Abstract
Fungal metabolites active for insects were obtained from fermentation products using okara media. The mechanisms of action of these compounds against insects were clarified using voltage clamp electrophysiology. The branching factor inducing hyphal branching in arbuscular mycorrhizal (AM) fungi was isolated from the root exudates of Lotus japonicus and identified as 5-deoxystrigol. Strigolactones were originally identified as seed germination stimulants of parasitic weeds; therefore, synthetic strigolactones were developed to exhibit the inducing activity of hyphal branching in AM fungi and diminish the stimulating activity of seed germination of parasitic weeds. Signaling molecules, acylhomoserine lactones (AHLs), in quorum sensing were identified in the fungal strain Mortierella alpina A-178, and the true producer of AHLs was clarified as symbiotic bacteria in the fungus. Since acyl-(S)-adenosylmethionine analogs may be good candidates for competitive inhibitors of AHL synthases, intermediate mimics in the biosynthesis of AHLs have been synthesized.
Structures of bioactive compounds
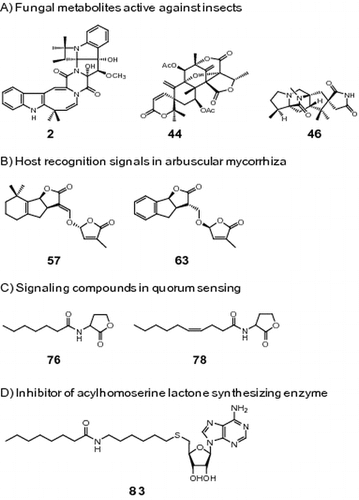
The importance of agricultural production for providing adequate food for mankind is of great significance. Inhibitory factors in crop production include attacks by pests, diseases caused by plant pathogens and viruses, and infestation by nematodes and parasitic weeds in roots. Nutrient depletion and alkalization in soil seriously damage crop growth, resulting in reductions in crop productivity. Synthetic insecticides and fungicides have given rise to the respective emergence of tolerant pests and drug-resistant fungal strains.Citation1) Furthermore, the excessive use of urea fertilizers results in soil alkalization, leading to the poor growth of crops.Citation2) On the basis of natural product chemistry, we attempted to use bioactive natural products and symbiosis between microbes and plants for the prevention of losses in agricultural production.
This review focused on the following subjects: bioactive fungal metabolites for pest control, arbuscular mycorrhizal formation for the uptake of nutrients, and the control of virulence in plant pathogens by quorum sensing (QS) system. This may pave the way toward sustainable crop production using bioactive natural compounds.
I. Fungal metabolites active against insects
Fungi produce diverse metabolites with various bioactivities and fungal secondary metabolites depend on the cultural medium. In the 1980s, our group began to screen microbes for insecticidal compounds that could be used in practice or become lead compounds for the generation of new carbon skeletons. We used okara, an insoluble residue in the whole soybean homogenate and a waste material in tofu (soybean curd) production, as a solid culture medium. It was the first to use this material to culture fungi.
As a result of random screening, various strains were found to exhibit not only insecticidal but also convulsive and paralytic activities against silkworm larvae.
1. Discovery of bioactive metabolites
Penicillium simplicissimum AK-40, Aspergillus aculeatus KF-428, and Penicilium expansum MK-57 were isolated as insecticide-producing fungi. Taralomyces sp. YO-2 also exhibited an insecticidal activity. During the screening process, some strains, P. expansum MY-57, Penicillium brasilianum JV-379, and P. brasilianum MG-11, exhibited convulsive activities. Paralytic activities were displayed by three isolates: Aspergillus japonicus JV-23, unidentified ascomycete OK 128, and Peniccilium griseofulvum OK-17.
1.1. Insecticidal compounds
1.1.1. Okaramines
P. simplicissimum AK-40 produced two novel bis-indole alkaloids, okaramines A (1) and B (2) (Fig. ).Citation3) Okaramines A (1) and B (2) exhibited insecticidal activities against silkworm larvae with LD50 values of 8 and 0.2 μg/g diet, respectively.
Fig. 1. Structures of the okaramine family. Notes: Okaramines A (1), B (2), C (3), D (4), E (5), F (6), G (7), H (8), I (9), J (10), K (11), L (12), M (13a: original; 13b: revised), N (14), O (15), P (16), Q (17), and R (18).
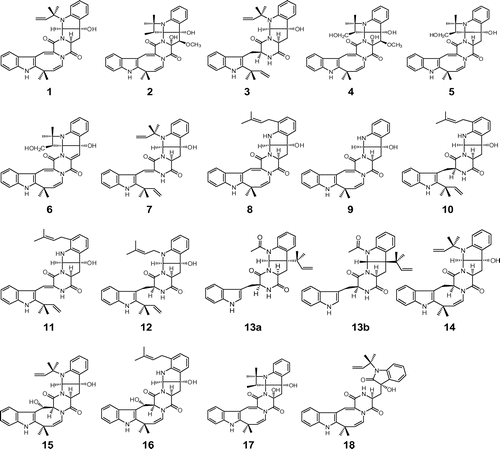
The unique structures of okaramines 1 and 2 prompted us to investigate whether okaramines or similar compounds were produced by other strains. P. simplicissimum AHU8402 was found to produce a new congener, okaramine C (3), together with 1 and 2.Citation4) Minor congeners of the okaramines were also investigated from the culture extracts of P. simplicissimum AK-40 in order to elucidate structure-activity relationships and clarify the biogenesis of okaramines. Four new okaramine congeners were isolated and termed okaramines D (4),Citation5) E (5),Citation5) F (6),Citation5) and G (7).Citation6)
In further screening, A. aculeatus KF-428 isolated from a soil sample produced two insecticidal compounds 1 and 2. The third compound also appeared to be an okaramine-related compound and was named okaramine H (8),Citation7) together with inactive okaramine analog okaramine I (9).Citation7)
Okaramines have attracted considerable attention due to their molecular complexity and biogenesis. We thoroughly searched the fermented material of P. simplicissimum AK-40 for new okaramine congeners, and isolated nine members of the okaramine family: okaramines J (10),Citation8) K (11),Citation8) L (12),Citation8) M (13a),Citation8) N (14),Citation9) O (15),Citation9) P (16),Citation9) Q (17),Citation9) and R (18).Citation9)
The unique and complex structures of okaramines inspired total chemical synthesis. The convergent total synthesis of (+)-okaramine J (10) was achieved by Roe et al. in 2003.Citation10) The first enantioselective total synthesis of a member of the okaramine family, okaramine N (14), was accomplished by Baran et al. in 2003.Citation11) In 2004, the total synthesis of okaramine C (3) was completed by Hewitt et al.Citation12) All stereoisomers of okaramine M were synthesized by Iizuka et al. and the reported structure of okaramine M (13a) was revised to 13b.Citation13)
1.1.2. Communesins
Three new communesin congeners, communesins D (19), E (20), and F (21), together with two known communesins A (22) and B (23) were obtained from okara medium fermented with P. expansum MK-57 (Fig. ).Citation14) These compounds exhibited insecticidal activities. Communesins 22 and 23 have been isolated from the mycelia of a strain of Penicillium sp. that adheres to the marine alga, Enteromorpha intestinalis, and were reported to exhibit cytotoxic activities in the P-388 lymphocytic leukemia test system in cell cultures.Citation15)
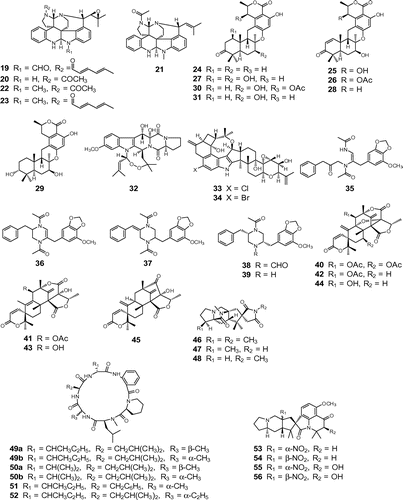
Fig. 2. Structures of bioactive fungal metabolites. Notes: Insecticidal compounds: communesins A (22), B (23), D (19), E (20), and F (21); chrodrimanins A (25), B (26), C (24), D (27), E (28), F (29), G (30), and H (31); convulsive compounds: verruculogen (32); penitrem A (33); 6-bromopenitrem E (34); brasiliamides A (35), B (36), C (37), D (38), and E (39); acetoxydehydroaustin (40), austin (41), dehydroaustin (42), austinol (43), dehydroaustinol (44), and neoaustin (45); paralytic compounds: asperparalines A (46), B (47), and C (48); PF1171 A (49a), epi-PF1171A (49b), PF1171B (50a), epi-PF1171B (50b), PF1171F (51), and PF1171 G (52); cyclopiamines A (53), B (54), C (55), and D (56).
