Abstract
Sugarcane is the most widely cultivated crop in the world, with equatorial developing nations performing most of this agriculture. Burning sugarcane is a common practice to facilitate harvest, producing extremely high volumes of respirable particulate matter in the process. These emissions are known to have deleterious effects on agricultural workers and nearby communities, but the extent of this exposure and potential toxicity remain poorly characterized. As the epidemicof chronic kidney disease of an unknown etiology (CKDu) and its associated mortality continue to increase along with respiratory distress, there is an urgent need to investigate the causes, determine viable interventions to mitigate disease andimprove outcomes for groups experiencing disproportionate impact. The goal of this review is to establish the state of available literature, summarize what is known in terms of human health risk, and provide recommendations for what areas should be prioritized in research.
Introduction
Sugarcane is a majorcrop in the global agricultural landscape, essential for meeting the world’s growing demand for sugar and bioenergy. Most of this agriculture is performed in equatorial developing nations with workers that have been economically marginalized performing the most difficult and hazardous jobs. Sugarcane agriculture workers often face extreme labor conditions including excessive physical exertion in extreme heat that results in dehydration and kidney injury (García-Trabanino et al. Citation2015; Leite, Zanetta, Trevisan, et al. Citation2018; Glaser et al. Citation2020). Furthermore, these workers are exposed to a variety of potential toxicants (i.e. pesticides, heavy metals, and ash) with minimal personal protective equipment (PPE)(Schaeffer et al. Citation2020; Imbulana and Oguma Citation2021). These occupational exposures have gained recent attention as rates of injury and disease among this population increase (Leite, Zanetta, Trevisan, et al. Citation2018). Concerns regarding worker conditions have grown as climate change and demand for more efficient harvests put added pressure on agricultural communities.
One such concern is exposure to sugarcane ash generated during the conventional practice of pre-harvest burns. Sugarcane fields are routinely burned to facilitate manual cropharvesting. Most of the ash from burning is respirable and represents a major respiratory exposure for both field workers and nearby communities. As such, it is no surprise that sugarcane ash has been suspected of being a contributor to poor health outcomes among nearbyagricultural communities (Le Blond et al. Citation2017). Determining this has proven difficult however, due to minimal scientific literature, variable sugarcane ash composition, limited understanding of physicochemical properties, unknown extent of human exposure, and a lack of medical resources in the areas where exposure is most common. Filling these gaps in knowledge is becoming increasingly urgent, as risks of exposure are likely to grow alongside the demand for sugar and interest in sugarcane biomass for green chemistry applications. To support public health and protect at-risk populations, it is crucial to understand the potential consequences of sugarcane ash exposure.
The goal of this review is to consolidate and summarize current literature on the properties of sugarcane ash, scope of human exposure and health outcomes, and potential mechanisms of toxicity. In doing so, the most pressing gaps in literature can be identified and recommendations can be made for how the scientific community can best proceed.
Sugarcane burning
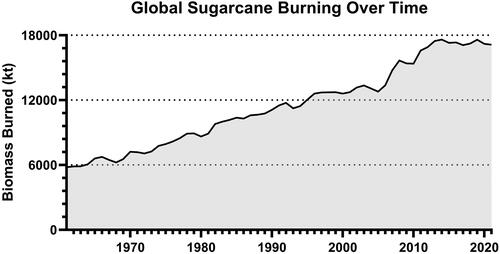
Sugarcane production has increased over the past several decades from roughly 0.6 million tons in the early 1970s to approximately 1,800 million tonsin 2017 and generating approximately 279 million metric tons of biomass () (Chandel et al. Citation2012; de Matos et al. Citation2020). Crops are primarily grown in tropical and subtropical regions between 22° N and S of the equator: with most growth in Brazil (40%), India (20%), and China (6%) but also many other countries in Central America, the United States and other parts of Asia (Souza et al. Citation2012; FAO FAOSTAT). To generate end-stage products (biomass and subsequent industrial/commercial materials), most sugarcane fields undergo preharvest burning, though shifts toward mechanical harvesting where burn is not required are beginning and utilized in more developed countries (Souza et al. Citation2012).
Figure 1. FAOSTAT estimation of global sugarcane crop biomass b-urned each year measuredaskilo tons of dry matter.
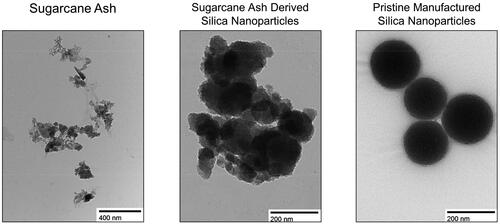
The burning process removes leaf surplusfrom the stalk and slows the degradation of sucrose allowing more time for harvest. This burning generates sugarcane straw ash (SCSA) and is responsible for notable increases in particulate matter (PM) into the atmosphere. Leaves, prior to burning, contain between 0.45 and 1.8% wt of silica (SiO2) depending on soil quality, concentrations of soil silicon, sugarcane cultivar, fertilizer, and climate (Le Blond et al. Citation2010; Bortolotto Teixeira et al. Citation2021). After burning, total silica content of SCSA by % weight is approximately 70% (Villar-Cociña et al. Citation2008). While most of the silica found in sugarcane crops is of the amorphous form, crystalline silica can be found up to 3.5% of total silica content with increases possible if burning reaches 1000°+C when amorphous silica can transition to crystalline forms (Le Blond et al. Citation2010).
Physicochemical properties
Of the major contributing components from the burning process, PM is critically important. Particulate matter can be roughly divided into three categories: (i) particulates capable of getting into the thoracic regions (nose and mouth), (ii) those inhalable and able to reach to the bronchi, (iii) and those respirable and able to reach the deepest parts of the lung where gas exchange occurs. Crop burning increases ultrafine PM (PM2.5)from approximately 8 µg/m3 prior to harvesting to roughly 23.58 µg/m3 in nearby towns and greater than 60 µg/m3 in the fields where most workers remain during the harvest process (Prado et al. Citation2012). Burning occurs in two combustion phases: flaming, where temperatures are higher and a reaction between a homogenous gas and an oxidizer occurs, releasing additional heat; and smoldering, where flameless, lower heat leads to a heterogenous reaction between a solid fuel and an oxidizer, usually leading to more toxic compounds getting released (Santoso et al. Citation2019). Particulate matter from preharvest burning contains silica, carbonaceous material, KCl, NaCl, oxides such as Mg and Fe, unburned hydrocarbons (UHC), and some fibrous materials, each emitted during both flaming and smoldering phases (Le Blond et al. Citation2017). A recent study, matching closely with other similarly performed analyses, showed that average values of emission factors (g/kg of burned dry biomass) of CO2, CO, NOx, UHC, and PM2.5 were 1303, 65, 1.5, 16, and 2.5, respectively (França et al. Citation2012). Of the total mass of PM, roughly 16% of SCSA is considered respirable while 2% of PM from bagasse ash is respirable (Le Blond et al. Citation2017).
The high concentration of silica in sugarcane ash is a primary concern as inhalation of silica is known to lead to adverse respiratory health, perturbations in kidney function, and potential cancer risk (Leung et al. Citation2012). Crystalline silica maintains long-range order throughout its atomic structure and therefore has a predictable density allowing for deformations to be precisely located and biochemical interactions to be better predicted (). Contrary to the crystalline form, amorphous silica contains short-range order where predictability of atomic structure is lost; deformations appear random; and reactivity, specifically at the surface, may increase ()(Lunt et al. Citation2018). Both crystalline and amorphous silica exist at nano and micron scales, with increased surface to volume ratios at the nano scale leading to increased reactivity in biological conditions.
Crystalline silica has been well-characterized and studied with a vast array of knowledge of physicochemical properties as well adverse health outcomes. Unlike crystalline forms, however, there is still comparatively little known of the potential adverse outcomes of amorphous silica (Dong et al. Citation2020). Recent studies have indicated a mostly size and dose-dependent cytotoxic response to amorphous silica, most likely related to the increased propensity for chemically reactive surface groups (Waters et al. Citation2009; Li et al. Citation2011; Li et al. Citation2016; Li et al. Citation2019).
Of the surface moieties onsilica, hydroxyl groups, termed silanols, are of increased interest in recent decades for their high chemical reactivity. Silanols are formed either through condensation polymerization or rehydroxylation of dehydroxylated silica (Zhuravlev Citation2000). New research has shown that disorganization of the long-range spatial arrangement of silanols on the silica surface could induce toxicity leading to membrane lysis, inflammatory cytokine release from macrophages, reactive oxygen species (ROS) generation, and lung damage in rats (Pavan et al. Citation2020).
Bioenergetic potential
The remaining biomass after burning can be further refined to produce liquid fuels, chemicals, and synthetic materials (e.g. ethanol, xylitol, acids, and enzymes) (Chandel et al. Citation2012; Zhu and Pan Citation2022). Because of the renewable and carbon neutral aspects of biomass, sugarcane production is expected to increase by 21.3% over the next decade (de Matos et al. Citation2020; Favero et al. Citation2020).
While sugar and ethanol are the primary products of sugarcane processing, the potential for additional streams of energy exist in residual biomass fractions like (i) straw ash, (ii) bagasse ash, (iii) vinasse, and (iv) filter cake from sugarcane juice clarification (Formann et al. Citation2020). The products of these biomasses have been studied and have diverse applications ranging from adsorbents, resins, cements, and polymers and they have the potential to drastically decrease global energy demand while improving sustainability and increasing environmental conservation (Ajala et al. Citation2021).
Occupational and community exposure
The harvest of sugarcane requires several distinct jobs with different degrees of sugarcane ash exposure risk. Field workers are generally considered to be at much higher risk of this occupational ash exposure than support staff or positions in subsequent processing and refinement, thus most public health studies have focused on this group. Among field workers, those performing the job of burned cane cutter have been found to experience the greatest inhalation burden due to initiating harvest burns, cutting/stacking burnt sugarcane stalks, and spreading the ash back over the field (). The height of PM exposure occured during burning, with a 1-h time weighted average (TWA) of 1807 µg/m3 for fine PM (PM10)(Le Blond et al. Citation2017). While workers do not exclusively labor during active burn times, PM10 concentrations remain relatively high during routine cutting with 1-h TWAs of 123 µg/m3(Le Blond et al. Citation2017). The majority of these particles seem to be respirable, with PM2.5 values found to be consistently in the range of 100 µg/m3 during harvest (Leite et al. Citation2015; Leite, Zanetta, Antonangelo, et al. Citation2018). It is important to note these exposures are chronic (recurring each harvest season) and further exacerbated by non-mechanized harvest methods, requiring heavy manual labor and elevated breathing rates in close proximity to ash sources.
Figure 2. Photograph of burnt cane harvesters in Nicaragua demonstrating the high potential for ash inhalation.

Figure 3. TEM Images of particles demonstrate the highly uniform nature of pristine manufactured SiNPs compared to the much more heterogeneous structure of environmentally relevant SiNPs derived from sugarcane ash.
Sugarcane fields are rarely far from agricultural communities, while this is beneficial for harvest efficiency and worker convenience, it also exposes the local population to emissions from sugarcane burning. Sugarcane burning has been found to negatively impact air quality in cities across Central America, South America, South Africa, and Asia (Flores-Jiménez et al. Citation2019; Shikwambana et al. Citation2021; Sahu et al. Citation2021; Souto-Oliveira et al. Citation2023). Ambient levels of PM10 in nearby cities during the harvest season have been found to be around ∼60µg/m3 and PM2.5 exceeding 45 µg/m3, with the majority of particles determined to be of sugarcane origin (Lara et al. Citation2005; Vasconcellos et al. Citation2010; Souza et al. Citation2014; Le Blond et al. Citation2017; Adegboye Citation2022). In more rural communities, PM10 averaged ∼45 µg/m3 with PM2.5 measuring ∼25µg/m3 in local towns and ∼60µg/m3 on the plantations themselves (Prado et al. Citation2012; Leite, Zanetta, Antonangelo, et al. Citation2018). Even in the southern United States, where more stringent regulations may mitigate effects, PM10increased ∼50% during the harvest season and sugarcane fires contributed an amount of PM2.5 comparable to motor vehicles (Gullett et al. Citation2006; Sevimoglu and Rogge Citation2015; Hiscox et al. Citation2015; Sevimoglu and Rogge Citation2016; Nowell et al. Citation2022; Adegboye Citation2022; Pinakana et al. Citation2023).
