ABSTRACT
This study evaluates nutritive, morphological and agronomic characteristics of forage maize predicted by using a high-quality near-infrared (NIR) spectrometer and an NIR hyperspectral-imaging technique using partial least squares (PLS) regression models. The study includes 132 samples of dried milled whole-plant homogenates of forage maize with variation in maturity, representing two growing seasons, three locations in Sweden and three commercial maize hybrids. The samples were measured by a classical sample cup NIR spectrometer and by a pushbroom hyperspectral-imaging instrument. The spectra and a number of variables (crude protein, CP, neutral detergent fibre, starch, water soluble carbohydrates (WSC) and organic matter digestibility), morphological variables (leaves, stems & ears) and crop yield were used to make PLS calibration models. Using PLS modelling allowed the determination of how well maize variables can be predicted from NIR spectra and a comparison of the two types of instruments. Most examined variables could be determined equally well, by both instruments, but the pushbroom technique gave slightly better predictions and had higher analytical capacity. Predictions of CP, starch, WSC and the proportions of ears in the maize gave robust. The findings open new possibilities to further utilise the technology in plant breeding, crop management, modelling and forage evaluation.
Introduction
Introduction of new hybrids has extended the area suitable for growing forage maize (Zea mays L.) to more northern latitudes (Mussadiq et al. Citation2013). Maize silage is an important feed for ruminants in many temperate parts of the world being increasingly cultivated in northern Europe with large potential for further northern expansion of the growing area (Nkurunziza et al. Citation2014). When maize is used as forage, the whole plant is cut at a stubble height of 20 cm, chopped into small pieces and ensiled prior to storage and feeding. Maize is different compared to other forages such as perennial grasses, due to its physiology and its large number of commercial hybrids, with large variation in morphology, relative maturity, yield and nutritive qualities. Thereby the maize silage shows large variation in chemical composition, nutritive characteristics and in morphological proportions in terms of contributions of stems, leaves, cobs and kernels (Mussadiq et al. Citation2013). The proportions of morphological fractions may affect the nutritional value of the forage (Cox & Cherney Citation2001a) and therefore represent traits that are of interest in terms of improvement in plant breeding and animal feeding. The proportions of morphological fractions are functions of the phenological development of the plants (e.g. Hetta et al. Citation2012), but also depend on the genetic background of the plant and on the agronomic practices, such as seeding rates and fertilisation (e.g. Cox & Cherney Citation2001b; Budakli Çarpici et al. Citation2010).
Maize hybrids are evaluated for their agronomic performance in national/regional field trials, where a large number of hybrids are harvested, collected and analysed for their nutritional qualities indirectly by near-infrared spectroscopy (NIRS), as the standard method for quality assessment. The results are used in plant breeding programmes for the evaluation of hybrids and by the farming community to select the most suitable hybrids (e.g. Hagman et al. Citation2016). Data of chemical composition, agronomic performance and morphological compositions may also be used in combination with metrological data for growth modelling (e.g. Nkurunziza et al. Citation2014) and development of decision support systems for efficient maize production. Fiorani and Schurr (Citation2013) have described the need for large databases that combine phenotypic data with environmental data to evaluate and compare genotypes. The NIRS technique is one of the spectral methods that can provide phenotypic data for such data bases. The NIRS analyses have mainly been focused on the prediction of the nutritional qualities such as concentrations of starch and neutral detergent fibre (NDF), and digestibility, but few applications have tried to predict the morphology of the plants or the agronomic performance from the NIRS information. Development of new NIR spectrometers and NIR hyperspectral-imaging instrument enables scanning of large number of samples in broad wavelength band with much less effort compared to previous techniques. Spectral information of crops in combination with new multivariate prediction models based on novel reference data such as phenotypic information may enable proximate predictions of plant characteristics such as morphology. The prediction models give instant and cost-efficient access to information that previously has been estimated mainly with laborious direct methods such as gravimetric proportions of morphological fraction (Mussadiq et al. Citation2013) and the dry matter (DM) yield.
