?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed to find the effects of several mycorrhiza species on agronomic characteristics and nutrient uptake of various sweet sorghum genotypes under the semi-arid Mediterranean soil conditions. Four sweet sorghum genotypes were inoculated with three mycorrhiza species under greenhouse conditions. The plants were evaluated for growth parameters such as shoot height, root length, root morphology (root surface, root diameter and volume), shoot and root dry weight, uptake of macro and micro mineral nutrients, root colonisation and mycorrhizal dependency. Mycorrhizal species significantly improved growth and productivity of sorghum genotypes. Inoculation of Funneliformis mosseae, Claroideoglomus claroideum and Claroideoglomus etunicatum resulted in the highest shoot and total dry matter biomass production in Ashana, Hereahri and Yellow genotypes, respectively. Mycorrhiza inoculated white genotype had higher root colonisation and root development, and shoot P, K, Ca and Mg contents. Yellow genotypes produced significantly higher shoot and total dry weight and also were highly mycorrhiza dependent among the four genotypes. Compared to the control treatment mycorrhizal inoculation increased shoot Zn concentration but had non-consistent effects on shoot Cu, Fe and Mn contents. Conclusively, our findings confirmed that sweet sorghum genotypes responded selectively to arbuscular mycorrhizal fungi (AMF) colonisation for their growth parameters and nutrients uptake.
Introduction
ssChallenges of the current era such as the climate changes, water scarcity, rapid world population growth, food safety and insurgency along with socio-economic factors are great threat to the global food security (Watts-Williams et al., Citation2022). Considering the current scenario of the increase in world population, more food is needed to feed the world under diminishing resources, and it is argued that these needs cannot be met sufficiently and problem of hunger and starvation may exist especially in developing and underdeveloped countries (Ortas, Citation2019). It is argued that people living in arid and semi-arid regions will be affected at the highest levels (Saleh et al., Citation2013). The livestock production is an integral component of global food security, and the forage and feed crop production is required to be expanded in terms of area and yield per unit area. It is reported that there will be no significant change in the demand for livestock products in developed countries in the future (Hocquette and Gigli, Citation2005). To increase the livestock and dairy products, they must be fed with sufficient and high-quality roughage feed which is vital in the feeding of ruminant animals. In this context, agricultural products grown in arid and semi-arid conditions have become more significant. Cereals such as maize, sorghum and wheat that are produced in tropical and semi-arid regions have different varieties and small grains under the taxonomy groups, which are believed to increase their economic importance in the future.
Sorghum (Sorghum bicolor L.) is often known as millet or broom grass in many parts of the world. Sorghum has a widespread cultivation area in Africa and is used for the production of ethanol. Sorghum is a drought-tolerant plant, grown for humans and livestock food and known as a food security plant. Sorghum is considered rich nutritious crop needed by human and animals for their growth, survival, maintenance and reproduction (Watts-Williams et al., Citation2022). Sorghum is consumed in the 5th class after the rice, wheat, corn and barley in the class of grains cultivated and produced. It has become the main food source of millions of people in the West African Region, which contains inefficient soils. At the same time, since it is a good C4 plant, it does a high level of photosynthesis and has high production capability. Sweet sorghum is one of the sugar-rich plants and has high biomass production containing both soluble (glucose and sucrose) and insoluble (cellulose and hemicellulose) carbohydrates (Dar et al., Citation2018).
Sweet sorghum cultivars have high biomass and carbohydrate and are recommended to be grown on marginal lands. The use of mycorrhizae as biofertiliser can reduce demand for chemical fertiliser and diminish chemical pollution. Soil beneficial microorganisms such as arbuscular mycorrhizal fungi (AMF) are known to stimulate sorghum growth and enhance plant productivity (Ortas et al., Citation1996). According to Sisaphaithong et al. (Citation2012), sorghum growth is strongly stimulated by mycorrhizae. Plant productivity and ecosystem stability are mainly supplied with AMF as a key component of ecosystems (Powell and Rillig, Citation2018). Arbuscular mycorrhizal fungi are known for their ability to absorb water and mineral nutrients and increase plant growth under fertile soil conditions. However, in many infertile soils, efficient nutrient acquisition depends on rhizosphere mechanisms such as mycorrhizal inoculation. Arbuscular mycorrhizal fungi inoculation enhances the adaptation of higher plants to a series of environmental stresses such as drought, heat, salinity and heavy metal contamination (Garg and Chandel, Citation2011). It has been demonstrated that mycorrhizal inoculation increased microbial populations in sorghum rhizosphere (Kumar and Fulekar, Citation2019), and this could improve the rhizosphere microbial dynamic to increase soil quality (Ortaş, Citation2017).
