginal area of the Kanto Mountains, central Japan (Part 2): sedimentary facies and stratigraphy of the Tsuchishio Formation. Bull Saitama Mus Nat Hist. 4:49–72, 3 plates.
- Homma T. 1987. Miocene series along the middle part of the River Ara-kawa in the northeastern marginal area of the Kanto Mountains, central Japan (Part 3): sedimentary facies and stratigraphy of the Yagii Formation. Bull Saitama Mus Nat Hist. 5:23–48, 3 plates.
- Hurst RJ, Baglet NW, McGregor GA, Francis MP. 1999. Movements of the New Zealand school shark, Galeorhinus galeus, from tag returns. New Zealand J Mar Fr Res. 33:29–48. doi:10.1080/00288330.1999.9516854
- Itoigawa J, Nishimoto N, Karasawa H, Okumura Y. 1985. Miocene fossils of the Mizunami group, central Japan. 3. Elasmobranchs. Monogr Mizunami Fossil Mus. 5:1–99.
- Jacoby DMP, Siriwat P, Freeman R, Carbone C. 2016. Scaling of swim speed in sharks: a reply to Morrison (2016). Biol Lett. 12:20160502. doi:10.1098/rsbl.2016.0502
- Kai M, Fujinami Y. 2020. Estimation of mean movement rates for blue sharks in the northwestern Pacific Ocean. Anim Biotelemetry. 8:35. doi:10.1186/s40317-020-00223-x
- Kast ER, Griffiths ML, Kim SL, Rao ZC, Shimada K, Becker MA, Maisch HM, Eagle RA, Clark CA, Neumann AN, et al. 2022. Cenozoic megatooth sharks occupied extremely high trophic positions. Sci Adv. 8(25):6529. doi:10.1126/sciadv.abl6529
- Kawano S, Tonomori W, Yoshizawa T. 2022. A pinniped canine from the Miocene Oogane Formation of the Arakawa Group in Nasukarasuyama, Tochigi Prefecture, Japan. Bull Tochigi Pref Mus. 39:1–6.
- Kent BW. 2018. The cartilaginous fishes (chimaeras, sharks, and rays) of Calvert Cliffs, Maryland, USA. In: Godfrey SJ, editor. The geology and vertebrate paleontology of Calvert Cliffs, Maryland. Washington D.C: Smithsonian Scholarly Press; p. 45–157.
- Kimura T, Hasegawa Y, Kashiwamura Y. 2014. A fossil mysticete skeleton from the upper Miocene of Tochigi Prefecture, Japan. Bull Gunma Mus Nat Hist. 18:87–100.
- Kohler NE, Turner PA. 2001. Shark tagging: a review of conventional methods and studies. Environ Biol Fish. 60:191–223. doi:10.1023/A:1007679303082
- Kurihara Y. 2010. Middle and Late Miocene marine Bivalvia from the northern Kanto region, central Japan. Nat Mus Nature Sci Monogr. 41:1–87.
- Lang A, Motta P, Hueter R, Habegger M. 2011. Shark skin separation control mechanisms. J Mar Technol Soc. 45:208–215. doi:10.4031/MTSJ.45.4.12
- Lauder GV, Di Santo V. 2015. Swimming mechanics and energetics of elasmobranch fishes. Fish Physiol. 34:219‒253. doi:10.1016/B978-0-12-801289-5.00006-7
- Lowe CG. 2001. Metabolic rates of juvenile scalloped hammerhead sharks (Sphyrna lewini). Mar Biol. 139:447–453. doi:10.1007/s002270100585
- Maisey JG. 2013. The diversity of tessellated calcification in modern and extinct chondrichthyans. Rev Paleobiol. 32:335–371.