In addition to particulate matter, sugarcane fires are known to produce a vast array of harmful emissions including volatile organic compounds (VOCs), polycyclic aromatic hydrocarbons (PAHs), carbon and nitrogen oxides, and various other compounds (de Andrade et al. Citation2010; Silva et al. Citation2010; Cristale et al. Citation2012; Pestana et al. Citation2017; Sahu et al. Citation2021; Nowell et al. Citation2022; Geldenhuys et al. Citation2023). Sugarcane fires have also been associated with elevated silica in nearby groundwater and silicon in the urine of local population (Smpokou et al. Citation2019; Nikagolla et al. Citation2020). This is unsurprising as amorphous silica is the primary component of sugarcane ash, but is concerning given silica’s posited role in anepidemic of kidney diseaseof unknown etiology (CKDu) and its impact on increased mortalityamong agricultural workers in these areas (Mascarenhas et al. Citation2017; Schaeffer et al. Citation2020; Imbulana and Oguma Citation2021; Seroka et al. Citation2022a, Citation2022b; Rogers et al. Citation2023).
Biomass use and increasing exposure risks
The process of sugarcane harvest produces a large volume of byproducts, the most abundant of these being the burnt remains of biomass not suitable for sugar or ethanol refinement. This residual waste ash is known as bagasse and is largely used to fertilize the fields in preparation for the next crop. Recently, however, there has been discussion about how this material could prove useful in a variety of green chemistry applications. Sugarcane bagasse has been used as a construction material for quite some time, serving as an environmentally friendly and economical supplement in cement (Xu et al. Citation2018; Bheel et al. Citation2022). This supplementation results in a cementitious material with durability and physical properties at least equivalent to standard cement, making it highly desirable (Ferreira-Ceccato et al. Citation2011; Amin et al. Citation2020; Farrant et al. Citation2022). Sugarcane bagasse biomass also has value in energy production, paper mills, and adsorbent applications (Ezeonuegbu et al. Citation2021; Mahmud and Anannya Citation2021; Ajala et al. Citation2021; Elshabrawy et al. Citation2023). Even the silica which has been investigated as a potential contributor toCKDu, has highly prized physicochemical properties for use in everything from medicine to dye once it is purified from bagasse (Chindaprasirt and Rattanasak Citation2020; Seroka et al. Citation2022a, Citation2022b; Malpani and Goyal Citation2023). While these are commendable applications for a material often considered “waste”, they also represent new potential inhalation exposures. If new industries begin to emerge centered around sugarcane bagasse processing, it could result in a new wave of occupational and community exposures with the accompanying risks discussed in previous sections. This is precisely why it is important that we achieve a better understanding of the potential toxicity of sugarcane ash exposure.
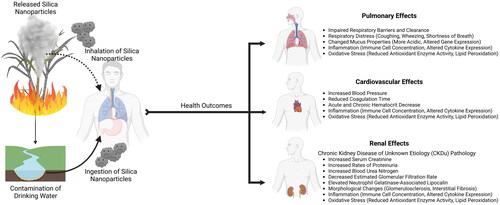
Sugarcane ash is already considered to be a substantial respiratory burden for sugarcane fieldworkers, but the extent of such exposures beyond those in the immediate vicinity and the full range of potential health effects are still being investigated. Initial studies focused on sampling respirable particles during sugarcane burning and ambiently throughout the harvest season, demonstrating clear occupational exposure risks for nearby workers. However, subsequent investigations found evidence of elevated particulate matter in cities miles away from sugarcane fires, revealing a greater scope of exposure than was originally considered. These sugarcane emissions have been linked to a range of poor health outcomes in the cardiopulmonary and renal systems of exposed populations (). It is feasible that sugarcane smoke could be carried a great distance from the source fires and still represent a significant health hazard. These exposure risks become increasingly salient as climate change results in increased temperatures and drought risks, particularlyin the areas where sugarcane is harvested, which could exacerbate heat stress, dehydration, PM exposure, and other conditions which compound the risk of ash exposure. At the same time, the growing demand for sugar and bioenergy places additional pressure on agricultural communities for more efficient harvests, expanding populations at risk of exposure. Together, these factors make for a potential health crisis, with most of this burden falling on under-resourced communities.
Health outcomes
Pulmonary effects
In the case of biomass burning and ash inhalation, the respiratory system is the first line of defense and lung damage is a common outcome. Sugarcane fires are thought to be linked to adverse pulmonary effects in local residents as they are often accompanied by symptoms of respiratory distress, acute respiratory illness, and increased hospital admissions (Arbex et al. Citation2007; de Aragão Umbuzeiro et al. Citation2008; Riguera et al. Citation2011; Mnatzaganian et al. Citation2015). These emissions are associated with increased mortality, contain mutagenic compounds, and seem to be of similar toxicity as those produced by traffic (de Aragão Umbuzeiro et al. Citation2008; Mazzoli-Rocha et al. Citation2008; Nowell et al. Citation2022). These effects are particularly pronounced among susceptible populations such as the young/elderly or those with preexisting respiratory disorders, further exacerbating episodes of respiratory distress (Long et al. Citation1998; Boopathy et al. Citation2002; Cançado et al. Citation2006; Mazzoli-Rocha et al. Citation2008). It is likely that this is a general response to PM exposure rather than a more specific allergic reaction, as workers in a sugarcane processing factory that were exposed to bagasse were found to demonstrate similar signs of respiratory distress (shortness of breath, rhinitis symptoms, etc.) and impaired pulmonary function without increased levels of IgE or indications of atopy (Patil et al. Citation2008; Gascon et al. Citation2012; Debela et al. Citation2023).
During the harvest season, sugarcane field workers were found to have significantly impaired nasal mucociliary clearance, altered mucus properties, and impaired respiratory system barriers that could increase susceptibility to particle exposure (Goto et al. Citation2011; Ferreira-Ceccato et al. Citation2011). In both sugarcane workers and nearby town residents, mucus became more acidic and mRNA levels of MUC5AC, MUC1, and MUC16 increased over the harvest season (Matsuda et al. Citation2020). Such changes are consistent with a protective response to PM exposure and have been found to occur following increased oxidative stress, resulting in pro-inflammatory responses (Kim et al. Citation2017). Indeed, antioxidant enzyme activity (i.e. catalase, glutathione peroxidase, glutathione S-transferase, and glutathione reductase) have all been found to decrease among sugarcane workers and nearby residents during the harvest season (Prado et al. Citation2012). This was accompanied by symptoms of respiratory distress, increased plasma malonaldehyde (a lipid peroxidation product), and, in the case of sugarcane workers, elevated urinary 1-hydroxypyrene indicating PAH exposure (Prado et al. Citation2012; Paula Santos et al. Citation2015). Sugarcane farmers also seem to be at increased risk of developing lung cancer (Amre et al. Citation1999).
Adverse pulmonary responses to sugarcane ash inhalation are worth monitoring and characterizing, but thus far do not seem to be distinct from general PM induced effects. Fine particulate matter (PM2.5) generated from fossil fuel combustion (e.g. automotive and factory emissions) can be deposited in the lungs following inhalation, induce respiratory distress, and exacerbate respiratory diseases (Pope et al. Citation2002; Kyung and Jeong Citation2020). The primary mechanisms of PM induced health effects are comparable to those seen in ash exposed populations, including ROS generation, reduced antioxidant activity, induction of lipid peroxidation, increased inflammation, and altered MUC5ACexpression (Rogers and Cismowski Citation2018; Leikauf et al. Citation2020; Hu et al. Citation2023). The one distinction between exposure to fine PM and sugarcane ash was change in concentration of CC16, an anti-inflammatory protein whose deficiency is associated with impaired lung function (Voraphani et al. Citation2023). Sugarcane harvesting was associated with reduced CC16 concentration in urine and plasma, while acute exposure to PM has been associated with increased serum CC16 concentration (Wang et al. Citation2017; Leite, Zanetta, Antonangelo, et al. Citation2018). This may be due to differences in chemical composition, whereas elemental composition of ambient PM is far more heterogeneous, while sugarcane ash is primarily comprised of amorphous silica (Xu et al. Citation2018; Zhang et al. Citation2020). Interestingly, reduced serum CC16 was found to occur in other silica-exposed workers, which could indicate the silica in sugarcane ash yields a distinct response and should be further investigated (Bernard et al. Citation1994).
Cardiovascular effects
While literature is limited, available studies investigating PM exposure and health effects among sugarcane workers have found some evidence of cardiovascular perturbation. Sugarcane workers have been found to lose weight and increase peak VO2 (cardiovascular fitness indicator) over the harvest season (Barbosa et al. Citation2012). Despite this, workers also demonstrated increased resting heart rates, higher blood pressure, and evidence of increased blood viscosity (Barbosa et al. Citation2012; Leite et al. Citation2015; Leite et al. Citation2018). Hematocrit was also found to decrease over the harvest season, both acutely and chronically, consistent with previous indications of alveolar inflammation (Leite et al. Citation2015; Leite, Zanetta, Antonangelo, et al. Citation2018). Pro-inflammatory state was further indicated by increased concentrations of monocytes and neutrophils (Paula Santos et al. Citation2015; Leite et al. Citation2015; Leite, Zanetta, Antonangelo, et al. Citation2018). Increased oxidative stress and inflammatory mediators, as demonstrated in both these studies, are known to induce negative cardiovascular effects (Barbosa et al. Citation2012; Basith et al. Citation2022). Thus it is unsurprising that sugarcane fires are associated with increased cardiovascular-related hospitalizations (Pestana et al. Citation2017). However, it is once again unclear whether any of these effects are specific to sugarcane burning or are just a function of the well documented cardiovascular risks of PM exposure (Du et al. Citation2016). Agricultural workers experience a wide range of potentially hazardous occupational exposures, because of this, more literature is desperately needed to determine the contribution of sugarcane ash inhalation to the potential cardiovascular impacts (Leite et al. Citation2018).
Renal effects
The majority of information regarding health effects of ash exposure in sugarcane workers is the association with deleterious effect on the kidney (Schaeffer et al. Citation2020). Sugarcane workers experience disproportionate rates of chronic kidney disease (CKD), particularly chronic kidney disease of unknown etiology (CKDu) which is unrelated to traditional risk factors such as age, diabetes or hypertension (López-Marín et al. Citation2014; Wesseling et al. Citation2015; Wesseling et al. Citation2016; Johnson et al. Citation2019). Kidney biopsies have found the disease presents with varying degrees of glomerulosclerosis, tubulointerstitial nephritis, fibrosis, and an absence of immune deposits (López-Marín et al. Citation2014; Selvarajah et al. Citation2016; Wijkström et al. Citation2017; Fischer et al. Citation2017). Field workers, especially cane cutters, have been found to have higher rates of proteinuria, increased serum creatinine, decreased estimated glomerular filtration rate (eGFR), and other biomarkers consistent with chronic kidney injury (Peraza et al. Citation2012; Laws et al. Citation2015; Paula Santos et al. Citation2015; Laws et al. Citation2016; Kupferman et al. Citation2018; López-Gálvez et al. Citation2021; Raines et al. Citation2023). Serum creatinine and eGFR are the most consistent reported markers, but neutrophil gelatinase-associated lipocalin (NGAL), blood urea nitrogen (BUN), and IL-18 were frequently found to be elevated as well (Laws et al. Citation2016; Wesseling, Aragón, González, Weiss, Glaser, Bobadilla, et al. Citation2016; Wesseling et al. Citation2016). Decreasing hematocrit, which has been associated with sugarcane harvest, is yet another feature which has been observed in CKDu and impaired renal function (Hsu et al. Citation2001; Leite et al. Citation2015; Leite, Zanetta, Trevisan, et al. Citation2018; Gunawickrama et al. Citation2022). The cause of these renal effects remains unclear, with heat stress, dehydration, and exertional injuries often cited as primary contributors (García-Trabanino et al. Citation2015; Wesseling, et al. Citation2016; Wijkström et al. Citation2017; Chapman et al. Citation2021).
