Abstract
Isoflavones play important roles in plant–microbe interactions in rhizospheres. Soybean roots secrete daidzein and genistein to attract rhizobia. Despite the importance of isoflavones in plant–microbe interactions, little is known about the developmental and nutritional regulation of isoflavone secretion from soybean roots. In this study, soybeans were grown in hydroponic culture, and isoflavone contents in tissues, isoflavone secretion from the roots, and the expression of isoflavone conjugates hydrolyzing beta-glucosidase (ICHG) were investigated. Isoflavone contents did not show strong growth-dependent changes, while secretion of daidzein from the roots dramatically changed, with higher secretion during vegetative stages. Coordinately, the expression of ICHG also peaked at vegetative stages. Nitrogen deficiency resulted in 8- and 15-fold increases in secretion of daidzein and genistein, respectively, with no induction of ICHG. Taken together, these results suggest that large amounts of isoflavones were secreted during vegetative stages via the hydrolysis of (malonyl)glucosides with ICHG.
Graphical abstract
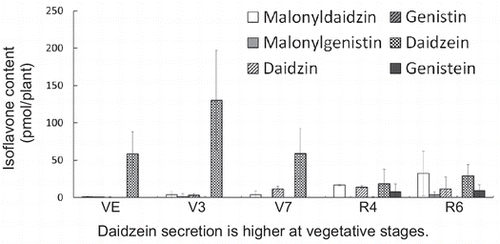
Daidzein secretion from soybean roots is higher at vegetative stages with higher isoflavone conjugates hydrolyzing beta-glucosidase expression.
Isoflavones, which occur mostly in legume plants, are biosynthesized from flavanones such as liquiritigenin and naringenin via 2-hydroxylation catalyzed by isoflavone synthase.Citation1) In humans, soybean isoflavones have been regarded as dietary components with important roles in reducing the incidence of breast and prostate cancersCitation2) promoting bone health,Citation3) relieving menopausal symptoms,Citation4) and preventing coronary heart disease.Citation5) In plants, isoflavones function in plant–microbe interactions, i.e., defense and symbiosis.Citation6–8) In defense, isoflavones serves as precursors for the production of phytoalexins, a group of plant metabolites possessing anti-microbial and/or anti-herbivore activities. Representative phytoalexins of soybean are glyceollins, prenylated pterocarpans derived from daidzein, which are induced in response to pathogens such as Phytophthora sojae and Macrophomina phaseolina.Citation9) In symbiosis, soybean roots secrete isoflavones such as daidzein and genistein into the rhizosphere, a small region around the roots, where these compounds signal rhizobia to form nodules on the roots.Citation10) In addition to signaling rhizobia to induce the NodD gene, isoflavones play various roles in biological communications with soil microbes.Citation11,12)
Although plants secrete a significant proportion of photosynthates into the rhizosphere from their roots, the mechanisms and genes involved in the root exudation are not fully understood.Citation13,14) Plant growth stages as well as the nutrient deficiency such as nitrogen, phosphate, and iron have been found to significantly affect the secretion of metabolites into the rhizosphere.Citation15–18) In rice, for example, the amount of root exudates was the lowest at seedling stage, gradually increased until flowering, and decreased at maturity.Citation19) In Arabidopsis, metabolites secreted from roots were dynamically altered during development, in correlation with the transcriptomes of rhizosphere microbial communities.Citation20) In soybean, however, little is known about the developmental and nutritional regulation of root exudation. Two pathways are suggested to be involved in the secretion of isoflavones: 1) ATP-dependent transport of isoflavone aglycones, especially genisteinCitation21) and 2) secretion of isoflavone glucosides into apoplasts followed by hydrolysis with ICHG (isoflavone conjugates hydrolyzing beta-glucosidase).Citation22) In this study, soybeans were grown in hydroponic culture medium, and tissue isoflavone contents, the secretion of isoflavones from soybean roots and the expression of ICHG gene at various growth stages and nutrient conditions analyzed.