The aim of this study was to evaluate predictions of nutritive, morphological and agronomic characteristics forage maize by using two applications of NIRS spectrometry, a high-quality NIR spectrometer and a NIR hyperspectral-imaging instrument.
Materials and methods
Forage maize samples
The plant material in this study originates from a previously published agronomic study on forage maize. A short summary of the treatment of the plant material is presented below and the full description can be found in the work of Mussadiq et al. (Citation2012, Citation2013). Three forage maize hybrids with increasing FAO indices (Zscheischler et al. Citation1990) for hybrid maturity were used in the study: Avenir (FAO 180) from Syngenta Seeds (Basel, Switzerland), and Isberi (FAO 190) and Burli (FAO 210), both from Caussade Semences (Caussade, France). The plants were grown during the summer of 2008 and 2009 at three locations in Sweden: Kristianstad (56°04′13″N; 14°19′11″E, 20 m above sea level), Skara (58°23′15″N; 13°29′03″E, 113 m above sea level) and Västerås (59°36′47″N; 16°38′07″E, 3 m above sea level). All hybrids were harvested at four occasions each year and on each location.
The plant material was treated as described by Mussadiq et al. (Citation2012, Citation2013) after harvest and the DM proportions of stems, leaves, kernels and cobs were determined by manual fractionation, and reconstituted whole-plant samples were produced from subsamples of the morphological fractions from each plot based on their mean DM proportion, as described by Fairey (Citation1983) and Hetta et al. (Citation2012). In order to simplify the predictions of the morphological parameters in this study, a new fraction, ears, was created for the present by combining the fractions cobs and kernels. This procedure resulted in four fractions representing stems, leaves, ears and whole plants (reconstituted).
Physical and chemical measurements
The samples (about 100 g each) were kept in glass jars (volume 1 l) with a circular metal lid (Diameter 85 mm and height 10 mm). Prior to any analytical procedure, each jar was carefully agitated by hand for about 60 s and representative subsamples were used for analysis. The maize was analysed for concentrations of crude protein (CP) (AOAC Citation1983), NDF with addition of sulphite and amylase (Chai & Udén Citation1998), and water soluble carbohydrates (WSC) and starch (Larsson & Bengtsson Citation1983). The in vivo organic matter digestibility (OMD) was estimated from in vitro OMD (Lindgren Citation1979). The expected standard deviation (SD) for repeated measurements with reference methods (Stdevref) are presented in . The expectations are based on the reported Stdevref in the descriptions of the analytical methods. The reported Stdevref values of the morphological proportions and the agronomic performance are based on estimates from previous studies of the material (Hetta et al. Citation2012; Mussadiq et al. Citation2013).
Table 1. The nutritional characteristics (g kg−1 DM unless otherwise stated), proportions of morphological fractions (% of DM) and agronomic performance (kg DM/ha) of the forage maize.
Near-infrared spectrophotometer measurements
The measurements were carried out on a Foss 6500 spectrometer (FOSS AS, Hillerød and Denmark) using standard Foss round cuvettes (diam. 32 mm) with a Teflon disc as backside lid. All measurements were conducted in triplicates. A standard instrument calibration with a Spectralon standard as white reference was done before each sample was scanned. The spinning cup (round cuvette) was collecting 32 scans, which were used to produce an average absorbance spectrum in the range 400–2498 nm. The measurements were done in triplicate by refilling the sample cup with same sample from the same jar. Each measurement took approximately 2 min, but filling and cleaning of the sampling cups was the slowest step (3 min). This means that each average spectrum obtained took 10–15 min to acquire. The average of the triple measurements was used for data analysis, resulting in a file of 132 samples × 1050 wavelengths. The data matrix was imported into a Matlab file. In order to have similar spectral data from both instruments, all wave lengths below 1000 nm were removed from the Foss recordings. The Hyperspectral system measures nominally between 1000 and 2500 nm.
