Abstract
Autoimmune hepatitis (AIH) is a chronic, inflammatory liver disease of unknown aetiology which requires lifelong immunosuppression. Most therapeutic and outcome studies of AIH have been conducted predominantly in Caucasian (European Ancestry, EA) cohorts, with the exclusion of African American (AA) patients due to inadequate sample size. It is known that AA patients have a severe phenotype of autoimmune diseases and demonstrate a poor response to conventional medical therapy. Understanding cellular and molecular pathways which determine AIH severity and progression in AA patients is likely to lead to the discovery of novel, personalised and better tolerated therapies. The aim of the study is to determine the distinct effector B cell phenotypes which contribute to disease severity and progression of AIH in AA children as compared to their EA cohorts. PBMCs were isolated from blood samples collected from patients visiting Children’s Healthcare of Atlanta (CHOA) and were grouped into AA, (n = 12), EA, (n = 11) and controls (n = 12) and were processed for flow cytometry. Markers of B cell development, maturation and activation were assessed namely CD19, CD21, IgD, CD27, CD38, CD11c, CD24, CD138. AA children with AIH demonstrated an expansion of CD19 + ve, Activated Naïve (aN), (CD19+ IgD-/CD27- Double Negative (DN2) ([CD19+/IgD-/CD27++CD38++) cells. Plasmablasts were significantly higher along with Signalling Lymphocytic activation molecule F7 (SLAMF7). Unswitched memory [CD19+] IgD+CD27+ (USM) B cells were significantly contracted in AA patients with AIH. B cell phenotyping reveals a distinct profile in AA AIH patients with a major skewing towards the expansion of effector pathways which have been previously characterised in severe SLE in AA patients. These results suggest that the quantification and therapeutic target of B cell pathway could contribute substantially to the clinical approach to AIH especially in the AA population.
Introduction
Autoimmune hepatitis is a chronic inflammatory liver disease of unknown aetiology which is believed to be due to a breakdown of immune tolerance against liver autoantigens. Although AIH presents both in adults and children, several studies have shown that the disease process may actually start early in life [Citation1–4]. Most therapeutic and outcome studies of AIH have been conducted predominantly in Caucasian cohorts; and though there are some adult studies reporting that clinical phenotype and outcome may vary by race, the focus of those studies have been Hispanic and Asians cohorts, with the exclusion of African American patients due to inadequate sample size [Citation5–7]. Historically, AIH has been thought to be a T-cell-mediated disease with disease onset driven by T helper cells directing attack against autoantigens and chronic disease mediated by impaired regulatory T cells [Citation8]. However, few anecdotal clinical reports have shown that anti-CD20 (B cell depleting antibody) may be an effective treatment for AIH patients who are refractory to conventional therapy, supporting a key role for B cells in pathogenesis of AIH [Citation9]. Plasma cells are terminally differentiated B lymphocytes that are primarily responsible for the production and secretion of antibodies. They contribute significantly to immune responses in various tissues including the liver [Citation10]. The liver represents a unique immunologic environment due to its continuous exposure to antigens derived from the gastrointestinal tract, circulation and resident immune cells. Several studies have highlighted the crucial role of plasma cells in hepatic immune surveillance, maintaining tissue integrity and combating pathogens [Citation11,Citation12]. Additionally, dysregulation of plasma cell function in the liver has been implicated in the pathogenesis of autoimmune liver disease [Citation9]. In this manuscript, we aim to review the intricate relationship between plasma cells and liver immunology, focusing on their roles in maintaining immune homeostasis and autoimmunity within the liver microenvironment. We will explore effector B cell phenotypes and plasma cells, highlighting their contribution to severity of AIH and disease progression in children of AA and EA ancestry. According to the 2019 US census bureau report, 44 million people in the United States identify themselves as black. Our recently published data has shown that there are significant racial disparities in outcomes of AIH in AA children as compared to their non-AA cohorts which were previously unreported [Citation13]. AA patients demonstrated a poor response to conventional medical therapy and were 4.5 times more likely to require a liver transplant or die within the first 12 months of diagnosis as compared to their non-AA cohorts. Liver transplantation was not a life-saving therapy either since AA patients had increased disease recurrence in the graft, leading to re-transplantation or death. Socioeconomic factors and access to healthcare have been extensively researched but it is abundantly clear, that after adjusting for these variables, AAs have severe autoimmune diseases, alluding to underlying biology and immune response as incriminating factors. In this research endeavour, we endeavour to ascertain whether individuals afflicted with autoimmune hepatitis (AIH) of African American descent exhibit a distinctive B cell profile characterised by varying proportions of markers indicative of B cell development, activation, maturation, and memory. This investigation aims to shed light on the potential correlation between alterations in these specific B cell subsets—namely, reduced levels of unswitched memory (USM), heightened presence of activated naïve B cells, and an increased prevalence of double negative 2 (DN2) cells—with the manifestation of a severe disease phenotype. This distinct immunological milieu may, in turn, elucidate the underlying factors contributing to a suboptimal response to contemporary therapeutic approaches, primarily designed to target T cells.
