Abstract
International trading markets of meat require the animal’s age information to prevent cross-contamination of ineligible meat products. Individual livestock age is either evaluated from physiological features or verified by breeding history. However, it remains impossible to perform age verification on meat when a suspicion of error occurred in the importing country. To investigate an age-related protein in skeletal muscle of livestock, we compared protein expression among chicken pectoralis major of different ages. Results indicated that the level of expression of chicken HSPB1, one of the small heat shock proteins, was increased in aged muscles. On the other hand, other heat shock proteins, heat shock factors, and myosin heavy chain isoform did not change the expression levels in aged chicken muscle. In addition, we identified that αB-crystallin interacted with HSPB1 in aged chicken muscle. These results suggest that HSPB1 protein forms complexes with αB-crystallin in aged chicken muscle and suppose to become the candidate of age-related bio-marker for verifying the age of chicken meat.
Graphical abstract
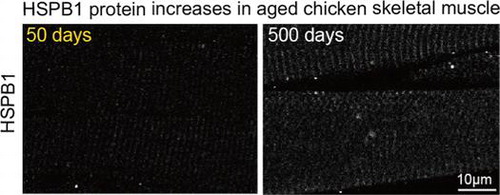
Immunohistochemical analysis of HSPB1 in chicken muscle (50 days and 500 days of age). Longitudinal sections show HSPB1 expression depending on the age in the muscle.
Following the bovine spongiform encephalopathy (BSE) outbreak, surveillance and traceability were demanded in the global meat trade.Citation1) For example, the export of beef and beef products in Japan are limited to only products derived from cattle 30 months of age or less. The age of livestock such as chicken, pigs, and cattle is verified by the breeding history records (birth date), or by studying physiological characteristics in the animal body that depend on senescence.Citation2) However, during the trading process, difference in age verification sometimes produces a big gap between exporting and importing countries. Chicken is also one of the most consumed meats, and two kinds of chicken, young chicken and culled adult hens, circulate in the world. Since culled hens have tough meat, the meat is disposed at a low price compared with that of young chicken.
However, there is few information about the meat of aged chicken, pigs, and cattle. It has remained to be difficult to evaluate the age of livestock from a piece of meat after slaughter. For safety management of food, it is strategically important to develop an age verification method based on cuts of meat in the importing country.
Recently, many proteomic analyses have clarified protein complement in the tissues, and two-dimensional gel electrophoresis (2-DE) method shows the possibility of the large-scale screening of proteins in skeletal muscle from various species.Citation1,3–5) The proteomic profiling represents a useful tool for the first screening of a novel bio-marker that is involved in some disease and physiological change.Citation6) Based on the proteomic profiling of aging-related change of protein expression in the muscle, new bio-markers for age-dependent muscle wasting have been identified in rodent and human.Citation7) To develop a suitable bio-index for the age verification of livestock, we analyzed protein expression in chicken breast muscle at different ages. Following proteomic analysis, we focused on the change in expression of the chicken heat shock protein (Hsp) family during muscle aging. Furthermore, to clarify the role of an age-dependent increase in HSPB1 protein, we identified the interactions of HSPB1 protein in chicken muscle. From these results, we suggest that HSPB1 increases in aged chicken muscle and may be protecting against the age-dependent stress.
Material and methods
Reagents
Most chemical reagents and Phos-tag acrylamide gel were purchased from Wako Pure Chemical Inc. (Osaka, Japan). The following antibodies were used for immunoblotting: Hsp25/27 #MAB3842 (Millipore, CA, USA) and #Hsp25–19 (GeneTex, CA, USA), Hsp70 (#610607, BD Bioscience, CA, USA), Hsp90 (#610418, BD Bioscience), αB-crystallin (SPC-126A, Biosciences Inc, Victoria, Canada), myosin fast type (#M4276, Sigma-Aldrich, MO, USA), dihydrodipicolinate reductase 1 (DHPR1; #ab58552, Abcam, Cambridge, UK), and β-actin (#612656, BD Bioscience) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH; #sc-32233, Santa Cruz, CA, USA) antibodies.
Experimental animals
White leghorn layer chickens and hens were obtained from a local poultry house. The chickens were maintained until slaughter in the animal institute of Kobe University. Experimental mice (ICR strain,>30 weeks old) were purchased from Nippon SLC, Inc. (Hamamatsu, Japan). All procedures using experimental animals were performed according to the Guidelines for the Care and Use of Laboratory Animals of Kobe University.
Sample preparation
Samples were dissected from the central portion of the pectoralis major and immediately stored at −80 °C. The frozen muscle was homogenized with a Waring blender in 10 volumes of Tris buffer (50 mM Tris–HCl pH 8.0, and 0.1% Nonited P40) containing various inhibitors for phosphatases and proteases (1 mM Na3O4 V, 1 mM disodium β-glycerophosphate, 1 mM NaF, 1 mM sodium pyrophosphate, and 1 mM PMSF). The homogenate was centrifuged at 3000×g for 20 min, and the pellet was suspended in detergent buffer (50 mM Tris–HCl, pH 8.0, and 1.0% Nonited P40) containing the same inhibitors and incubated for 1 h. The suspended lysates were centrifuged at 16000×g for 20 min, and the supernatants were carefully collected as the soluble fraction.Citation8) The protein concentration of each sample was assayed using the BCA Protein Assay kit (Thermo Fisher Scientific, MA, USA).