Hyperspectral-imaging system
The samples were measured using an Umbio Inspector (Umbio AB, Umeå, Sweden) linescan or pushbroom instrument equipped with a moving belt. The instrument measured images of size (variable length × 320 pixels width) in 256 wavelength bands 937–2542 nm. The resulting pixel size was 0.48 mm × 0.48 mm. The 22.5 mm Sisuchema objective gave a field of view of 155 mm. Illumination was with two rows of quartz-halogen lamps. The calibration was based on a rectangular piece of Spectralon for a white reference and closing a shutter for dark reference for each measured sample. Descriptions of the pushbroom principle are given in the literature (e.g. Grahn & Geladi Citation2007).
Each jar was carefully agitated by hand for about 60 s and a representative subsample of about 10 g was put in the lid of each jar. The lid with sample was put on the conveyor belt and measured. The image collection took about 15 s, and the complete procedure for sample preparation took about 90 s per sample. Each image had approximately 125,000 pixels, of which some 25,000 were maize pixels and the rest represent background. The average spectra over the images (maize pixels) were calculated in the Evince software (Umbio, Umeå, Sweden) and transferred to a Matlab file.
Sampling aspects
The Foss cuvettes had a surface of 800 mm2. Whether the complete surface was taken into account in the scanning is not known, but the cuvettes were rotated and 32 scans were corrected and averaged. By taking three replicates, an active area of 2400 mm2 was sampled. The lids imaged in the Umbio instrument presented an area of about 5600 mm2 that was completely used. The thickness was in both cases enough to prevent any radiation to be transmitted to the bottom of the vessel return and be picked up by the detector(s). Based on the above data, the sampled area was different for the two tested instruments. The imaging technique measured an area about twice as large as that of the Foss cuvette.
Data analysis
The obtained dataset for physical and chemical data contained 130 objects (two samples had each two missing data points) by with nine reference variables (). The data set was subjected to a principal component analysis (PCA) analysis after mean-centring and variance scaling. Data analysis was done using Matlab (MathWorks, Natick, MA, USA) and the PLS-Toolbox (Eigenvector, Wenatchee, WA, USA).
A number of spectral pre-treatments were tested for the NIR spectra and found not to produce much difference in the quality of the PLS models and they were therefore discarded (Beebe et al. Citation1998; Næs et al. Citation2002) .The multivariate scatter correction (MSC) and standard normal variance (Burger & Geladi Citation2007) were slightly better than using only mean-centring or unit variance scaling combined with mean-centring. Therefore only MSC correction with mean-centring was retained as the final procedure. This procedure simplifies the tables. The PLS models can be written as: y = Xb + f, where X is the matrix of calibration spectra, b is the vector of regression coefficients, y is the vector of response variables (the variables defined in ) and f is a vector of residuals.
A test set was made from each third of the 132 samples/spectra, giving a subset of 44 samples/spectra. The remaining 88 samples/spectra formed the training set. With 88 calibration samples, no more than 9–11 components should be used for modelling. Models with fewer components are to be preferred because they are more robust. Components were selected within the range 1–11 by looking at the percentage of variance explained for the response variable for each component, when the percentage of variance was much less than 1%, the component was not used for prediction.
The diagnostics of the selected PLS model are the coefficient of determination for the calibration (R2c) and the root mean squared error of calibration (RMSEC). For the test set similar coefficient of determination for the test (R2t) and root mean squared error of prediction (RMSEP) were given. An R2c of at least 2/3 (67%) should be obtained with five components or less. For R2t, at least 1/2 (50%) should be obtained after the number of components chosen. In general, values of R2c and R2t of 90% or more indicate good models. Values in the 80–90% range are acceptable. All PLS models with values below are open for improvement or just not good enough for robust predictions.