Methods
Patients
Whole blood samples were collected from paediatric patients diagnosed with Autoimmune hepatitis at Children’s Healthcare of Atlanta (CHOA). These patients were grouped into African American (AA, n = 12, aged 4-18 years old with 9 females and 3 males), and European Ancestry (EA, n = 7, aged 12–17-year-old, 4 females and 3 males) and healthy controls (n = 12) and their PBMCs were processed for flowcytometry as described below. All AA patients had severe disease and required liver transplantation in contrast to EA patients, none of whom required transplantation. Median follow-up was similar in both groups.
Isolation of peripheral blood mononuclear cells (PBMCs)
PBMC were isolated from whole blood samples collected in a CPT tube containing anticoagulants. Ficoll density gradient separation and Cell Preparation Tubes, The BD Vacutainer® CPT™ Cell Preparation Tube with Sodium Heparin were used for whole blood separation of mononuclear cells. Whole blood samples were centrifuged at room temperature (18-250C) for 25 min at 1500 RCF. After centrifugation, mononuclear cell layer was collected washed and resuspended, counted and processed for flowcytometry.
Staining samples with B cell antibody panel
One million cells were stained with antibody cocktail for several B cell markers including markers of B cell development (D19, CD21, CD24 (Naïve) markers of activation (CD38, CD11c, CD138) and markers of maturation and Memory (IgD-, CD27).
Antibody cocktail was added, CD19_ APC Cy7, CD3_ PercP Cy5.5, CD27_ BV605, CD38_ PECy7, CD24_PE, IgD_ FITC, CD138_ APC, CD21_ PECy5, CD11c_ PE and cells were incubated for 30 min at 4 °C in the dark. Then cells were washed in PBS and resuspended in 100 µL live/dead stain, incubated for 15 min at 4 °C in the dark. Then, 3 ml FACS buffer was added to quench the live/dead staining reaction and 200 µL of 0.8% Paraformaldehyde (PFA) was added to the cells and incubated for 15 min at room temperature in the dark. After 15 min 3 ml FACS buffer was added to wash the cells and then they were centrifuged. Cells were then resuspended in 250 µL FACS buffer and analysed on a Becton-Dickenson flow cytometer (LSR II) at the Emory Children’s Paediatric Flow Cytometry Core.
Immunofluorescence
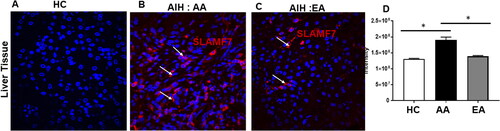
Paraffin sections of Liver tissues obtained from Autoimmune hepatitis patients were belonging to AA and EA race were subjected to immunofluorescence using antibody against Signalling Lymphocyte Activation Molecule Family (SLAMF7) and then Alexa 647 conjugated secondary antibody as shown by red positive stain, nuclear stain DAPI is in blue. Images were taken in fluorescent microscope Deltavision OMX Super resolution microscope.
Statistics
The abundance of different B cell populations between the groups was identified using t test for two groups, one-way ANOVA for three or more groups and Two-way Anova for four or more groups (GraphPad Prism 6). Wilcoxon tests and the fold-change (FC) calculated using mean differences among the groups. A probability value of p < 0.05 was considered significant.