Soil nutrient concentration especially phosphorus (P) availability control growth and development of many plants (Johnson and Graham, Citation2013). Kamaei et al. (Citation2019) showed that the highest rate of sorghum physiological growth indexes of root such as root area index and net assimilation rate was obtained after inoculation with mycorrhiza along with nitrogen (N) fertiliser treatment. McGowan et al. (Citation2019) found that the increases in soil organic carbon (SOC) were significantly correlated with greater root biomass and abundance of AMF. It is observed that some phosphorus-efficient cultivars have a capacity to increase P availability for root uptake than the other cultivars by converting non-available P forms into available ones (Rengel and Marschner, Citation2005; Subramanian et al., Citation2009). Sorghum plant is highly mycorrhizae dependent and its inoculation with mycorrhizal can promote plant growth (Ortas, Citation1996; Ortaş and Harris, Citation1996). After mycorrhizal inoculation, the direct competition of pathogens for uptake of nutrients decreases which improves plant nutrition to increase photosynthesis and carbohydrates production. Moreover, the mycorrhizal inoculation can help sorghum plant in efficient water uptake (Symanczik et al., Citation2020).
Arbuscular mycorrhizal fungi can enhance the growth, survival and nutrient uptake of sorghum by mitigating various biotic and abiotic stresses under droughts conditions (Badi et al., Citation2019). As a consequence of the increasing climate change effects, new plant species and genotypes would be needed that have greater yield potential and adaptability to ensure food and feed security. Under semi-arid climate conditions, it is expected that the increase in temperature will lead to water scarcity. Under those conditions, C4 plants such as sorghum genotypes will be suitable for food security and sustainable agricultural strategy. In this context, it is important to choose plant species or genotypes that require less fertiliser and water and show strong association with on mycorrhizae. It is expected that with mycorrhizal inoculation, some sorghum genotypes will get better benefit for nutrient uptake and growth under the Mediterranean climatic condition. The hypothesis is genotypes interact with different mycorrhiza species to increase sorghum growth under P and Zn deficiency soil conditions
The purpose of this study is to search the effects of several mycorrhizal species on the growth and mycorrhizal dependence of several sorghum genotypes under P and Zn deficiency soil conditions.
Material and Methods
Soil
Sultanönü soil was used as a growth material for the study. The soil was collected from GKTAEM Gateway Research Institute Area, Eskişehir-Turkey (‘39°45′ 43″ N, and ‘30°29′ 59 E″). Soil physical and chemical properties are given in .
Table 1. Physical, chemical and biological properties of Sultanönü soil.
Plant and Mycorrhizae Species
Four different sorghum genotypes (Ashana, Hereahri, White and Yellow) and three different mycorrhizal species (Funnelifomis mosseae (BEG 12), Claroideoglomus claroideum (BEG 31) and Claroideoglomus etunicatum (BEG 247)) along with control were used in the study. Mycorrhizae spores were provided by BEG collection and multiplied under the clover host plant.
Experimental Design, Treatments and Growth Conditions
The experiment was established under greenhouses conditions following a complete randomised design (CRD) with three replicates for each treatment. Before sowing, sorghum seeds were surface sterilised by immersion for 2 min in 2% of sodium hypochlorite solution and rinsed three times in distilled water; 2 mm sieved soil was sterilised by autoclaved for 2 h. Five seeds of different sorghum genotypes were planted in 3 L pots; and filter paper was placed in the middle of the pots, in order to keep mycorrhizae spores. After the sowing of the seeds water was given to the soil for germination. One week after germination thinning was performed per pot. After three weeks from sowing 100 mg N kg−1 soil was used as urea fertiliser to each pot. During the experiment, the plants were checked at regular intervals, and displacement was made according to the angle of sunlight. The inside of greenhouse temperature was at 26°C during the day and 20 during the evening. The relative humidity was at 70%.