Two-dimensional gel electrophoresis (2-DE)
Each sample (300 μg protein) was applied with rehydration buffer (8 M urea, 2% CHAPS, 0.28% dithiothreitol, and 0.5% ampholytes) onto Immobiline DryStrip gel pH 3.0–10 for 12 h and focused under the following conditions (100 V for 2 h, 500 V for 1 h, 1000 V for 1 h, 2500 V for 1 h, 5000 V for 1 h, and 8000 V for 10 h) using an IPGphor unit (GE Healthcare, Uppsala, Sweden).Citation9) After the first-dimensional separation, proteins were electrophoresed with a liner 12.5% acrylamide gel and stained with a silver staining kit (Wako). The expressed levels of major proteins were calculated using spot density with image-analysis software (Image J ver. 1.46; National Institutes of Health, MD, USA).
Immunoblotting
For Western blot analysis, equal amounts of protein were applied to SDS-PAGE and electrotransferred to a polyvinylidene difluoride membrane. After blocking with 5% skim milk in phosphate buffered saline (PBS) with 0.05% Tween20, the membranes were incubated with specific antibodies (Hsp25/27, 1:250; Hsp70, 1:1000; Hsp90, 1:1000; Myosin, 1:500; and αΒ-crystallin, 1:2000) and diluted in PBS with 0.05% Tween20 or Immuno-Enhancer solution (Wako). The bound antibodies on the membrane were detected by chemiluminescence with a horseradish peroxidase (HRP)-conjugated secondary antibody (Jackson Immunoresearch Laboratories Inc., PA, USA) and ImmunoStar LD detection reagents (Wako). Applied protein levels were confirmed by gel staining with Coomassie Brilliant Blue (CBB) or immunoblotting with β-actin (1:1000) or GAPDH (1:500) antibody.
Reverse transcription-PCR analysis
Reverse transcription-polymerase chain reaction (RT-PCR) was performed as described previously.Citation10) Briefly, the total RNA of chicken skeletal muscle was isolated using a Sepazol RNA super G Kit (Nacalai tesque, Kyoto, Japan). Equal amounts of RNA were reverse-transcribed with ReverTra Ace (Toyobo, Tokyo, Japan). The resulting cDNA was appropriately diluted and used as a template for PCR with KOD-FX Neo (Toyobo). The following primer pairs were used to amplify the specific chicken sequence: GAPDH, 5′-GGTTCAGAACAGGGCCAGGTAG-3′ and 5′-AGGGCTGTAGGCAGGCAAAC-3′ (NM_204305); HSPB1, 5′-GCTTCATCTCCAGGTGCTTC-3′ and 5′-TGGCTGGTTCTTCCTTCTTG-3′ (NCBI: NM_205290); αB-crystallin, 5′-TCCGCAGACCTCTGTTTTCT-3′ and 5′-TTTTAGCTCCTCAGGCGAGA-3′ (GGU26661); Hsp40, 5′-GATGCCGTGAAGAGGGTAAA-3′ and 5′-GCTTCTCGTCTCCAAACTGG-3′ (NM_001031224); Hsp60, 5′-CCAATAGTCACCGCAAACCT-3′ and 5′-CAAACCCTCCTCTCCAAACA-3′ (NM_001012916); Hsp70, 5′-AGACATTGAGCGGATGGTTC-3′ and 5′-GCTGGTGCTCAAACTCTTCC-3′ (NM_205003); Hsp90, 5′-ATGACTGGGAGGACCACTTG-3′ and 5′-TTCAGGTATTCGGGGATCAG-3′ (NM_001109785); HSF1, 5′-ACTCCAACCTGGACAACCTG-3′ and 5′-ACTGCTTCCCTGTGTCTGCT-3′ (L06098.1); HSF2, 5′-TTACAAGCTCCGTGCAGATG-3′ and 5′-GGAGCTCCCACTCTCAACAG-3′ (NM_001167764); HSF3, 5′-AGACGCAGACAGCTCTGGAT-3′ and 5′-ATCACTGACACGGGGTCTTC-3′ (XM_420166); HSF4, 5′-TGACAGTGGGTTGTCAGCTC-3′ and 5′-TGATCAGCATGAGGCACTTC-3′ (NM_001172374). The number of amplification cycles for Hsp isoforms were 30 or 33 cycles. Heat shock factor (HSF) isoforms underwent 35 cycles and GAPDH underwent 26 cycles. After electrophoretic separation, the amplified fragments were quantified with Image J software.
Quantification of the amount of HSPB1 protein
To standardize the reference standard of HSPB1 protein for the calibration curve, chicken HSPB1 DNA was amplified by PCR with first-strand cDNA library synthesized from chicken skeletal muscle RNA using the following primers: 5′-GCATGGATCCATGGCCGAGCGCCGCGTGC-3′ and 5′- AGTCGGATCCTTACTACTTCTTGGCTGGTTCTTCCTTC-3′. The amplified PCR fragment was subcloned into the pGEX-6p vector. Recombinant GST-HSPB1 was purified as described previously.Citation11,12) The concentration of purified GST-HSPB1 protein was assayed by BCA protein assay and confirmed with SDS-PAGE and CBB staining.