Results
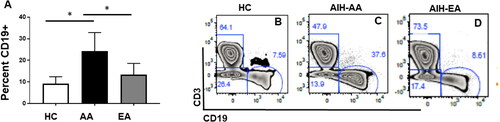
CD19+ B cell subpopulations were significantly increased in African American (AA) patients diagnosed with AIH compared to European ancestry (EA)
To determine markers of B cell development, maturation, and activation in paediatric Autoimmune Hepatitis we assessed the CD19+ cells in the PBMCs isolated from AA and EA patients along with controls. As shown in , AA patients with AIH had a significantly higher percentage of CD19 + ve cells compared to EA (p < 0.001) and healthy controls (p < 0.001). Figure B, C and D are representative Flowjo images of control and AIH patients respectively.
Figure 1. CD19+ B cell subpopulations were significantly increased in African American (AA) patients diagnosed with AIH compared to European ancestry (EA): whole blood was collected from Healthy controls (HC), African Americans (AA) European ancestry (EA) patients diagnosed with autoimmune hepatitis (AIH). PBMC were isolated and stained with live dead cells and stained with antibodies to CD3, CD19 and subjected to Flowcytometry. Bar graphs represent CD19 positive cells in total lymphocytes, B, C and D are representative flowjo charts from HC, AA, EA respectively. Bar plots represent mean and ± SD.
Unswitched memory [CD19+] IgD + CD27+ (USM) B cells are significantly reduced in AA patients with AIH
Based on CD27 and IgD expression, there are four types of B cells subsets which are detected in peripheral blood. Unswitched memory cells are antigen experienced B cells which express IgD + cells. Their role in paediatric onset autoimmune hepatitis is understudied. In this study, we demonstrate that patients with AIH displayed a significant contraction of unswitched memory (USM) (marginal zone) in the peripheral blood as compared to healthy controls. () AA-AIH patients compared to healthy controls (p < 0.0003) () and EA-AIH patients compared to Control (p < 0.02, ). Whereas AA-AIH patients are significantly different than EA-AIH (p < 0.007). This indicates that early depletion of CD19+ IgD + CD27+ (USM) B cells population may result in increased disease severity as shown in the AA-AIH patients. Importantly we demonstrated that USM cells were strikingly more contracted in AA patients.
Figure 2. DN2 cells [CD19+] IgD-CD27-CD11c+CD21- which are precursors of plasmablasts are increased in patients with AIH: whole blood was collected from Healthy controls (HC), African Americans (AA) European ancestry (EA) patients diagnosed with autoimmune hepatitis (AIH). PBMC were isolated and stained antibodies to CD3, CD19, IgD, CD27 and subjected to Flowcytometry. A, B, and C are representative flow charts and D, E, F are representative bar graphs of Unswitched memory [CD19+] IgD+CD27+) cells from HC, AA, EA respectively. Bar plots represent mean and ± SD.
![Figure 2. DN2 cells [CD19+] IgD-CD27-CD11c+CD21- which are precursors of plasmablasts are increased in patients with AIH: whole blood was collected from Healthy controls (HC), African Americans (AA) European ancestry (EA) patients diagnosed with autoimmune hepatitis (AIH). PBMC were isolated and stained antibodies to CD3, CD19, IgD, CD27 and subjected to Flowcytometry. A, B, and C are representative flow charts and D, E, F are representative bar graphs of Unswitched memory [CD19+] IgD+CD27+) cells from HC, AA, EA respectively. Bar plots represent mean and ± SD.](/cms/asset/5f1ed00c-cc4f-466a-82a8-266cb7c0b235/iaut_a_2356089_f0002_c.jpg)
DN2 cells [CD19+] IgD-CD27-CD11c+CD21- which are precursors of plasmablasts are increased in patients with AIH
DN2 cells are double negative population which are characterised by CD27- IgD- CXCR5- CD11c+ (DN2) B cells. DN2 cells do not express CXCR5 and CD21 but in turn have a high expression of CD19 and a high surface expression of the alpha integrin CD11c. We found that DN2 cells were more numerous in AA patients as compared to HC (p < 0.001) and EA patients with AIH (p < 0.04) (). This population is markedly dysregulated and has been shown before to become the predominant B cell population in active SLE [Citation14], produces autoantibodies, now alluding to a similar role in severe AIH.
