Abstract
This article discusses the ethical issues unique to the science and practice of chemistry. These issues arise from chemistry’s position in the middle between the theoretical and the practical, a science concerned with molecules that are of the right size to directly affect human life. Many of the issues are raised by the central activity of chemistry––synthesis. Chemists make thousands of new substances each year. Many are beneficial, but others are threats. Since the development of the chemical industry in the nineteenth century, chemistry has contributed to the deterioration of the environment but has also helped to reduce pollution. Finally, we discuss the role of codes of ethics and whether the current codes of conduct for chemists are adequate for the challenges of today’s world.
Editor’s Note
In this special issue of Accountability in Research, it is fitting and even necessary that the first article deals with important ethical issues facing the world, in general, and chemists and the chemical community, in particular. How do we preserve and improve the health and safety of the planet? What are the roles and consequences of the chemicals we produce, and how do we produce and manage them? What responsibilities do we have regarding the creation and manufacture of weapons—all of which are “chemical” in one way or another? In this article, we are privileged to have a professor of chemistry and an expert in ethics in science address these and related issues. Jeffrey Kovac has studied, lectured, and published extensively in this field for years. In addition, his CV includes theoretical research in the areas of thermodynamics and statistical mechanics of condensed matter—so, he’s a real scientist! He has also been active in advancing STEM (science, technology, engineering, and mathematics) to pre-collegiate students as director of Tennessee’s Governor’s School for the Sciences and Engineering and as director of the Tennessee Science Olympiad State Tournament. We are fortunate that Professor Kovac has joined our project.
Jeffrey I. Seeman
Guest Editor
University of Richmond
Richmond, Virginia 23173, USA
E-mail: [email protected]
What makes chemistry unique? And how does this uniqueness reflect on chemistry’s unique concerns with ethics?
As Roald Hoffmann argues, it is because chemistry is in the “tense middle,” occupying a space between several pairs of extremes (Hoffmann, Citation1995). Perhaps most importantly, chemistry has always inhabited a frontier between science and technology, the pure and the applied, the theoretical and the practical (Bensaude-Vincent and Simon, Citation2008). Unlike the other natural sciences, chemistry traces its origins to both philosophy and the craft tradition. Chemists are discoverers of knowledge and creators of new substances.
The objects of study in chemistry, molecules and the macroscopic systems made up of molecules, are intermediate between the very small, the elementary particles, and the very large, the cosmos. Chemical systems are the right size to affect humans directly, for better or worse. They are the building blocks of biological organisms; they are the substances we eat and drink; they are the drugs that have dramatically improved human health over the past century; they comprise the materials that we use to construct the products that we use daily; but they are also the environmental pollutants that can plague our world. Chemicals can also be used as weapons.
Being in the middle means that chemists face a unique set of ethical issues, which I will try to explicate in this article. These issues derive, in part, from the nature of chemistry as a science, a science that does not fit the neat picture that is drawn in the first chapter of textbooks. They also derive from the fact that ethics is an inquiry into right human conduct: What is a good life? Chemistry has contributed more to the betterment of human life than any other science, but at the same time has also contributed significantly to the deterioration of the environment. To develop a more complete picture of the nature of chemistry, we need to examine the distinction between pure and applied research more carefully.
PURE VS. APPLIED RESEARCH
As noted, chemistry has always been a mix of pure and applied science, a familiar distinction. The usual view is that pure science is undertaken for its own sake and that its outcome is knowledge or ideas as exemplified by the scientific article. Applied research, on the other hand, is practical. Its aim is to allow us to do things we could not otherwise do, and the outcome is some product or procedure. Applied research uses the results of pure research but does not contribute to the stock of fundamental knowledge.
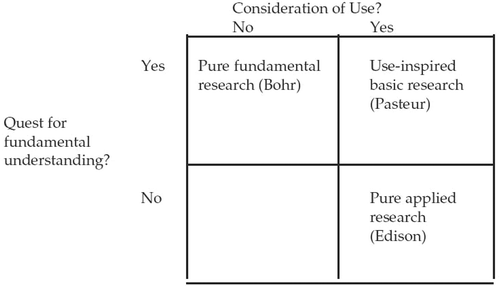
In his book, Pasteur’s Quadrant, Donald Stokes points out that this bipolar classification does not adequately describe the practice of science (Stokes, Citation1997). Instead of classifying research along a linear scale ranging from pure to applied, Stokes uses a quadrant model. He argues that there are two distinct motivations for research: the quest for fundamental understanding, traditionally called pure research, and the consideration for use, traditionally called applied research. Stokes puts these two motivations, both legitimate, on perpendicular axes as shown in . This leads to four distinct categories of research. The first category, where the primary motivation is the quest for fundamental understanding with no obvious use, is called Bohr’s quadrant. The work done in this quadrant is what is traditionally called pure research. Certainly, Bohr’s investigations into the fundamentals of quantum mechanics were not conducted with any practical end in sight. Traditional applied research lies in what Stokes called Edison’s quadrant where well-established science is used to solve practical problems. The most interesting category is Pasteur’s quadrant, where the quest for fundamental understanding is carried out in the context of a well-defined end use. Although much of Pasteur’s research was motivated by practical problems of French industry, in the process he essentially created the science of microbiology. The fourth quadrant, which Stokes did not name, is not empty, but it is not relevant to science policy.
