 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Inhalation of crystalline silica (cSiO2) in the workplace is etiologically linked to lupus and other autoimmune diseases. Exposing lupus-prone NZBWF1 mice to respirable cSiO2 unleashes a vicious cycle of inflammation and cell death in the lung that triggers interferon-regulated gene expression, ectopic lymphoid structure (ELS) development, elevation of local and systemic autoantibodies (AAbs), and glomerulonephritis. However, cSiO2-induced inflammation and onset of autoimmunity can be prevented by inclusion of the ω-3 polyunsaturated fatty acid docosahexaenoic acid (DHA) into the diet of these mice. Since cSiO2 both causes cell death and interferes with efferocytosis, secondary necrosis of residual cell corpses might provide a rich and varied autoantigen (AAg) source in the lung. While it is known that the particle induces anti-nuclear and anti-dsDNA AAbs in NZBWF1 mice, the full extent of the cSiO2-induced AAb response relative to specificity and isotype is not yet understood. The purpose of this study was to test the hypotheses that cSiO2 exposure induces a wide spectrum of AAbs in the pulmonary and systemic compartments, and that dietary DHA intervention prevents these changes. Archived tissue fluid samples were obtained from a prior study in which NZBWF1 mice were fed purified isocaloric diets containing no DHA (control) or DHA corresponding calorically to human doses of 2 and 5 g/day. Mice were intranasally instilled with 1 mg cSiO2 or saline vehicle weekly for 4 weeks, then groups euthanized 1, 5, 9, or 13 weeks post-instillation (PI) of the last cSiO2 dose. Bronchoalveolar lavage fluid (BALF) and plasma from each time point were subjected to AAb profiling using a microarray containing 122 AAgs. cSiO2 triggered robust IgG and IgM AAb responses against lupus-associated AAgs, including DNA, histones, ribonucleoprotein, Smith antigen, Ro/SSA, La/SSB, and complement as early as 1 week PI in BALF and 5 weeks PI in plasma, peaking at 9 and 13 weeks PI, respectively. Importantly, cSiO2 also induced AAbs to AAgs associated with rheumatoid arthritis (collagen II, fibrinogen IV, fibrinogen S, fibronectin, and vimentin), Sjögren’s syndrome (α-fodrin), systemic sclerosis (topoisomerase I), vasculitis (MPO and PR3), myositis (Mi-2, TIF1-γ, MDA5), autoimmune hepatitis (LC-1), and celiac disease (TTG). cSiO2 elicited comparable but more modest IgA AAb responses in BALF and plasma. cSiO2-induced AAb production was strongly associated with time dependent inflammatory/autoimmune gene expression, ELS development, and glomerulonephritis. AAb responses were dose-dependently suppressed by DHA supplementation and negatively correlated with the ω-3 index, an erythrocyte biomarker of ω-3 content in tissue phospholipids. Taken together, these findings suggest that cSiO2 exposure elicits a diverse multi-isotype repertoire of AAbs, many of which have been reported in individuals with lupus and other autoimmune diseases. Furthermore, induction of this broad AAb spectrum could be impeded by increasing ω-3 tissue content via dietary DHA supplementation.
Introduction
Systemic lupus erythematosus (lupus) is a heterogeneous, debilitating autoimmune disease typified by periods of remission and intermittent flaring that, over time, can inflict irreversible damage to multiple organs [Citation1–3]. Lupus results from loss of immune tolerance to self-antigens that is driven by unresolved chronic inflammation, defective clearance of dead cells, unmasking of autoantigens (AAgs), and aberrant autoantibody (AAb) responses. The resulting AAbs form immune complexes with AAgs that deposit in tissues, activate the complement system, promote mononuclear effector cell infiltration, elicit cytokine/chemokine release, induce cell death, and, collectively, promote organ damage. Importantly, immune complex deposition in the kidney can evoke glomerulonephritis in patients with lupus, often culminating in end-stage renal failure [Citation4].
AAbs are detectable in individuals several years prior to symptom onset and lupus diagnosis [Citation2]. While anti-nuclear AAbs are a critical hallmark of lupus [Citation5,Citation6], the American College of Rheumatology (ACR) classification criteria for this disease also includes anti-double-stranded DNA (dsDNA), anti-Smith antigen (Sm), and anti-phospholipid antibodies [Citation7]. Based on a review of published investigations, Yaniv et al. [Citation6] reported that at least 180 diverse AAbs have been associated with patients with lupus, 102 of which correlate with disease activity. Recently, a high-density protein array screening revealed over 300 novel AAbs related to cell death and DNA repair pathways in human lupus [Citation8]. Targeted AAgs were associated with several tissue and cellular compartments, including the nucleus, cytoplasm, cell membrane, phospholipid-associated proteins, blood cells, endothelial cells, nervous system antigens, plasma proteins, and matrix proteins. Together, these findings suggest that a wide spectrum of AAbs is evident in individuals with lupus that potentially contribute to both disease heterogeneity and symptom overlap with other autoimmune diseases.
Millions of workers in the construction, mining, and farming industries are exposed to crystalline silica (cSiO2) daily, and such exposures have been epidemiologically associated with lupus and other autoimmune diseases [Citation9,Citation10]. Inhalation of cSiO2 rapidly hastens both initiation and progression of autoimmunity in lupus-prone mice [Citation11,Citation12]. Intranasal instillation of cSiO2 into female NZBWF1 mice triggers autoimmunity and glomerulonephritis twelve weeks earlier than vehicle-instilled controls [Citation13,Citation14]. At the mechanistic level, exposure to respirable cSiO2 unleashes a vicious cycle of inflammation and cell death in the lung of these lupus-prone mice. This cycle promotes production of cytokines and chemokines, increased interferon-regulated gene expression, and ectopic lymphoid structure (ELS) development. Sometimes referred to as tertiary lymphoid organs, ELSs contain integrated networks of B-cells, T-cells, follicular dendritic cells, and antibody-secreting plasma cells that mimic germinal centres seen in secondary lymphoid organs. Accordingly, the lung serves as the nexus for cSiO2-triggered autoimmune flaring that is reflected by the initial elevation of local AAbs in the pulmonary compartment followed by increased AAbs in the plasma compartment [Citation15].
Exploration of cSiO2-triggered autoimmune pathogenesis in the NZBWF1 mouse provides a novel window for uncovering mechanisms by which this particle mediates lupus pathogenesis and for identifying interventions against this debilitating disease. A potential intervention against lupus is increasing consumption of long chain ω-3 polyunsaturated fatty acids (PUFAs) found in cold-water fish, including docosahexaenoic acid (C22:6 ω-3; DHA) and eicosapentaenoic acid (C20:5 ω-3; EPA). Many investigations in animals and humans have demonstrated that ω-3 PUFA intake can mitigate chronic inflammation and autoimmunity by modulating membrane function, interfering with proinflammatory gene expression, competing with inflammatory ω-6 PUFA-derived eicosanoids, and serving as substrates for metabolism to pro-resolving mediators (reviewed in [Citation16–19]). A valuable clinical biomarker for predicting ω-3 content in tissue phospholipids is the ω-3 index, which is the amount of DHA + EPA in the red blood cell membrane expressed as the percentage of total fatty acids [Citation20,Citation21].
Several clinical studies support the feasibility of using ω-3 PUFA supplementation as an intervention for lupus. Female patients with lupus have been reported to have a lower ω-3 index than age-matched healthy cohorts [Citation22]. Elkan et al. [Citation23] reported that low ω-3 intake among patients with lupus was associated with higher disease activity, adverse serum lipids, and atherosclerotic plaques. In 2019, a population study conducted by the Michigan Lupus Epidemiology & Surveillance (MiLES) programme found that high intake of ω-3 fatty acids and low dietary ω-6:ω-3 ratios were associated with more favourable patient-reported outcomes [Citation24]. Most intervention studies performed to date indicate that ω-3 supplementation provides some benefits to lupus patients (reviewed in [Citation19]). Consistent with clinical investigations, fish oil intake reduces dsDNA IgG AAb production, inflammatory gene signatures, kidney disease, and mortality in lupus-prone mouse models [Citation25–30]. Relevant to the present investigation, recent studies have demonstrated that DHA supplementation at realistic human dose equivalents of 2 and 5 g/day blocks cSiO2-triggered inflammation, ELS neogenesis, and AAb production in the lung, as well as glomerulonephritis in lupus-prone NZBWF1 female mice [Citation14,Citation26,Citation31].