- Maisey JG, Denton JSS, Burrow C, Pradel A. 2021. Architectural and ultrastructural features of tessellated calcified cartilage in modern and extinct chondrichthyan fishes. J Fish Biol. 98:919–941. doi:10.1111/jfb.14376
- Martin JE, Tacail T, Adnet S, Girard C, Balter V. 2015. Calcium isotopes reveal the trophic position of extant and fossil elasmobranchs. Chem Geol. 415:118–125. doi:10.1016/j.chemgeo.2015.09.011
- McCormack J, Griffiths ML, Kim SL, Shimada K, Karnes M, Maisch HM IV, Pederzani S, Bourgon N, Jaouen K, Becker MA, et al. 2022. Trophic position of Otodus megalodon and great white sharks through time revealed by zinc isotopes. Nat Comm. 13:2980. doi:10.1038/s41467-022-30528-9
- McKenzie RW, Motta PJ, Rohr JR. 2014. Comparative squamation of the lateral line canal pores in sharks. J Fish Biol. 84:1300–1311. doi:10.1111/jfb.12353
- Motta P, Habegger ML, Lang A, Hueter R, Davis J. 2012. Scale morphology and flexibility in the shortfin mako Isurus oxyrinchus and the blacktip shark Carcharhinus limbatus. J Morphol. 273:1096‒1110. doi:10.1002/jmor.20047
- Naylor GJP, de Lima A, Castro JI, Hubbell G. 2021. Comment on “An early Miocene extinction in pelagic sharks”. Science. 374(6573):8723. doi:10.1126/science.abj8723
- Nelms A, McIntosh AP, Shimada K. 2014. Fossil fishes from the Jetmore Chalk Member (Lower Turonian) of the Upper Cretaceous Greenhorn Limestone in north-central Kansas. Trans Kansas Acad Sci. 117:245–252. doi:10.1660/062.117.0310
- Nelson DR, McKibben JN, Strong WR, Lowe CG, Sisneros JA, Schroeder DM, Lavenberg RJ. 1997. An acoustic tracking of a megamouth shark, Megachasma pelagios: a crepuscular vertical migrator. Environ Biol Fish. 49:389‒399. doi:10.1023/A:1007369619576
- Nishimoto H, Okumura Y, Karasawa H. 1992. Dermal scales of Carcharocles megalodon (Agassiz) from the Miocene Mizunami Group, central Japan—Studies on dermal scales of some elasmobranchian fossils from Japan. Part 1. Bull Mizunami Fossil Mus. 19:269–271.
- Nowlin WH, Vanni MJ, Yang LH. 2008. Comparing resource pulses in aquatic and terrestrial ecosystems. Ecology. 89:647–659. doi:10.1890/07-0303.1
- Oliver SP, Turner JR, Gann K, Silvosa M, Jackson TDU. 2013. Thresher sharks use tail-slaps as a hunting strategy. PloS One. 8(7):e67380. doi:10.1371/journal.pone.0067380
- Pade NG, Queiroz N, Humphries NE, Witt MJ, Jones CS, Noble LR, Sims DW. 2009. First results from satellite-linked archival tagging of porbeagle shark, Lamna nasus: area fidelity, wider-scale movements and plasticity in diel depth changes. J Exp Mar Biol Ecol. 370:64–74. doi:10.1016/j.jembe.2008.12.002
- Paig-Tran EWM, Porter ME, Ferry LA, Whitenack LB. 2022. How to build a shark: biomechanics and bioinspiration. In: Carrier J, Simpfendorfer C, Heithaus MR, Yopak K, editors Biology of sharks and their relatives. 3 ed. Boca Raton (Florida): CRC Press; p. 59‒103.