The variance of prediction expressed as SD (e.g. RMSEC or RMSEP) has a limited usefulness. A small SD is no good if the data are in a small range. What we want is a small SD in a large range of results. The range error ratio (RER) and ratio of prediction to deviation (RPD) indexes are therefore useful indicators of model performance (Williams Citation1987). The RER index represents the ratio of the range and the error in the PLS prediction from cross validation or from a test set. If the prediction error is the same as the range (RER = 1), then no prediction is achieved because all results could have been random; therefore high RER values are preferred. The RER is calculated using the range of the values, which means, it is sensitive to outliers (Williams Citation1987). A robustified range procedure was used in the calculation where the three highest and three lowest response values in the calibration set were left out to compensate for potential outliers. There are no absolute rules, but an RER of 10 or more is to be considered very good, and an RER between 5 and 10 is a candidate for improvement and an RER below five is considered bad. Another indicator on how a good PLS model performs is the ratio of prediction to deviation (RPD). The RPD is the ratio of standard error of prediction corrected for bias to standard deviation of the reference data used in the validation (Cozzolino et al. Citation2006; Liu & Hana Citation2006).
Results and discussion
Correlations between variables and PCA
From the range of the starch concentration in the plant material, it is clear that the material analysed represents a large variation in plant maturity, depending on harvest occasion, hybrid and site, which is described in the previous papers (Hetta et al. Citation2012; Mussadiq et al. Citation2012, Citation2013).The nine variables describing the maize reported in are not independent to each other. The correlation coefficients (R) are presented in . The correlations are well in line with the biology of the maturation of forage maize, which has previously been reported for this plant material (Hetta et al. Citation2012; Mussadiq et al. Citation2012), even though slightly different nutritive and chemical analyses were conducted in the previous studies. The highest negative correlation was found between the proportion of ears and stems and the highest positive correlations were found between the proportions of ears and the concentrations of starch.
Table 2. Correlation coefficients (R) among proportions of morphological fractions, agronomic performance and nutritional characteristics.
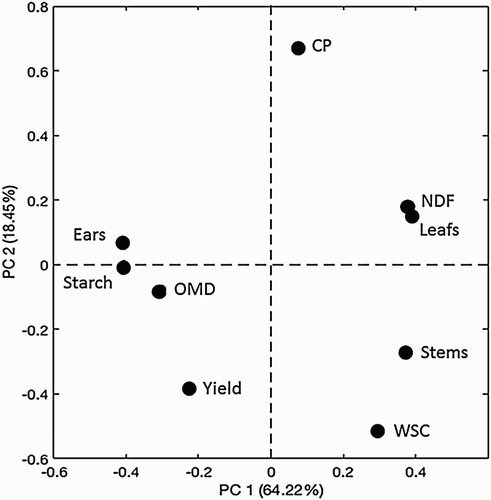
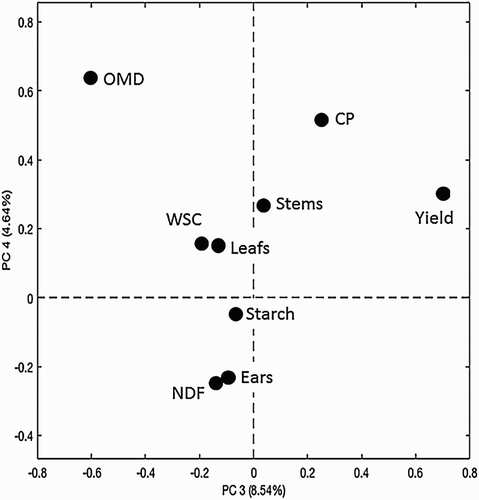
The PCA offered another overview of the relationship between parameters. The PCA of the dataset showed that the four principal components (PC 1–4) explained 64.2% (PC 1), 18.5% (PC 2), %, 8.5% (PC 3) and 4.5% (PC 4) of the total sum of squares in the material. The loading plots from this PCA analysis are presented in and . In the loading plots variables that are positively correlated, cluster together, and variables that are negatively correlated are on opposite sides of the quadrants. In , on the horizontal axis one can, for example, locate ears versus leaves and ears versus stems. The parameters OMD and starch are the variables that are closest to the variable ears, which make sense as starch is a very digestible component, which predominantly is stored in the ear of the plants (Van Soest Citation1994). The concentration of NDF is closest to the proportions of leaves and stems, which is expected as these morphological fractions contain almost no starch and are high in fibre (Van Soest Citation1994). In , the variables that have a unique meaning span the space. In other words, they are close to the outer borders of the panel (), for example, OMD, CP and Yield. Correlated variables are in the centre of , for example, we can interpret this as the concentration of CP, the Yield and OMD are somewhat independent to the other parameters analysed. Concerning the position