Identification of interactive protein and immunoprecipitation
Samples of the pectoralis major taken from aged chickens (500 day) were homogenized with a Waring blender in 10 volumes of lysis buffer (50 mM Tris–HCl pH 7.5, 2 mM MgCl2, 150 mM NaCl, 0.5% Nonited P40, and 1 mM EDTA) containing inhibitors (1 mM Na3O4 V, 1 mM NaF, and 1 mM PMSF), and centrifuged for 20 min at 16000×g to remove insoluble material. Supernatants (2 mL) were transferred to tubes containing 25 μl of pre-washed Protein G Sepharose slurry (GE Healthcare) and incubated for 30 min at 4 °C with rotation. Beads were washed twice with lysis buffer. After the elution of bound proteins with SDS-PAGE sample buffer, each sample was subjected to SDS-PAGE, and the gels were stained with a Silver stain MS kit (Wako). The bands were excised and subjected to in-gel tryptic digestion and peptide extraction. Peptides were analyzed by nanoLC/MS/MS using an LTQ Orbitrap Discovery mass spectrometer (Thermo Scientific). For protein identification, MS/MS data were searched against the SwissProt database using an in-house MASCOT server.
Lysates prepared from mouse hind-limb muscle were incubated with protein G-Sepharose beads and a specific antibody for 30 min at 4 °C. Subsequently, protein G Sepharose beads were washed three times with lysis buffer, and precipitated proteins were subjected to SDS-PAGE and immunoblotting with specific antibodies.
Immunofluorescence microscopy
Immunofluorescence staining was performed with specific antibodies (Hsp25/27; 1:100, DHPR1; 1:400) diluted with Can Get Signal immunostain solution A (Toyobo) using paraffin sections as described by Ueda et al.Citation10,13) The bound antibodies were visualized using anti-mouse antibody labeled with Dylight-488 or anti-rabbit antibody labeled with Alexa-546. Images were obtained using a confocal laser-scanning microscope (LSM 700; Carl Zeiss, Jena, Germany) and processed by the Zeiss LSM Image Browser.
Statistical analysis
The statistical significance of the p-value was analyzed using Student’s t-test (paired and two-sided). The correlation coefficient was calculated with the approximation curve in regression analysis. Results were expressed as means ± standard deviation (S.D.) or standard error (S.E.).
Results
Proteomic analysis of chicken muscle
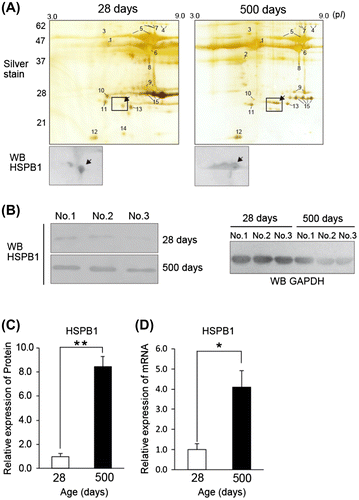
To identify an age-dependent protein increase, we compared the skeletal muscle protein from the pectoralis major of a young chicken (28 days post-hatching) and culled adult hens (500 days) by 2-DE. We used the subcellular fractions containing 1% Nonited P40 to avoid contamination of abundant myofibril and sarcoplasmic proteins. The silver staining of 2-DE gels (pH 3.0–10) was independently repeated three times, and the major spots were reproducibly observed, at least 43 spots at 28 days and 47 spots at 500 days (Fig. (A)). Major spots identified the proteins by comparison with an established 2-DE database.Citation14,15) The staining pattern almost agreed with a previous proteomic studyCitation4); apolipoprotein A1 (spot 10; 0.17 ± 0.10), aldolase C (spot 11; 0.38 ± 0.26), phosphoglycerate mutase (spot 9; 0.79 ± 0.27), and triosephosphate isomerase (spot 15; 0.63 ± 0.14) decreased at 500 days (relative value 28 days vs. 500 days, mean values ± S.D., n = 3). On the other hand, pyruvate kinase (spot 4), phosphoglucomutase (spot 7), α/β-enolase (spot 5), and creatine kinase (spot 6) persisted, or slightly decreased at 500 days. These characteristic expressions are recognized in other species,Citation7) and the expression change suggests a physiological difference in chicken skeletal muscle between the growth stage and mature stage.Citation4) In contrast to these spots, drastically increased spots (25 kDa and pI 5.0) in aged chicken muscle are indicated by an arrow in Fig. (A). Because sHsp expression in skeletal muscle has been detected in 2-DE map in a senescent studyCitation16), we predicted that the protein of the spot belonged to the sHsp family. To identify the spot, we performed Western blotting with prospective sHsp antibodies. Enlarged Western blotting membranes (Fig. (A)) indicate the spots of chicken Hsp, HSPB1 (alternative name: chicken Hsp24 which is homologous to human Hsp27 and mouse Hsp25) which works as an ATP-independent molecular chaperone to protect against protein denaturation.Citation17) To confirm the change of HSPB1 expression, we analyzed the expression by Western blotting (Fig. (B) and (C)) and semiquantitative RT-PCR (Fig. (D)). These data indicated an eightfold increase at the protein level and a fourfold increase at mRNA level at 500 days compared with those at 28 days.