Materials and methods
Chemicals
Malonyldaidzin and malonylgenistin were purchased from Nagara Science (Gifu, Japan). Acetonitrile of HPLC-grade was purchased from Junsei Chemical Co. (Tokyo, Japan) or Sigma-Aldrich (St. Louis, MO, USA). Other chemicals were obtained from Wako Pure Chemical Industries (Osaka, Japan) or Nacalai tesque (Kyoto, Japan).
Plant materials and growth conditions
The soybean seeds (cv. Enrei) used in this study were obtained from Tsurushin Shubyo (Matsumoto, Japan). Soybean seeds were surface sterilized with 70% ethanol for 1 min, 10% bleach for 5 min, followed by four washes with sterile water. Surface sterilized seeds were sown in vermiculite containing water and grown for 7 days at 25 °C in the dark. Seedlings were rinsed and transferred to a hydroponic culture system, in which soybeans were grown in 500 mL plastic containers filled with mineral nutrient medium consisting of 0.28 mM MgSO4, 0.6 mM KNO3, 0.084 mM KCl, 0.13 mM KH2PO4, 0.24 mM Ca(NO3)2, 2 μM Fe-EDTA, 4.5 μM KI, 28 μM MnCl2, 19 μM H3BO3, 2.3 μM ZnSO4, 0.5 μM CuSO4, and 0.003 μM Na2MoO4, pH 6.0. The soybeans were kept in a cultivation room set at 25 °C with 16/8 h photoperiod. Soybeans were transferred to new medium weekly, and isoflavones secreted into the hydroponic medium for 24 h were collected at different growth stages. The tissue and root exudates of each plant were sampled from 3-, 5-, 7-, and 9-week-old soybeans, corresponding to the V3, V7, R4, and R6 stages of soybean growth, respectively. One-week-old soybeans (VE stage) were grown in 5 mL tubes, instead of 500 mL containers. The VE stage was the stage of emergence before unifoliolate leaves were expanded; the V3 and V7 stages are the stages at which three and seven trifoliate are fully expanded at the main stem, respectively; the R4 stage is the stage at which pods are 3/4 in. long on one of the top four nodes; and the R6 stage is the stage at which the pods are filled with fully grown green seeds.Citation23) For growth under nitrogen-deficient conditions, 4-week-old plants were transferred to a nitrogen-deficient (-N) medium, consisting of 0.28 mM MgSO4, 0.42 mM KCl, 0.13 mM KH2PO4, 0.26 mM CaCl2, 0.002 mM Fe-EDTA, 4.5 μM KI, 28 μM MnCl2, 19 μM H3BO3, 2.3 μM ZnSO4, 0.5 μM CuSO4, and 0.003 μM Na2MoO4, pH 6.0; for growth under phosphate-deficient conditions, 4-week-old plants were transferred to a phosphate-deficient (-P) medium, consisting of 0.28 mM MgSO4, 0.6 mM KNO3, 0.61 mM KCl, 0.24 mM Ca(NO3)2, 0.002 mM Fe-EDTA, 4.5 μM KI, 28 μM MnCl2, 19 μM H3BO3,2.3 μM ZnSO4, 0.5 μM CuSO4, and 0.003 μM Na2MoO4, pH 6.0. Both sets of plants were grown for one week and transferred to the same medium for the sampling of tissues and root exudates.
Isoflavone extraction
Frozen soybean tissues were pulverized in liquid nitrogen with a mortar and a pestle, and lyophilized. The flavonoids were extracted from the lyophilized tissues with 80% methanol at 50 °C for 1 h, followed by centrifugation at 15,000 rpm for 5 min to remove the tissue debris. The supernatant was filtered through a Minisart 0.45 μm filter (Sartorius, Göttingen, Germany).