Figure 3. DN2 cells [CD19+] IgD-CD27-CD11c+CD21- which are precursors of plasmablasts are increased in patients with AIH: whole blood was collected from Healthy controls (HC), African Americans (AA) European ancestry (EA) patients diagnosed with autoimmune hepatitis (AIH). PBMC were isolated and stained with live dead cells and stained with antibodies to CD3, CD19, IgD, CXCR5, CD21, CD11c and subjected to Flowcytometry. Bar graphs represent [CD19+] IgD+CXCR5-CD11c + DN2 cells, B, C and D are representative flowjo charts from HC, AA, EA respectively. Bar plots represent mean and ± SD.
![Figure 3. DN2 cells [CD19+] IgD-CD27-CD11c+CD21- which are precursors of plasmablasts are increased in patients with AIH: whole blood was collected from Healthy controls (HC), African Americans (AA) European ancestry (EA) patients diagnosed with autoimmune hepatitis (AIH). PBMC were isolated and stained with live dead cells and stained with antibodies to CD3, CD19, IgD, CXCR5, CD21, CD11c and subjected to Flowcytometry. Bar graphs represent [CD19+] IgD+CXCR5-CD11c + DN2 cells, B, C and D are representative flowjo charts from HC, AA, EA respectively. Bar plots represent mean and ± SD.](/cms/asset/ccf4868a-2ed0-43ce-bb22-d53d8a58af21/iaut_a_2356089_f0003_c.jpg)
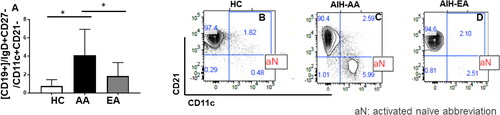
Activated naïve (an, CD19+/IgD+CD27-/CD11c+CD21-) cells are significantly higher in AA patients with AIH compared to EA
AIH is associated with circulating antibodies, and it has been shown in SLE that these antibodies arise from Antibody Secreting Cells (ASC) which increase during a flare. Although AIH is also associated with flares, there is complete lack of data delineating B cell responses and relationship to ASCs. At least in SLE, a large proportion of these ASCs originate from newly formed, circulating IgD+, CD27- naïve B cells with a distinct phenotype including high CD19 levels and loss of CD21. These cells are expanded in AIH. Since AIH is associated with circulating antibodies and clinical flares, we wanted to see if aN cells also had a role in AIH. As shown in , peripheral blood from patients with AIH showed that B cells were significantly increased in patients with AIH, as compared to healthy controls (p < 0.0001, ). There was a strikingly significant difference in the expansion of aN cells in patients of AA origin as compared to EA origin (p < 0.001, ).
Figure 4. Activated Naïve (aN, CD19+/IgD+CD27-/CD11c+CD21-) cells are significantly higher in AA patients with AIH compared to EA: whole blood was collected from Healthy controls (HC), African Americans (AA) European ancestry (EA) patients diagnosed with autoimmune hepatitis (AIH). PBMC were isolated and stained with live dead cells and stained with antibodies to CD3, CD19, IgD, CD11c, CD21 and subjected to Flowcytometry. Bar graphs represent activated naïve (CD19+/IgD+CD27-/CD11c+CD21-) cells, B, C and D are representative flowjo charts from HC, AA, EA respectively. Bar plots represent mean and ± SD.
Signalling lymphocytic activation molecule F7 (SLAMF7), a plasma cell marker is increased in AA patients with AIH
Since SLAMF7 is highly expressed by plasma cells and preponderance of plasma cells is a prominent phenotype on liver histology in patients with AIH, we wanted to see if SLAMF7 had a role in AIH and if it could be used as a biomarker for disease severity. On Immunofluorescent staining of liver biopsies, we demonstrated an increased presence of SLAMF7 in patients with AIH, particularly in patients of AA ancestry ().
Discussion
B cells are an integral part of the adaptive immune response, and they lead to the production of antibodies which are routinely used as diagnostic and prognostic biomarkers in several autoimmune diseases such as AIH, RA, and SLE. B cells are greatly understudied in AIH, which has traditionally been treated with immunosuppressants such as glucocorticoid derivatives, azathioprine, tacrolimus and mycophenolate mofetil. However recent literature has begun to shed light on the role of B cells in AIH, with the success of Rituximab (anti B cell therapy) in treating refractory cases of AIH [Citation15]. In this study we investigate the role of B cells in paediatric onset AIH and demonstrate that Activated Naïve and DN2 B cells are significantly expanded in AA patients with AIH as compared to their EA cohorts with contraction of the unswitched memory compartment alluding to a possible mechanism for severe disease presentation and progression in African American patients.