Fig. 1. Age-related expression changes in chicken muscle.
Notes: (A) Comparison of two-dimensional gel electrophoresis (2-DE) maps between young (28 days) and aged (500 days) chicken muscle. The lysates from the pectoralis major were separated by 2-DE and stained with silver staining. The remarkable spots were numbered on the gels. The significantly increased spots in aged muscle were marked by arrow on the staining gel and the immunoblotting membrane with heat shock protein (Hsp) 25/27 antibody. The 2-DE area surrounded by a rectangle corresponds to the Western blotting membrane. (B) and (C) Western blot analysis of HSPB1 expression with Hsp25/27 or GAPDH antibody in chicken muscle. Total lysate (3 μg/lane) was applied to SDS-PAGE gel. (D) Semiquantitative RT-PCR analysis of the mRNA of HSPB1. All values show mean ± S.E. from three samples. The amount of RNA was normalized by the expression level of GAPDH. Significant difference indicates **p < 0.01 and *p < 0.05.
Expression of HSPB1 in aged chicken muscle
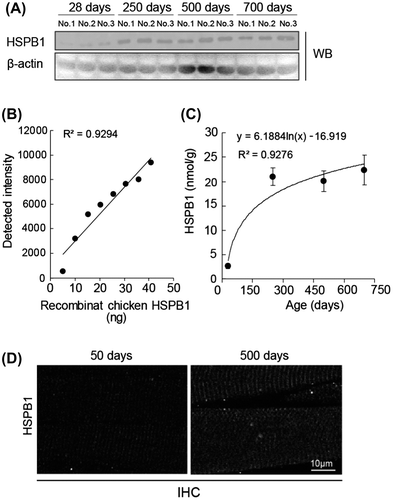
Members of the heat shock protein family are relatively abundant in muscle.Citation18) We analyzed the expression of major isoforms of Hsp protein in chicken muscle using RT-PCR. mRNA of αB-crystallin, Hsp40, Hsp60, Hsp70, and Hsp90 were detectable in the pectoralis major of chicken, but their expression levels did not change significantly at 500 days compared with those at 28 days (Fig. (A)). In agreement with this RT-PCR result, Western blotting indicated an almost equal expression of Hsp70 and Hsp90 protein at 28 days and 500 days (Fig. (B)). These data suggest that HSPB1 is likely a unique Hsp isoform with an expression that is drastically changed in culled chicken muscle. We estimated the amount of HSPB1 expression in chicken muscle using a quantification method for Hsp protein.Citation19) The lysates from chickens 28 days and 750 days muscle were applied to Western blotting (Fig. (A)), and the concentration of HSPB1 protein was evaluated using a calibration curve against the density of Western blotting which simultaneously detected the known concentration of recombinant chicken HSPB1 protein (Fig. (B)). The HSPB1 contents were estimated as 2.6 ± 0.5 nmol/g of wet muscle at 28 days, and 22.4 ± 3.1 nmol/g at 500 days (Fig. (C)). These results were supported by immunohistochemical analyses using paraffin sections. Fig. (D) shows the immune-labeled HSPB1 in longitudinal sections of chicken pectoralis major (50 days and 500 days). In contrast to 50 days, the immunofluorescence of HSPB1 was clearly localized at cross-striated patterns in aged (500 days) muscle and observed at relatively high intensity in aged muscle fibers (Fig. (D)). These results revealed that the amount of HSPB1 protein was drastically up-regulated depending on the age (days) of chicken muscle.
Fig. 2. Age-related expression change of Hsp isoforms.
Notes: (A) Semiquantitative RT-PCR analysis of major Hsp isoforms in young (28 days) and aged (500 days) chicken muscle. The amount of RNA was normalized by the expression level of GAPDH. (B) Western blot analysis of Hsp70 and Hsp90 with the specific antibodies. The amounts of lysate applied (3 μg/lane) were verified by Western blotting with actin antibody and also by CBB staining. All values show mean ± S.E. from three samples.
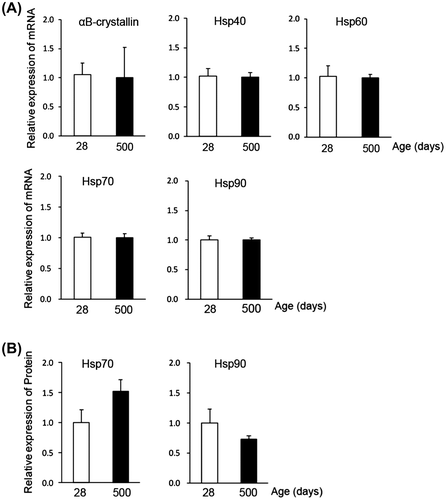
Fig. 3. Quantification of age-related HSPB1 protein.
Notes: (A) Western blot analysis of HSPB1 in chicken muscle from 28 to 750 days of age. The amounts of lysate applied (3 μg/lane) were verified by Western blotting with β-actin antibody. (B) A standard curve was calculated with the density of purified recombinant chicken HSPB1 protein (from 5 to 40 ng of GST-HSPB1). (C) Time course analysis of HSPB1 expression in chicken muscle. HSPB1 concentration was quantified with a standard curve on the same Western blot. All values are mean ± S.E. from three samples. R2 indicates Pearson’s product-moment correlation coefficient. (D) Immunohistochemical analysis of HSPB1 in chicken muscle (50 days and 500 days of age). Longitudinal sections were incubated with Hsp25/27 antibody and second antibody labeled with fluorescent Dylight-488 and imaged by confocal microscopy.