Much of chemistry lies in Pasteur’s quadrant (McGrayne, Citation2001). Although the landscape is changing with the rise of biotechnology, chemistry has always been more connected to the practical than the other sciences. A large fraction of chemists are employed by industry. There is a thriving chemical industry, but no comparable “physical industry,” for example. To be sure, physicists, biologists, geologists, and other scientists also contribute to industrial progress, but not to the same extent as chemists. The usual view is that physicists explore the deep secrets of the universe; biologists are concerned with the evolution of life; chemists make useful stuff.
Take for example the work of Wallace Hume Carothers on condensation or step-growth polymerization at DuPont. Although Carothers was looking for a way to demonstrate the existence of macromolecules using well-known organic reactions, a controversial hypothesis at the time, his work was motivated by the possibility of a commercial product. In the process, he and his research team developed nylon (Hermes, Citation1996).
Carrothers’s work is an example of chemical synthesis, which is perhaps the central activity of chemistry. Chemists make new substances, thousands every year, which means that synthetic chemists change the material world. Most scientists study nature; chemists actually create many of the objects they study. Chemical synthesis confounds the classic categories of pure and applied research. As noted, the usual view is that the outcome of pure research is an idea or knowledge, but synthesis can be undertaken for its own sake to make a new molecule that is interesting not because of its potential use, but because it is particularly symmetric or because the synthesis is particularly challenging. Certainly, new knowledge is gained, but there is another outcome, a new substance which might turn out to be useful.
Another core activity of chemistry is analysis: qualitative and quantitative. Having synthesized or isolated a substance, it is necessary to figure out what one actually has in hand, beginning with the molecular formula and ending with the structure and properties. Until fairly recently, this was done using chemical methods, but these have largely been replaced by spectroscopic techniques, particularly nuclear magnetic resonance (NMR). Analytical chemistry is also concerned with mixtures, both identifying what components are present and determining their concentrations. Analytical chemistry is a combination of science and technology because it often involves the development of a new technique or scientific instrument. An important advance in analytical chemistry during the 20th century was the development of the pH meter which is another example of use-inspired research. Arnold Beckman’s development of the pH meter was stimulated by the needs of the California citrus growers to quickly and accurately determine the acidity of lemons.
Is instrument development pure or applied research? If it is pure research, exactly what new knowledge has been gained? In his provocative book, Thing Knowledge, Davis Baird argues that the physical instrument is actually a form of knowledge, which he terms encapsulated knowledge (Baird, Citation2004). The instrument uses scientific principles, but it also has to work so real-world concerns with materials and how they fit together are important. The pH meter needs two electrodes, the glass electrode sensitive to the hydrogen ion concentration and a reference electrode. Constructing and calibrating reliable electrodes is challenging (Ives and Janz, Citation1961). Like so many things in chemistry, it is both an art and a science. A pH meter also needs appropriate electronics to measure and display the voltage. As Baird points out, in the development of instruments, one runs up against the “thing-y-ness of things,” practical, engineering-like constraints.
The geographic center of chemistry is in the laboratory, a place where scientific knowledge and technique meet. Whatever the chemical experiment, synthesis, analysis, determination of physical, or chemical properties—technique is important. Early chemistry books were filled with recipes, ways to make or isolate substances. Getting a chemical reaction to work is often not easy. There is an old saying that “chemists think with their hands.” Chemistry has often been compared to cooking, usually by those who want to criticize chemistry for its lack of rigorous theory (Bensaude-Vincent and Simon, Citation2008). But for chemists, the close connection to experiment is one of the strengths of the science and also part of the fascination (Sacks, Citation2001).
The practice of chemistry, and all other sciences, raises ethical questions on several levels. Many of these questions arise from the day-to-day work in the laboratory: the responsible conduct of research. Others are related to the relationships of chemists to their colleagues and to the relationship between science and society. To put these questions in context, we need to understand the nature of professional ethics and the moral ideals that underlie the profession of science.
Professionalism and Moral Ideals for Science
In several articles, I have argued that science should be considered a profession based on a definition given by Michael Davis:
A profession is a number of individuals in the same occupation voluntarily organized to earn a living by openly serving a moral ideal in a morally-permissible way beyond what law, market and morality would otherwise require. (Davis, Citation2002)
Davis goes on to say that a code of ethics is a central feature of a profession. Chemistry fits this definition and codes of ethics have been adopted by the American Chemical Society (American Chemical Society, Citation2012), American Institute of Chemists (American Institute of Chemists, Citation1983), and the Royal Society of Chemistry (Royal Society of Chemistry, Citation2012). For the classic professions such as law and medicine, it is relatively easy to identify a moral ideal that underlies the profession and its code of ethics. For science, which is not client or patient oriented, the moral ideals are less obvious, particularly in light of the dual motivations for research. There are at least three different ideals corresponding to different aspects of the scientific process (Kovac, Citation2001, Citation2007). In the search for fundamental understanding, there must be an ideal that ensures the integrity of the research process. I have called this the ideal of the habit of truth (Bronowski, Citation1956). Whatever the motivation for the research, the day-to-day practice of science requires an absolute integrity. There is an enormous literature on the responsible conduct of research, so I will say little about it in this article, not because it is unimportant, but rather because most of the ethical issues are common to all the sciences. There are several good textbooks that provide an introduction to ethics in science (Shamoo and Resnik, Citation2009; Steneck, Citation2004; Seebauer and Barry, Citation2001; Kovac, Citation2004).