Basic skeleton of communesin is composed of seven rings, and this ring system is very complicated and attractive for organic synthesis. The total synthesis of communesin F (21) was independently completed by Yang et al.Citation16), Zuo et al.Citation17), Liu et al.Citation18), and Belmar et al.Citation19)
1.1.3. Chrodrimanins
A new meroterpenoid chrodrimanin C (24) and the known compounds, chrodrimanins A (25) and B (26), were isolated from the fermentation products of Taralomyces sp. YO-2 (Fig. ).Citation20) Chrodrimanins 25 and 26 were first reported as metabolites produced by Penicillium variabilis, with insecticidal and insect-repelling effects of 26 on Lepidoptera.Citation21) As the absolute configuration of chrodrimanins remained unclear, we determined the absolute configuration of 26 using a modified Mosher’s method. The new chrodrimanin congeners, chrodrimanins D (27), E (28), F (29), G (30), and H (31), were also isolated from the strain YO-2 (Fig. ).Citation22) Chrodrimanins are considered to be meroterpenoids consisting of sesquiterpenoid and pentaketide moieties, and only the pentacecilide family has been reported to have the same carbon skeleton as chrodrimanins with a different stereochemistry.Citation23)
1.2. Convulsive compounds
1.2.1. Verruculogen
A strain P. expansum MY-57 exhibited convulsive activity against silkworm larvae, and verruculogen (32) was identified as the active principal (Fig. ).Citation24) Verruculogen (32) was originally isolated from a culture of Penicillium verruculosum as the agent responsible for tremor-producing activity in mice or 1-day-old cockerels.Citation25)
1.2.2. Penitrems
During the investigation of okarmines, P. simplicissimum AK-40 showed convulsive activity. Bioassay-guided fractionation from the fermentation product yielded penitrem A (33)Citation26) and the new congener 6-bromopenitrem E (34) (Fig. ).Citation27) Penitrem A (33) was first isolated from Penicillium cyclopium,Citation28) and the neurochemical effects of 33 were examined in sheep and rat synaptosomes.Citation29)
1.2.3. Brasiliamides
The tremorgenic mycotoxins, verruculogen and penitrems, exhibited similar activities in the bioassay system using silkworm larvae, strongly suggesting that this convenient bioassay was valuable as an initial screening tool in the search for active compounds on the nervous system. A soil isolate P. brasilianum JV-379 produced the novel convulsive compounds, brasiliamides A (35),Citation30) B (36),Citation30) C (37),Citation31) D (38),Citation31) and E (39) (Fig. ).Citation31) Feeding experiments using labeled phenylalanine indicated that two phenylalanine moieties formed a diketopiperazine ring and that some subsequent metabolic processes yielded brasiliamides (unpublished data).
1.2.4. Austins
P. brasilianum MG-11 showed convulsive activity and the active principal was identified as verruculogen (32). During this isolation process, we noted that the behavior of silkworm larvae ingesting 32 was different from those ingesting the crude extract; the latter exhibited markedly stronger convulsions than the former. This phenomenon strongly suggested the presence of another factor or factors synergistically enhancing the activity of 32. Acetoxydehydroaustin (40) and the two known compounds, austin (41)Citation32) and dehydroaustin (42),Citation33) were identified as active factors (Fig. ).Citation34) Two more compounds, austinol (43) and dehydroaustinol (44), were also identified together with a new compound named neoaustin (45) (Fig. ).Citation34) Members of the austin family are considered to be meroterpenoids, and 45 appears to be a key metabolite in austin biogenesis.
The convulsive activities of acetoxydehydroaustin (40), austin (41), and dehydroaustin (42) were examined in the presence or absence of verruculogen (32), which typically required a dose of more than 0.1 μg/g diet to cause convulsions. Dehydroaustin (42) alone at a dose of 1–100 μg/g diet did not cause convulsions. Two other compounds 40 and 41 also exhibited no effect. However, 40, 41, and 42 enhanced the convulsive effects of 32 when applied in combination with 32.
Another new function of dehydroaustinol (44) was discovered in a study on the sporulation of Aspergillus nidulans.Citation35) When growing A. nidulans hyphae encounter the atmosphere, they initiate a morphogenetic program that leads to the production of spores. Mutants that are defective in the fluG gene fail to undergo sporulation because they lack an endogenous diffusible factor that purportedly accumulates on aerial hyphae, thus signaling the initiation of development. This defect could be reversed by adding culture extracts from a wild-type strain onto a mutant colony. Dehydroaustinol (44) isolated from culture extracts was only active when administered in conjunction with the orsellinic acid derivative diorcinol. These two compounds formed an adduct, and this diorcinol–dehydroaustinol adduct prevented crystal formation of the signal on the surface of aerial hyphae and on an artificially prepared aqueous film and also increased signal lipophilicity.
1.3. Paralytic compounds
1.3.1. Asperparalines
The finding that prompted this investigation was the accidental observation of paralytic syndrome in silkworm larvae that had digested an extract of a certain isolate. Paralysis was observed in the strain A. japonicus JV-23. Asperparalines A (46), B (47), and C (48) were isolated as active constituents (Fig. ).Citation36,37) Asperparaline A (46) at 10 μg/g diet induced paralysis in silkworm larvae within 1 h and this effect lasted for 7–10 h after its oral administration.
Asperparalines have a bicyclo[2.2.2]diazaoctane core and spirosuccinimide moiety, and these two components are characteristic of asperparalines. Although some model compounds have been synthesized by Williams et al.Citation38) and Tanimori et al.Citation39) complete syntheses have not been completed until now.
Williams et al. determined the primary amino acid building blocks of 46 through the feeding and incorporation of 13C-labeled intermediates.Citation40) The β-methyl proline residue was constituted from L-isoleucine, the novel spiro-succinimide moiety was derived from the oxidative degradation of L-tryptophan, and S-adenosylmethionine contributed two N-methyl residues. Furthermore, the incorporation of 13C-labeled acetate into the single isoprene unit clearly demonstrated that the isoprene moiety was derived from the mevalonate pathway.