Sugarcane ash induction of renal damage is intriguing as the relationship between PM exposure and kidney function is not well explored. If PM is small enough, it does have the potential to penetrate the circulatory system and be distributed throughout the body (Magalhaes et al. Citation2018). Such particles could easily reach the nephron, evade glomerular filtration and be taken up by renal tubular cells, potentially damaging renal function (Kim Citation2017). While there is some association between PM2.5 exposure and occurrence of CKD, many studies demonstrate contradictory results and CKD events generally occur alongside the traditional risk factors of age and diabetes (Bowe et al. Citation2018; Rasking et al. Citation2022). This appears distinct from the increased risk of CKDu among sugarcane workers which occurs predominantly among young and otherwise healthy individuals, suggesting that increased CKDu prevalence may go beyond PM exposure or be unique to sugarcane ash. Heat stress, dehydration, and exertion are known to cause kidney injury and may be contributing to this disease, but such factors are not unique to sugarcane workers. Previous studies have investigated occupational heat stress and dehydration in similar occupations and found their CKDu risk to be significantly lower than farmers (De Silva et al. Citation2022). Wildland firefighters are exposed to high levels of smoke exposure, at risk for dehydration, and perform strenuous activities in extreme heat (Horn et al. Citation2013; Walker et al. Citation2016; Navarro et al. Citation2019). While this group has demonstrated increase cardiopulmonary risks consistent with sugarcane ash findings, there is no such evidence of increased risk of kidney disease (Navarro et al. Citation2019; Navarro Citation2020; Pinkerton et al. Citation2022). However, there are ongoing studies which are currently investigating this topic (Navarro et al. Citation2022). CKDu has also been found in individuals without significant heat stress. Interestingly, the histopathology appears distinct from chronic dehydration injuries and several studies have argued that an environmental toxicant is likely to contribute to pathogenesis (Herath et al. Citation2018; Broe and Vervaet Citation2020; Pett et al. Citation2022; Schreurs et al. Citation2023).
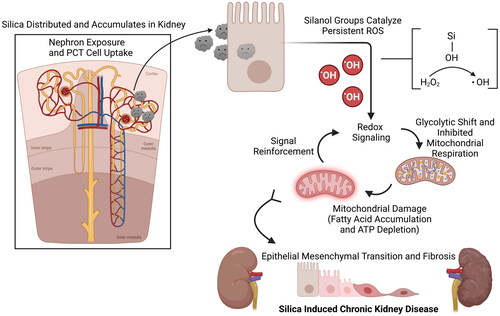
Silica content in sugarcane ash is far higher than wildfire ash making this a major distinction in the exposure among sugarcane workers anda potential contributor to increased risk of CKDu (Harper et al. Citation2019; Seroka et al. Citation2022a, Citation2022b). Indeed, silica nanoparticles have been recently reported to be found in the kidney of sugarcane agricultural workers (Rogers et al. Citation2023). These findings are consistent with elevated silica in agricultural communities’ water sources and higher silicon levels in the urine of individuals at risk of CKDu (Smpokou et al. Citation2019; Nikagolla et al. Citation2020). This also indicates that a major route of sugarcane ash exposure could be ingestion, such as groundwater contamination or ash deposition. Alternative exposures to ash inhalation may explain why observed pulmonary symptoms are often subclinical and relatively minor compared to CKD. The potential role of sugarcane ash in the CKDu epidemic is particularly concerning as kidney diseases are particularly devastating to those of low socio-economic status, who experience disproportionate exposure rates and financial barriers limiting access to appropriate medical care (Mckee and Paasche-Orlow Citation2012; Krinsky and Levine Citation2014; Aguilar and Madero Citation2019; Ranasinghe et al. Citation2019; Abdissa Citation2020; Kovesdy Citation2022).
Toxicological effects of sugarcane ash exposure
Current understanding of sugarcane ash toxicity relies heavily on observational studies and risk factor associations. Such investigations have been invaluable in the initial identification of an issue, but this body of literature is lacking in terms of mechanistic insights. As discussed in previous sections, occupational and public health studies have demonstrated clear hazards of sugarcane ash inhalation such as increased rates of cardiovascular disease, pulmonary distress, kidney injury, and systemic inflammation. Experimental studies are now required to further our understanding of potential mechanisms responsible for observed health changes. In vivo and clinical investigations will be needed to perform in-depth characterization of pathophysiological changes and to maintain physiologic relevance. While in vitro experimentation allows for a more controlled environment and is ideal for examining toxicity of different components of sugarcane ash as well as specific mechanisms and pathways. Bridging these knowledge gaps will help to identify key risk factors, early warning signs, and design effective interventions to better protect the disproportionately affected.
Clinical investigations
Most research into sugarcane ash toxicity is relatively recent, despite sugar production and processing having been a major industry for quite some time. Interestingly, medical concerns about bagasse exposure occurred as early as 1941, with clinicians reporting a respiratory condition in factory workers who experienced a high degree of bagasse inhalation (Lemone et al. Citation1947). The term “bagassosis” was used to describe this disease and it was thought to be due to particulate matter, microorganisms, silica, allergic reactions, or some combination thereof (Madu and Sharman Citation2023). Cases began to emerge across the world and were the subject of several clinical investigations, yet the specific cause remained unknown (Gillison and Taylor Citation1942; Sodeman Citation1967). This disease nearly vanished in the 1970s, likely due to different storage methods, improved ventilation, increased mask use, and other improved safety measures which reduced bagasse dust exposure (Lehrer et al. Citation1978; Madu and Sharman Citation2023). Similarly, interventions have been attempted with sugarcane workers, to reduce rates of kidney injury (Sorensen et al. Citation2019; Glaser et al. Citation2020). However, such approaches have not been fully successful, indicating the need for more experimental studies to improve mechanistic understanding and better designed workplace interventions.
Animal studies found that dosing mice or rats with sugarcane ash particles via intranasal installation resulted in pathophysiologic changes. Respiratory mechanics were impaired (promoting alveolar collapse), lung parenchyma were injured, and increased macrophage infiltration occurred in the lung (Mazzoli-Rocha et al. Citation2014). In another study alveolar spaces were reduced, conjunctive tissue thickness in the trachea reduced, and release of inflammatory cytokines was promoted (Ferreira et al. Citation2014). Direct inhalation exposure to sugarcane burning emissions in rats resulted in tracheal inflammation, interstitial edema, and cell infiltration (Matos et al. Citation2017). Sugarcane ash has also been found to induce liver toxicity in certain species of fish (Gonino et al. Citation2019). As discussed above, sugarcane ash is a highly heterogenous mixture and it remains unclear which components might be responsible for the observed effects. Amorphous silica nanoparticles (SiNPs) are often posited as a key toxicant, as silica is the predominant component and SiNPs have demonstrated similar toxicological effects (McCarthy et al. Citation2012). Administration of sugarcane ash in rats was found to induce CKD pathophysiology and result in accumulation of silica in several organs (Roncal-Jimenez et al. Citation2024). Exposure to SiNPs has resulted in liver and kidney damage in mice, as well as histopathological changes indicative of CKD in rats (Boudard et al. Citation2019; Sasai et al. Citation2022). Elevated SiNPs have even been seen in the kidney biopsies of sugarcane farmers, consistent with the increased risk of CKDu experienced by sugarcane agricultural workers (Rogers et al. Citation2023). Such findings suggest that silica may be a key factor in sugarcane ash toxicity and should be thoroughly investigated.
In vitro investigations
Toxicology studies of sugarcane ash in cell culture are rare, but available literature indicates varying biological response across ash components. Sugarcane ash in its natural state demonstrated minimal toxicity when directly exposed to cells, inducing some degree of ROS generation and hemolysis, but relatively low cytotoxicity (Le Blond et al. Citation2014). Ash was also found to reduce mitochondrial membrane potential and increase mitochondrial respiration in human kidney cell cultures (Stem et al. Citation2023). It is possible that this cell response is ameliorated by ash being in its initial state and that in-vivo biological reactions could digest the ash into more toxic components, requiring further investigation of these potential toxicants. PAHs and SiNPs are two such toxicants that have been thought to be responsible for adverse effects of sugarcane ash exposure. One study found that sugarcane ash induced significant ROS generation, DNA damage, and cell death in HeLa cells; suggesting that it could be due to the PAHs found in the ash samples (Verma et al. Citation2019). SiNPs purified from sugarcane ash have been found to be extremely cytotoxic, inhibiting mitochondrial respiration and perturbing energy metabolism (Stem et al. Citation2023). Interestingly, synthetic amorphous SiNPs did not demonstrate comparable cytotoxicity, suggesting different physicochemical properties which may limit relevance of invitro studies utilizing such SiNPs to determine potential toxicity of sugarcane ash. Synthetic amorphous SiNPs toxicity is highly variable and dependent on particle agglomeration, metabolic activity of cells, and particle size (Chang et al. Citation2007; Drescher et al. Citation2011). While smaller particles are consistently found to be more cytotoxic, overall understanding remains poor due to inconsistent standards and limited characterization in most literature (Murugadoss et al. Citation2017; Dong et al. Citation2020; Croissant et al. Citation2020). Determining silica’s contribution to sugarcane ash toxicity will require further investigation, ideally with particles derived from sugarcane ash directly.
Potential mechanisms
There are several mechanisms that likely contribute to the adverse health effects associated with sugarcane ash exposure (). Particulate matter, particularly PM2.5, is known to impair cardiopulmonary function, potentially through ROS generation and promotion of inflammation (Thangavel et al. Citation2022). There is also an increasing body of evidence suggesting deleterious effect on kidney function, though the mechanism remains unknown (Rasking et al. Citation2022). Sugarcane ash has also been found to be a source of PAHs, which are associated with lung, heart, and kidney damage, consistent with the primary health effects reported in sugarcane ash exposure (Mallah Manthar et al. Citation2022). The high carbon content in the ash has also been posited as a potential contributor, as carbonaceous particles are known to impair lung function (Le Blond et al. Citation2014). Silica is yet another component which has high potential for toxicity, sugarcane is primarily amorphous silica with crystalline silica remaining low despite the burning process (Le Blond et al. Citation2010). However amorphous silica is not without risk, amorphous SiNPs have a large surface area for their size and high adsorbent potential, making them quite capable of undesirable endogenous reactions and formation of a biocorona (Frost et al. Citation2017). Their structure also facilitates passage through biological barriers, such as cell membranes, which could be particularly harmful if carrying other toxicants (Häffner et al. Citation2021). One of the best predictors for silica nanoparticle toxicity appears to be presence of silanol groups, which can catalyze ROS generating reactions (Lehman et al. Citation2016). Nanoparticles with a high number of silanol groups on the surface have the potential for increased free radical production and subsequent oxidative stress (Rubio et al. Citation2019). If this oxidative stress is sufficient to perturb the redox environment of the cell (e.g. glutathione depletion), it can induce a range of harmful effects such as lipid peroxidation and DNA damage (Pizzino et al. Citation2017). Downstream effects of these could explain the symptoms observed in cardiopulmonary and renal systems of populations exposed to sugarcane ash but would require the SiNPs to be present in the tissue (). While such particles have been found in the kidney biopsies of sugarcane workers, no other such evidence yet exists. The toxicity of sugarcane ash is likely multifactorial and there remains much work to be done to determine key mechanisms.
Table 1. Summary of primary literature investigating sugarcane fire emissions and key findings.
Table 2. Summary of silica characteristics and sources.
