Abstract
Measurements of singlet oxygen (1O2) quenching rates (kQ (S)) and the relative singlet oxygen absorption capacity (SOAC) values were performed for seven rice bran extracts 1–7, which contained different concentrations of antioxidants (AOs) (such as α-, β-, γ-, and δ-tocopherols and -tocotrienols, three carotenoids (lutein, β-carotene, and zeaxanthin), and γ-oryzanol), in ethanol at 35 °C using UV–vis spectrophotometry. The concentrations of four tocopherols and four tocotrienols, three carotenoids, and γ-oryzanol contained in the extracts were determined using HPLC-MS/MS, UV-HPLC, and UV–vis absorption spectroscopy, respectively. Furthermore, comparisons of kQ (S) (Obsd.) values observed for the above extracts 1–7 with the sum of the product { [AO-i]} of the
values obtained for each AO-i and the concentration ([AO-i]) of AO-i contained in extracts 1–7 were performed. From the results, it has been ascertained that the SOAC method is applicable to general food extracts to evaluate their 1O2-quenching activity.
Graphical abstract
Measurements of the quenching rate (kQ) of singlet oxygen were performed for seven rice bran extracts in ethanol to evaluate their singlet oxygen quenching activity.
Lipid peroxyl radical (LOO) and singlet oxygen (1O2) are two well-known representative reactive oxygen species (ROS) generated in biological systems. In recent years, various methods to assess the total LOO
scavenging activity of foods and plants have been developed.Citation1–5) On the other hand, singlet oxygen absorption capacity (SOAC) assay methods to assess the total 1O2 quenching activity of foods and plants have not been developed. 1O2 reacts with many biological targets including lipids,Citation6) proteins,Citation7) and DNA,Citation8,9) as well as LOO
radical does. Reactions with 1O2 occur mainly by chemical reaction, inducing the degradation of biological systems. Carotenoids and vitamin E homologues are widely present in vegetables, fruits, and edible oilsCitation10–15) and may function as efficient 1O2 quenchers in biological systems.Citation16–18)
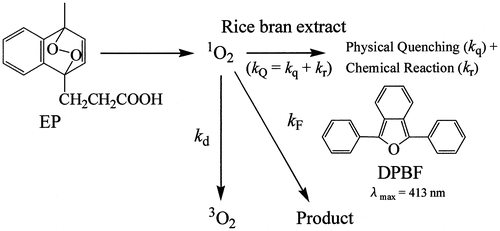
Recently, kinetic studies of the quenching reaction of 1O2 with naturally occurring antioxidants (AOs) (such as carotenoids, vitamin E homologues, catechins, and caffeic acids) were performed in ethanol/chloroform/D2O (50:50:1, v/v/v) and ethanol solutions at 35 °C.Citation19–22) The overall rate constants, kQ (= kq + kr, physical quenching + chemical reaction), for reaction of AOs with 1O2 were measured using a competition reaction method with endoperoxide (EP) as a singlet oxygen generator and 2,5-diphenyl-3,4-benzofuran (DPBF) as an UV–vis absorption probe (see Scheme ).Citation19–22)(1)
The second-order rate constants, kQ (S) and kQ (t1/2), were determined by analyzing the first-order rate constant (S) and half-life (t1/2) of the decay curve of DPBF, respectively, showing good accordance with each other. Furthermore, measurements of the kQ (S) and kQ (t1/2) values were performed for vegetable, fruit, and palm oil extracts.Citation22,23) From these results, a new assay method that could quantify the SOAC of AOs, including carotenoids, phenolic AOs, and food extracts, was proposed.Citation19–23) The relative SOAC value was defined in the following equation:(2)
where [α-Toc] and [AO] are molar concentrations (mol/L) (or weight concentrations (g/L)) of α-tocopherol (α-Toc) and AOs, respectively. α-Toc was used as a standard compound.Citation19)
In recent years, α-, β-, γ-, and δ-tocotrienols (α-, β-, γ-, and δ-Toc-3s) have received much attention,Citation24) because of reports of their beneficial biological properties such as cholesterol lowering,Citation25–27) anticancer,Citation28,29) anti-inflammatory,Citation30,31) antiangiogenic,Citation32,33), and neuroprotectiveCitation34) effects, which suggest that Toc-3s have a wide variety of health benefits. Generally, the amounts of α-, β-, γ-, and δ-Toc-3s found in foods are much lower than amounts of α-, β-, γ-, and δ-tocopherols (α-, β-, γ-, and δ-Tocs).Citation35) However, rice bran and palm oil contain comparatively high concentrations of Toc-3s, and are used as food sources of Toc-3s.Citation10,36,37)