Since cSiO2 both causes cell death [Citation32-36] and interferes with efferocytosis [Citation37], accumulation of residual cell corpses and their subsequent secondary necrosis likely evokes a rich and varied AAg source in the lung that could elicit a broad spectrum of AAbs. While induction of anti-nuclear and anti-dsDNA AAbs by the particle has been demonstrated by ELISA in NZBWF1 mice [Citation13,Citation26], it is not yet known how cSiO2 affects the overall AAb repertoire and how this might be influenced by dietary ω-3 supplementation. AAg microarrays offer a multiplexed approach for assessing AAb spectrum that have distinct advantages over ELISAs relative to minimal sample amounts required, sensitivity, range of detection, and throughput, and are therefore, amenable to preclinical mouse studies (reviewed in [Citation38]). Microarray technology has been used to relate AAb repertoires in both preclinical and clinical investigations of lupus pathogenesis [Citation39–42]. Here, microarrays were employed to test the hypotheses that cSiO2 exposure induces a wide spectrum of AAbs in the pulmonary and systemic compartments and that dietary DHA intervention prevents these changes. Specifically, a high-throughput microarray containing 122 AAgs was used to profile AAbs, specificity and isotype, in bronchioalveolar lavage fluid (BALF) and plasma retained from a prior investigation that assessed the effects of DHA on cSiO2-triggered lupus in NZBWF1 mice over time [Citation31]. AAb profiles were further related to ω-3 tissue content, inflammatory/autoimmune gene expression, and ELS neogenesis in the lung, as well as glomerulonephritis. The results point to the centrality of the lung for diverse, multi-isotype AAb production following airway exposure to cSiO2. Importantly, these AAb responses were ameliorated by translationally relevant dietary DHA supplementation to increase ω-3 tissue content.
Materials and methods
Experimental design
This investigation utilized tissues from a previous time course study that assessed the influence of DHA on cSiO2-induced autoimmune pathogenesis, focussing specifically on histopathology and mRNA signatures in the lung and kidney [Citation14,Citation26,Citation31]. Experimental protocols were approved by the Michigan State University Institutional Animal Care and Use Committee (AUF #01/15-021-00). The experimental design for the parent study is depicted in . Briefly, upon arrival, 6 week old female lupus-prone NZBWF1 mice (n = 8/group) (Jackson Laboratories Bar Harbour, ME) were fed one of three formulations based on the purified American Institute of Nutrition (AIN)-93G diet containing 70 g/kg fat [Citation43] as described previously [Citation31]. All diets were formulated with 10 g/kg corn oil to ensure adequate basal essential fatty acids. Control diet (CON) contained 60 g/kg high-oleic safflower oil (Hain Pure Food, Boulder, CO), whereas high-oleic safflower oil was replaced with 10 g/kg (DHA low) or 25 g/kg (DHA high) microalgal oil containing 40% DHA (DHASCO, provided by Dr. Kevin Hadley, DSM Nutritional Products, Columbia, MD) for DHA diets. The final DHA composition was 4 g/kg or 10 g/kg DHA for DHA low and DHA high diets, respectively. These diets are the human equivalent of 2 and 5 g of DHA per day on a caloric basis. Diets were prepared weekly and stored at −20 °C until use to avoid lipid oxidation products. Fresh feed was provided ad libitum to mice daily.
Figure 1. Experimental design. Groups (n = 8) of 6 week-old female NZBWF1 mice were initiated on either control (CON), low DHA, or high DHA AIN-93G diets. At 8 weeks of age, groups were then intranasally instilled with 1.0 mg cSiO2 or vehicle weekly for 4 weeks. Cohorts were euthanized at 12, 16, 20, or 24 weeks of age, corresponding to 1, 5, 9, or 13 weeks post final instillation (PI). Plasma and BALF were collected for AAb analysis using a high throughput AAg microarray panel for IgG, IgM, and IgA isotypes.
Mice (8 week old) were anaesthetized with 4% isoflurane and intranasally instilled with 1.0 mg cSiO2 (Min-U-Sil-5, 1.5–2.0 μm average particle size, U.S. Silica, WV) in 25 μl PBS or VEH. Exposures were repeated once per week for 4 weeks. All mice in this experiment were exposed to cSiO2 or VEH at the same time. At 1, 5, 9, and 13 weeks post-cSiO2 exposure, cohorts were euthanized by intraperitoneal injection with 56 mg/kg BW sodium pentobarbital and exsanguinated via the abdominal aorta. Blood was collected with heparin-coated syringes and centrifuged at 3500× g for 10 min at 4 °C for the separation of plasma. BALF was collected from whole lungs and centrifuged at 2400×g for 15 min. BALF supernatant and plasma were stored at −80 °C in single-use aliquots for future analysis. Lungs and kidneys were collected and analysed for gene expression and histopathology as described previously [Citation14,Citation31]. Red blood cells were analysed for fatty acid concentrations by gas liquid chromatography at Omega Quant Analytics (Sioux Falls, SD), and the ω-3 index determined using the formula
(1)
(1)
High-throughput protein microarray for AAb profiling
AAg-coated protein arrays were used for profiling three isotypes of AAb, including IgG, IgM, and IgA in plasma and BALF at the Genomics and Microarray Core Facility of the University of Texas Southwestern Medical Centre [Citation42]. summarizes the AAgs included in the panel. Briefly, samples were pre-treated with DNAse-I, then diluted at 1:50 (for serum) or 1:25 (for BALF) and hybridized to protein array plates coated with 122 antigens and 6 controls. The plates were stained with either (I) Cy3-conjugated anti-mouse IgG (1:2000, Jackson ImmunoResearch Laboratories, PA) and Cy5-conjugated anti-mouse IgM (1:2000, Jackson ImmunoResearch Laboratories), or (II) Cy3-conjugated anti-mouse IgA (1:1000, Jackson ImmunoResearch Laboratories), and fluorescent images acquired with a Genepix 4200 A scanner (Molecular Devices, San Jose, CA). Fluorescent images were converted to signal intensity values using GenePix 7.0 software (Molecular Devices). Background was subtracted and signal normalized to internal controls for IgG, IgM, or IgA, respectively. The final value for each AAb was expressed as antibody score (Ab-score), which is calculated based on the normalized signal intensity (NSI) and signal-to-noise ratio (SNR) using the formula:
(2)
(2)
Table 1. Autoantibody classification.
Data analysis
AAbs were grouped in relation to the following AAg classifications: i) nuclear, ii) phospholipid, iii) RNA/ribosome, iv) complement and circulating proteins, v) thyroid-specific proteins, vi) cytoplasmic/membrane proteins, vii) glomeruli-specific proteins, and viii) matrix Ags (). Summation values for each group were calculated based on the Ab-score of all AAbs within the group. Heat maps, Hierarchical Co-Clustering (HCC), and Principal Component Analyses (PCA) were performed using ClustVis [Citation44]. Normalized and unit variance-scaled Ab-score values were represented in heat maps, with samples organized by unsupervised HCC. In heat maps, values were centred by rows; imputation was used for missing value estimation. Rows were clustered using Euclidean distance and Ward linkage. Similarities among groups were calculated using PCA analysis. The singular value decomposition method with imputation was used to calculate principal components in PCA plots. Summation of AAb groups or selected AAb-score was presented as line graphs or scatter plots, generated using GraphPad Prism version 8.3.0 (GraphPad, San Diego, CA). One-way ANOVA was used to compare experimental groups at selected time points. Post hoc multiple comparisons were performed using Tukey’s HSD test (p < .05).
Correlation analyses were performed using cor and corrplot functions in R (www.R-project.org) and GraphPad. The Spearman rank test was used to correlate AAb scores to ω-3 indexes, mRNA signatures, ELS development, and glomerulonephritis in individual mice. Plasma to BALF correlation analysis in same animal was also performed using the Spearman rank test. Gene expression analyses were determined as described previously [Citation14] using the Nanostring nCounter. Expression signatures were summarized as pathway Z-scores for immune-related pathways, including interferon, dendritic cells, T cells, B cells, cytokines, chemokines, and complement. ELS neogenesis was previously assessed [Citation26] by histopathological scoring and digital morphometry of lung sections positively stained with antibodies to CD45R+ B cells, CD3+ T cells, and CD21/35+ follicular dendritic cells. Glomerulonephritis was determined by histopathological scoring of the kidney sections. A significant correlation was inferred when p ≤ .05 and the Spearman ρ > 0.5 or < −0.5.