Preparation of root exudates
Medium containing root exudates was filtered through Omnipore membrane filters (Millipore, Darmstadt, Germany), and its pH was adjusted to 3.0 using HCl. The medium was passed through a Sep-pak C18 Plus short cartridge (Waters, MA), which was washed with 3 mL water and eluted with 2 mL MeOH. The eluant was dried under nitrogen and reconstituted in 95% MeOH with 1% formic acid for reversed-phase HPLC analysis.
HPLC analysis of isoflavones
Flavonoids were analyzed using a HPLC apparatus (LC-10A, Shimadzu, Kyoto, Japan) under the following conditions: column, TSK-gel ODS-80TM (4.6 mm × 250 mm; Tosoh, Tokyo, Japan); solvent A, 0.3% (v/v) formic acid; solvent B, 0.3% (v/v) formic acid in acetonitrile; detection, 262 nm. Elution was at 0.8 mL/min with the solvent system A (water containing 0.3% (v/v) formic acid) and B (acetonitrile containing 0.3% (v/v) formic acid) with a linear gradient program from 15 to 22% B in 30 min, followed by a linear gradient from 22 to 35% B in 20 min, and a linear gradient from 35 to 70% B in 5 min.
RNA extraction and real-time quantitative PCR
Total RNA was prepared from soybean leaves and roots using RNeasy Plant Mini Kits (Qiagen, CA) according to the manufacture’s instruction. DNA in each total RNA sample was digested with DNase I, and, following inactivation of DNase I, the RNA was reverse transcribed to cDNA using ReverTra Ace (Toyobo, Osaka, Japan), according to the manufacturer’s instructions. Real-time qPCR of the GmICHG and ubiquitin genes was performed with the Rotor-Gene 3000A (Corbett Research, Australia), using Thunderbird SYBR qPCR Mix (Toyobo, Osaka, Japan) according to the manufacturers’ instructions. Briefly, each PCR reaction mixture consisted of 10 ng of cDNA template, 5 pmol of each primer, 1 μL of fluorescent probe, and 12.5 μL of Thunderbird Sybr Qpcr Mix in a total volume of 25 μL. The amplification protocol consisted of an initial denaturation at 95 °C for 2 min, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s. The primer sets were 5′ GCTTGCCATTTGGTGAACTT-3′ (forward) and 5′-AACTGGTTCATACCGCTTGC-3′ (reverse) for ICHG and 5′-GGGTTTTAAGCTCGTTGT-3′ (forward) and 5′-GGACACATTGAGTTCAAC -3′ (reverse) for ubiquitin.Citation24) Relative expression level was calculated based on the absolute quantification method using the standard curve.
Results
Isoflavone content and secretion at different growth stages
Fully expanded leaves and roots were collected for isoflavone analysis. In leaves at V3 stage, daidzin was the most predominant isoflavone, whereas at V7 to R6 stages, the most predominant isoflavone was malonylgenistin (Fig. (A)). Contents of daidzein and genistein were very small at V3 and V7 stages, and these aglycones were not detectable at R4 and R6 stages (Fig. (A)). In leaves, both isoflavones accumulated as malonylglucosides or glucosides; the ratio of daidzein derivatives to genistein derivatives was 3.5:1 at V3 stage, becoming approximately 1:2 at stages V7 to R6.
Fig. 1. Contents of isoflavones in soybean tissues and root exudates during growth.
Notes: Each bar represents the mean ± SD of five replicates. N.D.; not determined. Different letters indicate significant differences (p < 0.05) by one-way ANOVA with Tukey’s HSD test.
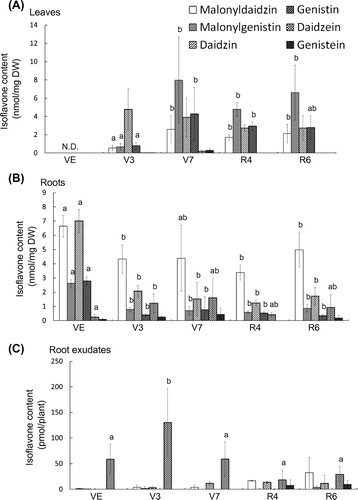
In roots, malonyldaidzin was the most predominant form at V3 to R6 stages, but the amounts of malonyldaidzin and daidzin were approximately the same at VE stage (Fig. (B)). Both daidzein and genistein were also detected in roots, with their amounts higher in roots than in leaves (Fig. (B)). In roots, the ratio of daidzein derivatives to genistein derivatives was 2.5:1 at VE stage, increasing to 4:1 to 5:1 at stages V3 to R6.