- Parsons G. 1990. Metabolism and swimming efficiency of the bonnethead shark Sphyrna tiburo. Mar Biol. 104:363–367. doi:10.1007/BF01314338
- Perez VJ, Godfrey SJ, Kent B, Weems R, Nance J. 2019. The transition between Carcharocles chubutensis and Carcharocles megalodon (Otodontidae, Chondrichthyes): lateral cusplet loss through time. J Vert Paleont. 38:6. doi:10.1080/02724634.2018.1546732
- Perez VJ, Leder RM, Badaut T. 2021. Body length estimation of Neogene macrophagous lamniform sharks (Carcharodon and Otodus) derived from associated fossil dentitions. Palaeontol Electron. 24(1):a09. doi:10.26879/1140
- Pimiento C, Balk MA. 2015. Body-size trends of the extinct giant shark Carcharocles megalodon: a deep-time perspective on marine apex predators. Paleobiol. 41:479‒490. doi:10.1017/pab.2015.16
- Pimiento C, Cantalapiedra JL, Shimada K, Field DJ, Smaers JB. 2019. Evolutionary pathways towards shark gigantism. Evolution. 73:588–599. doi:10.1111/evo.13680
- Popp M, White CF, Bernal D, Wainwright DK, Lauder GV. 2020. The denticle surface of thresher shark tails: three-dimensional structure and comparison to other pelagic species. J Morphol. 281:938–955. doi:10.1002/jmor.21222
- Purdy RW, Schneider VP, Applegate SP, McLellan JH, Meyer RL, Slaughter BH. 2001. The Neogene sharks, rays, and bony fishes from Lee Creek Mine, Aurora, North Carolina. Smithsonian Contrib Paleobiol. 90:71–202.
- Raschi W, Elsom J 1986. Comments on the structure and development of the drag reduction-type placoid scale. In: Uyeno T, Arai R, Taniuchi T, Matsuura K, editors. Proceedings of the second international conference on Indo-Pacific fishes. Tokyo: Ichthyological Society of Japan; p. 392–407.
- Raschi WG, Musick JA. 1986. Hydrodynamic aspects of shark scales. NASA Contract Rep. 3963:1–110.
- Raschi WG, Tabit C. 1992. Functional aspects of placoid scales: a review and update. Mar Fr Res. 43:123‒147. doi:10.1071/MF9920123
- Razak H, Kocsis L. 2018. Late Miocene Otodus (Megaselachus) megalodon from Brunei Darussalam: body length estimation and habitat reconstruction. Neues Jahrb Geol Palaontol Abh. 288:299–306. doi:10.1127/njgpa/2018/0743
- Reif W-E. 1978. Protective and hydrodynamic function of the dermal skeleton of elasmobranchs. Neues Jahrb Geol Paläontol, Abh. 157:133‒141.
- Reif W-E. 1982. Evolution of dermal skeleton and dentition in vertebrates. Evol Biol. 15:287–368. doi:10.1007/978-1-4615-6968-8_7
- Reif W-E. 1985a. Functions of scales and photophores in mesopelagic luminescent sharks. Acta Zool. 66:111–118. doi:10.1111/j.1463-6395.1985.tb00829.x
- Reif W-E. 1985b. Squamation and ecology of sharks. Frankfurt am Main: Courier Forschungsinstitut Senckenberg; 101 p.
- Reif W-E. 1985c. Morphology and hydrodynamic effects of the scales of fast swimming sharks. Fortschr Zool. 30:483‒485.
- Reif W-E, Dinkelacker A. 1982. Hydrodynamics of the squamation in fast swimming sharks. Neues Jahrb Geol Paläontol, Abh. 164:184–187. doi:10.1127/njgpa/164/1982/184
- Rudd JL, Exeter OM, Hall J, Hall J, Hall G, SM H, Kerry C, Witt MJ, Hawkes LA. 2021. High resolution biologging of breaching by the world’s second largest shark species. Sci Rep. 11:5236. doi:10.1038/s41598-021-84670-3
- Rygg AD, Cox JPL, Abel R, Webb AG, Smith NB, Craven BA. 2013. A computational study of the hydrodynamics in the nasal region of a hammerhead shark (Sphyrna tudes): implications for olfaction. PLoS ONE. 8(3):e59783. doi:10.1371/journal.pone.0059783.
- Sakamoto K, Uyeno T. 1992. Saitamapsetta nomurai gen. et sp. nov., a righteye flounder from a middle Miocene bed in Saitama Prefecture, Japan. Bull Nat Sci. Mus, Tokyo, Ser. C. 18(3):101–112.
- Schäfer W. 1972. Ecology and palaeoecology of marine environments (translated by I Oertel; edited by GY Craig). Edinburgh: Oliver and Boyd; 568 p.
- Schmidt-Nielsen K. 1972. Locomotion: energy cost of swimming, flying, and running. Science. 117:222–228. doi:10.1126/science.177.4045.222
- Schmidt-Nielsen K. 1984. Scaling: why is animal size so important? New York: Cambridge University Press; 241 p.