Results
cSiO2 triggers IgG and IgM AAb responses in BALF before those in plasma
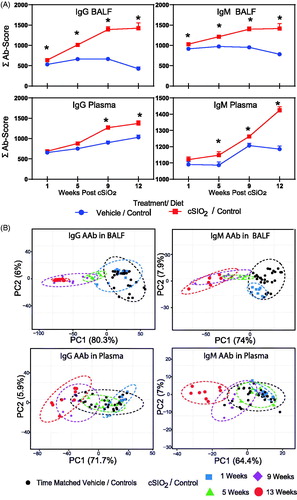
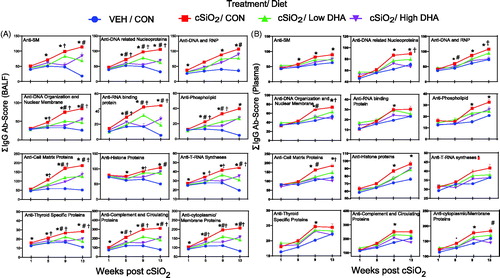
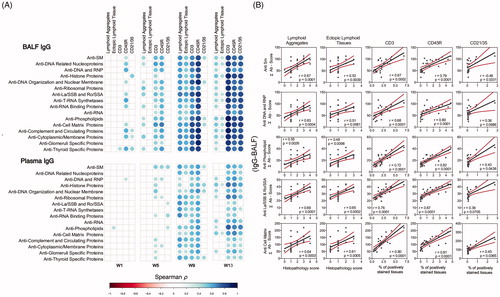
Heat mapping revealed that in mice fed control diets, intranasal instillation with cSiO2 elicited IgG AAbs in BALF () and plasma () with specificities for a diverse range of AAgs, including dsDNA, ssDNA, collagen VI, genomic DNA, LPS, α-fodrin, and chromatin. cSiO2 exposure similarly evoked IgM AAbs in BALF and plasma, but these responses were comparatively less robust than IgG AAbs (Supplementary Figures 1 and 2). Using summation values (ΣAb-Score) as a measure of overall AAb response, cSiO2-induced IgG and IgM AAb production were both found to be significantly elevated (p ≤ .05) in mice fed control diet beginning at 1 week PI and peaking at 9 week PI (). In plasma, IgG and IgM responses were significantly elevated (p ≤ .05) beginning at 9 and 5 weeks PI, respectively. PCA indicated that IgG and IgM AAb responses in cSiO2-treated mice at 9 and 13 weeks PI were distinctly separated from time-matched vehicle-treated mice in both BALF and plasma, regardless of isotype (). The samples segregated by cSiO2 treatment to a greater extent along the PC1 with 65% and 80% variation for BALF IgG and IgM AAbs suggests that the cSiO2 treatment explained a greater portion of the variation than did the timing of the sample collection irrespective of treatment.
Figure 2. cSiO2-induced IgG AAb responses in BALF are impeded by DHA intake. Heat maps with unsupervised clustering (Euclidian distance method) depict Ab-score values for IgG AAbs for 122 AAgs measured in the BALF. Top bar indicates the VEH/CON, cSiO2/CON, cSiO2/Low DHA, and cSiO2/High DHA groups, respectively, at 1, 5, 9, or 13 weeks PI. Scale bar values reflect the range of variance-stabilized Ab scores, which were centred across rows.
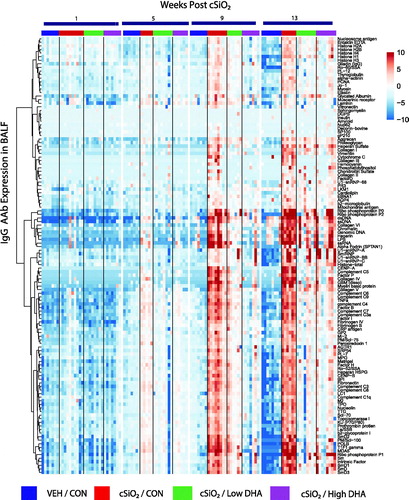
Figure 3. cSiO2-induced IgG AAb responses in plasma are inhibited by DHA intake. Heat maps with unsupervised clustering (Euclidian distance method) depict Ab-score values for IgG AAbs to 122 AAgs measured in the plasma. Top bar indicates the VEH/CON, cSiO2/CON, cSiO2/Low DHA, and cSiO2/High DHA groups, respectively, at 1, 5, 9, or 13 weeks PI. Scale bar values reflect the range of variance-stabilized Ab scores, which were centred across rows.
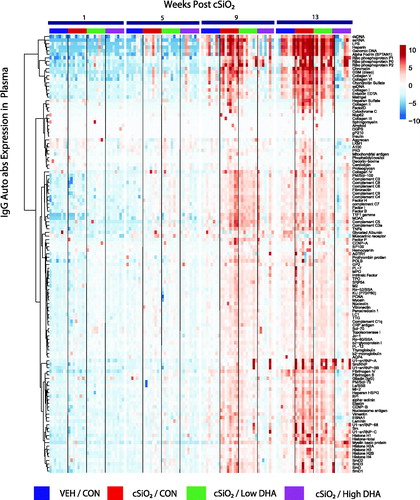
Figure 4. cSiO2-triggered IgG and IgM AAb responses in BALF precede those in plasma in NZBWF1 mice fed control diet. (A) Time course of total IgG and IgM AAb responses in BALF and plasma of mice. AAb expression data were calculated as the summation of 122 Ab-scores (Σ Ab-score) for VEH- and cSiO2-treated mice fed control diet at 1, 5, 9, or 13 weeks PI. Data are mean ± SEM. Asterisk indicates significant difference (p ≤ .05) between cSiO2/CON and VEH/CON mice as calculated by t-test. (B) PCA of differentially expressed IgG or IgM AAb in BALF or plasma of VEH/CON and cSiO2/CON mice. The dashed ellipses show 95% confidence intervals.
cSiO2 exposure triggers expression of IgG and IgM AAbs associated with lupus and other autoimmune diseases
When BALF AAb summation values were calculated based on AAg group classifications (), cSiO2 was found to induce detectable IgG responses to nuclear, phospholipid, RNA/ribosome, complement/circulating proteins, thyroid, membrane-specific, and glomerular AAgs in BALF by 1 or 5 weeks PI () and in plasma by 5 or 9 weeks PI (). These responses trended upward for the remainder of the study. IgM AAb responses to these AAg groups in BALF and to a lesser extent in plasma were temporally similar to those seen for IgG (Supplementary Figure 3(A,B)).
Figure 5. DHA supplementation dose dependently suppresses cSiO2-induced IgG AAb expression in BALF (A) and plasma (B) over time. Ab-score data were obtained using the microarray panel for vehicle (VEH)- or cSiO2-exposed mice fed CON, low DHA, or high DHA diets. Σ Ab-score refers to sum of Ab-scores for AAbs belonging to a particular category (). One-way ANOVA was used to compare experimental groups at selected time points followed by Tukey's HSD method as multiple comparison test. Data are mean ± SEM. Symbols indicate significant difference (p ≤ .05) as follows: * for cSiO2/CON vs. VEH/CON; # for cSiO2/CON vs. cSiO2/Low DHA; and † for cSiO2/CON vs cSiO2/High DHA.
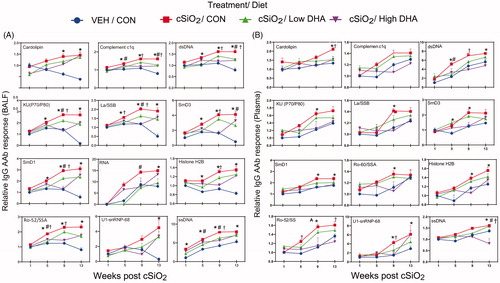
cSiO2 instillation induced many specific IgG AAbs in BALF () and plasma () that are highly associated with lupus, such as anti-SmD1, anti-chromatin, anti-histone H2B, anti-dsDNA, anti-Ro/SSA, anti-La/SSB, and anti-complement C1q. Comparable but more modest IgM AAb responses for lupus AAgs were observed in BALF and plasma (Supplementary Figure 4(A,B)).
Figure 6. DHA supplementation dose dependently suppresses cSiO2 induction of selected lupus-related IgG AAbs in BALF (A) and plasma (B) over time. Ab-score data were obtained using the microarray panel for vehicle (VEH)- or cSiO2-exposed mice fed CON, DHA low, or DHA high diets. Relative AAb responses for selected AAgs were determined by dividing individual Ab-score by the mean Ab-score for VEH-treated mice fed control diet at 1 week PI. One-way ANOVA was used to compare experimental groups at selected time points followed by Tukey's HSD method as multiple comparison test. Data are mean ± SEM. Symbols indicate significant difference (p ≤ .05) as follows: * for cSiO2/CON vs. VEH/CON; # for cSiO2/CON vs. cSiO2/Low DHA; and † for cSiO2/CON vs cSiO2/High DHA.