of the parameters, OMD and CP in may be explained by the fact that the digestibility of the crop and the concentration of protein is rather constant during the end of the maturation (e.g. Hetta et al. Citation2012), in contrast to the parameters in the centre of , which will vary during maturation of the plants (Hetta et al. Citation2012; Mussadiq et al. Citation2012, Citation2013). The crop yield is strongly dependent on site of cultivation for this plant material (Mussadiq et al. Citation2012, Citation2013). Site was not included as a factor in the present study, but the effects of sites may explain the peripheral position for yield in both the PCA plots ( and ). Overall, and and , all together give a good overview of the dependency of the analytical variables in relation to the underlying plant biology, crop management and growing conditions of the plant material, which is well in line to what is described by Mussadiq et al. (Citation2012). These relationships between the morphology of the plant and the nutritive values, justify further explorations of novel techniques to evaluate the phenotypic expressions of various genotypes of maize.
Calibration models and prediction results
The calibration models made between the NIRS spectra and the nine variables are presented in . The PLS prediction worked well for the traditional responses CP, WSC, starch and NDF, both for the Foss and Umbio techniques. These are all traditional characteristics of forage maize and it was expected that these parameters could be predicted well. More interesting is that the morphological proportions were accurately predicted from NIRS information, which may depend on the previously described correlations between nutritive values and morphology. In general, the Umbio results were slightly better than the Foss results. The results for parameters OMD, and DM yield showed room for improvement, as many PLS components were needed for achieving rather poor RER and RPD values. An RPD of above 3 can be considered as good model and a value of 2 and above is a model with potential for improvement and the models presented in should be judged in that perspective. The OMD in forages is often well predicted by NIRS techniques (e.g. Krizsan et al. Citation2014). Why the performance of the prediction of OMD in this study was a bit lower than expected in this study remains unknown.
Table 3. Calibration data for forage maize parameters using two instruments, Umbio and Foss.
Most of the predicted parameters in are well known for the usefulness of forage maize as a ruminant feed (Van Soest Citation1994; Krizsan et al. Citation2014), but the possibility to predict the morphological composition of the crop is a novel improvement of NIRS spectroscopy. The information provided by models predicting the morphology of the maize may improve the possibilities to classify hybrids into types (class variables e.g. leafy hybrids) or be used as a continuous variable in forage evaluation or plant breeding programmes. The potential of spectral phenotyping in plant breeding has been highlighted by Fiorani & Schurr (Citation2013). This aspect is interesting as there is a large interest in phenotyping of hybrids with different morphological traits, for example, leafy hybrids, with extra leaves above the ear (Shaver Citation1983). The variation in leafiness in this study though is mainly due to differences in maturity of the hybrids and the harvest occasions.
Another novel approach is the modelling of the yield of the crop, the agronomic performance. The parameter yield resulted in relative poor predictions with the models developed from both of the instruments (). Despite the relatively poor prediction, the agronomic performance is interesting to predict, as it is relatively expensive and difficult to do reliable estimate of in field trials. The accuracy of the parameter yield is dependent on other parameters as the estimates of DM concentration of the crop in the field and is very sensitive for sampling procedures, even with the present gravimetric direct methods.