Because science is a form of public knowledge, an ideal of open communication is needed, the ideal of the gift economy. The gifts of the scientist’s research through open communication are what sustain the growth of knowledge. Secrecy is an anathema to the health and progress of science. The ideal of open communication based on the concept of the gift economy is more problematic, particularly when commercial interests are involved, so this will be the topic of some discussion below. Finally, because science has uses there must be an ideal that governs the choice of applications, what I have called, following Norman Care, shared-fate individualism which states that in the conditions of today’s world, service to others should take preference to self-realization in important life decisions including career choice and the choice of research problems (Care, Citation2000; Kovac, Citation2007). Because chemistry is a complex mixture of the fundamental and the applied and because the products of chemistry substantially affect our daily lives, ethical questions related to the uses of chemicals are a central concern. Certainly, other sciences have ethical concerns. Physicists, for example, are involved in the debates regarding the use of nuclear power. Biotechnology has raised questions related to the use of genetically-modified organisms and human cloning. All of these are important, but the products of chemistry, drugs, food additives, personal care products, detergents, as well as the effects of environmental pollution, permeate our lives.
Moral Communities
All chemists simultaneously belong to several communities and each has its own set of responsibilities (Sinsheimer, Citation1990). Each of us is a citizen of a national society with a history, goals and ideals. With citizenship comes obligations. Second, the chemist is a member of a profession subject to the broad professional ethics of science and the more specific codes of ethics of chemistry. Third, almost all chemists are employed by an institution, a college or university, a government or private research laboratory, a government agency, or a corporation. Each of these has its own culture and expectations. Because a large fraction of chemists are employed by industry, the influence of the institution is a more important factor in the ethics of chemistry than for almost any other branch of science. Fourth, all chemists are members of the human community and have the same moral obligations as all other people. Simultaneous membership in these different communities can certainly give rise to moral dilemmas. For example, when does chemist’s moral responsibility as a member of the larger human community take precedence over obligations to an institution or country? The moral landscape might be further complicated by the scientist’s religious beliefs and practices. Because chemistry is a secular pursuit, I will not consider the moral demands of particular faith traditions, but it is important to remember that religious beliefs can strongly influence certain moral decisions.
With this background concerning the unique nature of chemistry as a science “in the middle,” and the nature of professional ethics, we can now turn to a discussion of specific ethical issues. Because it is arguably the central activity of chemistry, we will begin with synthesis, but move from there to a discussion of the laboratory, dangerous substances, especially chemical weapons, and chemistry and the environment. We will end with a critical discussion of the professional codes of ethics of chemistry asking the question of whether they are adequate to deal with the ethical problems of today’s world.
ETHICS AND CHEMICAL SYNTHESIS
Chemical synthesis is an activity that can be carried out in all three quadrants. Some of the ethical questions are the same in all three situations, but the commercial production of chemicals does raise additional issues. I will first consider Bohr’s quadrant where the synthesis is carried out with no particular use in mind. It is often assumed that pure research conducted to gain fundamental understanding is obviously good, but in the case of chemical synthesis there are some complications. Synthesis of a new compound certainly adds to knowledge, but, as pointed out by Joachim Schummer, the new compound also adds to what he calls non-knowledge because the number of substances in the universe has increased and all the properties and reactions of the compound are unknown (Schummer, Citation2001). This presents an interesting ethical question. The production of new knowledge is widely accepted as being a good thing to do, but in the synthesis of a new compound one is also creating uncertainty, which is generally not thought to be good. In addition, every new substance is a new potential threat. Even if the original synthesis only creates a few milligrams the new substance is now known and can be made by others. Perhaps the new compound is a potent neurotoxin or an explosive or a precursor to such a substance. Certainly, it is never possible to envision all the possible outcomes, but it is essential to try. As Roald Hoffmann remarks, “The invention or implementation of a tool without consideration of its use is deeply incomplete” (Hoffmann, Citation2012a). This ethical perspective has become a political principle, the precautionary principle, which states that “if an action or policy has a suspected risk of causing harm to the public or to the environment, in the absence of a scientific consensus that the action or policy is harmful, the burden of proof that it is not harmful falls on those taking an act” (Precautionary principle, Citation2013).