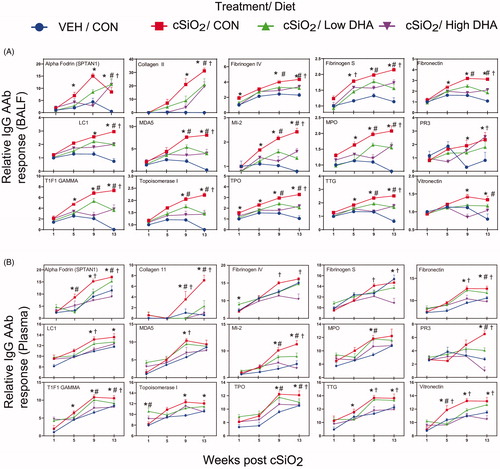
Remarkably, cSiO2 also elicited IgG AAbs in the BALF () and plasma () that are hallmarks of other autoimmune diseases, including rheumatoid arthritis (collagen II, fibrinogen IV, fibrinogen S, fibronectin, and vimentin), Sjögren’s syndrome (α-fodrin), systemic sclerosis (topoisomerase I and U1-snRNP), ANCA vasculitis (MPO and PR3), myositis (Mi-2, TIF1-γ, MDA5), autoimmune hepatitis (LC-1), Hashimoto thyroiditis (TPO), and coeliac disease (TTG). Similarly diverse IgM AAb profiles, but at lower magnitude, were observed in BALF and plasma (Supplementary Figure 5(A,B)).
Figure 7. DHA intake inhibits cSiO2-induced expression of IgG AAbs in BALF (A) and plasma (B) specific for AAgs associated other autoimmune diseases. AAbs included those associated with rheumatoid arthritis (collagen II, fibrinogen IV, AAbs fibrinogen S, fibronectin, and vimentin), Sjögren’s syndrome (α-fodrin), systemic sclerosis (topoisomerase I)), vasculitis (MPO and PR3), myositis (Mi-2, TIF1-γ, MDA5), autoimmune hepatitis (LC-1), and coeliac disease (TTG). Relative AAb responses for selected AAgs were determined by dividing individual Ab-score by the mean Ab-score for VEH-treated mice fed control diet at week 1 PI. One-way ANOVA was used to compare experimental groups at selected time points followed by Tukey's HSD method as multiple comparison test. Data are mean ± SEM. Symbols indicate significant difference (p ≤ .05) as follows: * for cSiO2/CON vs. VEH/CON; # for cSiO2/CON vs. cSiO2/Low DHA; and † for cSiO2/CON vs cSiO2/High DHA.
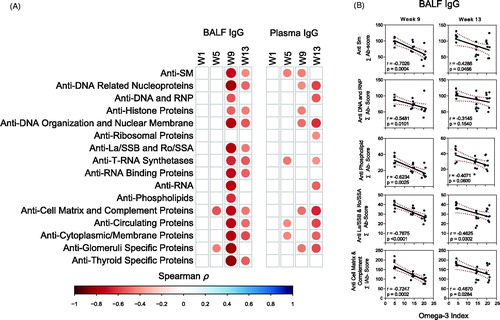
DHA suppression of cSiO2-triggered IgG and IgM AAbs is heralded by increased ω-3 index
DHA supplementation dose dependently suppressed or delayed almost all cSiO2-induced increases in BALF and plasma IgG AAbs () and IgM AAbs (Supplementary Figures 1–5). Both IgG AAb responses in BALF and plasma at 9 and 13 weeks negatively correlated with the red blood cell ω-3 index, which is a clinical indicator of ω-3 incorporation into tissue phospholipids (). Notably, BALF IgG AAbs at 9 weeks PI for all the AAb groups except anti-ribosomal proteins showed a stronger negative correlation with the red blood cell ω-3 index.
Figure 8. Relationship of IgG AAb responses to ω-3 index in erythrocytes. AAb responses negatively correlate with erythrocytes ω-3 index. (A) For all cSiO2-treated groups, Spearman ρ values were calculated by correlating Σ IgG Ab-score values for specific categories () in BALF and plasma with the ω-3 index in erythrocytes at 1, 5, 9, and 13 weeks PI. Significant correlation values (p ≤ .05) are represented as shaded circles; non-significant correlations are indicated by blank cells. (B) Scatter plots for Σ Ab-score values from selected IgG AAb groups in BALF vs. ω-3 index in erythrocytes at week 9 and week 13. Linear regression lines with 95% confidence intervals (dashed red line) are shown along with the Spearman ρ value and p-value.
IgG and IgM AAb responses positively correlate with lupus-related gene expression and disease pathogenesis
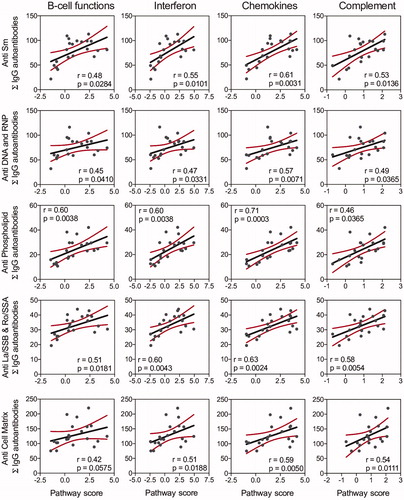
BALF and plasma IgG AAb responses in individual mice from all experimental groups were related to mRNA signatures and histopathologic markers of autoimmune disease activity determined in the same animal cohorts in prior studies [Citation14,Citation31]. IgG and IgM AAb responses in BALF in individual mice correlated positively with mRNA signatures associated with B-cell function, T-cell function, type I interferon, chemokines, cytokines, and complement pathways (Supplementary Figure 6(A)) These associations were strongest at 9 weeks PI and observed to a lesser extent at 5 and 13 weeks PI (). Plasma IgG and IgM AAbs also showed modest correlation with expression of some of these genes only at 13 weeks PI (Supplementary Figure 6(B)).
Figure 9. IgG AAb responses in BALF positively correlate with cSiO2-induced gene expression in lung. Scatter plots for Σ Ab-score from selected AAb groups in BALF vs. the Z scores of gene expression in selected immune pathways at week 9. Linear regression lines with 95% confidence intervals (curved lines) are shown along with the Spearman ρ value and p-value.
Moreover, histopathology scores for lymphoid aggregates and ELS in the lungs as well as percent CD45R+ (B-cells), CD3+ (T-cells), and CD21/35+ in lung tissue of cSiO2-exposed mice were strongly associated with IgG AAb in the BALF and plasma (). This was also observed with BALF IgM at 9 weeks and 13 weeks PI and, to a lesser extent, with IgM AAb at 13 weeks (Supplementary Figure 7). Lastly, IgG and IgM AAb responses in BALF, and to a smaller degree, in plasma, positively correlated with tubular proteinosis, a histologic marker of glomerulonephritis (Supplementary Figure 8).
Figure 10. IgG AAb responses in BALF and plasma positively correlate with markers for ELS development. (A) For all cSiO2-treated groups, Spearman ρ values were calculated by correlating Σ IgG Ab-score values with markers for ELS development assessed in lung tissues of mice collected 1, 5, 9, or 13 weeks PI. Σ Ab-score data calculated as the sum of AAbs belonging to a particular category (). Markers for ELS development in lung tissues of mice measured as the percentage of positively stained tissues (CD3, CD45R, and CD21/35) and scores of histopathology (lymphoid aggregates, ectopic lymphoid tissue). Significant correlation values (p ≤ .05) are represented as shaded circles; non-significant correlations are indicated by blank cells. (B) Scatter plots for Σ Ab-score from selected IgG groups in BALF vs. markers for ELS development at 13-week PI. Linear regression lines with 95% confidence intervals (curved lines) are shown along with the Spearman ρ value and p-value.
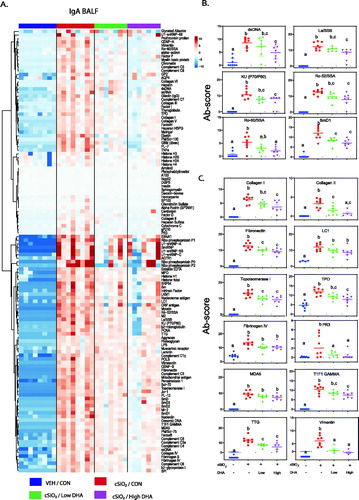
DHA consumption inhibits cSiO2-induced IgA AAb production
cSiO2 also induced IgA AAb elevations at 13 weeks PI in BALF, and these responses were suppressed by DHA intake (). Similar, though more subtle, trends were observed in the plasma (Supplementary Figure 9). Individual lupus-related IgA AAbs in BALF, namely dsDNA, La SSB, elastin, KU (P70/P80), RO-52/SSA, RO-60/SSA, SMD1 and SmD3, were significantly upregulated by cSiO2 at 13 weeks PI and downregulated by DHA (). Comparable effects were evident for BALF IgA AAbs associated with other autoimmune diseases ().
Figure 11. cSiO2-induced IgA AAb responses in BALF at 13 weeks PI are suppressed by DHA supplementation. (A) Heat maps with unsupervised clustering (Euclidian distance method) of 122 AAbs depict Ab-score values for IgA expression in BALF. Top key indicates the VEH/CON, cSiO2/CON, cSiO2/Low DHA, and cSiO2/High DHA experimental groups, respectively. Scale bar values reflect the range of variance-stabilized Ab scores, which were centred across rows. DHA intake suppressed cSiO2-induced IgA AAb responses against (B) selected lupus-related AAgs, and (C) AAgs associated with other autoimmune diseases. Data are Σ Ab-score or specific Ab-scores ± SEM. Different superscript letters on bar graph indicate significant difference (p ≤ .05) compared with the other treatment groups as assessed by one-way ANOVA followed by Tukey's HSD method as a multiple comparison test.