Reproducibility and usefulness of the instruments used in the study
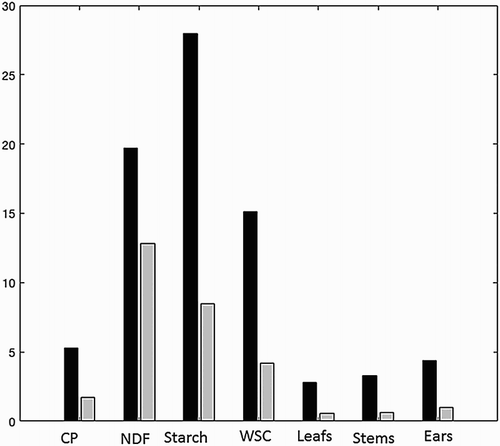
Given that the recordings with the Foss measurements were carried out in triplicate, it is possible to get an idea of inhomogeneity of the plant material by calculating a replicate error. This was done for predicted values for a few of the PLS models that worked well, shows such a comparison. For all response variables except concentrations of NDF the replicate error are much smaller than the RMSEP. From this we draw the conclusion that better calibrations can be achieved for all parameters evaluated.
Figure 3. Bar plot presenting the RMSEP (black) and the replicate error Srepl (grey) for selected number of variables of the forage maize using the Foss instrument. For abbreviations and units (see ).
The pushbroom procedure of the Umbio Inspector using the lids of the jars as sample cups was far more efficient compared with the procedure of the Foss instrument. Hybrid evaluation in plant breeding programmes and forage evaluation of on farm silages results in hundreds of thousands of samples and the feasibility of the scanning operation should therefore be taken in consideration when choosing instrumentation for a commercial application. An alternative for the Foss type of instrumentation is to invest in an improved spectrometer sampling solution using automatic filling of cuvettes to reduce measurement time for the Foss type instrument. In , robustified RER and RPD values were used to compare PLS models. Their use prevents misleading results produced by eventual outliers in the data. The use of the replicate spectra for the Foss instrument showed that the replicate error was much smaller than the modelling error. This means that the combination of sample handling and measurement was very reproducible in this study. The conclusions are that most variables can be determined equally well by both instruments, but the pushbroom imaging technique was faster and easier to operate both due to the mounting of the camera above the moving belt and the possibility to scan the samples with a minimum of preparation, for example, in the lid of the glass jars such as in this study. The hyper spectral instrument has several advantages over the Foss instrument as it may be used for advanced imaging and phenotyping of complete non-destructed plant material. Some of the future perspectives on this potential have been described by Fiorani and Schurr (Citation2013) and will be explored further in coming studies on forage maize at northern latitudes.
The study showed that it was possible to use NIRS and calibration models to obtain reasonable to good predictions of a number of parameters for whole crop maize. The predictions for the traditional composition parameters (e.g. concentrations of CP, NDF, Starch and WSC) were as good as those described in the literature (e.g. De Bouver et al. Citation1997; Volkers et al. Citation2003; Campo et al. Citation2013; Krizsan et al. Citation2014). Also, the morphological composition of the plants could be predicted very well, while the prediction quality for the crop yield was relatively poor. The comparison of both instruments gave slightly better predictions for the Umbio Inspector, which also was faster.
The findings that the phenology, for example, the morphological proportions of the maize plants tissues could be predicted by NIRS-based models, show new possibilities to further utilise the technology for providing useful information for plant breeding, growth modelling, crop management and forage evaluation.
Notes on contributors
Mårten Hetta is associate professor in animal nutrition and management with focus on the quality of grass and forages in livestock production. He works as a section leader for the animal science group at SLU Umeå and is also Head of the department for Agricultural research for Northern Sweden. Presently he is active as researcher in dairy and forage science in several projects related to analytical methods of feed quality.