In Pasteur’s and Edison’s quadrants, the synthesis is motivated by a possible use for the new substance. There are several ethical questions. First, and most obvious, what will the new substance be used for? There are at least six broad categories of chemicals used in modern society. First are structural chemicals, bulk plastics, and synthetic fibers. Second are agricultural products, pesticides, herbicides, and fertilizers. Third are drugs. The fourth category includes process chemicals, both for industrial and domestic uses. A fifth category is personal care products such as soaps and cosmetics. Finally, there are food-related chemicals. These would include bulk products such as salt and sugar but also food additives, such as flavorings and preservatives. Within these broad categories, however, there are a multitude of kinds of molecules that a chemist might try to make. The decision includes scientific, economic, and ethical considerations. These three factors will be weighed differently if the research is done in a university or government laboratory where the chemist has considerable control over what he or she does, or is it done in an industrial setting where the research agenda is largely determined by the company. My concern in this article is the ethical component.
I assume that the substance to be synthesized has a potential use that will be beneficial to society, perhaps a new drug. But, as noted earlier, every new molecule is a potential threat, so one important ethical question is whether the potential threat is sufficiently outweighed by the benefits. This can be a difficult problem. For example, all drugs have adverse effects—some of them minor, while others catastrophic. Some of these only affect a small percentage of those who take the drug; some affect essentially everybody. Depending on the disease to be treated, patients might be willing to suffer a side effect if the drug treats a terrible disease. Finally, the negative effects might not be easily foreseen.
A useful historical example is the chlorofluorcarbon refrigerants. At the time of their introduction, these were considered to be a great advance because they replaced toxic substances such as methyl chloride and sulfur dioxide as well as liquid ammonia. It was only much later that the negative environmental effects of the chlorofluorocarbons were discovered and a search for more benign alternatives initiated. What was originally considered a great advantage, chemical stability, was later shown to lead to a significant environmental problem, the destruction of ozone in the stratosphere. This is a common situation in the introduction of new chemicals. The evaluation is based on what they will replace. If the new substance has significant advantages and lacks most of the disadvantages of what is currently in use, it is accepted.
The problem of unforseen biological effects is complicated by the existence of chirality. Two compounds that are identical in every way expect that they are stereoisomers can have significantly different effects on an organism. The most familiar example is the sad story of (±)-thalidomide which was prescribed to help pregnant women with morning sickness between 1957 and 1962 but was withdrawn from the market when it was found to be a potent teratogen causing multiple kinds of birth defects. Thalidomide was sold as a racemic mixture as were most drugs at the time because of the cost of separating the left- and right-handed forms compared to the then lack of knowledge about the differences in physiological effects of the two enantiomers. Research done after the drug was removed from the market suggests that only one of the enantiomers is teratogenic, but the situation is complicated by the fact that the “harmless” enantiomer converts to the “harmful” form under physiological conditions. The thalidomide tragedy has led to stronger regulation on drug testing and to the increased production of single enantiomer drugs (Hoffmann, Citation1995, Chapter 27; DeCamp, Citation1989). Although drugs and some agricultural products are closely regulated, most chemicals are not, so there is significant risk of unforseen consequences with every new substance.
Purity is another serious issue, particularly in commercial products. Most chemical reactions do not result in a 100% pure product. Unwanted side products are usually formed, and they can be difficult and expensive to remove. Sometimes, these impurities, even when present in very low concentrations, are deadly. The familiar example is dioxin, a highly toxic compound that is an inevitable contaminant of the widely used herbicide 2,4,5-T. Dioxin is found in varying concentrations in all commercial preparations of the herbicide. In principle, it can be removed, but at what cost? At sufficient exposures dioxin is a serious health hazard, but the practical question is whether the level of contamination is large enough to pose a real danger to the public health.
What Should Chemists Synthesize?
Another ethical consideration for use-inspired research is based on the moral ideal of shared-fate individualism. Several years ago, Freeman Dyson published two articles in which he suggested that science was in trouble with the public because of a poor choice of goals (Dyson, Citation1993, Citation1997). Rather provocatively, he stated that scientists should be working on “necessities for the poor” rather than “toys for the rich.” A philosophical basis for this suggestion was given by Norman Care (Care, Citation2000). Care analyzes the situation in today’s world which includes vast disparities in quality of life particularly between the developed and developing countries but also between socioeconomic groups in our own country. To this, he adds a concern for future generations and for the future of the planet. (A similar analysis has been given by John Forge, in Forge, Citation2008). In light of the conditions of today’s world, Care suggests that it should be a moral imperative that what he calls competent individuals, those who have significant talent, education, and resources, to put service to others ahead of self-realization in significant life decisions. Chemists certainly meet Care’s criteria for being competent individuals based on their advanced educations and continuing professional experience. I have suggested that for scientists Care’s principle means that in the choice of research problems, necessities for the poor should take priority over toys for the rich (Kovac, Citation2007). Further, it means that doing research to ensure the long-term well-being of the population and the planet should take on the highest priority. For example, if one is synthesizing new drugs, treatments for diseases that affect large number of people, such as malaria, should take priority over designing a drug to treat a relatively rare disease suffered only by affluent people in developed countries of the world. On the other hand, it can be argued that it is necessary for pharmaceutical companies to produce high-value drugs to maintain their profitability so that they can engage in research on drugs to treat diseases in underdeveloped countries. This is certainly one of the ethical tensions of commercial science.