HSF and MHC expression
Physiological age-related changes in skeletal muscle are apparent in the decline of muscle fiber size and alteration of fiber type.Citation7,20) The transcription of Hsp family members is up-regulated via heat shock factor (HSF) which binds to heat shock elements on the Hsp genes through the heat shock response. Four members of the HSF family have been identified in vertebratesCitation21) and are also conserved in the genome of the chicken.Citation22) We analyzed the expression of all HSF members in 28 days and 500s day chicken muscle. At both stages, HSF1, HSF2, and HSF3 were detectable, but HSF4 was not detectable in chicken muscle by RT-PCR (Fig. (A)). The results indicated that expression of HSF1, HSF2, and HSF3 was not affected by aging up to 500 days in chicken muscle.
Fig. 4. Effect of the HSF and fast-to-slow fiber transition.
Notes: (A) Semiquantitative RT-PCR analysis of HSF in young and aged chicken muscle. The amount of RNA was normalized by the expression level of GAPDH. All values are mean ± S.E. from three samples. (B) Relative amount of myosin heavy chain (MHC) fast type in aged chicken muscle. The lysates from pectoral muscles were separated by SDS-PAGE and stained with CBB staining or analyzed by Western blot with specific antibody. The amounts of lysate applied (3 μg/lane) were verified by Western blotting with GAPDH antibody. CBB staining and Western blot revealed the amount of whole MHC and fast type MHC in aged chicken muscle, respectively.
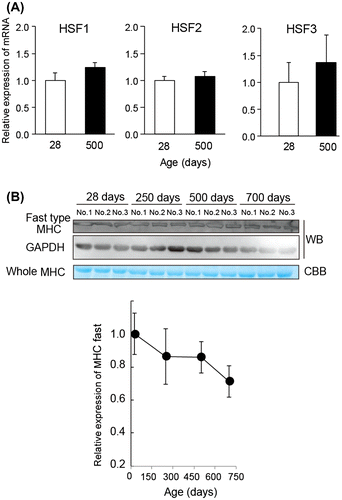
During natural aging of muscle, a remodeling of muscle fiber type occurs from fast type fiber to slow type fiber.Citation23) Furthermore, myosin heavy chain (MHC) isoform shifts from fast type MHC to slow type MHC with the senescent process in aged muscle.Citation24) Since HSPB1 is predominantly expressed in slow type muscle compared with fast type muscle in rats,Citation19,25) we further investigated the effect of muscle type toward HSPB1 expression during aging in chickens. Fig. (B) shows a relative expression of whole MHC protein with the CBB staining and fast type MHC by Western blotting with specific-MHC antibody (MY-32) for the fast type isoform.Citation24) The immunoblotting analysis indicated a slight decrease of fast type MHC expression with aging, but these differences were not enough to explain the change of HSPB1 expression through a remodeling of muscle fiber type.
Functional significance of HSPB1 in culled chicken muscle
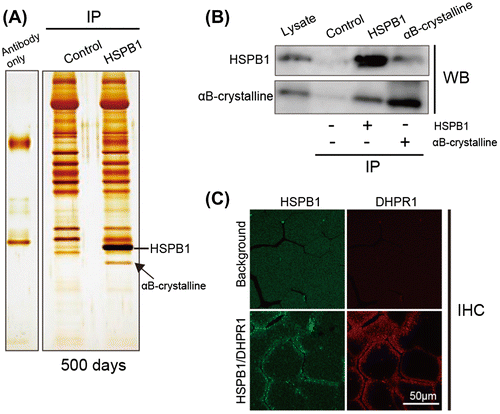
To reveal the functional role of increasing HSPB1, we investigated the proteins associated with HSPB1 in culled chicken muscle. Lysates of the muscle were immunoprecipitated with Hsp25/27 antibody and the immunoprecipitates were analyzed by nanoLC/MS/MS. An arrow in Fig. (A) indicates the identified band of αB-crystallin which is another member of the sHsp family and is known to form a complex with HSPB1 by in vitro assay.Citation26,27) To confirm the association of HSPB1 and αB-crystallin in culled chicken muscle, we performed co-immunoprecipitation using HSPB1 or αB-crystallin antibodies. A commercially available antibody for αB-crystallin did not recognize chicken αB-crystallin in our experimental condition; therefore, we used another skeletal muscle lysate prepared from retired breeder mice which were beyond the age of peak reproductive performance. In agreement with Fig. (A), the association between HSPB1 and αB-crystallin was confirmed by the co-immunoprecipitation assay (Fig. (B)). HSPB1 localization in transverse sections of chicken muscle was examined by confocal microscopy with a skeletal muscle marker (Fig. (C)). The immune-labeled HSPB1 was observed to be localized at the cell periphery of muscle fibers, and co-localized with DHPR1Citation28) at punctate structures near the sarcolemma and transverse tubule in aged muscle. These results suggest that up-regulated HSPB1 associates with αB-crystallin and contributes to preventing age-related protein aggregation in muscle.
Fig. 5. Association of HSPB1 and αB-crystallin in aged chicken muscle.