Plant Harvest and Analysis
Sorghum genotypes were grown under a greenhouse and plants were harvested at the end of 71 days from sowing. At harvest, plant height was measured. Root and above root fresh and dry weight were determined. Sorghum plants roots were washed and cleaned and scanned with an optical scanner reader. Total root length, root diameter and surface area have been determined by WinRHIZO Root Analysis System. Roots were cleaned and prepared for colonisation (Koske and Gemma, Citation1989) and were determined by using the grid-line intersect method under a dissecting microscope at 40× magnification (Giovannetti and Mosse, Citation1980).
Dependency on Mycorrhiza (MD) was determined according to Ortas (Citation2012b) using following formula:
At harvest, shoot and root were dried at 70°C for 48 h and weighed for dry weight (DW) determination. Dried shoot tissue was grinded, homogenised and digested with nitric and perchloric acid. The content of soil microelements (Cu, Fe, Mn and Zn) was extracted by diethylenetriaminepentaacetic acid (DTPA) and calcium, magnesium and potassium in the soil extracted NH4-Ac method and nutrient concentrations were determined by the ICP-OES. Soil P contents were determined by the Olsen method and measured calorimetrically by spectrophotometer (Murphy and Riley, Citation1962).
Statistical Analysis
Data were analyzed by analysis of variance (ANOVA) using the Statistical Analysis System (SAS 9.1.3) package program. The least-square means differences between applications were determined by the Tukey multiple comparison method.
The principal component analyses (PCA) biplots were constructed based on genotypes and mycorrhiza species selection for efficient genotypes and mycorrhizae. Data were analyzed by Excel-State computer program.
Results
In the conducted research, it was determined that sorghum genotypes reacted differently to selected mycorrhiza species inoculation in terms of growth and development (). Without considering the mycorrhizal inoculation, the highest plant length was measured in Hereahri, and the shortest length in Yellow genotypes was measured. Hereahri genotype seems to be early flowered than the other genotype. Without considering mycorrhizal inoculation Hereahri genotype has delayed flowering compared to other genotypes. The highest (Cl. etunicatum inoculated; 106.7 cm) and the lowest (control 55.0 cm) plant height were measured at the Ashana genotype ().
Figure 1. Effect of mycorrhiza species inoculation on different sorghum genotypes development. A: Fu. Mosseae, B: Cl. claroideum, C: Cl. etunicatum inoculated treatments.
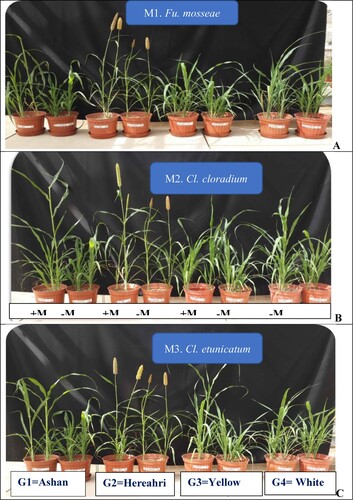
Table 2. Different mycorrhizae species effects on sweet sorghum genotypes plant length (cm) and plant dry weight.
Yellow genotype have the highest mean of total dry weight (TDW) than that of Ashana genotype which produced the lowest TDW in terms of dry weight production. Increase in shoot dry weight (SDW), Yellow genotypes produced the SDW. Hereahri genotype has the lowest while the Ashana genotype has the highest mean of root dry weight (RDW). It is observed that Fu. mosseae mycorrhiza species inoculated to the Ashana and Yellow genotype have high shoot and total dry weight with Cl. etunicatum species. Cl. claroideum inoculation produced the highest shoot and total DW than the other mycorrhiza species in the Hereahri genotype. At the same time, Hereahri genotype has the highest plant height, however, noted less root and total dry weight compared to the other three genotypes. Among the genotypes, Hereahri had the lowest root/shoot ratios, and Cl. etunicatum with the White genotypes produced the highest shoot and total dry matter. Yellow genotypes produced the higher shoot and total dry weight more than the other genotypes. It is observed that Cl. claroideum mycorrhiza species inoculated to the Yellow genotypes produced more root and total DW compared to other genotypes. Statistically, all mycorrhiza species significantly affected the development of different sorghum genotypes plants. And there are differences between mycorrhizae inoculated and non-inoculated plants (). Anova per single genotype and mycorrhizae spores showed significant (P < .0001) differences for plant length and drw weight (DW) parameters. Combined interaction also showed highly significant (P < .001) differences for genotype (G), mycorrhiza species (M) and their interaction effects for all measured traits.