BALF AAb responses positively correlate with plasma AAb responses
IgG, IgM and IgA AAb responses in BALF in individual mice correlated positively with plasma IgG, IgM and IgA AAb responses in the same animal at 13 weeks PI (Supplementary Figure 10).
Discussion
Several novel and important findings were made in the present study. First, cSiO2 exposure elicited a remarkably diverse repertoire of potentially pathogenic AAbs in BALF that mimicked profiles seen in individuals with lupus and other autoimmune diseases. Secondly, cSiO2-induced AAbs included IgM, IgG, and IgA isotypes in the pulmonary and systemic compartments, indicating that the particle induced class switching. Third, AAb responses among experimental groups positively correlated with inflammatory/autoimmune gene expression, pulmonary ectopic lymphoid neogenesis, and glomerulonephritis. Finally, the observations that realistic DHA supplementation strongly suppressed cSiO2-triggered AAb responses in BALF and plasma, and that this suppression correlated with the ω-3 index, are highly relevant from the perspective of clinical translation.
An important consideration is the relevance of the cSiO2 dose used here to actual human exposure. The U.S. Occupational Safety and Health Administration (OSHA) permissible exposure limit (PEL) for respirable cSiO2 is 50 µg/m3/day (https://www.osha.gov/Publications/OSHA3682.pdf). This PEL is routinely exceeded in many industries including demolition, crushing, chipping, and fracking activities [Citation45,Citation46]. Assuming human ventilation rate is 6.0 L/min, humans exposed to the OSHA PEL could inhale 1433 mg silica in 40 years of work (8 h/day for 5 days/week) with respirable cSiO2. Based on a estimated mice ventilation rate of 0.03 L/min, it can be predicted that an equivalent lifetime exposure to cSiO2 would be 8.28 mg in mouse. Accordingly, the cumulative cSiO2 doses employed in this investigation, 4 mg, equates to half of a human lifetime exposure to cSiO2 at the recommended OSHA exposure limit.
It is well-established that cSiO2 is a risk factor for lupus and other autoimmune diseases in occupational, residential, rural, and urban settings (reviewed in [Citation10,Citation47,Citation48]). Importantly, these studies suggest strong dose–response associations with increasing intensity or duration of exposure to the particle [Citation9,Citation49]. Due to cSiO2’s inability to be cleared effectively from the lung [Citation50] and its capacity to suppress efferocytosis [Citation37], this particle promotes a vicious cycle of unresolved inflammation characterized by cell death, increased expression of proinflammatory cytokine, and chemokine, interferon-regulated genes, ELS development, and AAb production. cSiO2’s capacity to elicit AAbs of wide-ranging specificity, first in the BALF and then in plasma, likely contributes to its strong epidemiological association with lupus. Particularly relevant was the induction of AAbs to nuclear antigens that include dsDNA [Citation7,Citation51,Citation52], Ku (p70/p80) [Citation53], Sm [Citation7,Citation52], RNP [Citation54,Citation55], histones [Citation56,Citation57], phospholipids [Citation58], ribosomal proteins [Citation59], and RNPs [Citation60]. cSiO2 also induced AAbs with specificities for neutrophil cytoplasmic proteins [Citation61,Citation62], collagen [Citation63], and complement [Citation64,Citation65]. Exposure to respirable cSiO2 has also been aetiologically linked to several autoimmune diseases besides lupus [Citation66–73]. Thus, it was noteworthy that cSiO2 induced AAbs associated with rheumatoid arthritis (collagen II, fibrinogen IV, fibrinogen S, fibronectin, and vimentin), Sjögren’s syndrome (α-fodrin), systemic sclerosis (topoisomerase I), vasculitis (MPO and PR3), myositis (Mi-2, TIF1-γ, MDA5), autoimmune hepatitis (LC-1), and coeliac disease (TTG) [Citation74–78]. Altogether, it might be speculated that these AAgs arise in the lung following chronic exposure to cSiO2, and subsequently drive the production of AAbs that play pathogenic roles in lupus and these other autoimmune diseases.
The observed induction of IgM, IgG, and IgA AAbs in BALF by cSiO2 suggests that pulmonary ELS are active sites of isotype switching. IgM is produced early in the humoral response preceding IgG, is secreted as a pentamer, and typically has high avidity but low affinity [Citation79]. Injection of DNA-specific monoclonal IgG causes robust lupus-like disease in normal mice, whereas DNA-specific IgM causes minimal disease [Citation80,Citation81]. Thus, isotype shifting from IgM to IgG in the lung likely exacerbates lupus pathogenesis. IgA AAbs might also have pathogenic roles, particularly in the context of glomerulonephritis [Citation82,Citation83]. While IgA nephropathy is the most common IgA-associated autoimmune disease [Citation18], this isotype has been linked to other autoimmune diseases, including lupus [Citation84–86]. Since cSiO2-induced IgA AAb responses were much higher in BALF than plasma, how this isotype might affect autoimmune pathogenesis in cSiO2-exposed mice requires more investigation. Moreover, the positive correlations between BALF IgG, IgM, and IgA AAb responses in individual mice from all experimental groups and plasma AAb responses in the same animal indicates that a relationship exists between the local and systemic distribution of AAbs in these animals.
The findings that dietary DHA dosedependently suppressed the full spectrum of cSiO2-induced IgG, IgM, and IgA AAbs in the BALF and plasma has important clinical implications. At the mechanistic level, the relative concentrations of ω-3 and ω-6 PUFA in the membrane likely influence inflammation and autoimmune pathogenesis in several ways. ω-3 PUFAs can interfere with lipid raft formation and suppress activation of transmembrane receptors associated with inflammation [Citation87]. Extracellular and intracellular phospholipases can cleave ω-3 PUFAs from the membrane [Citation88,Citation89], liberating DHA and EPA to activate transmembrane receptors that are capable of interfering with pro-inflammatory signalling pathways [Citation90,Citation91]. Both DHA and EPA are recognized ligands for PPARγ, which can suppress NF-kB-dependent transcription of inflammation-associated genes [Citation92,Citation93]. Additionally, these ω-3 PUFAs can be metabolized to specialized pro-resolving mediators (SPMs) that include resolvins, protectins, maresins, and anti-inflammatory epoxide metabolites [Citation94,Citation95]. SPMs promote both phagocytosis of bacteria and efferocytosis of dead cells [Citation96,Citation97], and have also been shown to inhibit inflammatory signalling [Citation98,Citation99]. Lastly, ω-3 PUFAs directly compete with ω-6 PUFAs for incorporation into the cell membrane, as well as for metabolism to downstream proinflammatory metabolites of the ω-6 PUFA arachidonic acid that include prostaglandins, thromboxanes, and leukotrienes [Citation100]. Accordingly, altering the balance of ω-3 PUFAs over ω-6 PUFAs in the membrane promotes resolution over inflammation.
While long chain ω-3 PUFAs cannot be synthesized in humans and animals, a number of marine algae proficiently elongate shorter chain ω-3 fatty acids to DHA and EPA. Fatty fish such as mackerel and salmon consume these algae and bioconcentrate ω-3 PUFAs, making them the primary source for these fatty acids in the human diet [Citation101]. However, Western diets typically contain high concentrations of proinflammatory ω-6 s, such as linoleic acid (C18:2 ω-6; LA) and arachidonic acid (C20:4 ω-6; ARA) found in the plant- and animal-derived lipids, thereby excluding the anti-inflammatory ω-3 s. Accordingly, Americans consume many times more ω-6 s than ω-3 s, causing tissue phospholipid fatty acid content to skew profoundly towards ω-6 s [Citation102,Citation103]. Thus decreasing the ratio of ω-6 s to ω-3 s is critical to decreasing inflammation. Individuals can correct ω-3 deficiency and increase DHA and EPA tissue incorporation into phospholipids by consuming dietary supplements made from fish or microalgal oil [Citation104]. The quantities of DHA used in this study, calorically equivalent to 2 and 5 g/day human consumption, were efficacious and would be consistent with realistic and safe doses [Citation105]. Some portion of this ω-3 intake can be achieved through diet by consuming oily fish daily. For example, contingent upon the source and the serving size, salmon contains 1.5–3 g EPA and DHA per serving [Citation106].
It was particularly notable that there was a strong negative correlation between the ω-3 index and both IgG and IgM AAb responses. The ω-3 index in humans ranges between 2% and 20%, with a range of 8–11% proposed to be optimal for several health outcomes including total mortality, myocardial infarction, and other cardiovascular diseases [Citation107]. It was observed here that mice fed the control diet exhibited a low ω-3 index (approximately 5%), whereas mice fed the low and high DHA diets had ω-3 indexes of approximately 15% and 20%, respectively. These findings suggest the desirability for investigators in future clinical trials assessing ω-3 interventions in lupus patients to consider relating the ω-3 index at the beginning and throughout the study to indicators of disease activity.