Notes: (A) Co-immunoprecipitation analysis of HSPB1 using Hsp25/27 antibody from pectoralis muscle of aged chicken (500 days). The lysates from aged muscle were incubated with Hsp25/27-conjugated protein G agarose. The immunoprecipitates were separated by SDS-PAGE and stained with silver staining. Hsp25/27 antibody only (left lane) and immunoprecipitation without antibody (control: middle lane) show a non-specific band. An arrow indicates the band of αB-crystallin identified by nanoLC/MS/MS. (B) Pull down assay with Hsp25/27 (lane 3) or αB-crystallin (lane 4) antibody using the lysate of mouse skeletal muscle. The immunoprecipitates were analyzed by Western blotting with specific antibodies. Means from the control samples without both antibodies. (C) Immunohistochemical analysis of HSPB1 in culled chicken muscle. Transverse sections were co-stained with Hsp25/27 and DHPR1 antibodies.
Discussion
The sHsp family are conserved in almost all vertebrae from prokaryotic and eukaryotic and there are ten isoforms in mammals.Citation29All sHsp isoforms contain α-crystallin domain which is essential for their oligomerization to protect protein aggregation. Several sHsp members have been reported to up- or down-regulate depending on a variety of stresses.Citation25,30) For example, increasing of αB-crystallin was shown in old skeletal muscle of rodent.Citation31) The expression patterns of sHsp are speculated to reflect the physiological conditions in a living body.Citation32) However, details of the relationships between sHsp and skeletal muscle conditions remain poorly understood.
In this study, we identified an increase in the level of HSPB1 protein in the pectoralis major muscle of chicken. We used specific antibody to recognize chicken HSPB1 using Hsp25/27 antibody (#MAB3842), which is a mouse monoclonal antibody raised against a recombinant chicken HSPB1. We double-checked the expression of chicken HSPB1 using two kinds of Hsp25/27 antibodies. 2-DE map analysis indicated that the multiple spots of HSPB1 increased in old chicken muscle (500 days; Fig. (A)). Since a variety of cellular stresses induces the phosphorylation of some serine residues in HSPB1, the multiple spots in gels were considered to be different number of phosphorylation of HSPB1.Citation30) Major sHsp isoforms are known to be expressed in skeletal muscle and change the transcriptional levels in skeletal muscle to protect cellular stress including reactive oxygen speciesCitation33) and sarcopenia in experimental animals.Citation7,16) Our results indicated that HSPB1 specifically increased the concentration and the transcriptional level (Fig. and Fig. ). These data support the phenomenon that increasing levels of HSPB1 are conserved in poultry such as culled chicken as well as other senescent animal models.Citation19,25) In addition, the levels of HSPB1 protein in aged muscle were significantly higher at 500 days (22.4 ± 3.1 nmol/g) than at 28 days (2.6 ± 0.5 nmol/g; Fig. (A) and (C)). We also checked the amount of HSPB1 at other stages (50 days, 2.0 ± 0.5 nmol/g; 100 days, 8.3 ± 6.1 nmol/g; 250 days, 18.4 ± 3.9 nmol/g; n = 3) by different Western blotting and confirmed that the amount of HSPB1 increased during the aging of chicken muscle. The amount of chicken HSPB1 protein is within a similar range of HSPB1 determined in the hind-limb muscle of rodents (extensor digitorum longus, 3.4 nmol/g; soleus, 8.9 nmol/g).Citation30)
Several studiesCitation7,34) have indicated the involvement of sHsp in the fast-to-slow transition process during skeletal muscle aging.Citation12,30) In this study, we expected to see an obvious difference in the senescent muscle of chicken, but we found no significant difference in the expression of fast type MHC and the HSF isoforms (Fig. (A) and (B)). Immunohistochemical analysis also did not reveal an unusual appearance of muscle fibers (Fig. (D) and (B)). Thus, even at the oldest age (700 days), drastic structural changes of skeletal muscle had not occurred in chicken muscle. Interestingly, chicken skeletal muscle is known to exist in multiple isoforms of MHC and myosin light chain (MLC) which are closely related with the subtypes of muscle fiber.Citation35,36) However, these expressions are still largely unknown during muscle aging in chicken. To understand this in detail, it will be necessary to determine the relationship of sHsp, MHC, and MLC expression using chicken muscle. Furthermore, our observations in this study suggest that chicken HSPB1 transcription employs a distinct mechanism from the typical regulation of HSP expression to react to age-related stress.Citation21)
Since up-regulated sHsp supposes to act as molecular chaperone in skeletal muscle,Citation25,37)we investigated the roles of HSPB1 in culled hens muscle. Co-immunoprecipitation analysis with Hsp25/27 antibody indicates that HSPB1 associated with αB-crystallin (Fig. (A)). The gel shifts of HSPB1 spots on the 2-DE map (Fig. (A)) and the drastic mobility shift of HSPB1 protein in Phos-tag acrylamide gel (unpublished data) demonstrated the phosphorylation of chicken HSPB1 in 500-day-old muscle. Since the phosphorylation of HSPB1 has been known to enhance the formation of hetero-oligomerization with sHsp including αB-crystallin,Citation29,30) these data may suggest that the phosphorylated HSPB1 associates with αB-crystallin in chicken muscle.