In contrast to tissue samples, daidzein was the predominant form of isoflavones in the root exudates of soybeans, especially at vegetative stages (VE to V7) (Fig. (C)). Only trace amounts of malonylglucosides and glucosides were present at VE stage, slightly increasing during development. At reproductive stages, the contents of malonylglucosides and glucosides were comparable to those of aglycones (Fig. (C)). Most isoflavones in root exudates at vegetative stages were daidzein derivatives.
Isoflavone content and secretion under nitrogen or phosphate-deficient condition
Contents of isoflavones in tissues and root exudates were analyzed under nitrogen- and phosphate-deficient conditions. When soybeans were grown under nitrogen-limited conditions for one week at V7 stage, the tissue contents and composition of isoflavones were not significantly changed (Figs. (A) and (B). In contrast, 8- and 15-fold increases in daidzein and genistein, respectively, were observed in the root exudates (Fig. (C)), along with reductions in malonyldaidzin and daidzin.
Fig. 2. Contents of isoflavones in soybean tissues and root exudates during growth under phosphate- and nitrogen-limited conditions.
Notes: Each bar represents the mean ± SD of five replicates. Different letters indicate significant differences (p < 0.05) by one-way ANOVA with Tukey’s HSD test.
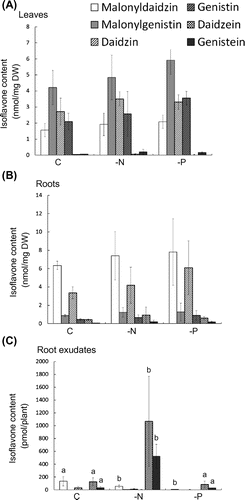
Under phosphate limited conditions, the amount of amino acids and organic acids in soybean root exudates was shown to be induced.Citation17) The tissue contents and composition of isoflavones were not significantly altered under phosphate deficiency (Fig. (A) and (B)). Root exudates showed approximately 10-fold reductions in malonyldaidzin and daidzin contents, while the aglycones contents were not significantly altered (Fig. (C)).
Expression of ICHG
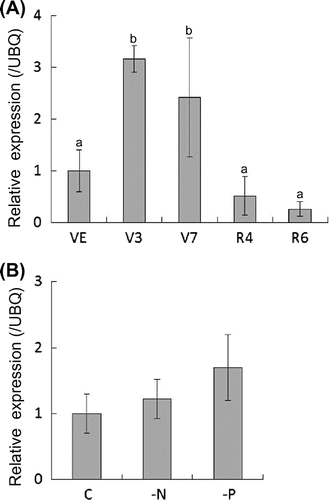
In the hydroponic growth system, the contents and composition of isoflavones in leaves and roots were not dynamically altered during development, while those in root exudates differed, depending on the developmental stages, especially from vegetative to reproductive stages and under nitrogen-deficient conditions. There are two possible pathways for isoflavone secretion from roots,Citation12) with ICHG thought to be a key regulator of the secretion of malonylglucosides and glucosidesCitation22,24 ).
Analysis of ICHG expression by real-time PCR showed that, during soybean development, ICHG expression was higher at vegetative stages, especially at V3 and V7, but was reduced during reproductive stages (Fig. (A)). These results are in agreement with the higher daidzein content in root exudates of soybeans at vegetative stages. Despite the large increase in daidzein and genistein secretion under nitrogen-deficient conditions, ICHG expression was not induced by nitrogen deficiency or phosphate deficiency (Fig. (B)).