Influence of climate change
Unfortunately, the very same communities that are experiencing the greatest sugarcane ash exposure are in the same regions which are poised to be most impacted by climate change (Sasai et al. Citation2021; Geladari et al. Citation2021). This has the potential to be even more challenging considering that agricultural communities are often resource-poor, and people living in poverty or at-risk of poverty are more likely to experience hardship from climate change (Shonkoff et al. Citation2011). Investigations into climate’s impact on sugarcane crop yield have found temperature to be non-significant, but do not consider worker conditions (Silva et al. Citation2020; Ali et al. Citation2021; Da Cruz and Machado Citation2023). Climate change also has the potential to increase crop water requirements and drought occurrences, which could prove difficult for agricultural communities that rely on groundwater and well water sources to meet drinking water needs (Gade and Khedkar Citation2023). Drought and increased temperatures also have the potential to concentrate toxicants already present in water, while perturbed rainfall patterns could redistribute toxicants in the environment. Another concern is how increased temperatures may increase the risk of heat stress and exertional injuries among sugarcane workers, which may be exacerbated by dehydration and put additional strain on water resources (Wegman et al. Citation2018; Dally et al. Citation2023). While these issues are not directly related to sugarcane burning, they place additional stress on biological systems and may compromise the ability to respond to respiratory challenges. The influence of climate change on sugarcane fires remains to be determined, but hotter and drier conditions have the potential to increase burn area and fire intensity (Littell et al. Citation2016). This could lead to more biomass burning and higher levels of particulate matter and other emissions. As climate change results in increased wildfire frequency and air quality hazards, the health effects of sugarcane burning may become even more pronounced (Rossiello and Szema Citation2019; Senande-Rivera et al. Citation2022). Thus, it becomes increasingly important to minimize greenhouse gas emissions through alternative non-burning harvest methods and support of environmentally friendly new sugarcane-based technologies (de Figueiredo et al. Citation2010; Moreira and Pacca Citation2020).
Transition to green harvesting
Concerns surrounding sugarcane burning emissions and long-term sustainability of the sugar industry have led to increased use of alternative ‘green harvesting’ methods to reduce the practice of sugarcane burning. Brazil began mandating the gradual elimination of pre-harvest burns in the 2000s and, while many regions are still mid-transition, there has already been some evidence of an improvement in local respiratory health (Nicolella and Belluzzo Citation2015). This method of harvesting also seems to have beneficial effects on soil conditions, with nutrients and other biochemical characteristics being improved under green harvesting conditions relative to conventional burning (Moitinho et al. Citation2021). Similar results have been reported in Ecuador, Costa Rica, and the United States, however overall harvest efficiency was reduced due to decreased yield and increased cost (Núñez and Spaans Citation2008; Sandhu et al. Citation2017). It is possible that improved soil conditions may improve subsequent yields and offset reductions to harvest efficiency, but initial investments in mechanical equipment and decreased cost efficiency are considerations which hinder transition away from pre-harvest burns (da Silva et al. Citation2021). It is also important to note that the transition to such harvest practices are significantly more difficult in areas where mechanical harvest may not be feasible due to terrain, costs or other factors (Núñez and Spaans Citation2008; Nicolella and Belluzzo Citation2015). Despite these difficulties, the shift to green harvesting has continued in Brazil and is gaining increased support in India (Bhuvaneshwari et al. Citation2019). The outcomes of such initiatives should be closely monitored as they have the potential to be highly valuable for environmental sustainability of the sugarcane industry and improve scientific understanding of sugarcane burning’s effect on public health.
Summary, recommendations, and conclusion
Sugarcane, a major global crop, serves as a critical source of sugar and bioenergy. However, its cultivation, predominantly in equatorial developing nations, poses significant health risks to workers and nearby communities due to the common practice of pre-harvest burning. This produces sugarcane ash, a complex mixture containing respirable particulate matter that is suspected of causing various health ailments. Despite its significance, the depth of exposure and potential hazards of sugarcane ash remain inadequately characterized. Research has shown that both direct (occupational) and indirect (community) exposures to this ash can lead to respiratory, cardiovascular, and renal health issues. The suspected role of sugarcane ash in the rising cases of chronic kidney disease, especially among field workers, highlights the urgency of elucidating toxicological mechanisms. Climate change exacerbates these concerns, potentially increasing the risks associated with sugarcane burning. With sugarcane ash finding new applications in green chemistry and other industries, understanding its health implications becomes even more crucial.
There are several approaches that should be considered to improve scientific understanding and mitigate the risks that such exposures pose, particularly to vulnerable populations. Increased funding and attention should be directed toward studies on the chemical composition of sugarcane ash and its specific toxicological effects on human health. Workplace interventions should be considered, including more thorough monitoring, stricter safety regulations, regular health checkups, and provision of PPE. Initiatives should be undertaken to educate communities residing near sugarcane fields about the potential hazards of sugarcane ash and ways to mitigate exposure (e.g. staying indoors during burning, using masks). Alternative harvest techniques should be employed when possible, minimizing the need for pre-harvest burning. Once more precise mechanisms of toxicity are elucidated, it may be feasible to develop therapeutic interventions to ameliorate the effects of sugarcane ash exposure or at least perform more targeted risk management and preventative care.
While initial studies have shed light on the potential dangers of sugarcane ash inhalation, there remains a pressing need for more in-depth research. Despite its economic importance, sugarcane cultivation brings significant health concerns. As our understanding grows regarding the extent of these health impacts, especially on the respiratory, cardiovascular, and renal systems, it’s evident that immediate actions are required. Combining targeted research with practical interventions will not only protect the health of millions but also ensure the sustainable growth of this crucial agricultural sector.
Acknowledgements
Figures were generated using Biorender.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
There is no data associated with this manuscript.
Additional information
Funding
References
- Abdissa D. 2020. Purposeful review to identify risk factors, epidemiology, clinical features, treatment and prevention of chronic kidney disease of unknown etiology. Int J Nephrol Renovasc Dis. 13:367–377. doi: 10.2147/IJNRD.S283161.
- Adegboye O. 2022. Field burning fallout: quantifying PM2.5 emissions from sugarcane fires. Environ Health Perspect. 130(8):84003. doi: 10.1289/EHP11533.
- Aguilar DJ, Madero M. 2019. Other potential CKD hotspots in the World: the cases of Mexico and the United States. Semin Nephrol. 39(3):300–307. doi: 10.1016/j.semnephrol.2019.02.008.
- Ajala EO, Ighalo JO, Ajala MA, Adeniyi AG, Ayanshola AM. 2021. Sugarcane bagasse: a biomass sufficiently applied for improving global energy, environment and economic sustainability. Bioresour Bioprocess. 8(1):87. doi: 10.1186/s40643-021-00440-z.
- Ali S, Zubair M, Hussain S. 2021. The combined effect of climatic factors and technical advancement on yield of sugarcane by using ARDL approach: evidence from Pakistan. Environ Sci Pollut Res Int. 28(29):39787–39804. doi: 10.1007/s11356-021-13313-x.
- Amin MN, Ashraf M, Kumar R, Khan K, Saqib D, Ali SS, Khan S. 2020. Role of sugarcane bagasse ash in developing sustainable engineered cementitious composites. Front Mater. doi: 10.3389/fmats.2020.00065.
- Amre DK, Infante-Rivard C, Dufresne A, Durgawale PM, Ernst P. 1999. Case-control study of lung cancer among sugar cane farmers in India. Occup Environ Med. 56(8):548–552. doi: 10.1136/oem.56.8.548.
- de Andrade SJ, Cristale J, Silva FS, Julião Zocolo G, Marchi MRR. 2010. Contribution of sugar-cane harvesting season to atmospheric contamination by polycyclic aromatic hydrocarbons (PAHs) in Araraquara city, Southeast Brazil. Atmos Environ. 44(24):2913–2919. doi: 10.1016/j.atmosenv.2010.04.026.
- de Aragão Umbuzeiro G, Franco A, Magalhães D, de Castro FJV, Kummrow F, Rech CM, Rothschild Franco de Carvalho L, de Castro Vasconcellos P. 2008. A preliminary characterization of the mutagenicity of atmospheric particulate matter collected during sugar cane harvesting using the Salmonella/microsome microsuspension assay. Environ Mol Mutagen. 49(4):249–255. doi: 10.1002/em.20378.
- Arbex MA, Martins LC, de Oliveira RC, Pereira LAA, Arbex FF, Cançado JED, Saldiva PHN, Braga ALF. 2007. Air pollution from biomass burning and asthma hospital admissions in a sugar cane plantation area in Brazil. J Epidemiol Community Health. 61(5):395–400. doi: 10.1136/jech.2005.044743.
- Azouz RA, Korany RMS. 2021. Toxic impacts of amorphous silica nanoparticles on liver and kidney of male adult rats: an in vivo study. Biol Trace Elem Res. 199(7):2653–2662. doi: 10.1007/s12011-020-02386-3.
- Barbosa CMG, Terra-Filho M, de Albuquerque ALP, Di Giorgi D, Grupi C, Negrão CE, Rondon MUPB, Martinez DG, Marcourakis T, dos Santos FA, et al. 2012. Burnt sugarcane harvesting - cardiovascular effects on a group of healthy workers, Brazil. PLOS One. 7(9):e46142. doi: 10.1371/journal.pone.0046142.
- Basith S, Manavalan B, Shin TH, Park CB, Lee W-S, Kim J, Lee G. 2022. The impact of fine particulate matter 2.5 on the cardiovascular system: a review of the invisible killer. Nanomaterials. 12(15):2656. doi: 10.3390/nano12152656.
- Bernard AM, Gonzalez-Lorenzo JM, Siles E, Trujillano G, Lauwerys R. 1994. Early decrease of serum Clara cell protein in silica-exposed workers. Eur Respir J. 7(11):1932–1937. doi: 10.1183/09031936.94.07111932.
- Bheel N, Khoso S, Baloch MH, Benjeddou O, Alwetaishi M. 2022. Use of waste recycling coal bottom ash and sugarcane bagasse ash as cement and sand replacement material to produce sustainable concrete. Environ Sci Pollut Res Int. 29(35):52399–52411. doi: 10.1007/s11356-022-19478-3.
- Bhuvaneshwari S, Hettiarachchi H, Meegoda JN. 2019. Crop residue burning in India: policy challenges and potential solutions. Int J Environ Res Public Health. [16(5)]. doi: 10.3390/ijerph16050832.
- Boopathy R, Asrabadi BR, Ferguson TG. 2002. Sugar cane (Saccharum officinarum L) burning and asthma in Southeast Louisiana, USA. Bull Environ Contam Toxicol. 68(2):173–179. doi: 10.1007/s001280235.
- Bortolotto Teixeira L, Guzi de Moraes E, Paolinelli Shinhe G, Falk G, Novaes de Oliveira AP. 2021. Obtaining Biogenic Silica from Sugarcane Bagasse and Leaf Ash. Waste Biomass Valor. 12(6):3205–3221. doi: 10.1007/s12649-020-01230-y.
- Boudard D, Aureli F, Laurent B, Sturm N, Raggi A, Antier E, Lakhdar L, Marche PN, Cottier M, Cubadda F, et al. 2019. Chronic oral exposure to synthetic amorphous silica (NM-200) results in renal and liver lesions in mice. Kidney Int Rep. 4(10):1463–1471. doi: 10.1016/j.ekir.2019.06.007.
- Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. 2018. Particulate matter air pollution and the risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 29(1):218–230. doi: 10.1681/ASN.2017030253.
- Broe MED, Vervaet BA. 2020. Is an environmental nephrotoxin the primary cause of CKDu (mesoamerican nephropathy)? PRO. Kidney360. 1(7):591–595. doi: 10.34067/KID.0003172020.
- Cançado JED, Saldiva PHN, Pereira LAA, Lara LBLS, Artaxo P, Martinelli LA, Arbex MA, Zanobetti A, Braga ALF. 2006. The impact of sugar cane–burning emissions on the respiratory system of children and the elderly. Environ Health Perspect. 114(5):725–729. doi: 10.1289/ehp.8485.
- Chandel AK, da Silva SS, Carvalho W, Singh OV. 2012. Sugarcane bagasse and leaves: foreseeable biomass of biofuel and bio-products. JChemTech Biotech. 87(1):11–20. doi: 10.1002/jctb.2742.