1.3.2. PF1171
The paralytic cyclic hexapeptides, PF1171 A (49a),Citation41) PF1171 C (50a),Citation41) PF1171 F (51), and PF1171 G (52), were produced by the unidentified ascomycete OK-128 (Fig. ).Citation42,43) Their absolute configurations were determined by Marfey’s method. These cyclic peptides have anthranilic acid and pipecolinic acid as common components, and 1171 G (52) had 2-aminobutyric acid as an additional component. The total synthesis of these cyclic hexapeptides was recently achieved by the solid-phase synthesis of a linear precursor and solution-phase macrolactamization.Citation44) The natural products exhibited potent paralytic activities against silkworm larvae, whereas epi-PF1171A (49b) and epi-PF1171C (50b), bearing l-Ala instead of d-Ala, were reported to be relatively less active than 49a and 50a.Citation44)
1.3.3. Cyclopiamines
P. griseofulvum OK-17 produced the nitro compounds, cyclopiamines A (53), B (54), C (55), and D (56) (Fig. ).Citation45) Cyclopiamines 53 and 54 were originally reported as oxindole metabolites produced by P. cyclopium,Citation46) and cyclopiamines 55 and 56 were determined to be new members of the cyclopiamine family.
2. Mechanism of bioactive fungal metabolites
The mode of action of the fungal metabolites obtained in this study remained unknown for a long period of time. Okaramine, asperparaline, and austin were selected as representatives and the mechanisms of action of these compounds against insects were recently clarified using voltage clamp electrophysiology.
2.1. OkaramineCitation47)
We examined the effects of okaramine B (2), a representative of okaramines with the highest insecticidal activity, on silkworm larval neurons using patch-clamp electrophysiology. Okaramine B (2) induced inward currents that were reversed close to the chloride equilibrium potential and were blocked by fibronil.Citation48) Thus, it was tested on the silkworm resistant-to dieldrin (RDL) γ-aminobutyric acid-gated chloride channel (GABACl) and silkworm L-glutamate-gated chloride channel (GluCl) expressed in Xenopus laevis oocytes. Okaramine B (2) activated GluCl, but not RDL. The activation of GluCl by okaramines correlated with their insecticidal activity. Unlike ivermectin,Citation49) 2 at 10 μM was inactive against human α1β2γ2 GABACl and α1β glycine-gated chloride channels, strongly indicating that 2 was a new lead for the development of safe insect control chemicals.
2.2. AsperparalineCitation50)
Asperparalines induce paralysis in silkworm larvae. We investigated the effects of asperparaline A (46) on ligand-gated ion channels expressed in the cultured larval brain neurons of the silkworm using patch-clamp electrophysiology. The application of 46 (10 μM) to the bath had no effect on the membrane current, but, when delivered for 1 min prior to the co-application of 10 μM acetylcholine (ACh), it completely blocked the ACh-induced current that was sensitive to mecamylamine,Citation51) a nicotinic acetylcholine receptor (nAChR)-selective antagonist. In contrast, 10 μM asperparaline A (46) was ineffective against the γ-aminobutyric acid- and L-glutamate-induced responses of silkworm larval neurons. The fungal alkaloid showed no-use dependency in blocking the ACh-induced response with distinct affinity for the peak and slowly desensitizing current amplitudes of the response to 10 μM ACh in terms of IC50 values of 20.2 and 39.6 nM, respectively. Asperparaline A (46) (100 nM) reduced the maximum neuron response to ACh with a minimal shift in EC50, suggesting that the alkaloid was non-competitive with ACh. In contrast to showing marked blocking effects on insect nAChRs, it only exhibited weak blocking effects on chicken α3β4, α4β2, and α7 nAChRs expressed in Xenopus laevis oocytes, suggesting high selectivity for insect over certain vertebrate nAChRs.
2.3. AustinCitation52)
We investigated the effects of austin family members on cockroach ACh, γ-aminobutyric acid (GABA), and L-glutamate receptors expressed in the American cockroach (Periplaneta americana) neuron. The U-tube application of austin (41) or its derivatives did not induce any current amplitudes, suggesting that they do not act as agonists of these three receptors. In the second step of these experiments, they were bath-applied for 1 min before the co-application of a corresponding ligand. We found that 41 and its derivatives had no effect on GABA and L-glutamate-induced currents, whereas they significantly reduced ACh- and epibatidine-induced currents, showing that these compounds act as selective antagonists of nAChRs. Of these compounds, dehydroaustin (42) showed the highest blocking potency for nAChRs, differentially attenuating the peak and slowly desensitizing the current amplitude of ACh-induced responses. Dehydroaustin (42) reduced the maximum normalized response to ACh, suggesting that this blocking effect was not competitive with ACh.
II. Host recognition signals in arbuscular mycorrhiza
Mycorrhizae are symbiotic associations between fungi and plant roots. Arbuscular mycorrhizal (AM) fungi form mutualistic, symbiotic associations with the roots of more than 80% of land plants.Citation53) Fossil records from the Ordovician and Devonian eras indicate the existence of AM symbioses over 460 million years ago,Citation4,50) suggesting that fungi played a crucial role in facilitating the colonization of land by plants.
AM fungi are incapable of completing their life cycle in the absence of a host root. They penetrate and colonize plant roots, in which they differentiate into highly branched structures known as arbuscules, which are thought to be the principal sites of nutrient exchange between the two organisms. The concomitant development of extra-radical hyphae outside the plants roots allows fungi to supply the host with essential nutrients such as phosphate, nitrate, and other minerals from the soil. In return, AM fungi receive carbohydrates derived from photosynthesis in the host. AM symbiosis also confers resistance to the plant against pathogens and environmental stresses. Since mycorrhizal formation is beneficial for agricultural production, AM fungi are used as agricultural materials. Their spores can germinate and grow in the absence of a host, but their hyphal growth is very limited. In one of the first stages of host recognition, the hyphae of AM fungi show extensive branching in the vicinity of host roots before formation of the appresorium.Citation55) Host roots are known to release signaling molecules that trigger hyphal branching; however, these branching factors had not been isolated.
Based on these findings, we attempted to isolate a branching factor from the root exudates of Lotus japonicus, and finally identified it as the strigolactone, 5-deoxystrigol.
1. Strigolactones as a host recognition signal
Lipophilic compounds released from L. japonicus into a hydroponic solution with a low phosphate concentration were extracted with ethyl acetate and then tested for hyphal branching activity in the germinating spores of Gigaspora margarita. Using the paper disk diffusion method developed by our own group, the ethyl acetate extract elicited strong activity at concentrations as low as 15 μg/disk. Bioassay-guided fractionation of the ethyl acetate extract afforded 5-deoxystrigol (57) as a branching factor (Fig. ).Citation56)
Fig. 3. Structures of natural and synthetic strigolactones. Notes: Natural strigolactones: 5-deoxystrigol (57), strigol (58), orobanchol (67), and orobanchyl acetate(68); synthetic strigolactones: (+)-GR24 (59), (−)-ent-GR24 (60), (+)-2′-epi-GR24 (61), (−)-ent-2′-epi-GR24 (62), (−)-3,6′-dihydro-GR24 (65), (+)-ent-3,6′-dihydro-GR24 (66), (−)-2′-epi-3,6′-dihydro-GR24 (63), and (+)-ent-2′-epi-3,6′-dihydro-GR24 (64).