Mycorrhiza species inoculated and non-inoculated genotypes root diameter, length, surface area and volume parameters were determined. Generally, without considering mycorrhizal inoculation the latest root parameters were measured in the Hereahri genotype, and the highest was measured in the White genotype. Statistically, genotypes were significantly different and mycorrhiza inoculation was not significant except root volume parameter (). There is significant interaction among the genotypes × mycorrhizal inoculation on root diameter and root length. Genotypes are also significantly different for plant growth and macronutrient concentrations.
Table 3. Different mycorrhizae species effects on sweet sorghum genotypes root diameter, length, surface, volume and mycorrhizal colonisation.
Root colonisation with AMF varied significantly between the sorghum genotypes (). Compared to the control treatment without root colonisation, the White genotype has the highest (39%) whereas the Ashana genotypes have comparatively least (31%) root colonisation. All genotypes inoculated with Cl. etunicatum produced higher colonisation than the other mycorrhiza species. White and Yellow sorghum genotypes showed higher mycorrhizal colonisation.
Shoot tissue P, K, Ca, Mg, Cu, Mn, Fe and Zn concentrations were determined. Results are presented in . All nutrient concentration is in the range of critical levels. Genotypes and mycorrhiza species have significant effects on phosphorus concentration. The White genotype had the highest and the Ashana genotypes had the lowest mineral nutrient concentrations. Mycorrhiza species and genotypes statistically significantly affected P concentration. Cl. etunicatum inoculated genotypes have the highest P concentration (%) (). Mycorrhiza species also significantly affected K, Ca and Mg mineral nutrient concentration among the genotypes. Usually, mycorrhizal species Fu. mosseae translocated a high concentration of Ca and Mg, when treated with the genotypes. Although other mycorrhiza species inoculation has not significant effects. Zn concentration was found maximum in all genotypes treated with mycorrhiza species compared to control. Similarly, micronutrients concentration was also varied with mycorrhiza inoculation with genotypes, and Cu, Fe and Mn concentrations were less than control plants. All data’s pear correlation results show that Cu, Mn and Fe concentrations have a negative correlation with mycorrhizal colonisation.
Table 4. Different mycorrhizae species effects on sweet sorghum genotypes shoot P, K, Ca, Mg, Fe, Zn, Cu and Mn concentration.
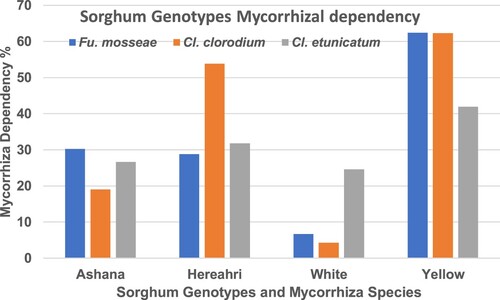
The sweet sorghum genotypes growth and mycorrhizae dependency (MD) was significantly differed. Regarding MD, maximum value was noted in Yellow (62%) genotype and Fu. mosseae, the least was determined in White (55.91%) genotype and Cl. claroideum treatments. Yellow genotypes have the highest mean of MD (56%), White have the lowest mean of MD (12%). Hereahri genotype MD is 38% and Ashana genotype MD is 25%. Mycorrhiza species dependency effects also were analysed and Cl. etunicatum, Fu. mosseae and Cl. claroideum species have mean of 31%, 32% and 35% MD, respectively ().
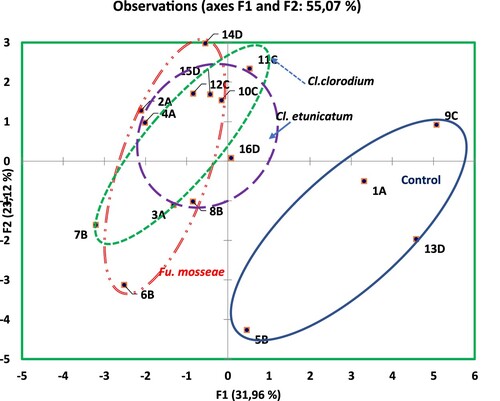
In order to determine the genotype and mycorrhiza relationship, all data were analysed for principal component analysis (PCA). PCA analysis successfully identified genotypes and mycorrhiza species effects on plant measured parameters separately ( and ). Also all measured data’s multiple correlations and genotypes and mycorrhiza species effectiveness were determined. To facilitate a less congested graphical presentation of many treatments, the second figure is used to make clear visualisation of the mycorrhiza species groups. Genotypes and mycorrhiza species 16 treatments for PCA analysis were used to determine the association of genotypes based on 17 measured parameter values. Grouping of mycorrhiza species on the base of all measured parameters is shown in . There is a significant difference between mycorrhiza species effect on measured parameters than control treatments. Fu. mosseae, Cl. claroideum and Cl. etunicatum mycorrhiza species were clustered separately than control on the base of all measured parameters. Cl. claroideum and Cl. etunicatum mycorrhizae species are significantly associated with measured parameters. Fu. mosseae species have a negative loading.