Multiplexed protein arrays have been validated extensively to be comparable with ELISA for antibody detection [Citation8,Citation39,Citation108,Citation109]. This approach has been proven to be a powerful and reliable technology and has been extensively utilized for high-throughput profiling of protein biomarkers in various immune-related disorders [Citation38,Citation46–49]. The AAg array employed here contains a group of 122 selected autoantigens shown to be targets of autoreactive antibodies in lupus and other autoimmune diseases [Citation41,Citation42,Citation110–113]. This specific platform has been used by other investigators for profiling a wide spectrum of autoantibodies in patients with lupus, as well as in genetically modified autoimmune mouse models [Citation8,Citation108,Citation114–117]. A key feature that made protein array particularly advantageous for this specific murine preclinical investigation is the low volume (<100 µl) required for the BALF and plasma samples. It should be noted that it was not possible to run additional confirmatory ELISAs because the available samples were expended for this microarray and other analyses. Nevertheless, in the prior parent study [Citation31], anti-dsDNA responses were measured by ELISA in BALF and plasma from same cohorts used here, and found these results to be highly consistent relative to time of appearance and magnitude with the microarray findings reported herein. Another limitation is that reporting ΣAb-score values for AAb groups raises the possibility that high expression AAb could bias the group expression. However, diagnosis of lupus in clinical setting already includes detecting positivity for the broad range of AAbs identified in the anti-nuclear antibody test. Furthermore, as illustrated graphically and by heat mapping, expression of individual AAbs tended to be similar to that of the category as a whole.
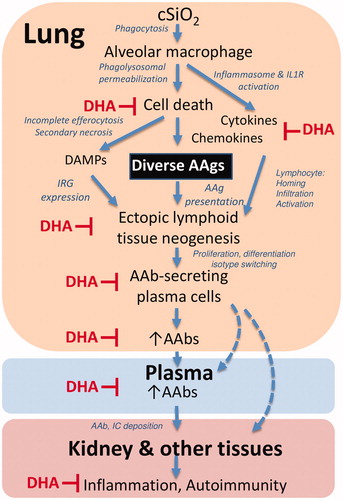
As depicted in , the results presented here and previously suggest that exposure of NZBWF1 mice to respirable cSiO2 promotes unresolved inflammation that results in uncovering diverse AAgs, ELS formation, B cells activation/differentiation, isotype switching, and expression of a broad repertoire of pathogenic AAbs in the lung. Importantly, inclusion of DHA in the mouse diet impedes many of these steps, consequently suppressing these pathogenic AAb responses. Additional investigation is needed to uncover the mechanisms by which cSiO2-induced cell death and/or inadequate clearance of cell corpses unmasks AAgs that drive production of pathogenic AAbs, and how these events are specifically impacted by dietary ω-3 intake. Further questions to be addressed include whether plasma AAbs solely arise from plasma cells in the lung, or whether long-lived plasma cells migrate via the blood to other tissues such as spleen, kidney, and bone marrow. Finally, in the future, clinical trials are needed to determine if raising the ω-3 index could be utilized as a precision health strategy for individuals at risk of or in remission from lupus and other autoimmune diseases.
Figure 12. Putative mechanisms for cSiO2 induction of broad autoantibody repertoire by in NZBWF1 mice and targets of DHA suppression. The results presented here and previously indicate that alveolar macrophages phagocytose cSiO2 in the lung, which induces a vicious cycle of phagolysosomal permeabilization, inflammasome activation, proinflammatory cytokine/chemokine release, and cell death. Inability to remove dying cells by efferocytosis results in secondary necrosis, exposure of damage-associated molecular patterns (DAMPs), and a diversity of autoantigens (AAgs). DAMPs stimulate type I interferon-related gene expression (IRG) from plasmacytoid dendritic cells, while cytokines and chemokines recruit and activate additional immune cells. Together, these actions promote the AAg presentation, formation of ectopic lymphoid tissue, and differentiation of B-cells to plasma cells that produce diverse AAbs in the lung fluid and plasma. Upon binding their cognate AAgs, AAbs can form immune complexes (IC) that ultimately deposit in the kidney inflicting damage. The symbol ⊥ indicates steps in this putative pathway that are impaired by dietary DHA supplementation.
Author contributions
LR: data analysis/interpretation, statistical analysis, figure preparation, manuscript preparation; QL: microarray, data analysis, manuscript preparation/submission; CZ: data analysis/interpretation; MY: microarray; PC: immunohistochemistry and manuscript preparation; KW: fatty acid analyses, data analysis/interpretation, manuscript preparation; MB: study design, animal study coordination, necropsy, manuscript preparation, project funding; JH: study design, lung/kidney histopathology, morphometry, data analyses, manuscript preparation, project funding; AB: data analysis, manuscript preparation; JP: planning, coordination, oversight, manuscript preparation/submission, project funding.
Supplemental Material
Download Zip (1.5 MB)SUBMITTED_SUPPLEMENTAL__MATERIALS__LEGENDs__FIGS__TABLE.pdf
Download PDF (6 MB)Acknowledgments
The authors thank Elizabeth Ross, Kristen Gilley, Augie Evered, Shamya Harris, Dr. James Wagner, Dr. Ning Li, Dr. Daven Humbles-Jackson, Amy Freeland, Lysie Eldridge, and Ryan Lewandowski for their excellent technical support.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Additional information
Funding
References
- Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533.
- Eriksson C, Kokkonen H, Johansson M, et al. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res Ther. 2011;13:R30.
- Sherer Y, Gorstein A, Fritzler MJ, et al. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum. 2004;34:501–537.
- Sterner RM, Hartono SP, Grande JP. The pathogenesis of lupus nephritis. J Clin Cell Immunol. 2014;5:205.
- Cross LS, Aslam A, Misbah SA. Antinuclear antibody-negative lupus as a distinct diagnostic entity-does it no longer exist? QJM. 2004;97:303–308.
- Yaniv G, Twig G, Shor DB, et al. A volcanic explosion of autoantibodies in systemic lupus erythematosus: a diversity of 180 different antibodies found in SLE patients. Autoimmun Rev. 2015;14:75–79.
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019;71:1400–1412.
- Luo H, Wang L, Bao D, et al. Novel autoantibodies related to cell death and DNA repair pathways in systemic lupus erythematosus. Gen Prot Bioinform. 2019;17:248–259.
- Parks CG, Cooper GS, Nylander-French LA, et al. Occupational exposure to crystalline silica and risk of systemic lupus erythematosus: a population-based, case-control study in the southeastern United States. Arthritis Rheum. 2002;46:1840–1850.
- Pollard KM. Silica, silicosis, and autoimmunity [perspective]. Front Immunol. 2016;7:97.
- Brown J, Archer A, Pfau J, et al. Silica accelerated systemic autoimmune disease in lupus‐prone New Zealand mixed mice. Clin Exp Immunol. 2003;131:415–421.
- Brown JM, Pfau JC, Holian A. Immunoglobulin and lymphocyte responses following silica exposure in New Zealand mixed mice. Inhal Toxicol. 2004;16:133–139.
- Bates MA, Brandenberger C, Langohr I, et al. Silica triggers inflammation and ectopic lymphoid neogenesis in the lungs in parallel with accelerated onset of systemic autoimmunity and glomerulonephritis in the lupus-prone NZBWF1 mouse. PLoS One. 2015;10:e0125481.
- Benninghoff AD, Bates MA, Chauhan PS, et al. Docosahexaenoic acid consumption impedes early interferon- and chemokine-related gene expression while suppressing silica-triggered flaring of murine lupus. Front Immunol. 2019;10:2851.
- Wierenga KA, Harkema JR, Pestka JJ. Lupus, silica, and dietary omega-3 fatty acid interventions. Toxicol Pathol. 2019;47:1004–1011.
- Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45:1105–1115.
- Ferreira HB, Pereira AM, Melo T, et al. Lipidomics in autoimmune diseases with main focus on systemic lupus erythematosus. J Pharm Biomed Anal. 2019;174:386–395.
- Pestka JJ. n-3 polyunsaturated fatty acids and autoimmune-mediated glomerulonephritis. Prostaglandins Leukot Essent Fatty Acids. 2010;82:251–258.
- Akbar U, Yang M, Kurian D, et al. Omega-3 fatty acids in rheumatic diseases: a critical review. J Clin Rheumatol. 2017;23:330–339.
- Fenton JI, Gurzell EA, Davidson EA, et al. Red blood cell PUFAs reflect the phospholipid PUFA composition of major organs. Prostaglandins Leukot Essent Fatty Acids. 2016;112:12–23.