- Chang J-S, Chang KLB, Hwang D-F, Kong Z-L. 2007. In vitro cytotoxicitiy of silica nanoparticles at high concentrations strongly depends on the metabolic activity type of the cell line. Environ Sci Technol. 41(6):2064–2068. doi: 10.1021/es062347t.
- Chapman CL, Hess HW, Lucas RAI, Glaser J, Saran R, Bragg-Gresham J, Wegman DH, Hansson E, Minson CT, Schlader ZJ. 2021. Occupational heat exposure and the risk of chronic kidney disease of nontraditional origin in the United States. Am J Physiol Regul Integr Comp Physiol. 321(2):R141–R151. doi: 10.1152/ajpregu.00103.2021.
- Chindaprasirt P, Rattanasak U. 2020. Eco-production of silica from sugarcane bagasse ash for use as a photochromic pigment filler. Sci Rep. 10(1):9890. doi: 10.1038/s41598-020-66885-y.
- Cristale J, Silva FS, Zocolo GJ, Marchi MRR. 2012. Influence of sugarcane burning on indoor/outdoor PAH air pollution in Brazil. Environ Pollut. 169:210–216. doi: 10.1016/j.envpol.2012.03.045.
- Croissant JG, Butler KS, Zink JI, Brinker CJ. 2020. Synthetic amorphous silica nanoparticles: toxicity, biomedical and environmental implications. Nat Rev Mater. 5(12):886–909. doi: 10.1038/s41578-020-0230-0.
- Da Cruz TV, Machado RL. 2023. Measuring climate change’s impact on different sugarcane varieties production in the South of Goiás. Sci Rep. 13(1):11637. doi: 10.1038/s41598-023-36582-7.
- Dally M, Suresh K, Van Dyke M, James KA, Bauer AK, Krisher L, Newman LS. 2023. Occurrence of occupational injuries and within day changes in wet bulb temperature among sugarcane harvesters. J Agromedicine. 28(3):523–531. doi: 10.1080/1059924X.2023.2169425.
- De Silva PMCS, Ekanayake EMDV, Gunasekara T, Thakshila WAKG, Sandamini PMMA, Abeysiriwardhana PA, Nishara KGD, Harishchandra A, De Silva PHC, Siribaddana N, et al. 2022. Occupational heat exposure alone does not explain chronic kidney disease of uncertain aetiology (CKDu) in Sri Lanka. J Climate Change Health. 8:100143. doi: 10.1016/j.joclim.2022.100143.
- Debela M, Kebeta ND, Begosaw AM, Okello G, Azage M. 2023. Bagasse dust exposure and chronic respiratory symptoms among workers in the Metehara and Wonji sugar factories in Ethiopia: a longitudinal study design. BMJ Open Resp Res. 10(1):e001511. doi: 10.1136/bmjresp-2022-001511.
- Dong X, Wu Z, Li X, Xiao L, Yang M, Li Y, Duan J, Sun Z. 2020. The size-dependent cytotoxicity of amorphous silica nanoparticles: a systematic review of in vitro studies. Int J Nanomedicine. 15:9089–9113. doi: 10.2147/IJN.S276105.
- Drescher D, Orts-Gil G, Laube G, Natte K, Veh RW, Österle W, Kneipp J. 2011. Toxicity of amorphous silica nanoparticles on eukaryotic cell model is determined by particle agglomeration and serum protein adsorption effects. Anal Bioanal Chem. 400(5):1367–1373. doi: 10.1007/s00216-011-4893-7.
- Du Y, Xu X, Chu M, Guo Y, Wang J. 2016. Air particulate matter and cardiovascular disease: the epidemiological, biomedical and clinical evidence. J Thorac Dis. 8(1):E8–E19. doi: 10.3978/j.issn.2072-1439.2015.11.37.
- Elshabrawy SO, Elhussieny A, Taha MM, Pal K, Fahim IS. 2023. Wastewater treatment via sugarcane bagasse pulp. Int J Environ Sci Technol. 20(11):12405–12416. doi: 10.1007/s13762-023-04831-x.
- Ezeonuegbu BA, Machido DA, Whong CMZ, Japhet WS, Alexiou A, Elazab ST, Qusty N, Yaro CA, Batiha GE-S. 2021. Agricultural waste of sugarcane bagasse as efficient adsorbent for lead and nickel removal from untreated wastewater: biosorption, equilibrium isotherms, kinetics and desorption studies. Biotechnol Rep. 30:e00614. doi: 10.1016/j.btre.2021.e00614.
- FAO FAOSTAT. [accessed 2023 Sep 26]. http://www.fao.org/faostat/en/#data/QC.
- Farrant WE, Babafemi AJ, Kolawole JT, Panda B. 2022. Influence of sugarcane bagasse ash and silica fume on the mechanical and durability properties of concrete. Materials. 15(9):3018. doi: 10.3390/ma15093018.
- Favero A, Daigneault A, Sohngen B. 2020. Forests: carbon sequestration, biomass energy, or both? Sci Adv. 6(13):eaay6792. doi: 10.1126/sciadv.aay6792.
- Ferreira LEN, Muniz BV, Bittar TO, Berto LA, Figueroba SR, Groppo FC, Pereira AC. 2014. Effect of particles of ashes produced from sugarcane burning on the respiratory system of rats. Environ Res. 135:304–310. doi: 10.1016/j.envres.2014.07.030.
- Ferreira-Ceccato AD, Ramos EMC, de Carvalho LCS, Xavier RF, Teixeira MFdS, Raymundo-Pereira PA, Proença CdA, de Toledo AC, Ramos D. 2011. Short-term effects of air pollution from biomass burning in mucociliary clearance of Brazilian sugarcane cutters. Respir Med. 105(11):1766–1768. doi: 10.1016/j.rmed.2011.08.003.
- de Figueiredo EB, Panosso AR, Romão R, La Scala N. 2010. Greenhouse gas emission associated with sugar production in southern Brazil. Carbon Balance Manage. 5(1)3. doi: 10.1186/1750-0680-5-3.
- Fischer RSB, Mandayam S, Chavarria D, Vangala C, Nolan MS, Garcia LL, Palma L, Garcia F, García-Trabanino R, Murray KO. 2017. Clinical evidence of acute mesoamerican nephropathy. Am J Trop Med Hyg. 97(4):1247–1256. doi: 10.4269/ajtmh.17-0260.
- Flores-Jiménez DE, Carbajal N, Algara-Siller M, Aguilar-Rivera N, Álvarez-Fuentes G, Ávila-Galarza A, García AR. 2019. Atmospheric dispersion of methane emissions from sugarcane burning in Mexico. Environ Pollut. 250:922–933. doi: 10.1016/j.envpol.2019.04.025.
- Formann S, Hahn A, Janke L, Stinner W, Sträuber H, Logroño W, Nikolausz M. 2020. Beyond sugar and ethanol production: value generation opportunities through sugarcane residues. Front Energy Res. doi: 10.3389/fenrg.2020.579577.
- França DdA, Longo KM, Neto TGS, Santos JC, Freitas SR, Rudorff BFT, Cortez EV, Anselmo E, Carvalho JA. 2012. Pre-harvest sugarcane burning: determination of emission factors through laboratory measurements. Atmosphere. 3(1):164–180. doi: 10.3390/atmos3010164.
- Frost R, Langhammer C, Cedervall T. 2017. Real-time in situ analysis of biocorona formation and evolution on silica nanoparticles in defined and complex biological environments. Nanoscale. 9(10):3620–3628. doi: 10.1039/C6NR06399C.
- Gade SA, Khedkar DD. 2023. Implication of climate change on crop water requirement in the semi-arid region of Western Maharashtra, India. Environ Monit Assess. 195(7):829. doi: 10.1007/s10661-023-11429-w.
- García-Trabanino R, Jarquín E, Wesseling C, Johnson RJ, González-Quiroz M, Weiss I, Glaser J, José Vindell J, Stockfelt L, Roncal C, et al. 2015. Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador–A cross-shift study of workers at risk of Mesoamerican nephropathy. Environ Res. 142:746–755. doi: 10.1016/j.envres.2015.07.007.
- Gascon M, Kromhout H, Heederik D, Eduard W, van Wendel de Joode B. 2012. Respiratory, allergy and eye problems in bagasse-exposed sugar cane workers in Costa Rica. Occup Environ Med. 69(5):331–338. doi: 10.1136/oemed-2011-100029.
- Geladari E, Vallianou N, Geladari C, Aronis K, Vlachos K, Andreadis E, Theocharopoulos I, Dourakis S. 2021. Failing kidneys in a failing planet; CKD of unknown origin. Rev Environ Health. 38(1):125-135. doi: 10.1515/reveh-2021-0109.
- Geldenhuys G, Orasche J, Jakobi G, Zimmermann R, Forbes PBC. 2023. Characterization of gaseous and particulate phase polycyclic aromatic hydrocarbons emitted during preharvest burning of sugar cane in different regions of Kwa-Zulu Natal, South Africa. Environ Toxicol Chem. 42(4):778–792. doi: 10.1002/etc.5579.
- Gillison JA, Taylor F. 1942. Four cases of Bagassosis. Br Med J. 2(4271):577–578. doi: 10.1136/bmj.2.4271.577.
- Glaser J, Hansson E, Weiss I, Wesseling C, Jakobsson K, Ekström U, Apelqvist J, Lucas R, Arias Monge E, Peraza S, et al. 2020. Preventing kidney injury among sugarcane workers: promising evidence from enhanced workplace interventions. Occup Environ Med. 77(8):527–534. doi: 10.1136/oemed-2020-106406.
- Gonino GMR, Figueiredo BRS, Manetta GI, Zaia Alves GH, Benedito E. 2019. Fire increases the productivity of sugarcane, but it also generates ashes that negatively affect native fish species in aquatic systems. Sci Total Environ. 664:215–221. doi: 10.1016/j.scitotenv.2019.02.022.
- Goto DM, Lança M, Obuti CA, Galvão Barbosa CM, Nascimento Saldiva PH, Trevisan Zanetta DM, Lorenzi-Filho G, de Paula Santos U, Nakagawa NK. 2011. Effects of biomass burning on nasal mucociliary clearance and mucus properties after sugarcane harvesting. Environ Res. 111(5):664–669. doi: 10.1016/j.envres.2011.03.006.
- Gullett BK, Touati A, Huwe J, Hakk H. 2006. PCDD and PCDF emissions from simulated sugarcane Field Burning. Environ Sci Technol. 40(20):6228–6234. doi: 10.1021/es060806k.
- Gunawickrama SHNP, Silva ARN, Nanayakkara PGCL, Gunawickrama KBS, Jayasekara JMKB, Chandrasekharan NV. 2022. Metals and metallothionein expression in relation to progression of chronic kidney disease of unknown etiology (CKDu) in Sri Lanka. Diseases. 10(2):34. doi: 10.3390/diseases10020034.
- Guo C, Liu Y, Li Y. 2021. Adverse effects of amorphous silica nanoparticles: focus on human cardiovascular health. J Hazard Mater. 406:124626. doi: 10.1016/j.jhazmat.2020.124626.
- Häffner SM, Parra-Ortiz E, Browning KL, Jørgensen E, Skoda MWA, Montis C, Li X, Berti D, Zhao D, Malmsten M. 2021. Membrane interactions of virus-like mesoporous silica nanoparticles. ACS Nano. 15(4):6787–6800. doi: 10.1021/acsnano.0c10378.
- Harper AR, Santin C, Doerr SH, Froyd CA, Albini D, Otero XL, Viñas L, Pérez-Fernández B, Harper AR, Santin C, et al. 2019. Chemical composition of wildfire ash produced in contrasting ecosystems and its toxicity to Daphnia magna. Int J Wildland Fire. 28(10):726–737. doi: 10.1071/WF18200.
- Herath C, Jayasumana C, De Silva PMCS, D, Silva PHC, Siribaddana S, De Broe ME. 2018. Kidney diseases in agricultural communities: a case against heat-stress nephropathy. Kidney Int Rep. 3(2):271–280. doi: 10.1016/j.ekir.2017.10.006.