Zohaib Mussadiq is a doctor of crop science. He has research articles on crop production and forage quality as well as scope of digital image analysis in agriculture.
Johanna Wallsten has a PhD in Animal Science and is working as a researcher at the Swedish University of Agricultural Sciences. Her main area of research is cultivation and feeding of whole crop silages to ruminants.
Magnus Halling is a senior research officer of agricultural sciences in the field of agronomy and physiology of forage crops. He works at the Department of Crop Production Ecology, Swedish University of Agricultural Science in Uppsala, Sweden. He has more than 50 research articles on forage crop management. Presently he is involved in research on modeling the growth of maize and improving the variety testing of maize.
Christian Swenson is a professor emeritus of animal husbandry in the field of dairy production. He works in the Department of Biosystems and Technology, Swedish University of Agricultural Sciences in Alnarp, Sweden. He has been working with environmental issues in dairy production. Presently he is working with nitrogen efficiency in dairy production.
Paul Geladi is professor emeritus in chemometrics at the University of Agricultural Sciences in Umeå, Department of Forest Biomaterials and Technology. He has over 150 publications in chemometrics and spectroscopy applied to bioenergy, electrochemistry, clinical studies and plant material characterization.
Additional information
Funding
References
- AOAC. 1983. Official methods of analysis of AOAC. Washington, DC: Association of Official Analytical Chemists.
- Beebe KR, Pell R, Seasholtz MB. 1998. Chemometrics: a practical guide. New York: John Wiley & Sons, Inc.
- Budakli Çarpici E, Çelik N, Bayram G. 2010. Yield and quality of forage maize as influenced by plant density and nitrogen rate. Tur J Field Crops. 15:128–132.
- Burger J, Geladi P. 2007. Spectral pre-treatments of hyperspectral near infrared images: analysis of diffuse reflectance scattering. J Near Infrared Spectros. 15:29–38. doi: 10.1255/jnirs.717
- Campo L, Monteagudo AB, Salleres B, Castro P, Moreno-Gonzalez J. 2013. NIRS determination of non-structural carbohydrates, water soluble carbohydrates and other nutritive quality traits in whole plant maize with wide range variability. Span J Agricult Res. 11:463–471. doi: 10.5424/sjar/2013112-3316
- Chai WH, Udén P. 1998. An alternative oven method combined with different detergent strengths in the analysis of neutral detergent fibre. Anim Feed Sci Technol. 74:281–288. doi: 10.1016/S0377-8401(98)00187-4
- Cox WJ, Cherney DJR. 2001a. Influence of brown midrib, leafy and transgenic hybrids on corn forage production. Agron J. 93:790–796. doi: 10.2134/agronj2001.934790x
- Cox WJ, Cherney DJR. 2001b. Yield and quality of forage maize as influenced by plant density and nitrogen rate effects on corn silage. Agron J. 93:597–602. doi: 10.2134/agronj2001.933597x
- Cozzolino D, Fassio A, Fernandez E, Restaino E, La Manna A. 2006. Measurement of chemical composition in wet whole maize silage by visible and near infrared reflectance spectroscopy. Anim Feed Sci Technol. 129:329–336. doi: 10.1016/j.anifeedsci.2006.01.025
- De Boever JL, Cottyn BG, De Brabander DL, Vanacker JM, Boucqué C. 1997. Prediction of the feeding value of maize silages by chemical parameters, in vitro digestibility and NIRS. Anim Feed Sci Technol. 66:211–222. doi: 10.1016/S0377-8401(96)01101-7
- Fairey NA. 1983. Yield, quality and development of forage maize as influenced by dates of planting and harvesting. Can J Plant Sci. 63:157–168. doi: 10.4141/cjps83-015
- Fiorani F, Schurr U. 2013. Future scenarios for plant phenotyping. Annu Rev Plant Biol. 64:267–91. doi: 10.1146/annurev-arplant-050312-120137
- Grahn H, Geladi P, editors. 2007. Techniques and applications of hyperspectral image analysis. Chichester: Wiley.