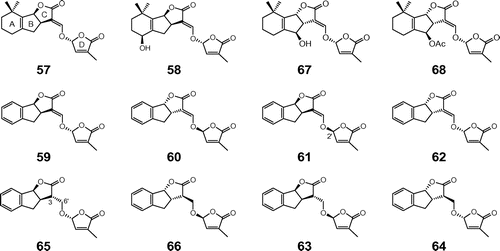
Strigolactones are a group of apocarotenoid lactones that were previously isolated as seed germination stimulants for the parasitic weeds Striga and Orobanche. Striga and Orobanche are among the most damaging agricultural pests. Most species are obligate parasites incapable of completing their life cycle in the absence of a host. Strigol (58) was first isolated from the false host cotton (Fig. ).Citation57) The discovery of 5-deoxystrigol as the branching factor for AM fungal hyphae also provided a clear answer to the long-standing question in parasitic plant biology of “what is the natural role for germination stimulants?” This could also provide a new strategy for the management and control of beneficial fungal symbionts and devastating parasitic weeds in both agriculture and natural ecosystems.
The natural strigolactones, 5-deoxystrigol, sorgolactone, and strigol and the synthetic analog GR24 induced extensive hyphal branching in the germinating spores of the AM fungus G. margarita at very low concentrations. Since strigolactones are janus-faced molecules in the plant rhizosphere, it is essential to balance the disruption of parasite germination signaling with the preservation of vital AM symbiosis.
To clarify the structural requirements of strigolactones for hyphal branching in AM fungi, we tested a series of natural and synthetically modified lactones for hyphal branching-inducing activity in germinating spores of the AM fungus G. margarita. We found that the structural requirements of strigolactones for hyphal branching in AM fungi were very similar, but not identical to those observed in root parasitic weeds, especially with respect to the enol ether bridge in the C-D part.Citation58)
The synthetic strigolactone analog, GR24, was prepared as a mixture of two racemic diastereomers, and subsequent chiral HPLC separation gave four stereoisomers: (+)-GR24 (59), (−)-ent-GR24 (60), (+)-2′-epi-GR24 (61), and (−)-ent-2′-epi-GR24 (62) (Fig. ).Citation58) The activity of (+)-GR24 (59), which has the same configuration as (+)-5-deoxystrigol (57), was markedly higher than that of its enantiomer 60. This difference in activity amounted to a factor of 100 (100 pg vs. 10 ng per disk). The two enantiomeric isomers 61 and 62 were less active than 59, but more active than 60, exhibiting activity at 1 ng/disk.
The reduced analog of GR24, 3,6′-dihydro-GR24, in which the carbon double bond in the C-D connecting enol ether was reduced to a single bond, was totally inactive in inducing the germination of root parasitic weeds. To determine the necessity of the enol ether bond in the C-D part for hyphal branching-inducing activity in AM fungi, we synthesized reduced GR24 and tested it for hyphal branching. The four stereoisomers were separated into optically pure forms by successive normal-phase and chiral HPLC. The absolute configuration of (−)-2′-epi-3,6′-dihydro-GR24 (63), obtained from the (−)-3a(R),8b(S)-tricyclic lactone, was determined as 3(S), 3a(R), 8b(S), and 2’(S) by X-ray crystallographic analysis (Fig. ). Consequently, the configuration of the remaining stereoisomers could be assigned on the basis of the synthetic sequence and chiral HPLC analyses. Among the four isomers tested, only one diastereomer, (+)-ent-2′-epi-3,6′-dihydro-GR24 (64) with the same configuration as (−)-ent-2′-epi-GR24 (62) at the corresponding chiral centers, showed hyphal branching activity in the AM fungus. The activity of 64 was similar to that of 62 (3 ng/disk).
We investigated the effects of the reduced GR24s (63, 64, 65, and 66) on seed germination by the parasitic weeds Striga hermonthica and Orobanche minor. In the case of S. hermonthica, the reduced GR24s did not stimulate seed germination and also inhibited seed germination induced by GR24. In the case of O. minor, (−)-2′-epi-3,6′-dihydro-GR24 (63) inhibited the growth of the radicle and exhibited the malformation of appresorium, resulting in the inhibition of plant growth.Citation59) This finding strongly suggested that the reduced GR24 (63) played a very significant role in the control of devastating parasitic weeds.
2. Production of strigolactones in non-host plants
AM fungi exhibit symbiosis with more than 80% of land plants. The mechanisms that determine the non-host nature of the remaining 20% of land plants are almost unknown. Strigolactones have been indicated to play a crucial role in mycorrhizal formation. We evaluated the productivity of strigolactones in non-host plants to determine whether the lack of strigolactones in exudates from non-host plant roots hindered the infection of AM fungi to plants. White lupin (Lupinus albus) and rapeseed (Brassica napus) were used as non-host plants of AM fungi for this study, and in the case of rapeseed (B. napus), the amount of branching factors released from the roots was markedly lower than that of the host plant L. japonicus (unpublished data).
Conversely, strigolactones in root exudates from hydroponically grown white lupin (L. albus) were characterized by comparing the retention times of germination stimulants on reverse-phase HPLC with those of standards and using tandem mass spectrometry. Even the non-mycotrophic L. albus exuded orobanchol (67), orobanchyl acetate (68), and 5-deoxystrigol (57) (Fig. ).Citation60) The root exudates contained small but sufficient amounts of strigolactones capable of inducing AM fungal hyphal branching in an in vitro bioassay. These findings strongly indicated that a diffusible factor emitted from Lupinus roots into the rhizosphere inhibits hyphal branching in AM fungi by counteracting the effects of strigolactones.
In order to clarify the chemical factors that confer the non-host nature of Lupinus species, we investigated the root exudates of white lupin for the inhibitors of hyphal development in AM fungi. Seven compounds were isolated as germ tube growth inhibitors.Citation61) Three of them were novel compounds, which were named lupindipyranoisoflavone A (69), 10′-hydroxylicoisoflavone B (70), and 10’-hydroxysophoraisoflavone A (71) (Fig. ). The other four compounds were identified as the pyranoisoflavones, licoisoflavone B (72), sophoraisoflavone A (73), alpinumisoflavone (74), and 3′-hydroxy-4′-O-methylalupinumisoflavone (75) (Fig. ). Isoflavones 72, 73, and 74 strongly inhibited germ tube growth in a concentration-dependent manner.
Fig. 4. Structures of isoflavones isolated from L. albus. Notes: Lupindipyranoisoflavone A (69), 10’-hydroxylicoisoflavone B (70), 10′-hydroxysophoraisoflavone A (71), licoisoflavone B (72), sophoraisoflavone A (73), alpinumisoflavone (74), and 3′-hydroxy-4′-O-methylalpinumisoflavone (75).