- Hiscox AL, Flecher S, Wang JJ, Viator HP. 2015. A comparative analysis of potential impact area of common sugar cane burning methods. Atmos Environ. 106:154–164. doi: 10.1016/j.atmosenv.2015.02.005.
- Horn GP, Blevins S, Fernhall B, Smith DL. 2013. Core temperature and heart rate response to repeated bouts of firefighting activities. Ergonomics. 56(9):1465–1473. doi: 10.1080/00140139.2013.818719.
- Hsu CY, Bates DW, Kuperman GJ, Curhan GC. 2001. Relationship between hematocrit and renal function in men and women. Kidney Int. 59(2):725–731. doi: 10.1046/j.1523-1755.2001.059002725.x.
- Hu L, Xu C, Tang X, Yu S, Wang L, Li Q, Zhou X. 2023. Fine particulate matter promotes airway inflammation and mucin production by activating endoplasmic reticulum stress and the IRE1α/NOD1/NF‑κB pathway. Int J Mol Med. 52(4):1–13. doi: 10.3892/ijmm.2023.5299.
- Imbulana S, Oguma K. 2021. Groundwater as a potential cause of chronic kidney disease of unknown etiology (CKDu) in Sri Lanka: a review. J Water Health. 19(3):393–410. doi: 10.2166/wh.2021.079.
- Johnson RJ, Wesseling C, Newman LS. 2019. Chronic kidney disease of unknown cause in agricultural communities. N Engl J Med. 380(19):1843–1852. doi: 10.1056/NEJMra1813869.
- Kim E-A. 2017. Particulate matter (fine particle) and urologic diseases. Int Neurourol J. 21(3):155–162. doi: 10.5213/inj.1734954.477.
- Kim S-S, Kim CH, Kim JW, Kung HC, Park TW, Shin YS, Kim JD, Ryu S, Kim W-J, Choi YH, et al. 2017. Airborne particulate matter increases MUC5AC expression by downregulating Claudin-1 expression in human airway cells. BMB Rep. 50(10):516–521. doi: 10.5483/BMBRep.2017.50.10.100.
- Kovesdy CP. 2022. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011). 12(1):7–11. doi: 10.1016/j.kisu.2021.11.003.
- Krinsky LM, Levine WJ. 2014. An island of widows: the human face of Mesoamerican endemic nephropathy. Kidney Int. 86(2):221–223. doi: 10.1038/ki.2014.133.
- Kupferman J, Ramírez-Rubio O, Amador JJ, López-Pilarte D, Wilker EH, Laws RL, Sennett C, Robles NV, Lau JL, Salinas AJ, et al. 2018. Acute kidney injury in sugarcane workers at risk for mesoamerican nephropathy. Am J Kidney Dis. 72(4):475–482. doi: 10.1053/j.ajkd.2018.04.014.
- Kyung SY, Jeong SH. 2020. Particulate-matter related respiratory diseases. Tuberc Respir Dis. 83(2):116–121. doi: 10.4046/trd.2019.0025.
- Lara LL, Artaxo P, Martinelli LA, Camargo PB, Victoria RL, Ferraz ESB. 2005. Properties of aerosols from sugar-cane burning emissions in Southeastern Brazil. Atmos Environ. 39(26):4627–4637. doi: 10.1016/j.atmosenv.2005.04.026.
- Laws RL, Brooks DR, Amador JJ, Weiner DE, Kaufman JS, Ramírez-Rubio O, Riefkohl A, Scammell MK, López-Pilarte D, Sánchez JM, et al. 2015. Changes in kidney function among Nicaraguan sugarcane workers. Int J Occup Environ Health. 21(3):241–250. doi: 10.1179/2049396714Y.0000000102.
- Laws RL, Brooks DR, Amador JJ, Weiner DE, Kaufman JS, Ramírez-Rubio O, Riefkohl A, Scammell MK, López-Pilarte D, Sánchez JM, et al. 2016. Biomarkers of kidney injury among nicaraguan sugarcane workers. Am J Kidney Dis. 67(2):209–217. doi: 10.1053/j.ajkd.2015.08.022.
- Le Blond JS, Horwell CJ, Williamson BJ, Oppenheimer C. 2010. Generation of crystalline silica from sugarcane burning. J Environ Monit. 12(7):1459–1470. doi: 10.1039/c0em00020e.
- Le Blond JS, Tomatis M, Horwell CJ, Dunster C, Murphy F, Corazzari I, Grendene F, Turci F, Gazzano E, Ghigo D, et al. 2014. The surface reactivity and implied toxicity of ash produced from sugarcane burning. Environ Toxicol. 29(5):503–516. doi: 10.1002/tox.21776.
- Le Blond JS, Woskie S, Horwell CJ, Williamson BJ. 2017. Particulate matter produced during commercial sugarcane harvesting and processing: a respiratory health hazard? Atmos Environ. 149:34–46. doi: 10.1016/j.atmosenv.2016.11.012.
- Lehman SE, Morris AS, Mueller PS, Salem AK, Grassian VH, Larsen SC. 2016. Silica nanoparticle-generated ROS as a predictor of cellular toxicity: mechanistic insights and safety by design. Environ Sci Nano. 3(1):56–66. doi: 10.1039/C5EN00179J.
- Lehrer SB, Turer E, Weill H, Salvaggio JE. 1978. Elimination of bagassosis in Louisiana paper manufacturing plant workers. Clin Allergy. 8(1):15–20. doi: 10.1111/j.1365-2222.1978.tb00442.x.
- Leikauf GD, Kim S-H, Jang A-S. 2020. Mechanisms of ultrafine particle-induced respiratory health effects. Exp Mol Med. 52(3):329–337. doi: 10.1038/s12276-020-0394-0.
- Leite MR, Ramos D, Trevisan IB, Freire APCF, Silva BSdA, Tacao GY, David RM, Burdmann EA, Santos UDP. 2015. Work in burnt sugar cane harvesting: chronic and acute change on inflammatory markers and blood pressure. Eur Respir J [Internet]. [accessed 2023 Oct 16] 46(suppl 59). doi: 10.1183/13993003.congress-2015.PA1177.
- Leite MR, Zanetta DMT, Antonangelo L, Marçal LJ, Ramos D, Almeida Burdmann E, Paula Santos U. 2018. Burnt sugarcane harvesting work: effects on pulmonary and systemic inflammatory markers. Inhal Toxicol. 30(6):205–212. doi: 10.1080/08958378.2018.1494765.
- Leite MR, Zanetta DMT, Trevisan IB, Burdmann E de A, Santos U de P. 2018. Sugarcane cutting work, risks, and health effects: a literature review. Rev Saúde Pública. 52:80. doi: 10.11606/s1518-8787.2018052000138.
- Lemone DV, Scott WG, Moore S, Koven AL. 1947. Bagasse disease of the lungs. Radiology. 49(5):556–567. doi: 10.1148/49.5.556.
- Leung CC, Yu ITS, Chen W. 2012. Silicosis. Lancet. 379(9830):2008–2018. doi: 10.1016/S0140-6736(12)60235-9.
- Li Q, Hu H, Jiang L, Zou Y, Duan J, Sun Z. 2016. Cytotoxicity and autophagy dysfunction induced by different sizes of silica particles in human bronchial epithelial BEAS-2B cells. Toxicol Res. 5(4):1216–1228. doi: 10.1039/c6tx00100a.
- Li Y, Duan J, Chai X, Yang M, Wang J, Chen R, Sun Z. 2019. Microarray-assisted size-effect study of amorphous silica nanoparticles on human bronchial epithelial cells. Nanoscale. 11(47):22907–22923. doi: 10.1039/c9nr07350g.
- Li Y, Sun L, Jin M, Du Z, Liu X, Guo C, Li Y, Huang P, Sun Z. 2011. Size-dependent cytotoxicity of amorphous silica nanoparticles in human hepatoma HepG2 cells. Toxicol in Vitro. 25(7):1343–1352. doi: 10.1016/j.tiv.2011.05.003.
- Littell JS, Peterson DL, Riley KL, Liu Y, Luce CH. 2016. A review of the relationships between drought and forest fire in the United States. Glob Chang Biol. 22(7):2353–2369. doi: 10.1111/gcb.13275.
- Long W, Tate RB, Neuman M, Manfreda J, Becker AB, Anthonisen NR. 1998. Respiratory symptoms in a susceptible population due to burning of agricultural residue. Chest. 113(2):351–357. doi: 10.1378/chest.113.2.351.
- López-Gálvez N, Wagoner R, Canales RA, Ernst K, Burgess JL, de Zapien J, Rosales C, Beamer P. 2021. Longitudinal assessment of kidney function in migrant farm workers. Environ Res. 202:111686. doi: 10.1016/j.envres.2021.111686.
- López-Marín L, Chávez Y, García XA, Flores WM, García YM, Herrera R, Almaguer M, Orantes CM, Calero D, Bayarre HD, et al. 2014. Histopathology of chronic kidney disease of unknown etiology in Salvadoran agricultural communities. MEDICC Rev. 16(2):49–54. doi: 10.37757/MR2014.V16.N2.8.
- Lunt AJG, Chater P, Korsunsky AM. 2018. On the origins of strain inhomogeneity in amorphous materials. Sci Rep. 8(1):1574. doi: 10.1038/s41598-018-19900-2.
- Madu A, Sharman T. 2023. Bagassosis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; [accessed 2023 Oct 23]. http://www.ncbi.nlm.nih.gov/books/NBK554444/.
- Magalhaes S, Baumgartner J, Weichenthal S. 2018. Impacts of exposure to black carbon, elemental carbon, and ultrafine particles from indoor and outdoor sources on blood pressure in adults: a review of epidemiological evidence. Environ Res. 161:345–353. doi: 10.1016/j.envres.2017.11.030.
- Mahmud M, Anannya FR. 2021. Sugarcane bagasse - A source of cellulosic fiber for diverse applications. Heliyon. 7(8):e07771. doi: 10.1016/j.heliyon.2021.e07771.
- Mallah Manthar A, Changxing L, Mallah Mukhtiar A, Noreen S, Liu Y, Saeed M, Xi H, Ahmed B, Feng F, Mirjat AA, et al. 2022. Polycyclic aromatic hydrocarbon and its effects on human health: an overeview. Chemosphere. 296:133948. doi: 10.1016/j.chemosphere.2022.133948.
- Malpani SK, Goyal D. 2023. Synthesis, analysis, and multi-faceted applications of solid wastes-derived silica nanoparticles: a comprehensive review (2010-2022). Environ Sci Pollut Res Int. 30(11):28321–28343. doi: 10.1007/s11356-022-23873-1.
- Mascarenhas S, Mutnuri S, Ganguly A. 2017. Deleterious role of trace elements - Silica and lead in the development of chronic kidney disease. Chemosphere. 177:239–249. doi: 10.1016/j.chemosphere.2017.02.155.
- de Matos M, Santos F, Eichler P. 2020. Chapter 1 - Sugarcane world scenario. In: Santos F, Rabelo SC, De Matos M, Eichler P, editors. Sugarcane Biorefinery, Technology and Perspectives [Internet]. [place unknown]: Academic Press; [accessed 2023 Nov 17p. 1–19. doi: 10.1016/B978-0-12-814236-3.00001-9.
- Matos VSB, Gomes FdS, Oliveira TM, Schulz R da S, Ribeiro LCV, Gonzales ADF, Lima JM, Guerreiro MLdS 2017. Effects of emissions from sugar cane burning on the trachea and lungs of Wistar rats. J Bras Pneumol. 43(3):208–214. doi: 10.1590/S1806-37562016000000144.
- Matsuda M, Braga ALF, Marquezini MV, Monteiro MLR, Saldiva PHN, de Santos U. 2020. Occupational effect of sugarcane biomass burning on the conjunctival mucin profile of harvest workers and residents of an adjacent town - A Brazilian panel study. Exp Eye Res. 190:107889. doi: 10.1016/j.exer.2019.107889.