In the previous study, Doran et al. (2006)Citation7) demonstrated that αB-crystallin clearly localized at the sarcolemma in the senescent rat muscle. In agreement with their results, our immunohistochemical analysis revealed that HSPB1 localized at the sarcolemma in chicken muscle fiber, and suggests the co-localization of HSPB1 with αB-crystallin at the sarcolemma (Fig. (C)). HSPB1 is down-regulated during muscle atrophy triggered by sarcopenia and muscle disuse.Citation16,20) Conversely, an increase in HSPB1 by overexpression with recombinant HSPB1 attenuates muscle atrophy in an experimental model.Citation39) By combining our results with those studies, we suggest that chicken HSPB1 formed complexes with αB-crystallin and acts as a molecular chaperon in aged chicken muscle.
In conclusion, we demonstrated that HSPB1 is a candidate protein for verifying the age of cuts of meat. HSPB1 associates with αB-crystallin in culled chicken muscle, and the complex seems to prevent the cellular stresses that take place with aging. Extensive analysis of the role of the sHsp in skeletal muscle and the meat of other livestock will clarify the effectiveness of HSPB1 as biomarker for the evaluation of meat quality.
Author contribution
SU designed the study and wrote the draft of the manuscript. SS and YK carried out the experimental procedures. KY carried out the LC-MS analysis. KH and HK provided valuable advice. YS and MY assisted with the study. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Grants-in-aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan [Research Project Numbers: 23700832 and 26350889].
Acknowledgment
The authors thank Innami Poultry Agricultural Cooperative in Hyogo for donating many chickens for this study.
Notes
Abbreviations: BSE, bovine spongiform encephalopathy; CBB, coomassie brilliant blue; BCA, bicinchoninic Acid; DHPR1, dihydrodipicolinate reductase 1; PMSF, phenylmethylsulfonyl fluoride; PCR, polymerase chain reaction; RT-PCR, reverse transcription-polymerase chain reaction; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GST, glutathione S-transferase; Hsp, heat shock protein; HSF, Heat shock factor; LC-MS, Liquid chromatography–mass spectrometry; MHC, myosin heavy chain; sHsp, small heat shock protein; 2-DE, two-dimensional gel electrophoresis; PVDF, polyvinylidene difluoride; PBS, phosphate buffered saline; HRP, horseradish peroxidase; S.D., standard deviation; S.E., standard error.
References
- Yamanouchi K, Yoshikawa Y. Bovine spongiform encephalopathy (BSE) safety measures in Japan. J. Vet. Med. Sci. 2007;69:1–6.10.1292/jvms.69.1
- Koizumi N, Iguchi H, Smith TE. Comparison and verification of BSE surveillance in USA and Japan. Environ. Health Prev. Med. 2005;10:130–137.10.1007/BF02900805
- Bouley J, Chambon C, Picard B. Mapping of bovine skeletal muscle proteins using two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2004;4:1811–1824.10.1002/(ISSN)1615-9861
- Doherty MK, McLean L, Hayter JR, et al. The proteome of chicken skeletal muscle: Changes in soluble protein expression during growth in a layer strain. Proteomics. 2004;4:2082–2093.10.1002/(ISSN)1615-9861
- Kim NK, Joh JH, Park HR, Kim OH, Park BY, Lee CS. Differential expression profiling of the proteomes and their mRNAs in porcine white and red skeletal muscles. Proteomics. 2004;4:3422–3428.10.1002/(ISSN)1615-9861
- Doran P, Donoghue P, O’Connell K, Gannon J, Ohlendieck K. Proteomic profiling of pathological and aged skeletal muscle fibres by peptide mass fingerprinting (Review). Int. J. Mol. Med. 2007;19:547–564.