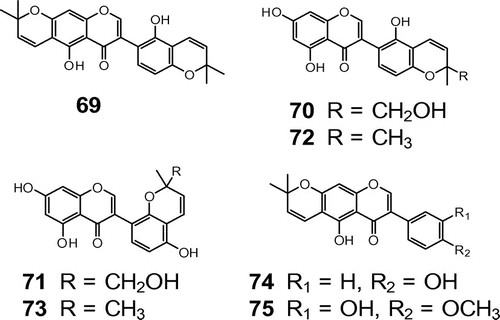
To investigate whether germ tube growth-inhibiting pyranoisoflavones prevented strigolactone-induced hyphal branching in AM fungi, the secondary hyphae of the germinating spores of G. marugarita were treated with pyranoisoflavones in the presence of the lupin strigolactone, (+)-orobanchyl acetate (68) at 10 ng/disk. At this concentration, 68 induced clusters of hyphal branches consisting of third and fourth hyphae from the treated secondary hyphae located proximal to the paper disks. Isoflavones 72 and 73 completely inhibited hyphal branching at the minimum concentrations of 0.16 and 0.63 μg/disk, respectively. This is the first study to show that flavonoids exuded from roots inhibited the function of strigolactones. Alpinumisoflavone (74), which was one of the most potent inhibitors of germ tube growth, did not inhibit the formation of new branching hyphae at 0.31, 0.63, or 1.25 μg/disk. Unexpectedly, high-order branches up to sixth or seventh hyphae were formed by the treatment with 74 at the three tested concentrations in the presence of 68. No hyphal branching inhibition was observed for 69 and 70, but, as in the case of 74, these compounds also promoted hypha branching to form branches up to sixth hyphae at 1.9–1.5 and 3.8–15 μg/disk, respectively. 10′-Hydroxysophoraisoflavone A (71) neither inhibited nor promoted hyphal branching at 7.5, 15, or 30 μg/disk.
III. Signaling compounds in QS
Gram-negative bacteria communicate with one another using N-acylhomoserine lactones (AHLs) as signaling molecules. This mechanism, known as QS, is needed to develop pathogenicity.Citation62) When cell density increases and the concentration of AHLs reaches a threshold level, AHLs bind to cognate LuxR-type receptors, and these complexes then activate the expression of target genes. In many pathogenic bacteria, this process results in pathogenic events such as the formation of biofilms and production of virulence factors.Citation62) Therefore, QS modulators have potential uses in agrochemical fields by preventing microbial infection in hosts.Citation63)
A compound that specifically regulates QS has been sought in medical and agrochemical pharmacology because it could prevent pathogenicity without killing bacteria,Citation64) thereby avoiding the emergence of drug-resistant strains. Therefore, we screened fungal strains isolated from soil for agonists or antagonists of AHLs using the biosensor Agrobacterium tumefaciens NTL4.
1. Isolation of Mortierella alpine A-178
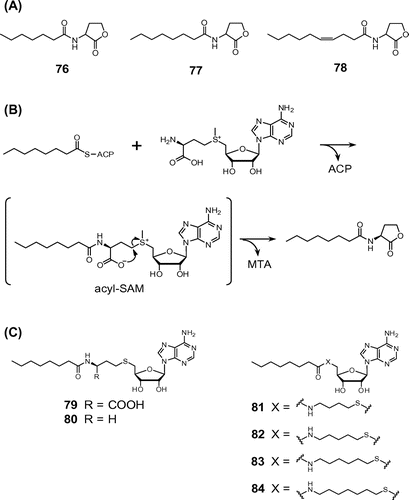
To identify fungal metabolites that were active in bacterial QS, we used the reporter A. tumefaciens NTL4, an AHL-synthase-deficient mutant that harbors a plasmid with lacZ fused to the gene traG, which is regulated by AHL-dependent QS.Citation65) This strain showed broad sensitivity to various AHLs, even at low concentrations, and the response was easily confirmed by the AHL-induced galactosidase that hydrolyzes X-Gal to give a blue color. We screened the ethyl acetate extracts of the culture broths of many fungi isolated from soil, and found that strain Mortierella alpina A-178 produced compounds capable of activating the reporter gene in A. tumefaciens NTL4. Bioassay-based purification, a spectroscopic analysis, and chemical synthesis revealed that the A-178 strain produced N-heptanoylhomoserine lactone (76)Citation66) and N-octanoylhomoserine lactone (77)Citation66) together with the novel AHL, (Z)-N-(4-decenoyl)homoserine lactone (78) (Fig. ).Citation67)
Fig. 5. Structures of AHLs isolated from M. alpina A-178 (A), mechanism of AHL production by LuxI-type AHL synthases (B), and structures of acyl-SAM analogs (79 and 80) and intermediate analogs (81–84) (C).
This finding raised the possibility that endobacteria are present in M. alpina A-178 because AHLs are conserved QS signaling molecules in Gram-negative bacteria. To examine this, we performed a PCR assay with genomic DNA and universal primers for bacterial 16S rRNA genes,Citation68) fluorescence in situ hybridization (FISH) with a bacterial 16S-rRNA-targeting oligonucleotide probe, EUB-338,Citation69) conjugated with the fluorescence dye Cy3, and transmission electron microscopy. The findings obtained showed that endobacteria, one of which was provisionally identified as the β-proteobacterium Castellaniella defragrans, were present in the cytoplasm of M. alpina and, thus, suggested that these were the true producers of AHLs. Moreover, the endobacteria in M. alpine A-178 were suggested to be uncultivable with the general methodology.
We then attempted to generate an endobacteria-free fungus. Repeated treatments with antibiotics generated a strain of bacteria-free M. alpina, and this gave negative results in both reporter gene and PCR assays. Thus, AHLs were shown to be synthesized by the endobacteria in M. alpina A-178.
It was unknown whether the fungus-endobacterium interaction was unique to strain A. alpina A-178 among Mortierella fungi. Therefore, we carried out reporter and PCR assays with M. alpina NBRC8573 and other Mortierella species: M. minutissima NBRC8573, M. globalpina NBRC32282, M. longicollis NBRC32293, and M. pilulefera NBRC104548. The expression of the reporter gene in A. tumefaciens NTL4 was induced by culture extracts of these Mortierella species. The expected 1.5 kb bands (16S rRNA gene fragments) were amplified from all strains by universal primers. These findings suggested that endobacteria were widely present in Mortierella fungi.
Our study demonstrated for the first time that endobacteria produce AHLs in fungi. We proposed that QS is a process more basic, general, and important than previously thought, allowing bacteria to establish themselves in their niche environment. Furthermore, we speculated that AHLs provide a mechanism by which the intimate associations between Mortierellas and endobacteria are established and maintained. The identification of QS signaling molecules in endofungal bacteria will help to unveil the molecular basis of fungus-endobacterium symbiosis.
2. Inhibitors of acylhomoserine lactone-synthesizing enzyme
AHLs are synthesized from the acyl-acyl carrier protein (acyl-ACP) and S-adenosylmethionine (SAM) by LuxI-type synthases.Citation70) As shown in Fig. , this catalytic reaction is thought to be proceeded by a two-step mechanism involving the intermediate acyl-ACP to the amino group of SAM and lactonization of the methionine part, concomitant with the release of methylthioadenosine (MTA).Citation71)
We speculated that acyl-SAM analogs may be good candidates for competitive inhibitors of AHL synthases because many examples of catalytic intermediate analogs were previously reported to be good inhibitors of target enzymes,Citation72) and the intermediate acyl-SAM was shown to be specific to the synthesis of AHL in Gram-negative bacteria.Citation71) Furthermore, acyl-SAM has not yet been detected in other organisms; thus, these analogs may also be useful in crop protection from plant pathogens.