- Mazzoli-Rocha F, Carvalho GMC, Lanzetti M, Valença SS, Silva LFF, Saldiva PHN, Zin WA, Faffe DS. 2014. Respiratory toxicity of repeated exposure to particles produced by traffic and sugar cane burning. Respir Physiol Neurobiol. 191:106–113. doi: 10.1016/j.resp.2013.11.004.
- Mazzoli-Rocha F, Magalhães CB, Malm O, Saldiva PHN, Zin WA, Faffe DS. 2008. Comparative respiratory toxicity of particles produced by traffic and sugar cane burning. Environ Res. 108(1):35–41. doi: 10.1016/j.envres.2008.05.004.
- McCarthy J, Inkielewicz-Stępniak I, Corbalan JJ, Radomski MW. 2012. Mechanisms of toxicity of amorphous silica nanoparticles on human lung submucosal cells in vitro: protective effects of Fisetin. Chem Res Toxicol. 25(10):2227–2235. doi: 10.1021/tx3002884.
- Mckee MM, Paasche-Orlow M. 2012. Health literacy and the disenfranchised: the importance of collaboration between limited english proficiency and health literacy researchers. J Health Commun. 17 Suppl 3(Suppl 3):7–12. doi: 10.1080/10810730.2012.712627.
- Merget R, Bauer T, Küpper HU, Philippou S, Bauer HD, Breitstadt R, Bruening T. 2002. Health hazards due to the inhalation of amorphous silica. Arch Toxicol. 75(11–12):625–634. doi: 10.1007/s002040100266.
- Mnatzaganian CL, Pellegrin KL, Miyamura J, Valencia D, Pang L. 2015. Association between sugar cane burning and acute respiratory illness on the island of Maui. Environ Health. 14(1):81. doi: 10.1186/s12940-015-0067-y.
- Moitinho MR, Ferraudo AS, Panosso AR, Bicalho EdS, Teixeira DDB, Barbosa MdA, Tsai SM, Borges BMF, Cannavan FdS, Souza J d, et al. 2021. Effects of burned and unburned sugarcane harvesting systems on soil CO2 emission and soil physical, chemical, and microbiological attributes. CATENA. 196:104903. doi: 10.1016/j.catena.2020.104903.
- Moreira JR, Pacca SA. 2020. The climate change mitigation potential of sugarcane based technologies for automobiles; CO2 negative emissions in sight. Transportation Res Part D: transp Environ. 86:102454. doi: 10.1016/j.trd.2020.102454.
- Murugadoss S, Lison D, Godderis L, Van Den Brule S, Mast J, Brassinne F, Sebaihi N, Hoet PH. 2017. Toxicology of silica nanoparticles: an update. Arch Toxicol. 91(9):2967–3010. doi: 10.1007/s00204-017-1993-y.
- Navarro K. 2020. Working in smoke: wildfire impacts on the health of firefighters and outdoor workers and mitigation strategies. Clin Chest Med. 41(4):763–769. doi: 10.1016/j.ccm.2020.08.017.
- Navarro KM, Butler CR, Fent K, Toennis C, Sammons D, Ramirez-Cardenas A, Clark KA, Byrne DC, Graydon PS, Hale CR, et al. 2022. The wildland firefighter exposure and health effect (WFFEHE) study: rationale, design, and methods of a repeated-measures study. Ann Work Expo Health. 66(6):714–727. doi: 10.1093/annweh/wxab117.
- Navarro KM, Kleinman MT, Mackay CE, Reinhardt TE, Balmes JR, Broyles GA, Ottmar RD, Naher LP, Domitrovich JW. 2019. Wildland firefighter smoke exposure and risk of lung cancer and cardiovascular disease mortality. Environ Res. 173:462–468. doi: 10.1016/j.envres.2019.03.060.
- Nicolella AC, Belluzzo W. 2015. The effect of reducing the pre-harvest burning of sugar cane on respiratory health in Brazil. Envir Dev Econ. 20(1):127–140. doi: 10.1017/S1355770X14000096.
- Nikagolla C, Meredith KT, Dawes LA, Banati RB, Millar GJ. 2020. Using water quality and isotope studies to inform research in chronic kidney disease of unknown aetiology endemic areas in Sri Lanka. Sci Total Environ. 757:144152. doi: 10.1016/j.scitotenv.2020.140896.
- Nowell HK, Wirks C, Val MM, van DA, Martin RV, Uejio CK, Holmes CD. 2022. Impacts of sugarcane fires on air quality and public health in South Florida. Environ Health Perspect. 130(8):87004. doi: 10.1289/EHP9957.
- Núñez O, Spaans E. 2008. Evaluation of green-cane harvesting and crop management with a trash-blanket. Sugar Tech. 10(1):29–35. doi: 10.1007/s12355-008-0005-1.
- Patil SN, Somade PM, Joshi AG. 2008. Pulmonary function tests in sugar factory workers of Western Maharashtra (India). J Basic Clin Physiol Pharmacol. 19(2):159–166. doi: 10.1515/jbcpp.2008.19.2.159.
- Paula Santos U, Zanetta DMT, Terra-Filho M, Burdmann EA. 2015. Burnt sugarcane harvesting is associated with acute renal dysfunction. Kidney Int. 87(4):792–799. doi: 10.1038/ki.2014.306.
- Pavan C, Santalucia R, Leinardi R, Fabbiani M, Yakoub Y, Uwambayinema F, Ugliengo P, Tomatis M, Martra G, Turci F, et al. 2020. Nearly free surface silanols are the critical molecular moieties that initiate the toxicity of silica particles. Proc Natl Acad Sci U S A. 117(45):27836–27846. doi: 10.1073/pnas.2008006117.
- Peraza S, Wesseling C, Aragon A, Leiva R, García-Trabanino RA, Torres C, Jakobsson K, Elinder CG, Hogstedt C. 2012. Decreased kidney function among agricultural workers in El Salvador. Am J Kidney Dis. 59(4):531–540. doi: 10.1053/j.ajkd.2011.11.039.
- Pestana PRdS, Braga ALF, Ramos EMC, de Oliveira AF, Osadnik CR, Ferreira AD, Ramos D. 2017. Effects of air pollution caused by sugarcane burning in Western São Paulo on the cardiovascular system. Rev Saúde Pública. 51(0):13. doi: 10.1590/s1518-8787.2017051006495.
- Pett J, Mohamed F, Knight J, Linhart C, Osborne NJ, Taylor R. 2022. Two decades of chronic kidney disease of unknown aetiology (CKDu) research: existing evidence and persistent gaps from epidemiological studies in Sri Lanka. Nephrology. 27(3):238–247. doi: 10.1111/nep.13989.
- Pinakana SD, Robles E, Mendez E, Raysoni AU. 2023. Assessment of air pollution levels during sugarcane stubble burning event in La Feria, South Texas, USA. Pollutants. 3(2):197–219. doi: 10.3390/pollutants3020015.
- Pinkerton LE, Bertke S, Dahm MM, Kubale TL, Siegel MR, Hales TR, Yiin JH, Purdue MP, Beaumont JJ, Daniels RD. 2022. End-stage renal disease incidence in a cohort of US firefighters from San Francisco, Chicago, and Philadelphia. Am J Ind Med. 65(12):975–984. doi: 10.1002/ajim.23435.
- Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. 2017. Oxidative stress: harms and Benefits for Human Health. Oxid Med Cell Longev. 2017:8416763–8416713. doi: 10.1155/2017/8416763.
- Pope CA, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. 2002. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 287(9):1132–1141. doi: 10.1001/jama.287.9.1132.
- Prado GF, Zanetta DMT, Arbex MA, Braga AL, Pereira LAA, de Marchi MRR, de Melo Loureiro AP, Marcourakis T, Sugauara LE, Gattás GJF, et al. 2012. Burnt sugarcane harvesting: particulate matter exposure and the effects on lung function, oxidative stress, and urinary 1-hydroxypyrene. Sci Total Environ. 437:200–208. doi: 10.1016/j.scitotenv.2012.07.069.
- Raines NH, Leone DA, O'Callaghan-Gordo C, Ramirez-Rubio O, Amador JJ, Lopez Pilarte D, Delgado IS, Leibler JH, Embade N, Gil-Redondo R, et al. 2023. Metabolic features of increased gut permeability, inflammation, and altered energy metabolism distinguish agricultural workers at risk for mesoamerican nephropathy. Metabolites. 13(3):325. doi: 10.3390/metabo13030325.
- Ranasinghe AV, Kumara GWGP, Karunarathna RH, De Silva AP, Sachintani KGD, Gunawardena JMCN, Kumari SKCR, Sarjana MSF, Chandraguptha JS, De Silva MVC. 2019. The incidence, prevalence and trends of chronic kidney disease and chronic kidney disease of uncertain aetiology (CKDu) in the North Central Province of Sri Lanka: an analysis of 30,566 patients. BMC Nephrol. 20(1):338. doi: 10.1186/s12882-019-1501-0.
- Rasking L, Vanbrabant K, Bové H, Plusquin M, De Vusser K, Roels HA, Nawrot TS. 2022. Adverse effects of fine particulate matter on human kidney functioning: a systematic review. Environ Health. 21(1):24. doi: 10.1186/s12940-021-00827-7.
- Riguera D, André PA, Zanetta DMT. 2011. Sugar cane burning pollution and respiratory symptoms in schoolchildren in Monte Aprazível, Southeastern Brazil. Rev Saude Publica. 45(5):878–886. doi: 10.1590/s0034-89102011005000052.
- Rogers KL, Roncal-Jimenez CA, Leiva R, Stem A, Wijkstrom J, Serpas L, González-Quiroz MA, Sasai F, Wernerson A, Schaeffer J, et al. 2023. Silica nanoparticles and Mesoamerican nephropathy: a case series. Am J Kidney Dis. [accessed 2023 Oct 23]. doi: 10.1053/j.ajkd.2023.06.010.
- Rogers LK, Cismowski MJ. 2018. Oxidative stress in the lung – the essential paradox. Curr Opin Toxicol. 7:37–43. doi: 10.1016/j.cotox.2017.09.001.
- Roncal-Jimenez CA, Rogers KL, Stem A, Wijkstrom J, Wernerson A, Fox J, Garcia Trabanino R, Brindley S, Garcia G, Miyazaki M, et al. 2024. Intranasal administration of sugarcane ash causes chronic kidney disease in rats. Am J Physiol Renal Physiol. doi: 10.1152/ajprenal.00251.2023.
- Rossiello MR, Szema A. 2019. Health effects of climate change-induced wildfires and heatwaves. Cureus. 11(5):e4771. doi: 10.7759/cureus.4771.
- Rubio L, Pyrgiotakis G, Beltran-Huarac J, Zhang Y, Gaurav J, Deloid G, Spyrogianni A, Sarosiek KA, Bello D, Demokritou P. 2019. Safer-by-design flame-sprayed silicon dioxide nanoparticles: the role of silanol content on ROS generation, surface activity and cytotoxicity. Part Fibre Toxicol. 16(1):40. doi: 10.1186/s12989-019-0325-1.
- Sahu S K, Mangaraj, P, Beig G, Samal A, Dash S, Tyagi B, Chinmay Pradhan,. 2021. Quantifying the high resolution seasonal emission of air pollutants from crop residue burning in India. Environ Pollut. 286:117165. doi: 10.1016/j.envpol.2021.117165.
- Sandhu HS, Singh MP, Gilbert RA, Subiros-Ruiz F, Rice RW, Shine JM. 2017. Harvest management effects on sugarcane growth, yield and nutrient cycling in Florida and Costa Rica. Field Crops Res. 214:253–260. doi: 10.1016/j.fcr.2017.09.002.
- Santoso MA, Christensen EG, Yang J, Rein G. 2019. Review of the transition from smouldering to flaming combustion in wildfires. Front Mech Eng. 5:49. doi: 10.3389/fmech.2019.00049.
- Sasai F, Rogers KL, Orlicky DJ, Stem A, Schaeffer J, Garcia G, Fox J, Ray MS, Butler-Dawson J, Gonzalez-Quiroz M, et al. 2022. Inhaled silica nanoparticles cause chronic kidney disease in rats. Am J Physiol Renal Physiol. 323(1):F48–F58. doi: 10.1152/ajprenal.00021.2022.