Figure 3. Biplot analysis for F1 (on x-axis) and F2 (y-axis) for all genotypes and mycorrhizae species interaction of 16 treatments based on all measured plant and nutrients analysed values.
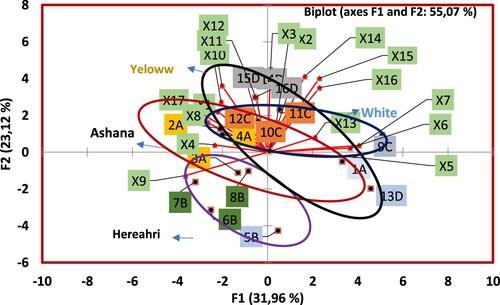
Figure 4. Biplot analysis for F1 (on x-axis) and F2 (y-axis) without considering the genotypes 16 treatments of mycorrhizae species interactions based on all measured parameters values. Codes 1A, 5B, 9C and 13D are no inoculated control treatments; codes 2A, 6B, 10C and 14D are Fu. Mosseae inoculated treatments; codes 3A, 7B, 11C, 15D are Cl. claroideum inoculated treatments; 4A, 8B, 12C, 16D codes are Cl. etunicatum inoculated treatments.
In F1 axes, Mn, Fe, Cu, K, Mg and root parameters had high positive loading. Plant growth parameters and root colonisation had negative loading. Mycorrhizal species inoculated to genotype Ashana and Hereahri were clustered separately and were different from each other; however, both genotypes were close to each other and they have less effects on measured parameters compared to White and Yellow genotypes. White and Yellow weighted data are appeared to be clustered in a different group and have higher effects on measured parameters. Hereahri genotype was associated with plant length, dry weight and mineral nutrients. Genotypes Yellow and White were specifically associated with shoot and root parameters, Cu, Mn and Fe concentrations. Also, root parameters were positively correlated with Cu, Mn and Fe concentrations.
Discussion
We investigated the effects of AMF species on sorghum genotype under greenhouse conditions in P and Zn limited soil. Variance analyses results showed that all AM species inoculated sorghum genotypes increased plant growth, root colonisation and nutrient concentration compared with controls. It seems that sweet sorghum genotypes are very selective with mycorrhizal inoculations. Data presented in showed that the highest shoot, root and total dry weight were obtained for the Yellow sorghum genotype inoculated with Cl. claroideum and Fu. mosseae. The Ashana genotypes inoculated with Cl. etunicatum and Cl. claroideum resulted in highest plant length followed Fu. mosseae inoculation. Mehraban et al. (Citation2009) and Abdelhameid (Citation2020) showed significant differences between sorghum genotypes and their interactions with mycorrhizae for plant height, biomass and root colonisation. Mycorrhizal colonisation rate was significantly positively correlated with plant length, P concentrations, shoot length and total dry weight. The research findings showed that all genotypes performed higher root infection with Cl. etunicatum. Root parameters of sorghum genotypes differ statistically significantly and the data in are consistent with findings by Liang et al. (Citation2017).