- Wierenga KA, Strakovsky RS, Benninghoff AD, et al. Requisite omega-3 HUFA biomarker thresholds for preventing murine lupus flaring. Front Immunol. 2020;11:1796.
- Aghdassi E, Ma DWL, Morrison S, et al. Alterations in circulating fatty acid composition in patients with systemic lupus erythematosus: a pilot study. JPEN J Parenter Enteral Nutr. 2011;35:198–208.
- Elkan AC, Anania C, Gustafsson T, et al. Diet and fatty acid pattern among patients with SLE: associations with disease activity, blood lipids and atherosclerosis. Lupus. 2012;21:1405–1411.
- Charoenwoodhipong P, Harlow SD, Marder W, et al. Dietary omega polyunsaturated fatty acid intake and patient-reported outcomes in systemic lupus erythematosus: The Michigan Lupus Epidemiology & Surveillance (MILES) Program. Arthr Care Res (Hoboken). 2020;72:874–881.
- Pestka JJ, Vines LL, Bates MA, et al. Comparative effects of n-3, n-6 and n-9 unsaturated fatty acid-rich diet consumption on lupus nephritis, autoantibody production and CD4+ T cell-related gene responses in the autoimmune NZBWF1 mouse. PLoS One. 2014;9:e100255.
- Bates MA, Brandenberger C, Langohr II, et al. Silica-triggered autoimmunity in lupus-prone mice blocked by docosahexaenoic acid consumption. PLoS One. 2016;11:e0160622.
- Bhattacharya A, Lawrence RA, Krishnan A, et al. Effect of dietary n-3 and n-6 oils with and without food restriction on activity of antioxidant enzymes and lipid peroxidation in livers of cyclophosphamide treated autoimmune-prone NZB/W female mice. J Am Coll Nutr. 2003;22:388–399.
- Chandrasekar B, Troyer DA, Venkatraman JT, et al. Dietary omega-3 lipids delay the onset and progression of autoimmune lupus nephritis by inhibiting transforming growth factor beta mRNA and protein expression. J Autoimmun. 1995;8:381–393.
- Halade GV, Williams PJ, Veigas JM, et al. Concentrated fish oil (Lovaza(R)) extends lifespan and attenuates kidney disease in lupus-prone short-lived (NZBxNZW)F1 mice. Exp Biol Med (Maywood). 2013;238:610–622.
- Halade GV, Rahman MM, Bhattacharya A, et al. Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs median and maximal life span of autoimmune lupus-prone mice. J Immunol. 2010;184:5280–5286.
- Bates M, Akbari P, Gilley K, et al. Dietary docosahexaenoic acid prevents silica-induced development of pulmonary ectopic germinal centers and glomerulonephritis in the lupus-prone NZBWF1 mouse. Front Immunol. 2018;9:2002.
- Joshi GN, Gilberti RM, Knecht DA. Single cell analysis of phagocytosis, phagosome maturation, phagolysosomal leakage, and cell death following exposure of macrophages to silica particles. Meth Mol Biol. 2017;1519:55–77.
- Desai J, Foresto-Neto O, Honarpisheh M, et al. Particles of different sizes and shapes induce neutrophil necroptosis followed by the release of neutrophil extracellular trap-like chromatin. Sci Rep. 2017;7:15003.
- Hamilton RF, Jr., Thakur SA, Mayfair JK, et al. MARCO mediates silica uptake and toxicity in alveolar macrophages from C57BL/6 mice. J Biol Chem. 2006;281:34218–34226.
- Wierenga KA, Wee J, Gilley KN, et al. Docosahexaenoic acid suppresses silica-induced inflammasome activation and IL-1 cytokine release by interfering with priming signal. Front Immunol. 2019;10:2130.
- Rajasinghe LD, Chauhan PS, Wierenga KA, et al. Omega-3 docosahexaenoic acid (DHA) impedes silica-induced macrophage corpse accumulation by attenuating cell death and potentiating efferocytosis. Front Immunol. 2020;11:2179.
- Lescoat A, Ballerie A, Lelong M, et al. Crystalline silica impairs efferocytosis abilities of human and mouse macrophages: implication for silica-associated systemic sclerosis. Front Immunol. 2020;11:219.
- Zhu H, Luo H, Yan M, et al. Autoantigen microarray for high-throughput autoantibody profiling in systemic lupus erythematosus. Genom Prot Bioinform. 2015;13:210–218.
- Chong BF, Tseng LC, Lee T, et al. IgG and IgM autoantibody differences in discoid and systemic lupus patients. J Invest Dermatol. 2012;132:2770–2779.
- van der Meulen PM, Barendregt AM, Cuadrado E, et al. Protein array autoantibody profiles to determine diagnostic markers for neuropsychiatric systemic lupus erythematosus. Rheumatol. 2017;56:1407–1416.
- Li QZ, Zhou J, Lian Y, et al. Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clin Exp Immunol. 2010;159:281–291.
- Li QZ, Xie C, Wu T, et al. Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J Clin Invest. 2005;115:3428–3439.
- Reeves WH, Lee PY, Weinstein JS, et al. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009;30:455–464.
- Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43:W566–W570.
- Quail MT. Overview of silica-related clusters in the United States: will fracking operations become the next cluster? J Environ Health. 2017;79:20–27.
- Bello A, Mugford C, Murray A, et al. Characterization of occupational exposures to respirable silica and dust in demolition, crushing, and chipping activities. Ann Work Expos Health. 2019;63:34–44.
- Miller FW, Alfredsson L, Costenbader KH, et al. Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J Autoimmun. 2012;39:259–271.
- Parks CG, de Souza Espindola Santos A, Barbhaiya M, et al. Understanding the role of environmental factors in the development of systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2017;31:306–320.
- Parks CG, Conrad K, Cooper GS. Occupational exposure to crystalline silica and autoimmune disease. Environ Health Perspect. 1999;107(Suppl 5):793–802.
- Vacek PM, Hemenway DR, Absher MP, et al. The translocation of inhaled silicon dioxide: an empirically derived compartmental model. Fund Appl Toxicol. 1991;17:614–626.
- Ceppellini R, Polli E, Celada F. A DNA-reacting factor in serum of a patient with lupus erythematosus diffusus. Proc Soc Exp Biol Med. 1957;96:572–574.
- Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthr Rheum. 2012;64:2677–2686.
- Reeves WH. Antibodies to the p70/p80 (Ku) antigens in systemic lupus erythematosus. Rheum Dis Clin North Am. 1992;18:391–414.
- Greidinger EL, Hoffman RW. The appearance of U1 RNP antibody specificities in sequential autoimmune human antisera follows a characteristic order that implicates the U1–70 kd and B′/B proteins as predominant U1 RNP immunogens. Arthr Rheum. 2001;44:368–375.
- Cozzani E, Drosera M, Gasparini G, et al. Serology of lupus erythematosus: correlation between immunopathological features and clinical aspects. Autoimmune Dis. 2014;2014:321359.
- Cutter AR, Hayes JJ. A brief review of nucleosome structure. FEBS Lett. 2015;589:2914–2922.
- Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541.
- McClain M, Scofield R, Kurien B, et al. Selective small antigenic structures are capable of inducing widespread autoimmunity which closely mimics the humoral fine specificity of human SLE. Scand J Immunol. 2002;56:399–407.
- Elkon KB, Parnassa AP, Foster CL. Lupus autoantibodies target ribosomal P proteins. J Exp Med. 1985;162:459–471.
- Harley JB, Alexander EL, Bias WB, et al. Anti-Ro (SS-A) and anti-La (SS-B) in patients with Sjögren’s syndrome. Arthritis Rheum. 1986;29:196–206.
- Alba P, Bento L, Cuadrado M, et al. Anti-dsDNA, anti-Sm antibodies, and the lupus anticoagulant: significant factors associated with lupus nephritis. Ann Rheum Dis. 2003;62:556–560.
- Sen D, Isenberg DA. Antineutrophil cytoplasmic autoantibodies in systemic lupus erythematosus. Lupus. 2003;12:651–658.
- Haddon DJ, Diep VK, Price JV, et al. Autoantigen microarrays reveal autoantibodies associated with proliferative nephritis and active disease in pediatric systemic lupus erythematosus. Arthr ResTher. 2015;17:162–162.
- Trendelenburg M, Lopez-Trascasa M, Potlukova E, et al. High prevalence of anti-C1q antibodies in biopsy-proven active lupus nephritis. Nephrol Dial Transplant. 2006;21:3115–3121.
- Dragon-Durey MA, Blanc C, Marinozzi MC, et al. Autoantibodies against complement components and functional consequences. Mol Immunol. 2013;56:213–221.
- Yahya A, Bengtsson C, Larsson P, et al. Silica exposure is associated with an increased risk of developing ACPA-positive rheumatoid arthritis in an Asian population: evidence from the Malaysian MyEIRA case-control study. Mod Rheumatol. 2014;24:271–274.