- Sasai F, Roncal-Jimenez C, Rogers K, Sato Y, Brown JM, Glaser J, Garcia G, Sanchez-Lozada LG, Rodriguez-Iturbe B, Dawson JB, et al. 2021. Climate change and nephrology. Nephrol Dial Transplant. 38(1):41–48. doi: 10.1093/ndt/gfab258.
- Schaeffer JW, Adgate JL, Reynolds SJ, Butler-Dawson J, Krisher L, Dally M, Johnson RJ, James KA, Jaramillo D, Newman LS. 2020. A pilot study to assess inhalation exposures among sugarcane workers in Guatemala: implications for chronic kidney disease of unknown origin. Int J Environ Res Public Health. 17(16):5708. doi: 10.3390/ijerph17165708.
- Schreurs G, Maudsley S, Nast C, Praet M, Da Silva Fernandes S, Boor P, D'Haese P, De Broe ME, Vervaet BA. 2023. Chronic dehydration induces injury pathways in rats, but does not mimic histopathology of chronic interstitial nephritis in agricultural communities. Sci Rep. 13(1):18119. doi: 10.1038/s41598-023-43567-z.
- Selvarajah M, Weeratunga P, Sivayoganthan S, Rathnatunga N, Rajapakse S. 2016. Clinicopathological correlates of chronic kidney disease of unknown etiology in Sri Lanka. Indian J Nephrol. 26(5):357–363. doi: 10.4103/0971-4065.167280.
- Senande-Rivera M, Insua-Costa D, Miguez-Macho G. 2022. Spatial and temporal expansion of global wildland fire activity in response to climate change. Nat Commun. 13(1):1208. doi: 10.1038/s41467-022-28835-2.
- Seroka S, Taziwa R, Khotseng L. 2022a. Green synthesis of crystalline silica from sugarcane bagasse ash: physico-chemical properties. Nanomaterials. 12(13):2184. doi: 10.3390/nano12132184.
- Seroka NS, Taziwa RT, Khotseng L. 2022b. Extraction and synthesis of silicon nanoparticles (SiNPs) from sugarcane bagasse ash: a mini-review. Appl Sci. 12(5):2310. doi: 10.3390/app12052310.
- Sevimoglu O, Rogge WF. 2015. Organic compound concentrations of size-segregated PM10 during sugarcane burning and growing seasons at a Rural and an Urban Site in Florida, USA. Aerosol Air Qual Res. 15(5):1720–1736. doi: 10.4209/aaqr.2015.02.0069.
- Sevimoglu O, Rogge WF. 2016. Seasonal size-segregated PM10 and PAH concentrations in a rural area of sugarcane agriculture versus a coastal urban area in Southeastern Florida, USA. Particuology. 28:52–59. doi: 10.1016/j.partic.2015.09.013.
- Shikwambana L, Ncipha X, Sangeetha SK, Sivakumar V, Mhangara P. 2021. Qualitative study on the observations of emissions, transport, and the influence of climatic factors from sugarcane burning: a south african perspective. Int J Environ Res Public Health. 18(14):7672. doi: 10.3390/ijerph18147672.
- Shonkoff SB, Morello-Frosch R, Pastor M, Sadd J. 2011. The climate gap: environmental health and equity implications of climate change and mitigation policies in California—a review of the literature. Clim Change. 109(S1):485–503. doi: 10.1007/s10584-011-0310-7.
- Silva FS, Cristale J, André PA, Saldiva PHN, Marchi MRR. 2010. PM2.5 and PM10: the influence of sugarcane burning on potential cancer risk. Atmos Environ. 44(39):5133–5138. doi: 10.1016/j.atmosenv.2010.09.001.
- da Silva MJ, O. Neves L de, Correa MHF, de Souza CHW. 2021. Quality indexes and performance in mechanized harvesting of sugarcane at a burnt cane and green cane. Sugar Tech. 23(3):499–507. doi: 10.1007/s12355-021-00957-9.
- Silva WKdM, Medeiros SEL, da Silva LP, Coelho Junior LM, Abrahão R. 2020. Sugarcane production and climate trends in Paraíba state (Brazil). Environ Monit Assess. 192(6):392. doi: 10.1007/s10661-020-08358-3.
- Smpokou E-T, González-Quiroz M, Martins C, Alvito P, Le Blond J, Glaser J, Aragón A, Wesseling C, Nitsch D, Pearce N, et al. 2019. Environmental exposures in young adults with declining kidney function in a population at risk of Mesoamerican nephropathy. Occup Environ Med. 76(12):920–926. doi: 10.1136/oemed-2019-105772.
- Sodeman WA. 1967. Bagasse disease of the lungs—after 25 years. Dis Chest. 52(4):505–507. doi: 10.1378/chest.52.4.505.
- Sorensen CJ, Butler-Dawson J, Dally M, Krisher L, Griffin BR, Johnson RJ, Lemery J, Asensio C, Tenney L, Newman LS. 2019. Risk factors and mechanisms underlying cross-shift decline in kidney function in Guatemalan sugarcane workers. J Occup Environ Med. 61(3):239–250. doi: 10.1097/JOM.0000000000001529.
- Souto-Oliveira CE, Marques MTA, Nogueira T, Lopes FJS, Medeiros JAG, Medeiros IMMA, Moreira GA, da Silva Dias PL, Landulfo E, Andrade MdF 2023. Impact of extreme wildfires from the Brazilian Forests and sugarcane burning on the air quality of the biggest megacity on South America. Sci Total Environ. 888:163439. doi: 10.1016/j.scitotenv.2023.163439.
- Souza DZ, Vasconcellos PC, Lee H, Aurela M, Saarnio K, Teinilä K, Hillamo R. 2014. Composition of PM2.5 and PM10 Collected at Urban Sites in Brazil. Aerosol Air Qual Res. 14(1):168–176. doi: 10.4209/aaqr.2013.03.0071.
- Souza RA, Telles TS, Machado W, Hungria M, Filho JT, Guimarães MdF 2012. Effects of sugarcane harvesting with burning on the chemical and microbiological properties of the soil. Agric, Ecosyst Environ. 155:1–6. doi: 10.1016/j.agee.2012.03.012.
- Stem AD, Rogers KL, Roede JR, Roncal-Jimenez CA, Johnson RJ, Brown JM. 2023. Sugarcane ash and sugarcane ash-derived silica nanoparticles alter cellular metabolism in human proximal tubular kidney cells. Environ Pollut. 332:121951. doi: 10.1016/j.envpol.2023.121951.
- Thangavel P, Park D, Lee Y-C. 2022. Recent insights into particulate matter (PM2.5)-mediated toxicity in humans: an overview. Int J Environ Res Public Health. 19(12):7511. doi: 10.3390/ijerph19127511.
- Toxicological Profile for Silica. 2019. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); [accessed 2024 Jan 18]. http://www.ncbi.nlm.nih.gov/books/NBK592827/.
- Vasconcellos PC, Souza DZ, Sanchez-Ccoyllo O, Bustillos JOV, Lee H, Santos FC, Nascimento KH, Araújo MP, Saarnio K, Teinilä K, et al. 2010. Determination of anthropogenic and biogenic compounds on atmospheric aerosol collected in urban, biomass burning and forest areas in São Paulo, Brazil. Sci Total Environ. 408(23):5836–5844. doi: 10.1016/j.scitotenv.2010.08.012.
- Verma A, Meena R, Maurya A, Gaharwar US, Rajamani P. 2019. Identification, quantification and in-vitro genotoxicity of major polyaromatic hydrocarbons produced by Sugarcane Fly Ash emitted from Sugarmill. JEP. 10(10):1244–1261. doi: 10.4236/jep.2019.1010074.
- Villar-Cociña E, Frías M, Valencia-Morales E. 2008. Sugar cane wastes as pozzolanic materials: applications of mathematical model [Internet]. [accessed 2023 Nov 17]. https://digital.csic.es/handle/10261/30458.
- Voraphani N, Stern DA, Ledford JG, Spangenberg AL, Zhai J, Wright AL, Morgan WJ, Kraft M, Sherrill DL, Curtin JA, et al. 2023. Circulating CC16 and asthma: a population-based, multicohort study from early childhood through adult life. Am J Respir Crit Care Med. 208(7):758–769. doi: 10.1164/rccm.202301-0041OC.
- Walker A, Pope R, Orr RM. 2016. The impact of fire suppression tasks on firefighter hydration: a critical review with consideration of the utility of reported hydration measures. Ann of Occup and Environ Med. 28(1):63. doi: 10.1186/s40557-016-0152-x.
- Wang C, Cai J, Chen R, Shi J, Yang C, Li H, Lin Z, Meng X, Liu C, Niu Y, et al. 2017. Personal exposure to fine particulate matter, lung function and serum club cell secretory protein (Clara). Environ Pollut. 225:450–455. doi: 10.1016/j.envpol.2017.02.068.
- Waters KM, Masiello LM, Zangar RC, Tarasevich BJ, Karin NJ, Quesenberry RD, Bandyopadhyay S, Teeguarden JG, Pounds JG, Thrall BD. 2009. Macrophage responses to silica nanoparticles are highly conserved across particle sizes. Toxicol Sci. 107(2):553–569. doi: 10.1093/toxsci/kfn250.
- Wegman DH, Apelqvist J, Bottai M, Ekström U, García-Trabanino R, Glaser J, Hogstedt C, Jakobsson K, Jarquín E, Lucas RAI, et al. 2018. Intervention to diminish dehydration and kidney damage among sugarcane workers. Scand J Work Environ Health. 44(1):16–24. doi: 10.5271/sjweh.3659.
- Wesseling C, Aragón A, González M, Weiss I, Glaser J, Bobadilla NA, Roncal-Jiménez C, Correa-Rotter R, Johnson RJ, Barregard L. 2016. Kidney function in sugarcane cutters in Nicaragua–A longitudinal study of workers at risk of Mesoamerican nephropathy. Environ Res. 147:125–132. doi: 10.1016/j.envres.2016.02.002.
- Wesseling C, Aragón A, González M, Weiss I, Glaser J, Rivard CJ, Roncal-Jiménez C, Correa-Rotter R, Johnson RJ. 2016. Heat stress, hydration and uric acid: a cross-sectional study in workers of three occupations in a hotspot of Mesoamerican nephropathy in Nicaragua. BMJ Open. 6(12):e011034. doi: 10.1136/bmjopen-2016-011034.
- Wesseling C, van Wendel de Joode B, Crowe J, Rittner R, Sanati NA, Hogstedt C, Jakobsson K. 2015. Mesoamerican nephropathy: geographical distribution and time trends of chronic kidney disease mortality between 1970 and 2012 in Costa Rica. Occup Environ Med. 72(10):714–721. doi: 10.1136/oemed-2014-102799.
- Wijkström J, González-Quiroz M, Hernandez M, Trujillo Z, Hultenby K, Ring A, Söderberg M, Aragón A, Elinder C-G, Wernerson A. 2017. Renal morphology, clinical findings, and progression rate in Mesoamerican nephropathy. Am J Kidney Dis. 69(5):626–636. doi: 10.1053/j.ajkd.2016.10.036.
- Xu Q, Ji T, Gao S-J, Yang Z, Wu N. 2018. Characteristics and applications of sugar cane bagasse ash waste in cementitious materials. Materials. 12(1):39. doi: 10.3390/ma12010039.
- Zhang G, Ding C, Jiang X, Pan G, Wei X, Sun Y. 2020. Chemical compositions and sources contribution of atmospheric particles at a typical steel industrial urban site. Sci Rep. 10(1):7654. doi: 10.1038/s41598-020-64519-x.
- Zhu JY, Pan X. 2022. Efficient sugar production from plant biomass: current status, challenges, and future directions. Renew Sust Energ Rev. 164:112583. doi: 10.1016/j.rser.2022.112583.
- Zhuravlev LT. 2000. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf, A. 173(1-3):1–38. doi: 10.1016/S0927-7757(00)00556-2.