Shared-fate individualism is a moral ideal, not a moral rule. Ideals represent our best aspirations, but no one should be blamed for not reaching an ideal. The choice of research problems is not always in the hands of the individual, particularly for chemists who work in industry or even in government laboratories. A second constraint is that the individual chemist may not have the background or research competence required to tackle a socially important problem. Finally, it is often hard to predict the ultimate effects of a particular invention. A drug designed to cure a particular disease might turn out to be effective for a completely different condition. A “toy for the rich” might eventually become something that makes life better for large numbers of non-affluent people. Many modern electronic devices, such as the personal computer and the cell phone, which were once only available to the affluent, have become so inexpensive that they are available to large numbers of people world-wide and have changed their lives for the better.
Ethics in the Laboratory
Chemistry is rooted in the laboratory where ideas, knowledge, and technique come together. Getting a chemical reaction to work satisfactorily, in a reasonable amount of time, and with a good yield can be tricky. A classic volumetric analysis requires careful use of glassware: volumetric flasks, pipettes, and burettes. Accurate weighing is an essential part of chemistry.
One of the core principles of science, and an important moral rule, is to describe experimental procedures completely and carefully so that another person can reproduce the results. Anyone who has tried to reproduce an experiment described in the chemical literature knows that this is easier said than done. Sometimes, an experiment cannot be reproduced because it really did not happen that way; the results are fabricated. Usually, however, the reasons do not involve scientific misconduct. Often experimental details are unintentionally omitted due to carelessness or because they seem obvious or are part of the usual routine of a particular research group or because of poor record keeping. A more ethically interesting reason is that some people are better at doing experiments than others. They are more careful or just seem to have a knack for making things work. For example, apparently the only person who could get Robert Boyle’s air pump to work properly was the man who made it, Robert Hooke (Shapin, Citation2010). In chemistry, we say that some people just have “magic hands” (Stemwedel, Citation2006). The difficult ethical issue is the following one. Can we call an experiment reproducible if the only people who can get it to work properly are those with magic hands? What is the responsibility of the original research group to ensure that the procedure can be reproduced by an average chemist? This is a problem in all of laboratory science, but it is perhaps most important in chemistry which involves as much art as science.
Some related issues have been recently discussed by Carlson and Hudicky who discuss malpractice in organic synthesis (Carlson and Hudlicky, Citation2012). They distinguish malpractice from scientific misconduct. Malpractice is not a deliberate attempt to deceive, but instead it is a result of improper practice. They list three classes of improper practice in organic synthesis: improper experimental protocols, improper methods used in characterization of compounds, and the lack of proper citations to previous work. Probably the greatest concern is improper characterization which can lead to errors in structural assignments. The primary method used is NMR, a powerful tool, but not always definite. When syntheses are carried out in microscale, it may be impossible to obtain some of the classic data, such as a percent composition by combustion analysis, or even a good melting point. Without a definitive characterization of the product, the results may not be trustworthy.
Analytical chemistry also presents ethical challenges in the laboratory. As discussed above, many synthetic commercial chemical products contain impurities, some of which are dangerous. Products derived from natural sources are usually complex mixtures. Sea salt, which has recently gained popularity in gourmet cooking, is mainly sodium chloride, but also contains small concentrations of other cations and anions. Beverages such as coffee, tea, or wine can contain hundreds of components in widely varying concentrations. The task of the analytical chemist is to determine what components are present, which involves separation and identification, and then to determine how much is present. Knowing what is present is an important question in product safety for example.
There are several aspects of the analysis process that can raise ethical questions. One is the problem of detection limit. All chemical analyses have a lower limit of detection so the question of whether a trace component is part of a mixture can only be answered by saying that, if it is there, it is present in a quantity less than the detection limit. If the suspected contaminant is pernicious, this can put the analyst in a difficult position. Here is a simple example (Kovac, Citation2000). Suppose the analytical chemist is called to testify in a lawsuit where the crucial issue is whether a particular contaminant was present. The question from the lawyer would be, “Was substance A present?” In an adversary proceeding, the expected answer is either “yes” or “no.” But the professional ethics of science require a more nuanced answer like, “It was not detected, but the method used can only detect concentrations greater than X.” Although the analyst could answer “no” without committing perjury, such an answer would not be scientifically responsible.
Another issue is chemical calibration (Edmonds, Citation1999). Quantitative determination of the concentration of a substance often requires a chemical calibration using known amounts of the substance to be determined, which may have to be synthesized, in a matrix that is sufficiently similar to that of the unknown sample. The question of what is sufficiently similar is a matter of judgment and, as I and others have argued, scientific judgments have both a technical and a moral component. Depending on the circumstances, getting it wrong can have serious consequences.