- Ilar A, Klareskog L, Saevarsdottir S, et al. Occupational exposure to asbestos and silica and risk of developing rheumatoid arthritis: findings from a Swedish population-based case-control study. RMD Open. 2019;5:e000978.
- Vihlborg P, Bryngelsson IL, Andersson L, et al. Risk of sarcoidosis and seropositive rheumatoid arthritis from occupational silica exposure in Swedish iron foundries: a retrospective cohort study. BMJ Open. 2017;7:e016839.
- De Decker E, Vanthuyne M, Blockmans D, et al. High prevalence of occupational exposure to solvents or silica in male systemic sclerosis patients: a Belgian cohort analysis. Clin Rheumatol. 2018;37:1977–1982.
- Englert H, Small-McMahon J, Davis K, et al. Male systemic sclerosis and occupational silica exposure-a population-based study. Aust N Z J Med. 2000;30:215–220.
- Rubio-Rivas M, Moreno R, Corbella X. Occupational and environmental scleroderma. Systematic review and meta-analysis. Clin Rheumatol. 2017;36:569–582.
- Gomez-Puerta JA, Gedmintas L, Costenbader KH. The association between silica exposure and development of ANCA-associated vasculitis: systematic review and meta-analysis. Autoimmun Rev. 2013;12:1129–1135.
- Makol A, Reilly MJ, Rosenman KD. Prevalence of connective tissue disease in silicosis (1985-2006)-a report from the state of Michigan surveillance system for silicosis. Am J Ind Med. 2011;54:255–262.
- Martucciello S, Paolella G, Esposito C, et al. Anti-type 2 transglutaminase antibodies as modulators of type 2 transglutaminase functions: a possible pathological role in celiac disease. Cell Mol Life Sci. 2018;75:4107–4124.
- Trouw LA, Mahler M. Closing the serological gap: promising novel biomarkers for the early diagnosis of rheumatoid arthritis. Autoimmun Rev. 2012;12:318–322.
- Murakami K, Mimori T. Recent advances in research regarding autoantibodies in connective tissue diseases and related disorders. Intern Med. 2019;58:5–14.
- Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Intern Med. 2016;280:8–23.
- Muratori L, Deleonardi G, Lalanne C, et al. Autoantibodies in autoimmune hepatitis. Dig Dis. 2015;33:65–69.
- Casali P. IgM. In: Delves PJ, editor. Encyclopedia of immunology. 2nd ed. Oxford: Elsevier; 1998. p. 1212–1217.
- Korganow AS, Ji H, Mangialaio S, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461.
- Vaughan JH. 1992 Joseph J. Bunim Lecture. Pathogenetic concepts and origins of rheumatoid factor in rheumatoid arthritis. Arthritis Rheum. 1993;36:1–6.
- Wu MY, Chen CS, Yiang GT, et al. The emerging role of pathogenesis of IgA nephropathy. J Clin Med 2018;7:225.
- Hongyan L, Yi Z, Bao D, et al. A study on clinical and pathologic features in lupus nephritis with mainly IgA deposits and a literature review. Clin Dev Immunol. 2013;2013:1–5.
- Jost SA, Tseng LC, Matthews LA, et al. IgG, IgM, and IgA antinuclear antibodies in discoid and systemic lupus erythematosus patients. ScientificWorldJournal. 2014;2014:171028.
- Villalta D, Bizzaro N, Bassi N, et al. Anti-dsDNA antibody isotypes in systemic lupus erythematosus: IgA in addition to IgG anti-dsDNA help to identify glomerulonephritis and active disease. PLoS One. 2013;8:e71458.
- Li QZ, Zhao HJ, Yan M, et al. Profiling autoreactive IgA antibodies against 124 autoantigens in Systemic lupus erythematosus, systemic scleroderma and idiopathic inflammatory myositis. J Immunol. 2016;196:209.9.
- Wong SW, Kwon MJ, Choi AM, et al. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–27392.
- Norris PC, Dennis EA. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proc Natl Acad Sci USA. 2012;109:8517–8522.
- Norris PC, Gosselin D, Reichart D, et al. Phospholipase A2 regulates eicosanoid class switching during inflammasome activation. Proc Natl Acad Sci USA. 2014;111:12746–12751.
- Li X, Yu Y, Funk CD. Cyclooxygenase-2 induction in macrophages is modulated by docosahexaenoic acid via interactions with free fatty acid receptor 4 (FFA4). FASEB J. 2013;27:4987–4997.
- Yan Y, Jiang W, Spinetti T, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38:1154–1163.
- Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771:926–935.
- Chang HY, Lee HN, Kim W, et al. Docosahexaenoic acid induces M2 macrophage polarization through peroxisome proliferator-activated receptor γ activation. Life Sci. 2015;120:39–47.
- Serhan CN. Systems approach to inflammation resolution: identification of novel anti-inflammatory and pro-resolving mediators. J Thromb Haemost. 2009;7(Suppl 1):44–48.
- Ostermann AI, Schebb NH. Effects of omega-3 fatty acid supplementation on the pattern of oxylipins: a short review about the modulation of hydroxy-, dihydroxy-, and epoxy-fatty acids. Food Funct. 2017;8:2355–2367.
- Chiang N, Fredman G, Backhed F, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528.
- Fredman G, Hellmann J, Proto JD, et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun. 2016;7:12859.
- Sham HP, Walker KH, Abdulnour RE, et al. 15-Epi-lipoxin a4, resolvin d2, and resolvin d3 induce NF-κB regulators in bacterial pneumonia. J Immunol. 2018;200:2757–2766.
- Titos E, Rius B, Lopez-Vicario C, et al. Signaling and immunoresolving actions of resolvin d1 in inflamed human visceral adipose tissue. J Immunol. 2016;197:3360–3370.
- Lands B. Highly unsaturated fatty acids (HUFA) mediate and monitor food’s impact on health. Prostaglandins Other Lipid Mediat. 2017;133:4–10.
- Adarme-Vega TC, Thomas-Hall SR, Schenk PM. Towards sustainable sources for omega-3 fatty acids production. Curr Opin Biotechnol. 2014;26:14–18.
- Lands B, Bibus D, Stark KD. Dynamic interactions of n-3 and n-6 fatty acid nutrients. Prostaglandins Leukot Essent Fatty Acids. 2018;136:15–21.
- Harris WS. The omega-6:omega-3 ratio: A critical appraisal and possible successor. Prostaglandins Leukot Essent Fatty Acids. 2018;132:34–40.
- Walker RE, Jackson KH, Tintle NL, et al. Predicting the effects of supplemental EPA and DHA on the omega-3 index. Am J Clin Nutr. 2019;110:1034–1040.
- EFSA Panel on Dietetic Products N, Allergies. Scientific Opinion on the extension of use for DHA and EPA-rich algal oil from Schizochytrium sp. as a Novel Food ingredient. EFSA J. 2014;12:3843.
- Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645–662.
- von Schacky C. Confusion about the effects of omega-3 fatty acids: contemplation of study data taking the omega-3 index into consideration. Internist (Berl). 2019;60:1319–1327.
- Wang T, Marken J, Chen J, et al. High TLR7 expression drives the expansion of CD19 + CD24hiCD38hi transitional B cells and autoantibody production in SLE patients. Front Immunol. 2019;10:1243.
- Henjes F, Lourido L, Ruiz-Romero C, et al. Analysis of autoantibody profiles in osteoarthritis using comprehensive protein array concepts. J Proteome Res. 2014;13:5218–5229.
- Arai S, Maehara N, Iwamura Y, et al. Obesity-associated autoantibody production requires AIM to retain the immunoglobulin M immune complex on follicular dendritic cells. Cell Rep. 2013;3:1187–1198.
- Li QZ, Karp DR, Quan J, et al. Risk factors for ANA positivity in healthy persons. Arthr Res Ther. 2011;13:R38.
- Li QZ, Zhou J, Wandstrat AE, et al. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol. 2007;147:60–70.
- Packard TA, Li QZ, Cosgrove GP, et al. COPD is associated with production of autoantibodies to a broad spectrum of self-antigens, correlative with disease phenotype. Immunol Res. 2013;55:48–57.
- Martinez J, Cunha LD, Park S, et al. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature. 2016;533:115–119.
- Cao Q, Zhao X, Bai J, et al. Circadian clock cryptochrome proteins regulate autoimmunity. Proc Natl Acad Sci USA. 2017;114:12548–12553.
- Chen Y, Yu M, Zheng Y, et al. CXCR5 + PD-1+ follicular helper CD8 T cells control B cell tolerance. Nat Commun. 2019;10:4415.
- Preite S, Cannons JL, Radtke AJ, et al. Hyperactivated PI3Kδ promotes self and commensal reactivity at the expense of optimal humoral immunity. Nat Immunol. 2018;19:986–1000.