In terms of sorghum plant growth, pronounced genetic variations were determined within genotypes. Previously, Leiser et al. (Citation2016) reported there they detected a genetic difference for AMF root colonisation; however, the trait appears to be highly polygenic. Our findings divulged that the White genotype response to mycorrhizal dependency is low. White genotype had the highest root length and root surface area. Root surface is directly related to nutrient uptake. White et al. (Citation2013) indicated that under infertile soil conditions the development of crop genotypes with root traits increasing mineral nutrient acquisition should increase yields as well. The lower mycorrhizal growth response of the White genotype was combined based on shoot high Ca, K, Mg, Cu, Fe and Mn concentrations. Previously reported that genotypes are different from each other in terms of nutrient uptake (Clark Citation2002). Also, Clark and Reinhard (Citation1991) reported that sorghum genotypes differed in most growth traits, especially dry matter yields, nutrient uptake and root lengths. But there was non-significant relationship between mycorrhizal species on root parameters. However, plant nutrient concentrations were not much different in general as shown previously high contents of plant-available soil P may have inhibited the symbiotic contribution plants. Similarly, Cobb et al. (Citation2016) showed that sorghum cultivars were significantly more responsive to mycorrhizal colonisation than commercial hybrids for mineral nutrient content.
The genetic diversity of wild sorghum genotypes that come from sweet sorghum needs to be searched for other beneficial rather than their shoot DM production and P acquisition efficiency. Similar work was done by Abdelhalim et al. (Citation2019) and Abdelhalim et al. (Citation2020) first time, they found that Sudanese sorghums genotypes and their growth responsiveness to AMF and P acquisition. In another work by Abdelhalim et al. (Citation2019), it has been indicated that mycorrhiza response and phosphorus acquisition efficiency of sorghum genotypes differing in plant’s roots produced. de Oliveira et al. (Citation2021) also indicated that root exudates such as strigolactone composition in sorghum genotypes have effects on growth and P uptake. It probably showed less need for mycorrhiza because it absorbs better nutrients. Therefore, it is critical that nutrient use efficient sorghum genotypes be assessed with mycorrhiza species to found potential mycorrhizal partnership. Also, there is a significant difference in terms of MD within genotypes. White genotype has the lowest MD, Yellow genotype has the highest MD. Previously, Janos (Citation2007) and Ortas (Citation2012a) suggested that there is a great difference in species in terms of their dependence and responsiveness to mycorrhizas. Identification and introduction of genotypes that are highly addicted to mycorrhizae and produce more biomass are important in ecological and economic aspects.
PCA also showed that Yellow genotype inoculated to Cl. claroideum and Cl. etunicatum mycorrhiza species can be potentially be used to provide higher plant growth and nutrient uptake. Also, there is a significant correlation between nutrient uptake and root parameters. Mehraban et al. (Citation2009) and Badi et al. (Citation2019) showed that only a few Rhizoglomus species dominated without host plant genotype specificity in a semi-arid Sudanese cropland region.
Conclusion
Our study reported that the sweet sorghum genotypes were selectively associated with AMF inoculations for growth, root parameters and nutrient concentrations. The sorghum genotypes were significantly and highly responsive to AMF dependency. Fu. mosseae and Cl. claroideum inoculated Yellow sorghum genotype produced the highest total dry weight and MD. Early flowering Hereahri genotypes showed significantly higher plant height than the other genotypes. It is of great interest to search further for the best combination of genotype and mycorrhizal species under marginal soil conditions. Also, it is important to find the relationship between sweet sorghum genotypes' sugar content and mycorrhiza species preferences. Successful and high levels of root colonisation are an important step to further experiment to explore the best mycorrhizal colonisation with sorghum genotype growth and nutrient uptake in terms of the root exudate content of each sorghum genotype. Future work should be focused on sweet sorghum genotypes carbon sequestration, higher sugar content and mycorrhizae carbon demand related to soil quality and biofuel production as well.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
Acknowledgements
The authors thank Dr İbrahim Malik for providing the genotypes. The authors thank Research assistant Mehmet Işık and Feyzullah Oztur for their help on root analyses. Also the authors thank Dr Muhammad Riaz for proof reading.