Chemical Weapons and Other Dangerous Substances
A very difficult ethical question for chemists is whether to conduct research on chemical weapons. These substances are banned under the Chemical Weapons Convention of 1993, which has been signed by nearly every country in the world including the United States. The Chemical Weapons convention prohibits the development, production, stockpiling, and use of chemical weapons. It also mandated the destruction of all chemical weapons and destruction or conversion of all production facilities by 2007. Progress in accomplishing this latter goal has been delayed, but most of the stockpiles of these weapons have been destroyed and the production plants deactivated.
In recent years, there has been less fear of the use of chemical weapons in wars between nations, but more concern about their production and use by terrorist organizations or by what are sometimes called rogue states. Research on chemical weapons continues, for example at the U.S. Army Medical Institute of Chemical Weapons Defense (Chemical Weapons Defense, Citation2013). Of course, much of this research is classified, but even defensive research might involve the development of new chemical weapons with the goal of finding appropriate counter measures.
There is also research on what are being called non-lethal chemical weapons which include anti-traction agents and malodorants or other novel chemical agents (Guardian, Citation2008). There is some question as to whether these new non-lethal agents violate the Chemical Weapons Convention because it is not known whether they are toxic at high doses. Malodorants might also have adverse effects other than toxicity, adverse psychological effects for example.
The broader ethical question as to whether scientists should engage in war-related research is complex, but chemical weapons raise that question in stark terms (Kovac, Citation2013). The use of chemical weapons, initially chlorine gas by Germany in World War I and subsequently chlorine and other compounds by the Allies, produced enormous moral outrage. One reason is that chemical weapons pose a greater risk to innocent bystanders than conventional weapons. Unlike bullets, they cannot be precisely aimed. Once the cloud of poison is released, everyone who encounters it is affected. Even in the just war tradition, there is a prohibition against using weapons that are “evil in themselves” (no means mala in se). Chemical weapons such as nerve agents seem to fit this description, but so do weapons such as napalm that was extensively used in the Vietnam War. The broad scale use of herbicides such as the infamous “Agent Orange,” also used in Vietnam, also seem to be inhumane. Because chemists are the ones that develop these substances, chemists must confront the ethical implications.
Weapons research is an area where the obligations of the different moral communities to which the chemist belongs can come into conflict. As citizens, chemists may feel an obligation to contribute to the national defense of their country including weapons development. As employees of a particular institution, they may be pressured to work on weapons related research. On the other hand, as a member of the human community, the chemist might feel that use of chemical weapons is immoral. Unfortunately, the professional codes of ethics of chemists do not help to resolve this dilemma. This is a question that will be discussed in more detail below.
Weapons research is a particularly challenging issue, but there are others. For example, tobacco use is a serious health risk. Should a chemist engage in research to produce safer cigarettes? On the one hand, it might be argued that such research would reduce the risks of smoking. On the other hand, the chemist is helping to perpetuate an industry that produces an intrinsically harmful product. Another example is a better abortion drug, the so-called “morning-after pill.” Here is where personal morals, perhaps derived from religious beliefs, come into play.
Environmental Pollution and Green Chemistry
Whatever the substance to be made, there are ethical issues related to the method of production. Since its inception, the modern chemical industry has been responsible for widespread environmental degradation (Bensaude-Vincent and Simon, Citation2008). Well publicized accidents such as the disaster in Bhopal, where thousands of Indians were poisoned by methyl isocyanate leaking from a Union Carbide plant, have added to the negative public image of chemistry.
There are practical, economic, and ethical reasons to improve safety at chemical plants and to reduce the environmental impact of the production of chemicals. The effort to make chemical production more environmentally benign is usually called Green Chemistry. I think we can stipulate that the chemical industry will adopt cleaner and greener methods of production if they make economic sense or are required by government regulation or severe public pressure. The question is who will develop the new chemistry. Certainly, some of the research will take place in industrial laboratories, but as recently argued by Roald Hoffmann, “the spiritual center of chemistry in our country remains in the research universities. Where people are taught, values are formed” (Hoffmann. Citation2012b). The ideal of shared fate individualism suggests that chemists in research universities should focus more of their research efforts on green chemistry: atom and energy economy and benign solvents. It will be a hard sell because there is a prejudice that such research is boring, routine industrial-style research to find a new way to make something ordinary. What self-respecting academic organic chemist would want to find a greener synthesis of ibuprofen? What glory in that?
As Hoffmann points out, polymer chemistry once had a similar reputation of being a mundane, mainly industrial field. Today, because of the research accomplishments of some outstanding academic chemists polymer chemistry has become a respectable academic endeavor. If a few prominent academic chemists responded to the moral ideal of shared-fate individualism and showed that Green Chemistry is both scientifically interesting and attractive to graduate students, the field might blossom. Adding case studies of the successes of green chemistry to the curriculum in textbooks and laboratory courses would also help interest students in this field.