B cell memory is complex and consists of many different subsets. It is critical to elucidate human memory B cell heterogeneity, as certain subsets are preferentially expanded in various clinical conditions. These variations create unique signatures and may have a prognostic significance. Although AIH is also associated with flares, there is complete lack of data delineating B cell responses and relationship to ASCs. Large proportion of these antibody circulating cells originate from activated naïve B cells, which have a distinct phenotype, characterised by high CD19 and loss of CD21 [Citation16]. These cells are expanded and are precursors to the development of plasma cells which secrete antibodies. Our study is the first report to provide evidence for involvement of the extrafollicular B cell pathway in AIH. SLE has been extensively studied in the African American population and studies of SLE showed a distinct differentiation fate of autoreactive naive B cells into PC precursors and though germinal centre derived memory cells have an important role, the extrafollicular B cells could possibly identify patients with an extra follicular B cell endotype, hence directing therapy [Citation14]. Expansion of Double Negative (DN) cells, especially DN2 were recently reported by [Citation14] Sanz et al. in patients with SLE. These cells were autoreactive, derived from naïve B cells and poised to generate plasmablasts [Citation14]. Our data demonstrates that patients with AIH exhibited an expansion of B cell and plasma cell subtypes along with DN2 expansion alluding to the fact that a distinct extrafollicular B cell pathway may be operational in AIH especially in AA. Though preliminary and additional studies are needed, we postulate that this may be one of the underlying mechanisms leading to worse outcomes in AA with AIH as compared to their EA cohorts.
Though diagnostic hallmarks of AIH, plasma cell differentiation, cell fate, and immunoglobulin secretion are greatly understudied. Plasma cells are non-dividing sessile cells which secrete large amounts of antibodies which are seen in the terminal stage of B cell differentiation [Citation17]. There is agreement that even after CD20 depletion and low expression of CD20 [Citation18] long lived PC survive, which could serve as individual, and possibly additional targets for therapy. Our data has shown that AA patients with AIH have resident plasma cells in their liver tissue and expanded plasma cell precursor population in their peripheral blood. Plasma cells have been most extensively studied in patients with Multiple myeloma, a malignant disease of monoclonal plasma cells and in a recent phase 3 study, it was shown that Elotuzumab, a monoclonal antibody targeting SLAMF7 [Citation19] when used in combination in refractory multiple myeloma showed reduction of disease progression and death. Despite liver tissue invasion of plasma cells in patients with AIH and a prominent phenotype on routine liver histology, there is negligible data on the clinical utility of PCs in AIH in terms of disease progression, severity and outcomes. Medical literature remains marked by ongoing controversy surrounding the involvement of autoantibodies in the pathogenesis of AIH [Citation20–23]. Furthermore, divergent research findings persist regarding the impact of B cell depletion within AIH models, with some studies underscoring its significance [Citation24] while others assert no discernible distinctions [Citation25,Citation26]. Additionally, the ongoing discussion within the field acknowledges the potential influence of non-antibody-related functions, which could potentially underlie the severity of autoimmune hepatitis. We wanted to see if SLAMF7 had a role in AIH, providing a biomarker for disease outcome. Utilising immunofluorescence staining of liver biopsies, we demonstrated increased presence of SLAMF7 in patients with AIH who were of AA origin. Thus, B cells play an important role in AIH with a higher DN2 and PB population in AA patients who exhibited a more severe phenotype compared to EA patients with AIH.
In conclusion, our study provides compelling evidence of distinct differences in B cell populations between AA and EA children with autoimmune hepatitis. These findings emphasise the importance of understanding the unique immunological profiles of different racial and ethnic groups, particularly in the context of autoimmune liver diseases. Although asymptomatic early disease cannot be fully excluded in healthy controls, a clear difference is evident. Additionally, causation for poor outcomes is likely to be multifactorial, but delineation of biological targets remains important. The identification of these differences underscores the need for personalised therapeutic approaches that specifically target the B cell subsets implicated in AIH in AA children. In light of these considerations, we propose that the observations made in this study warrant further in-depth investigation, to better comprehend the underlying mechanisms at play in AIH and to ensure equitable access to effective treatments for all patient groups.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data will be available upon request.