In the case of Burkholderia glumae TofI, N-octanoylhomoserine lactone (C8-HSL) was synthesized from octanoyl-ACP and SAM. Hence, we firstly designed and synthesized N-octanoyl-S-adenosyl-L-homocysteine (79) as an octanoyl-SAM analog, which lacked the methyl group at the 5′-sulfur atom of the intermediate. The activity of 79 was tested for its ability to inhibit AHL synthase using octanoyl-ACP (10 μM), which was prepared from the ACP of Escherichia coli K-12 by chemical acylation,Citation73) with SAM (10 μM) as the substrate and the TofI enzyme (1 μM), which was heterogeneously expressed in E. coli strain BL21(DE3)pLysS.Citation74) Analog 79 showed 91% and 51% inhibitory activities at concentrations of 100 and 10 μM, respectively. A kinetic analysis indicated that 79 acted as a competitive inhibitor of TofI activity with a Ki value of 4.8 μM. The decarboxy analog 80 could simplify the structure 79, making it easy to prepare the derivative of the octanoyl-SAM analog. Analog 80 was able to inhibit the TofI reaction with a Ki value of 6.7 μM, which was similar to that of 79. These results indicated that the carboxy group of analog 79 was not necessary for inhibition of TofI.Citation75)
We next designed and synthesized analogs 81–84, in which the methylene chains between the sulfur atom and amide in 80 were extended to respective C4–C7 (Fig. ). As expected, the inhibitory activities of these analogs were higher than that of 80, and almost completely inhibited the production of C8-HSL by TofI at a concentration of 10 μM, while 81, 82, and 83 were effective to a similar degree and at 1 μM.Citation75) Analogs 82 and 83 showed strong inhibitory activities against TofI with respective Ki values of 0.22 and 0.11 μM.Citation74) Because analog 84 showed lower activity than analogs 81–83, the methylene chains between the sulfur atom and amide in 84 were too long to fit well into TofI. Acyl-SAM analogs are expected to be the lead compounds for anti-virulence agents because they bound to AHL synthases with high specificity.
IV. Summary and perspective
Pest and disease control is currently a major issue in crop cultivation. The fungal metabolites, okaramine and asperparaline, functioned in a highly selective manner against insects, which is indicative of the importance of these metabolites for pest control. Strigolactones, which have been identified as seed germination stimulants of parasitic weeds, were identified as branching factors for AM fungi. The synthetic strigolactone analogs, 3,6′-dihydro-GR24s, prepared in this study exhibited hyphal branching-inducing activity in the germinating spores of mycorrhizal fungi while inhibited the root growth of parasitic weeds, strongly suggesting that these compounds are useful for agricultural production. QS signaling molecules in endofungal bacteria were determined. We obtained catalytic intermediate analogs that exhibited competitive inhibition against AHL synthases. These inhibitors can interfere with the QS system in plant pathogens without killing them.
The use of the bioactive natural products discussed in this review is considered to provide an insight into novel crop protection technology. Moreover, the molecular mechanisms of mycorrhizal formation between AM fungi and plants and the molecular basis of fungus–endobacterium symbiosis are not only useful for obtaining a better understanding of the complex interactions among plants, fungi, and bacteria, but also applicable to establishing new research fields in the future.
Acknowledgments
I wish to express my thanks to the late Prof. Tetsuo Mitsui and Prof. Emeritus Koichi Koshimizu of Kyoto University for guiding me to the fascinating field of natural product chemistry. I am also grateful to the late Prof. Sawao Murao and Prof. Emeritus Motoo Arai of Osaka Prefecture University for their insightful guidance and providing me with an opportunity to work in the field of applied microbiology. I am deeply grateful to Prof. Kazuhiro Irie of Kyoto University, Prof. Hiroshi Nozaki of Okayama University of Science, Prof. Kohichi Yoneyama of Utsunomiya University, and Prof. Yukihiro Sugimoto of Kobe University for their analytical help and continuous guidance. I thank all members of my laboratory, especially Prof. Kohki Akiyama and Dr Kenji Kai, who have shared valuable time for studying bioactive natural products and symbiosis. I cannot express enough my sincere thanks to Prof. Kazuhiko Matsuda of Kinki University and Prof. Emeritus Akikazu Hatanaka of Yamaguchi University for their continuous encouragement and courteous help with my research.
Notes
This review was written in response to receipt of The Japan Prize for Agricultural Science in 2014.
References
- Harker KN, O’Donovan JT. Recent weed control, weed management, and integrated weed management. Weed Technol. 2013;27:1–11.10.1614/WT-D-12-00109.1
- Mobley HLT, Hausinger RP. Microbial urease: significance, regulation, and molecular characterization. Microbial. Rev. 1989;53:85–108.
- Hayashi H, Takiuchi K, Murao S, Arai M. Structure and insecticidal activity of new indole alkaloids, okaramines A and B, from Penicillium simplicissimum AK-40. Agric. Biol. Chem. 1989;53:461–469.10.1271/bbb1961.53.461
- Hayashi H, Fujiwara T, Murao S, Arai M. Okaramine C, a new insecticidal indole alkaloid from Penicillium simplicissimum. Agric. Biol. Chem. 1991;55:3143–3145.10.1271/bbb1961.55.3143
- Hayashi H, Asabu Y, Murao S, Arai M. New okaramine congeners, okaramines D, E, and F, from Penicillium simplicissimum ATCC 90288. Biosci. Biotechnol. Biochem. 1995;59:246–250.10.1271/bbb.59.246
- Hayashi H, Sakaguchi A. Okaramine G, a new okaramine congener from Penicillium simplicissimum ATCC 90288. Biosci. Biotechnol. Biochem. 1998;62:804–806.10.1271/bbb.62.804
- Hayashi H, Furutsuka K, Shiono Y. Okaramines H and I, new okaramine congeners, from Aspergillus aculeatus. J. Nat. Prod. 1999;62:315–317.10.1021/np9802623
- Shiono Y, Akiyama K, Hayashi H. New okaramine congeners, okamines J, K, L, M and related compounds, from Penicillium simplicissimum ATCC 90288. Biosci. Biotechnol. Biochem. 1999;63:1910–1920.10.1271/bbb.63.1910
- Shiono Y, Akiyama K, Hayashi H. Okaramines N, O, P, Q and R, new okaramine congeners, from Penicillium simplicissimum ATCC 90288. Biosci. Biotechnol. Biochem. 2000;64:103–110.10.1271/bbb.64.103
- Roe JM, Webster RAB, Ganesan A. Total synthesis of (+)-okaramine J featuring an exceptionally facile N-reverse-prenyl to C-prenyl aza-Claisen rearrangement. Org. Lett. 1994;5:2825–2827.10.1021/ol034822n
- Baran PS, Guerrero CA, Corey EJ. Short, enantioselective total synthesis of okaramine N. J. Am. Chem. Soc. 2003;125:5628–5629.10.1021/ja034491+
- Hewitt PR, Cleator E, Ley SV. A concise total synthesis of (+)-okaramine C. Org. Biomol. Chem. 2004;2:2415–2417.10.1039/b410180d
- Iizuka T, Takiguchi S, Kumakura Y, Tsukioka N, Higuchi K, Kawasaki T. First total synthesis and stereochemical revision of okaramine M. Tetrahedron Lett. 2010;51:6003–6005.10.1016/j.tetlet.2010.09.026
- Hayashi H, Matsumoto H, Akiyama K. New insecticidal compounds, communesins C, D and E, from Penicillium expansum Link MK-57. Biosci. Biotechnol., Biochem. 2004;68:753–756.10.1271/bbb.68.753
- Numata A, Takahashi C, Ito Y, Takada T, Kawai K, Usami Y, Matsumura E, Imachi M, Ito T, Hasegawa T. Communesins, cytotoxic metabolites of a fungus isolated from a marine alga. Tetrahedron Lett. 1993;34:2355–2358.10.1016/S0040-4039(00)77612-X
- Yang J, Wu H, Shen L, Qin Y. Total synthesis of (±)-communesin F. J. Am. Chem. Soc. 2007;129:13794–13795.10.1021/ja075705g
- Zuo Z, Xie W, Ma D. Total synthesis and absolute stereochemical assignment of (−)-communesin F. J. Am. Chem. Soc. 2010;132:13226–13228.10.1021/ja106739g
- Liu P, Seo JH, Weinreb SM. Total synthesis of the polycyclic fungal metabolite (±)-communesin F. Angew. Chem. Int. Ed. 2010;49:2000–2003.10.1002/anie.200906818
- Belmar J, Funk RL. Total synthesis of (±)-communesin F via a cycloaddition with indol-2-one. J. Am. Chem. Soc. 2012;134:16941–16943.10.1021/ja307277w
- Hayashi H, Oka Y, Kai K, Akiyama K. A New meroterpenoid, chrodrimanin C, from YO-2 of Talaromyces sp. Biosci. Biotechnol. Biochem. 2012;76:745–748.10.1271/bbb.110858
- Nakajima M, Takahashi H, Furuya K, Sato S, Itoi K, Kagasaki T, Hara M. Japan patent 02221277, 1990 Sep.