- Hagman J, Halling MA, Dryler K. 2016. Stråsäd, trindsäd, oljeväxter och potatis (Cereals, pulses, oilseeds and potaoes). Sortval 2016 (Vaiety selection 2016). Institutionen för växtproduktionsekologi (Department of Crop Production Ecology), Sveriges lantbruksuniversitet (Swedish University of Agriculture), Uppsala in Swedish.
- Hetta M, Mussadiq Z, Gustavsson A-M, Swensson C. 2012. Effects of hybrid and maturity on performance and nutritive characteristics of forage maize at high latitudes, estimated using the gas production technique. Anim Feed Sci Technol. 171:20–30. doi: 10.1016/j.anifeedsci.2011.09.015
- Krizsan SJ, Mussadiq Z, Hetta M, Ramin M, Nyholm L, Huhtanen P. 2014. Predicting feeding value of forage maize hybrids harvested at different maturities and sites. J Anim Feed Sci. 23:269–278. doi: 10.22358/jafs/65690/2014
- Larsson K, Bengtsson S. 1983. Bestämning av lätttillgängliga kolhydrater i växtmaterial. Metodbeskrivning, Vol.22. Statens Lantbrukskemiska Laboratorium, Uppsala. (in Swedish).
- Lindgren E. 1979. The nutritional value of roughages determined in vitro and by laboratory methods. Swedish University of Agricultural Sciences, Department of Animal Nutrition, Report No. 45. Uppsala.
- Liu X, Hana L. 2006. Prediction of chemical parameters in maize silage by near infrared reflectance spectroscopy. J Near Infrar Spectros. 14:333–339. doi: 10.1255/jnirs.685
- Mussadiq Z, Gustavsson A-M, Geladi P, Swensson C, Hetta M. 2013. Multivariate models of the predicted milk yield and forage quality in maize depending on growing site, plant maturity, proportion and nutritional quality of morphological fractions. Acta Agricult Scand A Anim Sci. 20:407–413.
- Mussadiq Z, Hetta M, Swensson C, Gustavsson A-M. 2012. Plant development, agronomic performance and nutritive value of forage maize depending on hybrid and marginal site conditions at high latitudes. Acta Agricult Scand, B Plant Sci. 62:420–430.
- Næs T, Isaksson T, Fearn T, Davies, T. 2002. A user friendly guide to multivariate calibration and classification. Chichester: NIR Publications.
- Nkurunziza L, Kornher A, Hetta M, Halling M, Weih M, Eckersten H. 2014. Crop genotype-environment modelling to evaluate forage maize cultivars under climate variability. Acta Agricult Scand B-Soil Plant Sci. 64:56–70.
- Shaver, DL. 1983. Genetics and breeding of maize with extra leaves above the ear. Paper presented at: Proceedings of the 38th Annual Corn and Sorghum Research Conference; 1983 Dec. 7–8; Washington, DC: American Seed Trade Association.
- Van Soest PJ. 1994. Nutritional ecology of the ruminant. Ithaca (NY): Cornell University Press.
- Volkers KC, Wachendorf M, Loges R, Jovanovic NJ, Taube F. 2003. Prediction of the quality of forage maize by near-infrared reflectance spectroscopy. Animal Feed Sci Technol. 109:183–194. doi: 10.1016/S0377-8401(03)00173-1
- Williams PC. 1987. Implementation of near-infrared technology. In: Williams P, Norris K, editors. Near-infrared technology. In the agricultural and food industries. 2nd ed. St. Paul (MN): American Association of Cereal Chemists; p. 145–169.
- Zscheischler J, Estler MC, Staudacherm W, Gross F, Burgstaller G, Streyl H, Rechmann T. 1990. Maize handbook: environmentally-friendly cultivation, cost-effective utilization. Frankfurt Main: DLG-Verlag; p. 320.