- Schumacher JF, Carman ML, Estes TG, Feinberg AW, Wilson LH, Callow ME, Callow JA, Finlay JA, Brennan AB. 2007. Engineered antifouling microtopographies—effect of feature size, geometry, and roughness on settlement of zoospores of the green alga Ulva. Biofouling. 23:55–62. doi:10.1080/08927010601136957
- Seidel R, Jayasankar AK, Dean MN. 2021. The multiscale architecture of tessellated cartilage and its relation to function. J Fish Biol. 98:942–955. doi:10.1111/jfb.14444
- Shimada K. 1997. Skeletal anatomy of the Late Cretaceous lamniform shark, Cretoxyrhina mantelli, from the Niobrara Chalk in Kansas. J Vert Paleontol. 17:642–652. doi:10.1080/02724634.1997.10011014
- Shimada K. 2006. (date of imprint 2005). Types of tooth sets in the fossil record of sharks, and comments on reconstructing dentitions of extinct sharks. J Fossil Res 38:141–145.
- Shimada K. 2019. The size of the megatooth shark, Otodus megalodon (Lamniformes: Otodontidae), revisited. Hist Biol. 33(7):904–911. doi:10.1080/08912963.2019.1666840
- Shimada K, Becker MA, Griffiths ML. 2020. Body, jaw, and dentition lengths of macrophagous lamniform sharks, and body size evolution in Lamniformes with special reference to ‘off-the-scale’ gigantism of the megatooth shark, Otodus megalodon. Hist Biol. 33(11):2543–2559. doi:10.1080/08912963.2020.1812598
- Shimada K, Bonnan MF, Becker MA, Griffiths ML. 2021. Ontogenetic growth pattern of the extinct megatooth shark Otodus megalodon—implications for its reproductive biology, development, and life expectancy. Hist Biol. 33(12):3254–3259. doi:10.1080/08912963.2020.1861608
- Shimada K, Chandler RE, Lam OLT, Tanaka T, Ward DJ. 2017. A new elusive otodontid shark (Lamniformes: Otodontidae) from the lower Miocene, and comments on the taxonomy of otodontid genera, including the ‘megatoothed’ clade. Hist Biol. 29(5):704–714. doi:10.1080/08912963.2016.1236795
- Shimada K, Cicimurri DJ. 2005. Skeletal anatomy of the Late Cretaceous shark, Squalicorax (Neoselachii: Anacoracidae). Palaeontol Z. 79:241–261. doi:10.1007/BF02990187
- Shimada K, Everhart MJ. 2019. A new large Late Cetaceous lamniform shark from North America with comments on the taxonomy, paleoecology, and evolution of the genus Cretodus. J Vert Paleontol. 39:e1673399. doi:10.1080/02724634.2019.1673399
- Shimada K, Maisch HM IV, Perez VJ, Becker MA, Griffiths ML. 2022. Revisiting body size trends and nursery areas of the Neogene megatooth shark, Otodus megalodon (Lamniformes: Otodontidae) reveals Bergmann’s rule possibly enhanced its gigantism in cooler waters. Hist Biol. doi:10.1080/08912963.2022.2032024
- Sibert EC, Rubin LD. 2021. An early Miocene extinction in pelagic sharks. Science. 372:1105–1107. doi:10.1126/science.aaz3549
- Sims DW. 2000. Filter-feeding and cruising swimming speeds of basking sharks compared with optimal models: they filter-feed slower than predicted for their size. J Exp Mar Biol Ecol. 249:65–76. doi:10.1016/S0022-0981(00)00183-0
- Sternes PC, Shimada K. 2020. Body forms in sharks (Chondrichthyes: Elasmobranchii), and their functional, ecological, and evolutionary implications. Zoology. 140:125799. doi:10.1016/j.zool.2020.125799
- Sternes PC, Wood JJ, Shimada K. 2022. Body forms of extant lamniform sharks (Elasmobranchii: Lamniformes), and comments on the morphology of the extinct megatooth shark, Otodus megalodon, and the evolution of lamniform thermophysiology. Hist Biol. doi:10.1080/08912963.2021.2025228