- Hayashi H, Oka Y, Kai K, Akiyama K. New chrodrimanin congeners, chrodrimanins D-H, from YO-2 of Talaromyces sp. Biosci. Biotechnol. Biochem. 2012;76:1765–1768.10.1271/bbb.120365
- Yamazaki H, Ugaki N, Matsuda D, Tomoda H. Absolute stereochemistry of pentacecilides, new inhibitors of lipid droplet formation in mouse macrophages, produced by Penicillium cecidicola FKI-3765-1. J. Antibiot. 2010;63:315–318.10.1038/ja.2010.39
- Hayashi H, Murao S, Arai M. Verruculogen, as a convulsive factor against silkworm, from Penicillium simplicissimum MF-24. Chem. Express. 1991;6:989–992.
- Fayos J, Lokensgard D, Clardy J, Cole RJ, Kirksey JW. Structure of verruculogen, a tremor producing peroxide from Penicillium verruculosum. J. Am. Chem. Soc. 1974;96:6785–6787.10.1021/ja00828a054
- Hayashi H, Asabu Y, Murao S, Nakayama M, Arai M. Penitrem A, as a convulsive factor against silkworm, from Penicillium simplicissimum AK-40. Chem. Express. 1993;8:177–180.
- Hayashi H, Asabu Y, Murao S, Nakayama M, Arai M. A new congener of penitrems, 6-bromopenitrem E, from Penicillium simplicissimum AK-40. Chem. Express. 1993;8:233–236.
- Wilson BJ, Wilson CH, Hayes AW. Tremorgenic toxin from Penicillium cyclopium grown on food materials. Nature. 1968;220:77–78.10.1038/220077b0
- Norris PJ, Smith CCT, De Beleroche J, Bradford HF, Mantle PC, Thomas AJ, Penny RHC. Actions of tremorgenic fungal toxins on neurotransmitter release. J. Neurochem. 1980;34:33–42.10.1111/jnc.1980.34.issue-1
- Fujita T, Makishima D, Akiyama K, Hayashi H. New convulsive compounds, brasiliamides A and B, from Penicillium brasilianum Batista JV-379. Biosci. Biotechnol. Biochem. 2002;66:1697–1705.10.1271/bbb.66.1697
- Fujita T, Hayashi H. New brasiliamide congeners, brasiliamides C, D and E, from Penicillium brasilianum Batista JV-379. Biosci. Biotechnol. Biochem. 2004;68:820–826.10.1271/bbb.68.820
- Chexal KK, Springer JP, Clardy J, Cole RJ, Kirksey JW, Dorner JW, Cutler HG, Strawter BJ. Austin, a novel polyisoprenoid mycotoxin from Aspergillus ustus. J. Am. Chem. Soc. 1976;98:6748–6750.10.1021/ja00437a081
- Simpson TJ, Stenzel DJ, Bartlett AJ, O’Brien E, Holker JSE. Studies on fungal metabolites. Part 3. 13C N.m.r. spectral and structural studies on austin and new related meroterpenoids from Aspergillus ustus, Aspergillus variecolor, and Penicillium diversum. J. Chem. Soc. Perkin Trans. 1. 1982;2687–2692.10.1039/p19820002687
- Hayashi H, Mukaihara M, Murao S, Arai M, Lee AY, Clardy J. Acetoxydehydroaustin, a new bioactive compound, and related compound neoaustin from Penicillium sp. MG-11. Biosci. Biotechnol. Biochem. 1994;58:334–338.10.1271/bbb.58.334
- Rodríguez-Urra AB, Jiménez C, Nieto MI, Rodríguez J, Hayashi H, Ugalde U. Signaling the induction of sporulation involves the interaction of two secondary metabolites in Aspergillus nidulans. ACS Chem. Biol. 2012;7:599–606.10.1021/cb200455u
- Hayashi H, Nishimoto Y, Nozaki H. Asperparaline A, a new paralytic alkaloid from Aspergillus japonicus JV-23. Tetrahedron Lett. 1997;38:5655–5658.10.1016/S0040-4039(97)01231-8
- Hayashi H, Nishimoto Y, Akiyama K, Nozaki H. New paralytic alkaloids, asperparalines A, B and C, from Aspergillus japonicus JV-23. Biosci. Biotechnol. Biochem. 2000;64:111–115.10.1271/bbb.64.111
- Adams LA, Gray CR, Williams RM. Concise synthesis of the core bicyclo[2.2.2]diazaoctane ring common to asperparaline, paraherquamide, and stephacidin alkaloids. Tetrahedron Lett. 2004;45:4489–4493.10.1016/j.tetlet.2004.04.054
- Tanimori S, Sunami T, Fukubayashi K, Kirihata M. An efficient construction of bridged chiral tetracyclic indolidines, a core structure of asperparaline, via stereocontrolled catalytic Pauson–Khand reaction. Tetrahedron. 2005;61:2481–2492.10.1016/j.tet.2004.12.057
- Gray CR, Sanz-Cervera JF, Silks LA, Williams RM. Studies on the biosynthesis of asperparaline A: origin of the spirosuccinimde ring system. J. Am. Chem. Soc. 2003;125:14692–14693.10.1021/ja037687i
- Umagome K, Nagase K, Harimaya K, Nakayama F, Yaguchi T, Sato E, Hoshido S, Kamito N, Soneda T, Hachisu M. Japan patent 11021297, 1999.