Discussion
Genetic and environmental variations of isoflavone contents in soybean seeds have been widely studied due to the agricultural importance of this crop.Citation25) In contrast, little is known about changes in isoflavones during soybean growth stages. A recent analysis of flavonoids in leaves during development showed that daidzein and genistein are increased in leaves of R5 to R6 stages.Citation26) This study investigated tissue isoflavone contents and secretion from roots during development and nutritional stress. Variations in isoflavone contents of leaves and roots during development were relatively small, suggesting that steady-state levels of isoflavone contents were maintained throughout development. The ratios of daidzein derivatives to genistein derivatives were reversed in roots and leaves, being high in roots but low in leaves, except for V3 stage. These inter-tissue differences suggest the tissue-specific expression of chalcone synthase and chalcone reductase, which are key enzymes involved in the 5-deoxyflavonoid synthesis.Citation27) In contrast to tissue isoflavone contents, the secretion of isoflavones showed growth-stage-dependent changes; i.e. the secretion of isoflavones into the rhizosphere reached a maximum at V3 stage then gradually decreased, suggesting that the biological interactions mediated by isoflavones are important during vegetative stages. At VE stage, daidzein was the predominant isoflavones secreted into the rhizosphere, and the secreted amount was 225 ± 99 pmol/g FW, which was the highest amount per gram biomass during growth stage (V3; 37 ± 18, V7; 9.9 ± 5.6, R4; 2.4 ± 2.4, R6; 3.5 ± 1.7 pmol/g FW). The higher amount of daidzein secreted into the rhizosphere at seedling stages would contribute the symbiosis with rhizobia at the vegetative stage. At reproductive stages, the expression of ICHG was reduced, and coordinately malonyldaidzin and other glucosides were present in root exudates. Daidzein and genistein from the seed and root exudates of soybeans were found to induce the expression of the nod gene.Citation10) When soybeans are grown under nitrogen-deficient conditions, isoflavone contents in roots are increased, suggesting that the secretion of isoflavones into the rhizosphere was also increasedCitation28). These secreted isoflavones have been analyzed in young seedlings,Citation10,29) but not in plants during active growth under nitrogen-deficient conditions. In this study, tissue isoflavone contents and secreted isoflavones were analyzed in soybeans at V7 stage. Nitrogen deficiency for one week did not increase the isoflavone contents of leaves or roots, but induced the secretion of isoflavones, in particular daidzein and genistein, approximately 8- and 15-fold, respectively. Nitrogen deficiency did not induce the expression of ICHG in root, suggesting that an ATP-dependent transporter contributes the secretion of isoflavones during the nitrogen deficiency in addition to the ICHG-mediated secretion. In soybeans, the root exudation of amino acids and organic acids was induced by phosphate deficiency,Citation17) whereas isoflavone secretion was somewhat reduced and the tissue isoflavone contents were not significantly altered.
In conclusion, the results presented here showed the developmental and nutritional regulation of isoflavone secretion from soybean roots. Higher amounts of isoflavones were secreted into the rhizosphere at vegetative stages, when ICHG expression was increased. These findings suggest the secretion of the isoflavone-(malonyl)glucosides that may have accumulated in vacuoles. Under nitrogen-deficient conditions, genistein was also highly secreted, along with daidzein, suggesting that an ATP-dependent transporter contributes to the secretion of genistein biosynthesized in response to nitrogen deficiency. Two pathways for isoflavone secretion in soybean roots are expected to have distinct physiological roles, i.e. ICHG-mediated secretion during vegetative growth and ATP-dependent transport during nitrogen deficiency, although proteins functioning in these processes are still unknown.
The metabolites secreted from roots are collectively called root exudates and are one of the primary factors influencing rhizosphere microbial communities,Citation14,30) which play important roles in plant growth and health.Citation31,32) We recently showed that the structures of soybean rhizosphere microbial communities, especially of bacteria, were altered during growth,Citation33,34) suggesting that plant growth, including root exudates, affects the regulation of rhizosphere bacterial communities. The effects of alterations in isoflavone secretion during development on the structure and function of rhizosphere microbial communities, not only on rhizobia, are the focus of future research.