Additional information
Funding
References
- Mack CL, Adams D, Assis DN, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American association for the study of liver diseases. Hepatology. 2020;72(2):671–722.
- Gregorio GV, Portmann B, Reid F, et al. Autoimmune hepatitis in childhood: a 20-year experience. Hepatology. 1997;25(3):541–547.
- Gregorio GV, Portmann B, Karani J, et al. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology. 2001;33(3):544–553.
- Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6:635–647.
- Wong RJ, Gish R, Frederick T, et al. The impact of race/ethnicity on the clinical epidemiology of autoimmune hepatitis. J Clin Gastroenterol. 2012;46(2):155–161.
- Zolfino T, Heneghan MA, Norris S, et al. Characteristics of autoimmune hepatitis in patients who are not of european caucasoid ethnic origin. Gut. 2002;50(5):713–717.
- Lee WS, Lum SH, Lim CB, et al. Characteristics and outcome of autoimmune liver disease in Asian children. Hepatol Int. 2015;9(2):292–302.
- Than NN, Jeffery HC, Oo YH. Autoimmune hepatitis: progress from global immunosuppression to personalised regulatory T cell therapy. Can J Gastroenterol Hepatol. 2016;2016:7181685.
- Taylor SA, Assis DN, Mack CL. The contribution of B cells in autoimmune liver diseases. Semin Liver Dis. 2019;39(4):422–431.
- Patel AM, Liu YS, Davies SP, et al. The role of B cells in adult and paediatric liver injury. Front Immunol. 2021;12:729143.
- Chen K, Xu W, Wilson M, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10(8):889–898.
- Liu C, Zhang P, Zhang W. Immunological mechanism of IgG4-related disease. J Transl Autoimmun. 2020;3:100047.
- Palle SK, Naik KB, McCracken CE, et al. Racial disparities in presentation and outcomes of paediatric autoimmune hepatitis. Liver Int. 2019;39:976–984.
- Jenks SA, Cashman KS, Zumaquero E, et al. Distinct effector B cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity. 2018;49(4):725–739 e6.
- Burak KW, Swain MG, Santodomingo-Garzon T, et al. Rituximab for the treatment of patients with autoimmune hepatitis who are refractory or intolerant to standard therapy. Can J Gastroenterol. 2013;27(5):273–280.
- Tipton CM, Fucile CF, Darce J, et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol. 2015;16(7):755–765.
- Oracki SA, Walker JA, Hibbs ML, et al. Plasma cell development and survival. Immunol Rev. 2010;237(1):140–159.
- Hammarlund E, Thomas A, Amanna IJ, et al. Plasma cell survival in the absence of B cell memory. Nat Commun. 2017;8(1):1781.
- Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373(7):621–631.
- Vergani D, Mieli-Vergani G, Mondelli M, et al. Immunoglobulin on the surface of isolated hepatocytes is associated with antibody-dependent cell-mediated cytotoxicity and liver damage. Liver. 1987;7(6):307–315.
- Manns M, Zanger U, Gerken G, et al. Patients with type II autoimmune hepatitis express functionally intact cytochrome P-450 db1 that is inhibited by LKM-1 autoantibodies in vitro but not in vivo. Hepatology. 1990;12(1):127–132.
- Muratori L, Parola M, Ripalti A, et al. Liver/kidney microsomal antibody type 1 targets CYP2D6 on hepatocyte plasma membrane. Gut. 2000;46(4):553–561.
- Schramm C, Herkel J, Beuers U, et al. Pregnancy in autoimmune hepatitis: outcome and risk factors. Am J Gastroenterol. 2006;101:556–560.
- Liu X, Jiang X, Liu R, et al. B cells expressing CD11b effectively inhibit CD4+ T-cell responses and ameliorate experimental autoimmune hepatitis in mice. Hepatology. 2015;62(5):1563–1575.
- Lübbering D, Preti M, Schlott L, et al. Autoantigen-selected B cells are bystanders in spontaneous T cell-driven experimental autoimmune hepatitis. Immunology. 2023;170(2):214–229.
- Buitrago-Molina LE, Dywicki J, Noyan F, et al. Anti-CD20 therapy alters the protein signature in experimental murine AIH, but not exclusively towards regeneration. Cells. 2021;10(6):1471.