- Doran P, Donoghue P, O’Connell K, Gannon J, Ohlendieck K. Proteomics of skeletal muscle aging. Proteomics. 2009;9:989–1003.10.1002/pmic.v9:4
- Nishiumi S, Ashida H. Rapid preparation of a plasma membrane fraction from adipocytes and muscle cells: application to detection of translocated glucose transporter 4 on the plasma membrane. Biosci. Biotechnol. Biochem. 2007;71:2343–2346.10.1271/bbb.70342
- Yamanoue M, Ueda S, Ohashi A, Yoshimura Y, Norioka S. The N-terminal sequence of paratropomyosin binding fragments from β-connectin. Biosci. Biotechnol. Biochem. 2003;67:563–569.10.1271/bbb.67.563
- Ueda S, Tu-Sekine B, Yamanoue M, Raben DM, Shirai Y. The expression of diacylglycerol kinase theta during the organogenesis of mouse embryos. BMC Dev. Biol. 2013;13:35.10.1186/1471-213X-13-35
- Ueda S, Kataoka T, Satoh T. Role of the Sec14-like domain of Dbl family exchange factors in the regulation of Rho family GTPases in different subcellular sites. Cell Signal. 2004;16:899–906.10.1016/j.cellsig.2004.01.007
- Ueda S, Kataoka T, Satoh T. Activation of the small GTPase Rac1 by a specific guanine-nucleotide-exchange factor suffices to induce glucose uptake into skeletal-muscle cells. Biol. Cell. 2008;100:645–661.10.1042/BC20070160
- Ueda S, Kitazawa S, Ishida K, et al. Crucial role of the small GTPase Rac1 in insulin-stimulated translocation of glucose transporter 4 to the mouse skeletal muscle sarcolemma. FASEB J. 2010;24:2254–2261.10.1096/fj.09-137380
- Hoogland C, Mostaguir K, Sanchez JC, Hochstrasser DF, Appel RD. SWISS-2DPAGE, ten years later. Proteomics. 2004;4:2352–2356.10.1002/(ISSN)1615-9861
- Sato Y, Shimizu M, Mizunoya W, et al. Differential expression of sarcoplasmic and myofibrillar proteins of rat soleus muscle during denervation atrophy. Biosci. Biotechnol. Biochem. 2009;73:1748–1756.10.1271/bbb.90085
- Calderwood SK, Murshid A, Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging—a mini-review”. Gerontology. 2009;55:550–558.10.1159/000225957
- Arrigo AP, Simon S, Gibert B, et al. Hsp27 (HspB1) and αB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–3674.10.1016/j.febslet.2007.04.033
- Liu Y, Steinacker JM. Changes in skeletal muscle heat shock proteins: pathological significance. Front Biosci. 2001;6:D12–25.10.2741/Liu
- Larkins NT, Murphy RM, Lamb GD. Absolute amounts and diffusibility of HSP72, HSP25, and alphaB-crystallin in fast- and slow-twitch skeletal muscle fibers of rat. Am. J. Physiol. Cell Physiol. 2012;302:C228–C239.10.1152/ajpcell.00266.2011
- Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br. Med. Bull. 2010;95:139–159.10.1093/bmb/ldq008
- Åkerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010;11:545–555.10.1038/nrm2938
- Nakai A, Morimoto RI. Characterization of a novel chicken heat shock transcription factor, heat shock factor 3, suggests a new regulatory pathway. Mol. Cell Biol. 1993;13:1983–1997.
- Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011;91:1447–1531.10.1152/physrev.00031.2010
- Gannon J, Doran P, Kirwan A, Ohlendieck K. Drastic increase of myosin light chain MLC-2 in senescent skeletal muscle indicates fast-to-slow fibre transition in sarcopenia of old age. Eur. J. Cell Biol. 2009;88:685–700.10.1016/j.ejcb.2009.06.004
- Golenhofen N, Perng MD, Quinlan RA, Drenckhahn D. Comparison of the small heat shock proteins alphaB-crystallin, MKBP, HSP25, HSP20, and cvHSP in heart and skeletal muscle. Histochem. Cell Biol. 2004;122:415–425.
- Fu L, Liang JJ. Detection of protein-protein interactions among lens crystallins in a mammalian two-hybrid system assay. J. Biol. Chem. 2002;277:4255–4260.10.1074/jbc.M110027200
- Fu L, Liang JJ. Enhanced stability of alpha B-crystallin in the presence of small heat shock protein Hsp27. Biochem. Biophys. Res. Commun. 2003;302:710–714.10.1016/S0006-291X(03)00257-2
- Piétri-Rouxel F, Gentil C, Vassilopoulos S, et al. DHPR α1S subunit controls skeletal muscle mass and morphogenesis. EMBO J. 2010;29:643–654.10.1038/emboj.2009.366
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat. Struct. Mol. Biol. 2005;12:842–846.10.1038/nsmb993
- Kostenko S, Moens U. Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell Mol. Life Sci. 2009;66:3289–3307.10.1007/s00018-009-0086-3
- O’Connell K, Gannon J, Doran P, Ohlendieck K. Proteomic profiling reveals a severely perturbed protein expression pattern in aged skeletal muscle. Int. J. Mol. Med. 2007;20:145–153.
- Piec I, Listrat A, Alliot J, Chambon C, Taylor RG, Bechet D. Differential proteome analysis of aging in rat skeletal muscle. FASEB J. 2005;19:1143–1145.
- Escobedo J, Pucci AM, Koh TJ. HSP25 protects skeletal muscle cells against oxidative stress. Free Radical Biol. Med. 2004;37:1455–1462.10.1016/j.freeradbiomed.2004.07.024
- Doran P, Gannon J, O’Connell K, Ohlendieck K. Aging skeletal muscle shows a drastic increase in the small heat shock proteins alphaB-crystallin/HspB5 and cvHsp/HspB7. Eur. J. Cell Biol. 2007;86:629–640.10.1016/j.ejcb.2007.07.003
- Robbins J, Horan T, Gulick J, Kropp K. The chicken myosin heavy chain family. J. Biol. Chem. 1986;261:6606–6612.
- Bandman E. Functional properties of myosin isoforms in avian muscle. Poult. Sci. 1999;78:729–734.10.1093/ps/78.5.729
- Lindquist S, Craig EA. The heat-shock proteins. Annu. Rev. Genet. 1988;22:631–677.10.1146/annurev.ge.22.120188.003215
- Glass DJ. A signaling role for dystrophin: inhibiting skeletal muscle atrophy pathways. Cancer Cell. 2005;8:351–352.
- Dodd SL, Hain B, Senf SM, Judge AR. Hsp27 inhibits IKKbeta-induced NF-kappaB activity and skeletal muscle atrophy”. FASEB J. 2009;23:3415–3423.10.1096/fj.08-124602