Author contribution
AS, ST, TN, and KY conceived and designed the experiments. AS, YY, and KY*: Kazuaki Yamashita performed the experiments. AS, ST, TN, and KY analyzed the data. AS, and KY wrote the paper.
Supplementary material
The supplementary material for this article is available online at http://dx.doi.10.1080/09168451.2015.1062714.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan (Genomics-based Technology for Agricultural Improvement, SFC2001) (AS) and JSPS KAKENHI [grant number 26712013] and [grant number 26660279] (AS).
Acknowledgments
We would like to thank Ms. Yuko Kobayashi and Dr. Saya Shirai for technical assistance. We also thank DASH/FBAS, Research Institute for Sustainable Humanosphere, Kyoto University.
References
- Aoki T, Akashi T, Ayabe S. Flavonoids of leguminous plants: structure, biological activity, and biosynthesis. J. Plant Res. 2000;113:475–488.10.1007/PL00013958
- Sarkar FH, Li YW. Soy isoflavones and cancer prevention. Cancer Invest. 2003;21:744–757.10.1081/CNV-120023773
- Messina M. Soyfoods and soybean isoflavones for promoting bone health. Agro Food Ind. Hi-Tec. 2005;16:30–32.
- Faraj A, Vasanthan T. Soybean isoflavones: effects of processing and health benefits. Food Rev. Int. 2004;20:51–75.10.1081/FRI-120028830
- Xiao CW. Health effects of soy protein and isoflavones in humans. J. Nutr. 2008;138:1244S–1249S.
- Cesco S, Neumann G, Tomasi N, Pinton R, Weisskopf L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant and Soil. 2010;329:1–25.10.1007/s11104-009-0266-9
- Cesco S, Mimmo T, Tonon G, et al. Plant-borne flavonoids released into the rhizosphere: impact on soil bio-activities related to plant nutrition. A review. Biol. Fert. Soils. 2012;48:123–149.10.1007/s00374-011-0653-2
- Pii Y, Mimmo T, Tomasi N, Terzano R, Cesco S, Crecchio C. Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fert. Soils. 2015;51:403–415.10.1007/s00374-015-0996-1
- Tzi BN, Ye XJ, Wong JH, et al. Glyceollin, a soybean phytoalexin with medicinal properties. Appl. Microbiol. Biotech. 2011;90:59–68.
- Kosslak RM, Bookland R, Barkei J, Paaren HE, Appelbaum ER. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc. Natl. Acad. Sci. USA. 1987;84:7428–7432.10.1073/pnas.84.21.7428
- Weston LA, Mathesius U. Flavonoids: their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol. 2013;39:283–297.10.1007/s10886-013-0248-5
- Sugiyama A, Yazaki K. Flavonoids in plant rhizospheres: secretion, fate and their effects on biological communication. Plant Biotech. 2014;31:431–443.
- Sugiyama A, Yazaki K. Root exudates of legume plants and their involvement in interactions with soil microbes. In: Vivanco JM, Baluska F, editors. Secretions and exudates in biological systems. Heidelberg: Springer; 2012. p. 27–48.
- Haichar FE, Santaella C, Heulin T, Achouak W. Root exudates mediated interactions belowground. Soil Biol. Biochem. 2014;77:69–80.10.1016/j.soilbio.2014.06.017
- Kuzyakov Y. Carbon input by plants into the soil. Review. J. Plant Nutr. Soil Sci. 2000;163:421–431.10.1002/(ISSN)1522-2624
- Valentinuzzi F, Cesco S, Tomasi N, Mimmo T. Influence of different trap solutions on the determination of root exudates in Lupinus albus L. Biol. Fert. Soils. Forthcoming.