- Kai K, Yoshikawa H, Kuo YH, Akiyama K, Hayashi H. Determination of absolute structures of cyclic peptides, PF1171A and PF1171C, from unidentified ascomycete OK-128. Biosci. Biotechnol. Biochem. 2010;74:1309–1311.10.1271/bbb.100098
- Kuo YH, Kai K, Akiyama K, Hayashi H. Novel bioactive peptides, PF1171F and PF1171G, from unidentified ascomycete OK-128. Tetrahedron Lett. 2012;53:429–431.10.1016/j.tetlet.2011.11.068
- Masuda Y, Tanaka R, Kai K, Ganesan A, Doi T. Total synthesis and biological evaluation of PF1171A, C, F, and G, cyclic hexapeptides with insecticidal activity. J. Org. Chem. 2014;79:7844–7853.10.1021/jo500861k
- Miki S, Kai K, Akiyama K, Hayashi H. Cyclopiamines produced by P. griseofulvum OK-17. The Annual Meeting of Kansai Branch of Japan Society for Bioscience, Biotechnology, and Agrochemistry; 2012 Sep; Kameoka.
- Bond RF, Boeyens JCA, Holzapfel CW, Steyn PS. Cyclopiamines A and B, novel oxindole metabolites of Penicillium cyclopium westling. J. Chem. Soc., Perkin Trans. 1. 1979;1751–1761.10.1039/p19790001751
- Furutani S, Nakatani Y, Miura Y, Ihara M, Kai K, Hayashi H, Matsuda K. GluCl a target of indole alkaloid okaramines: a 25 year enigma solved. Sci. Rep. 2014;4:6190.10.1038/srep06190
- Ikeda T, Zhao X, Kono Y, Yeh JZ, Narahashi T. Fipronil modulation of glutamate-induced chloride currents in cockroach thoracic ganglion neurons. NeuroToxicology. 2003;24:807–815.10.1016/S0161-813X(03)00041-X
- Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LH. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371:707–711.10.1038/371707a0
- Hirata K, Kataoka S, Furutani S, Hayashi H, Matsuda K. A fungal metabolite asperparaline A strongly and selectively blocks insect nicotinic acetylcholine receptors: the first report on the mode of action. PLoS ONE. 2011;6:e18354.10.1371/journal.pone.0018354
- Ascher P, Large WA, Rang HP. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J. Physiol. 1979;295:139–170.10.1113/jphysiol.1979.sp012958
- Kataoka S, Furutani S, Hirata K, Hayashi H, Matsuda K. Three austin family compounds from Penicillium brasilianum exhibit selective blocking action on cockroach nicotinic acetylcholine receptors. NeuroToxicology. 2011;32:123–129.10.1016/j.neuro.2010.10.003
- Gianinazzi-Pearson V. Plant cell responses to arbuscular mycorrhizal fungi: getting to the roots of the symbiosis. Plant Cell. 1996;8:1871–1883.10.1105/tpc.8.10.1871
- Redecker D, Kodner R, Graham LE. Glomalean fungi from the Ordovician. Science. 2000;289:1920–1921.10.1126/science.289.5486.1920
- Powell CL. Development of mycorrhizal infections from Endogone spores and infected root segments. Trans. Br. Mycol. Soc. 1976;66:439–445.10.1016/S0007-1536(76)80214-8
- Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827.10.1038/nature03608
- Cook CE, Whichard LP, Turner B, Wall ME, Egley GH. Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science. 1966;154:1189–1190.10.1126/science.154.3753.1189
- Akiyama K, Ogasawara S, Ito S, Hayashi H. Structural requirements of strigolactones for hyphal branching in AM fungi. Plant Cell Physiol. 2010;51:1104–1117.10.1093/pcp/pcq058
- Akiyama K, Hayashi H. Strigolactones as a host-derived signal in the arbuscular mycorrhizal symbiosis. J. Pestic. Sci. 2009;34:306–309.10.1584/jpestics.34.306
- Yoneyama K, Xie X, Sekimoto H, Takeuchi Y, Ogasawara S, Akiyama K, Hayashi H, Yoneyama K. Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol. 2008;179:484–494.10.1111/nph.2008.179.issue-2
- Akiyama K, Tanigawa F, Kashihara T, Hayashi H. Lupin pyranoisoflavones inhibiting hyphal development in arbuscular mycorrhizal fungi. Phytochem. 2010;71:1865–1871.10.1016/j.phytochem.2010.08.010
- Galloway WRJD, Hodgkinson JT, Bowden SD, Welch M, Spring DR. Quorum sensing in Gram-negative bacteria: small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem. Rev. 2011;111:28–67.10.1021/cr100109t
- Galloway WRJD, Hodgkinson JT, Bowden S, Welch M, Spring DR. Applications of small molecule activators and inhibitors of quorum sensing in Gram-negative bacteria. Trends Microbiol. 2012;20:449–458.10.1016/j.tim.2012.06.003
- Rasmussen TM, Givskov M. Quorum sensing inhibitors: a bargain of effects. Microbiol. 2006;152:895–904.10.1099/mic.0.28601-0
- Shaw PD, Ping G, Daly SL, Cha C, Cronan JE Jr, Rinehart KL, Farrand SK. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. U.S.A. 1997;94:6036–6041.10.1073/pnas.94.12.6036
- Kai K, Furuyabu K, Tani A, Hayashi H. Production of the quorum-sensing molecules N-acylhomoserine lactones by endobacteria associated with Mortierella alpina A-178. ChemBioChem. 2012;13:1776–1784.10.1002/cbic.201200263
- Kai K, Kasamatsu K, Hayashi H. (Z)-N-(4-Decenoyl)homoserine lactone, a new quorum-sensing molecule, produced by endobacteria associated with Mortierella alpina A-178. Tetrahedron Lett. 2012;53:5441–5444.10.1016/j.tetlet.2012.07.133
- Partida-Martinez LP, Hertweck C. Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature. 2005;437:884–888.10.1038/nature03997
- Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 1990;56:1919–1925.
- Watson WT, Minogue TD, Val DL, von Bodman SB, Churchill MAE. Structural basis and specificity of acyl-homoserine lactone signal production in bacterial quorum sensing. Mol. Cell. 2002;9:685–694.10.1016/S1097-2765(02)00480-X
- Raychaudhuri A, Tullock A, Tipton PA. Reactivity and reaction order in acylhomoserine lactone formation by Pseudomonas aeruginosa RhlI. Biochem. 2008;47:2893–2898.10.1021/bi702009n
- Oikawa H, Yagi K, Ohashi S, Watanabe K, Mie T, Ichihara A, Honma M, Kobayashi K. Potent inhibition of macrophomate synthase by reaction intermediate analogs. Biosci. Biotechnol. Biochem. 2000;64:2368–2379.10.1271/bbb.64.2368
- Cronan JE Jr, Klages AL. Chemical synthesis of acyl thioesters of acyl carrier protein with native structure. Proc. Natl. Acad. Sci. U.S.A. 1981;78:5440–5444.10.1073/pnas.78.9.5440
- Chung J, Goo E, Yu S, Choi O, Lee J, Kim J, Kim H, Igarashi J, Suga H, Moon JS, Hwang I, Rhee S. Small-molecule inhibitor binding to an N-acyl-homoserine lactone synthase. Proc. Natl. Acad. Sci. U.S.A. 2011;108:12089–12094.10.1073/pnas.1103165108
- Kai K, Fujii H, Ikenaka R, Akagawa M, Hayashi H. An acyl-SAM analog as an affinity ligand for identifying quorum sensing signal synthases. Chem. Commun. 2014;50:8586–8589.10.1039/C4CC03094J