The current environmental crisis raises other scientific and ethical questions. First is environmental remediation, cleaning up the messes that industrial chemistry has made over the past century and more. A second one is what has come to be called sustainability, finding ways to use renewable resources rather than petroleum as both fuels and feedstocks. There is an obvious link to global warming, reducing the production of greenhouse gases. There has been progress; the development of the modern catalytic converter has done much to reduce air pollution, but much more needs to be done. Bensaude-Vincent and Simon argue that we need a new chemical culture in relationship to the environment, one that proscribes the causes of dangers to human and environmental health (Bensaude-Vincent and Simon, Citation2008). They suggest that chemists embrace the Hippocratic principle of medical ethics: first, do no harm. They extend Hoffmann’s argument that the chemistry needs to consider the possible uses of a new substance or process to say that chemists should anticipate the long-term negative consequences of their actions.
Codes of Ethics
The Chemist’s Code of Conduct of the American Chemical Society presumably applies to all chemists (American Chemical Society, Citation2012). It lists the responsibilities of chemists to various groups beginning with the responsibilities of chemists to the public:
Chemists have a professional responsibility to serve the public interest and welfare and to further knowledge of the science. Chemists should actively be concerned with the health and welfare of coworkers, consumers and the community. Public comments on scientific matters should be made with care and precision, without unsubstantiated, exaggerated, or premature statements. (American Chemical Society, Citation2012)
Michael Davis contrasts this statement with several of the provisions of the code of ethics for engineers adopted by the Accreditation Board of Engineering and Technology (ABET) which presumably applies to all engineers (Davis, Citation2002). One of the fundamental principles of the ABET code is the following one:
Engineers uphold and advance the integrity, honor, and dignity of the engineering profession by:
1. using their knowledge and skill for the advancement of human welfare.
The first “fundamental canon” of the ABET code states as follows:
1. Engineers shall hold paramount the health, safety, and welfare of the public in the performance of their professional duties (ABET, Citation1977).
An identical statement is the first requirement of the Code of Ethics of the American Institute of Chemical Engineers (AIChE, Citation2006).
Although the two codes are similar, there are important differences. Chemists are supposed to be “actively concerned with the health and welfare of coworkers, consumers and the community” (American Chemical Society, Citation2012), but engineers are to “hold paramount the health, safety, and welfare of the public” (ABET, Citation1977). Further, engineers should advance human welfare, not just be actively concerned with it. As Davis points out, the ACS code lists various responsibilities with no guidance as to how to deal with conflicts. If the chemist’s responsibility for welfare comes in conflict with the chemist’s responsibility to an employer, the code does not say which should have priority. For the engineer, the priorities are clear. Take care of the public first; everything else comes second.
Davis raises an important issue for the chemical profession. In a world where the public health and welfare is threatened by the effects of chemicals, particularly environmental pollution, should the code of ethics be revised to make the chemist’s responsibility to the health and well-being of the public and to the environment a higher priority. Should chemists also be encouraged to use their talents for the advancement of human welfare? The profession needs to be held to a higher standard, and the codes of ethics for chemists need to be revised.
CONCLUDING REMARKS
Throughout its history, chemistry has made significant contributions to human progress but with those successes have come problems, especially problems of environmental pollution. Synthetic chemicals have become a major part of our lives. The circumstances of today’s world provide both scientific and ethical challenges for chemistry. In this article, I have outlined many of those challenges focusing on ethical questions that derive from the unique nature of chemistry as a science. Perhaps the most important ethical issues involve chemical synthesis. When a new substance is created, chemists need to think about the long-term effects of that compound. If the substance is made commercially, we need to develop “greener” methods of production that conserve nonrenewable resources and minimize the effects on the environment. On a broader level we need to consider the problems of today’s world and work on problems that will improve the human condition, particularly the lives of those in underdeveloped countries. Chemists also need to think carefully about their role in preserving the health and safety of the planet, including their role in the creation of weapons.
More than the other sciences, chemistry is centered in the laboratory so it is important that laboratory practice adhere to the highest professional and ethical standards. Finally, the chemical profession through the professional societies needs to reexamine the codes of ethics to ensure that they respond to the practical and ethical challenges of today’s world in which synthetic chemicals touch essentially every part of our daily lives.
Of course, chemists face the same ethical challenges that all other scientists, and indeed all human beings must confront. Our future depends on our willingness to ask and answer these crucial ethical questions.
ACKNOWLEDGMENTS
I am grateful to Jeffrey I. Seeman, Roald Hoffmann, Stan Guffey, and Christopher Pynes for reading drafts of this article and providing suggestions for its improvement.
FUNDING
The author thanks the Innovative Grant Program (IGP) of the American Chemical Society. The IGP has provided the funds that permit permanent open access to this article and all articles in this special issue of Accountability in Research titled, “Ethics and Responsible Conduct of Research within the Chemical Community. Ideas and Experiences Worth Sharing.”
REFERENCES
- ABET. 1977. Code of Ethics of Engineers, https://engineering.purdue.edu/MSE/Academics/Undergrad/ethics.pdf (Accessed June 3, 2015).
- AIChe. 2006. Code of ethics. http://sts.aiche.org/content/code-ethics (accessed August 1, 2013).