- Tawaraya K, Horie R, Shinano T, Wagatsuma T, Saito K, Oikawa A. Metabolite profiling of soybean root exudates under phosphorus deficiency. Soil Sci. Plant Nutr. 2014;60:679–694.10.1080/00380768.2014.945390
- Pii Y, Penn A, Terzano R, Crecchio C, Mimmo T, Cesco S. Plant-microorganism-soil interactions influence the Fe availability in the rhizosphere of cucumber plants. Plant Physiol. Biochem. 2015;87:45–52.10.1016/j.plaphy.2014.12.014
- Aulakh MS, Wassmann R, Bueno C, Kreuzwieser J, Rennenberg H. Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Biol. 2001;3:139–148.10.1055/s-2001-12905
- Chaparro JM, Badri DV, Vivanco JM. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2014;8:790–803.10.1038/ismej.2013.196
- Sugiyama A, Shitan N, Yazaki K. Involvement of a soybean ATP-binding cassette-type transporter in the secretion of genistein, a signal flavonoid in legume-Rhizobium symbiosis. Plant Physiol. 2007;144:2000–2008.10.1104/pp.107.096727
- Suzuki H, Takahashi S, Watanabe R, et al. An isoflavone conjugate-hydrolyzing beta-glucosidase from the roots of soybean (Glycine max) seedlings: purification, gene cloning, phylogenetics, and cellular localization. J. Biol. Chem. 2006;281:30251–30259.10.1074/jbc.M605726200
- Fehr WR, Caviness CE, Burmood DT, Pennington JS. Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Sci. 1971;11:929.10.2135/cropsci1971.0011183X001100060051x
- Yoo D, Hara T, Fujita N, et al. Transcription analyses of GmICHG, a gene coding for a beta-glucosidase that catalyzes the specific hydrolysis of isoflavone conjugates in Glycine max (L.) Merr. Plant Sci. 2013;208:10–19.10.1016/j.plantsci.2013.03.006
- Chennupati P, Seguin P, Liu WC. Effects of high temperature stress at different development stages on soybean isoflavone and tocopherol concentrations. J. Agric. Food Chem. 2011;59:13081–13088.10.1021/jf2037714
- Song HH, Ryu HW, Lee KJ, Jeong IY, Kim DS, Oh SR. Metabolomics investigation of flavonoid synthesis in soybean leaves depending on the growth stage. Metabolomics. 2014;10:833–841.10.1007/s11306-014-0640-3
- Yan Y, Huang L, Koffas MA. Biosynthesis of 5-deoxyflavanones in microorganisms. Biotechnol. J. 2007;2:1250–1262.10.1002/(ISSN)1860-7314
- Cho MJ, Harper JE. Effect of inoculation and nitrogen on isoflavonoid concentration in wild-type and nodulation-mutant soybean roots. Plant Physiol. 1991;95:435–442.10.1104/pp.95.2.435
- Smit G, Puvanesarajah V, Carlson RW, Barbour WM, Stacey G. Bradyrhizobium japonicum nodD1 can be specifically induced by soybean flavonoids that do not induce the nodYABCSUIJ operon. J. Biol. Chem. 1992;267:310–318.
- Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006;57:233–266.10.1146/annurev.arplant.57.032905.105159
- Berendsen RL, Pieterse CMJ, Bakker P. The rhizosphere microbiome and plant health. Trends in Plant Sci. 2012;17:478–486.10.1016/j.tplants.2012.04.001
- Schlaeppi K, Bulgarelli D. The plant microbiome at work. Mol. Plant-Microbe Interact. 2015;28:212–217.10.1094/MPMI-10-14-0334-FI
- Sugiyama A, Ueda Y, Zushi T, Takase H, Yazaki K. Changes in the bacterial community of soybean rhizospheres during growth in the field. PLoS One. 2014;9:e100709.10.1371/journal.pone.0100709
- Sugiyama A, Ueda Y, Takase H, Yazaki K. Pyrosequencing assessment of rhizosphere fungal communities from a soybean field. Can. J. Microbiol. 2014;60:687–690.10.1139/cjm-2014-0443