Additional information
Notes on contributors
Ibrahim Ortas
Prof. D. Ibrahim Ortas is a full-time professor in work University of Cukurova, Faculty of Agriculture, Department of Soil Science and Plant Nutrition, Adana/TURKEY. He got his PhD at University of Reading, UK. Presently he is doing research and teaching at Çukurova University. He is responsible from rhizosphere laboratuvar and field of research and implementation. His research area(s): 1. The effect of mycorrhizae on mineral nutrient uptake. 2. Using mycorrhiza in sustainable agriculture systems 3. Producing mycorrhizae inoculated seedling under microporogated conditions. 4. Long term field experiment Such as a) ‘the effect of organic manure, compost, mycorrhizal inoculum and inorganic fertilizer on pepper growth and nutrient uptake under long-term experiments. c) The effect of long-term P fertilization on soil quality', c) Long term citrus mycorrhizae inoculation on tree plant nutrient uptake and yield. 5. Agricultural management and soil protection 6. Mycorrhizae and belowground carbon sequestration on forestry ecosystem 7. Climate change and food sequestration 8. Biochar and mycorrhiza contribution on soil organic carbon pool and atmospheric carbon mitigation. Recently he has been working on belowground C pools. Under long-term field conditions, soil organic C and N mineralization and related to CO2 flux is the main working area. At the moment, he is working the effect of vouchers on citrus tree plant’s C budget and C sequestration.
Gizem Bilgili
Agricultural Engineer Gizem Bilgili She was graduated in Soil Science and Plant Nutrition department as an agricultural engineer. She is working as an agricultural consultant.
References
- Abdelhalim T, Jannoura R, Joergensen RG. 2019. Mycorrhiza response and phosphorus acquisition efficiency of sorghum cultivars differing in strigolactone composition. Plant Soil. 437:55–63.
- Abdelhalim T, Jannoura R, Joergensen RG. 2020. Arbuscular mycorrhizal dependency and phosphorus responsiveness of released, landrace and wild Sudanese sorghum genotypes. Arch Agron Soil Sci. 66:706–716.
- Abdelhameid NM. 2020. Effect of mycorrhizal inoculation and potassium fertilization on grain yield and nutrient uptake of sweet sorghum cultivated under water stress in calcareous soil. Egypt J Soil Sci. 60:17–29. Win
- Badi OBM, Abdelhalim TS, Eltayeb MM, Gorafi YSA, Tsujimoto H, Taniguchi T. 2019. Dominance of limited arbuscular mycorrhizal fungal generalists of Sorghum bicolor in a semi-arid region in Sudan. Soil Sci Plant Nutr. 65:570–578.
- Clark RB. 2002. Differences among mycorrhizal fungi for mineral uptake per root length of switchgrass grown in acidic soil. J Plant Nutr. 25:1753–1772.
- Clark RB, Reinhard N. 1991. Effects of soil-temperature on root and shoot growth traits and iron-deficiency chlorosis in sorghum genotypes grown on a low iron calcareous soil. Plant Soil. 130:97–103.
- Cobb AB, Wilson GWT, Goad CL, Bean SR, Kaufman RC, Herald TJ, Wilson JD. 2016. The role of arbuscular mycorrhizal fungi in grain production and nutrition of sorghum genotypes: enhancing sustainability through plant-microbial partnership. Agric Ecosyst Environ 233:432–440.
- Dar RA, Dar EA, Kaur A, Phutela UGJR, Reviews SE. 2018. Sweet sorghum-a promising alternative feedstock for biofuel production. Renew Sustain Energy Rev. 82:4070–4090.
- de Oliveira IF, Simeone MLF, de Guimaraes CC, Garcia NS, Schaffert RE, de Sousa SM. 2021. Sorgoleone concentration influences mycorrhizal colonization in sorghum. Mycorrhiza 31:259–264.
- Garg N, Chandel S. 2011. Arbuscular mycorrhizal networks: process and functions. In: Sustainable agriculture volume 2. Springer; p. 907–930.
- Giovannetti M, Mosse B. 1980. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 84:489–500.
- Hocquette J-F, Gigli S. 2005. The challenge of quality. Indicators of Milk and Beef Quality EAAP Publication. 112:13–22.
- Janos DP. 2007. Plant responsiveness to mycorrhizas differs from dependence upon mycorrhizas. Mycorrhiza 17:75–91.
- Johnson NC, Graham JH. 2013. The continuum concept remains a useful framework for studying mycorrhizal functioning. Plant Soil. 363:411–419.
- Kamaei R, Faramarzi F, Parsa M, Jahan M. 2019. The effects of biological, chemical, and organic fertilizers application on root growth features and grain yield of sorghum. J Plant Nutr. 42:2221–2233.
- Koske RE, Gemma JN. 1989. A modified procedure for staining roots to detect VA-mycorrhizas. Mycol Res. 92:486–488.
- Kumar P, Fulekar MH. 2019. Mycorrhizal soil development using sorghum bicolor for rhizospheric bioremediation of heavy metals. Biosci Biotechnol Res Commun 12:688–697.