- Stevens ED, McLeese JM. 1984. Why bluefin tuna have warm tummies: temperature effect on trypsin and chymotrypsin. Am J Physiol. 246:487–494. doi:10.1152/ajpregu.1984.246.4.R487
- Stone NR, Shimada K. 2019. Skeletal anatomy of the bigeye sandtiger shark, Odontaspis noronhai (Lamniformes: Odontaspididae), and its implications to lamniform phylogeny, taxonomy, and conservation biology. Copeia. 107:632–652. doi:10.1643/CG-18-160
- Summers AP. 2000. Stiffening the stingray skeleton—an investigation of durophagy in myliobatid stingrays (Chondrichthyes, Batoidea, Myliobatidae). J Morphol. 243:113–126. doi:10.1002/(SICI)1097-4687(200002)243:2<113::AID-JMOR1>3.0.CO;2-A
- Sundström LF, Gruber SH. 1998. Using speed sensing transmitters to model the bioenergetics of subadult lemon sharks, Negaprion brevirostris (Poey), in the field. Hydrobiologia. 371:241–247. doi:10.1023/A:1017031406947
- Suto I, Takahashi M, Yanagisawa Y. 2003. Diatom biostratigraphy of the Miocene Tsuchishio Formation in the Hiki Hills area (Aketo Section), Saitama Prefecture, central Japan. J Geol Soc Japan. 109:48–62. doi:10.5575/geosoc.109.48
- Swinsburg W, Kohler NE, Turner PA, McCandless CT. 2012. Mark/recapture data for the blacktip shark, Carcharhinus limbatus, in the Gulf of Mexico from the NEFSC Cooperative Shark Tagging Program. SEDAR29-WP-16. North Charleston (South Carolina): Southeast Data, Assessment and Review Program, South Atlantic Fishery Management Council; p. 25.
- Takeda M, Fujiyama I. 1984. A new majid crab from the Miocene Matsuyama Group. Saitama Prefecture, central Japan. Bull Nat Sci Mus, Tokyo, Ser C. 10(2):49–53.
- Trif N, Ciobanu R, Codrea V. 2016. The first record of the giant shark Otodus megalodon (Agassiz, 1835) from Romania. Brukenthal, Acta Musei. 11:507–526.
- Uyeno T, Sakamoto O. 1984. Lamnoid shark Carcharodon from Miocene beds of Chichibu Basin, Saitama Prefecture, Japan. Bull Saitama Mus Nat Hist. 2:47–65, 10 plates.
- Uyeno T, Sakamoto O, Sekine H. 1989. Description of an almost complete tooth set of Carcharodon megalodon from a middle Miocene bed in Saitama Prefecture, Japan. Bull Saitama Mus Nat Hist. 7:73–85, 16 plates.
- Videler JJ, Nolet BA. 1990. Costs of swimming measured at optimum speed: scale effects, differences between swimming styles, taxonomic groups and submerged and surface swimming. Comp Biochem Physiol. 97:91–99. doi:10.1016/0300-9629(90)90155-L
- Watanabe YY, Goldman KJ, Caselle JE, Chapman DD, Papastamatiou YP. 2015. Comparative analyses of animal-tracking data reveal ecological significance of endothermy in fishes. Proc Nat Acad Sci. 112(19):6104–6109. doi:10.1073/pnas.1500316112
- Yanagisawa Y, Akiba F. 1998. Refined Neogene diatom biostratigraphy for the northwest Pacific around Japan, with introduction of code numbers for selected diatom biohorizons. J. Geol Soc Japan. 104:395–414. doi:10.5575/geosoc.104.395
- Yanagisawa Y, Ando H. 2020. Neogene Taga and Hitachi groups in the Kitaibaraki-Takahagi area, Ibaraki Prefecture, Japan: sedimentary complexes of shelf to slope deposits, submarine channel fills and submarine slide scar fills, reconstructed from lithostratigraphy and diatom biostratigraphy. Bull Geol Surv Japan. 71:85–199. doi:10.9795/bullgsj.71.85