- American Chemical Society. 2012. The chemical professional’s code of conduct. http://www.acs.org/content/acs/en/careers/profdev/ethics/the-chemical-professionals-code-of-conduct.html (accessed July 29, 2013).
- American Institute of Chemists. 1983. Code of ethics. http://www.theaic.org/about_ethics.html (accessed July 29, 2013).
- Baird, D. 2004. Thing knowledge: A philosophy of scientific instruments. Berkeley and Los Angeles: University of California Press.
- Bensaude-Vincent, B., and J. Simon. 2008. Chemistry: The impure science. London: Imperial College Press.
- Bronowski, J. 1956. Science and human values, Revised Edition. New York: Harper Torchbooks.
- Care, N. S. 2000. Decent people. Lanham, MD: Rowman and Littlefield.
- Carlson, R. and T. Hudlicky. 2012. On hype, malpractice, and scientific misconduct in organic synthesis. Helvetica Chimica Acta 95:2052–2062.
- Chemical Weapons Defense. 2013. Research for the warfighter. http://chemdef.apgea.army.mil/ (accessed January 5, 2015).
- Davis, M. 2002. Do the professional ethics of chemists and engineers differ?. Hyle 8:21–34.
- DeCamp, W. H. 1989. The FDA perspective on the development of stereoisomers. Chirality 1:2–6.
- Dyson, F. 1993. Science in trouble. The American Scholar 62: 513–522.
- Dyson, F. 1997. Can science be ethical. New York Review of Books 44 (6).
- Edmonds, T. E. 1999. A philosophical approach to analytical chemistry. Unpublished manuscript.
- Forge, J. 2008. The responsible scientist: A philosophical inquiry, Pittsburgh: University of Pittsburgh.
- Guardian. 2008. US weapons research is raising a stink. http://www.guardian.co.uk/science/2008/jul/10/weaponstechnology.research (accessed July 1, 2013).
- Hermes, M. E. 1996. Enough for one lifetime: Wallace carothers, inventor of Nylon, Washington, DC: American Chemical Society and Chemical Heritage Foundation.
- Hoffmann, R. 1995 The Same and not the same, New York: Columbia University Press.
- Hoffmann, R. 2012a. Ethics and science. In Roald Hoffmann on the Philosophy, Art, and Science of Chemistry, eds. J. Kovac and M. Weisberg, 360–376. New York: Oxford.
- Hoffmann, R. 2012b. The material and spiritual rationales are inseparable. In Roald hoffmann on the philosophy, art, and science of chemistry, eds. J. Kovac and M. Weisberg, 339–346. New York: Oxford.
- Ives, D. J. G., and G. J. Janz, eds. 1961. Reference electrodes. New York and London: Academic Press.
- Kovac, J. 2000. Science, law, and the ethics of expertise. Tennessee law review 67:397–408.
- Kovac, J. 2001. Gifts and commodities in chemistry. Hyle 7:141–153.
- Kovac, J. 2004. The ethical chemist: Professionalism and ethics in science. Upper Saddle River: Pearson Education
- Kovac, J. 2007. Moral rules, moral ideals and use-inspired research. Science and Engineering Ethics 13:159–169.
- Kovac, J. 2013. Science, ethics and war: A pacifist’s perspective. Science and Engineering Ethics 19:449–460
- McGrayne, S. B. 2001. Prometheans in the lab: Chemistry and the making of the modern world. New York: McMillan
- Precautionary principle. 2013. Precautionary principle. http://en.wikipedia.org/wiki/Precautionary_principle. (accessed August 12, 2013).
- Royal Society of Chemistry. 2012. Regulation of the profession and code of conduct. www.rsc.org/images/code-of-conduct_tcm18-5101.pdf (accessed June 3, 2015)
- Sacks, O. 2001. Uncle tungsten. New York: A. A. Knopf.
- Schummer, J. 2001. Ethics of chemical synthesis. Hyle 7:103–124.
- Seebauer, E. G. and R. L. Barry. 2001. Fundamentals of ethics for scientists and engineers. New York and Oxford: Oxford University Press.
- Shamoo, A. E. and D. B. Resnik. 2009. Responsible conduct of research, second edition. Oxford, New York: Oxford University Press.
- Shapin, S. 2010. Who was Robert Hooke?” In Never Pure, ed. Steven Shapin, Baltimore: Johns Hopkins Press.
- Sinsheimer, R. L. 1990. Scientists and Research. In Preventing a Biological Arms Race, ed. S. Wright, 71–77. Cambridge: MIT Press.
- Stemwedel, J. 2006. Reproducibility and retracted papers. http://scientopia.org/blogs/ethicsandscience/2006/06/15/reproducibility-and-retracted-papers/ (accessed June 3, 2015).
- Steneck, N. H. 2004. Introduction to the responsible conduct of research. Washington, D.C.: U. S. Government Printing Office.
- Stokes, D. E. 1997. Pasteur’s quadrant. 46–49. Washington, D.C.: Brookings Institution Press