- Leiser WL, Olatoye MO, Rattunde HFW, Neumann G, Weltzien E, Haussmann BIG. 2016. No need to breed for enhanced colonization by arbuscular mycorrhizal fungi to improve low-P adaptation of West African sorghums. Plant Soil. 401:51–64.
- Liang X, Erickson JE, Vermerris W, Rowland DL, Sollenberger LE, Silveira ML. 2017. Root architecture of sorghum genotypes differing in root angles under different water regimes. J Crop Improv. 31:39–55.
- McGowan AR, Nicoloso RS, Diop HE, Roozeboom KL, Rice CW. 2019. Soil organic carbon, aggregation, and microbial community structure in annual and perennial biofuel crops. J Agron. 111:128–142.
- Mehraban A, Vazan S, Rad MRN, Ardakany AR. 2009. Effect of vesicular-arbuscular mycorrhiza (VAM) on yield of sorghum cultivars. J Food Agric Environ. 7:461–463.
- Murphy J, Riley JP. 1962. A modified single solution method for determination of phosphate in natural waters. Anal Chim Acta. 27:31–36.
- Ortas I. 1996. The influence of use of different rates of mycorrhizal inoculum on root infection, plant growth, and phosphorus uptake. Commun Soil Sci Plant Anal. 27:2935–2946.
- Ortas I. 2012a. Do maize and pepper plants depend on mycorrhizae in terms of phosphorus and zinc uptake? J Plant Nutr. 35:1639–1656.
- Ortas I. 2012b. The effect of mycorrhizal fungal inoculation on plant yield, nutrient uptake and inoculation effectiveness under long-term field conditions. Field Crops Res. 125:35–48.
- Ortas I. 2019. Role of Microorganisms (Mycorrhizae) in Organic Farming. In: Organic Farming: Global Perspectives and Methods. p. 181–211.
- Ortas I, Harris PJ, Rowell DL. 1996. Enhanced uptake of phosphorus by mycorrhizal sorghum plants as influenced by forms of nitrogen. Plant Soil. 184:255–264.
- Ortas I. 2017. Mycorrhizae: soil quality. In: Lal R, editor. Encyclopedia of soil science, Vols. I–III. New York: Taylor & Francis; p. 1505–1510.
- Ortaş I, Harris PJ. 1996. The effect of partial soil sterilization and seasonal change on soil degradation (N-minerilization and soil chemical properties). In: Kapur S, editor. 1st international conference on land degradation. Adana: Çukurova Universitey; p. 204–207.
- Powell JR, Rillig MC. 2018. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 220:1059–1075.
- Rengel Z, Marschner P. 2005. Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytol. 168:305–312.
- Saleh AS, Zhang Q, Chen J, Shen Q. 2013. Millet grains: nutritional quality, processing, and potential health benefits. Compr Rev Food Sci Food Saf. 12:281–295.
- Sisaphaithong T, Kondo D, Matsunaga H, Kobae Y, Hata S. 2012. Expression of plant genes for arbuscular mycorrhiza-inducible phosphate transporters and fungal vesicle formation in sorghum, barley, and wheat roots. Biosci Biotechnol Biochem 76:2364–2367.
- Subramanian KS, Tenshia V, Jayalakshmi K, Ramachandran V. 2009. Biochemical changes and zinc fractions in arbuscular mycorrhizal fungus (glomus intraradices) inoculated and uninoculated soils under differential zinc fertilization. Appl Soil Ecol. 43:32–39.
- Symanczik S, Krutzmann J, Nehls U, Boller T, Courty PE. 2020. Expression of major intrinsic protein genes in sorghum bicolor roots under water deficit depends on arbuscular mycorrhizal fungal species. Soil Biol Biochem 140:5.
- Watts-Williams SJ, Gill AR, Jewell N, Brien CJ, Berger B, Tran BTT, Mace E, Cruickshank AW, Jordan DR, Garnett T, et al. 2022. Enhancement of sorghum grain yield and nutrition: A role for arbuscular mycorrhizal fungi regardless of soil phosphorus availability. Plants People Planet. 4:143–156.
- White PJ, George TS, Dupuy LX, Karley AJ, Valentine T. 2013. Root traits for infertile soils. Front Plant Sci. 4:7.