As rice is the staple food of many Asian peoples, including Japanese, species improvement is often practiced, and many kinds of rice are cultivated.Citation10) Rice bran includes a variety of AOs, such as vitamin E homologues (4 Tocs and 4 Toc-3s), carotenoids (Cars), γ-oryzanol (γ-Ory) (i.e. 10 phytosterols), and polyphenols.Citation10,37–43) As the amounts of AOs in rice brans differ from one another, we recently carried out a detailed kinetic study of free radical scavenging activity of seven rice bran extracts to compare their free radical scavenging activity.Citation44)
In the present study, measurements of the rate constants ( and
) and the relative SOAC values were performed for seven rice bran extracts 1–7 (Table ), which were prepared from Japanese and Korean rice varieties, in ethanol, to evaluate their 1O2-quenching activity. Measurement of the rate constants (
and
) for γ-Ory was also performed, as rice brans generally contain high concentrations of γ-Ory.Citation37–39,42)
Table 1.  and
and  values for rice bran extracts 1–7 in ethanol at 35.0 °C, relative rate constants
values for rice bran extracts 1–7 in ethanol at 35.0 °C, relative rate constants  , and relative SOAC Values.
, and relative SOAC Values.
The concentrations of α-, β-, γ-, δ-Tocs and -Toc-3s, and γ-Ory contained in rice bran extracts 1–7 were reported in a previous study.Citation44) In addition, the concentrations (in mg/100 g) of three carotenoids (lutein, β-carotene, and zeaxanthin) contained in rice bran extracts 1–7 were determined using UV-HPLC.Citation45,46) Furthermore, comparison of values observed for the above extracts 1–7 with the sum of the product {
[AO-i]/105} of the
value obtained for each AO-i and the concentration ([AO-i]/105) of AO-i contained in extracts 1–7 was performed, in order to ascertain the validity of the developed SOAC method.Citation19–23)
Materials and methods
Materials
DPBF was obtained from Tokyo Kasei Chemicals, Japan. 3-(1,4-Epidioxy-4-methyl-1,4-dihydro-1-naphthyl) propionic acid (endoperoxide, EP) was obtained from Wakenyaku Co. Ltd., Japan. The UV spectrum of EP indicated that the commercially available EP includes 95% EP and 5% unreacted EP-precursor.Citation19)
Preparations of rice bran samples and rice bran extracts 1–7
Details of the preparation of 109 kinds of rice and rice bran samples were reported by Sookwong et al. (including three of the present authors).Citation10) Sookwong et al. determined the concentrations of 4 Tocs and 4 Toc-3s contained in 109 kinds of rice bran extracts. In the present study, seven kinds of rice brans 1–7 (Okka Modoshi (1), Wataribune (2), Nipponbare (3), Milyang-23 (4), γ-Rich Koshihikari (5), Koshihikari (6), and Moritawase (7)) (see Table ) were chosen from the above 109 rice brans to measure the and SOAC values, because these rice brans contain remarkably different concentrations of 4 Tocs and 4 Toc-3s one another.Citation10) γ-Rich Koshihikari (extract 5), which includes high concentrations of γ-Toc and -Toc-3 (Supplementary Table S1), is a mutant of Koshihikari (extract 6).
Rice cultivation was performed at the experiment farm of Toyama Agricultural Research Center, following the method of Sookwong et al.Citation10) Seed rice obtained was stored at a cold dark place (5–15 °C). Rice bran samples (1–4 and 7) and (5 and 6) were prepared from the seed rice of the year 2010 and 2012 products, respectively, using a Toyo-Tester grain-polishing machine (Toyo Rice Corporation, Tokyo, Japan).Citation10) The weight of each sample was approximately 10% of the dehulled rice. The rice brans 1–7 were stored at −30 °C with controlled humidity. Measurements of the values of rice bran extracts 1–7 and the concentrations of AOs contained were performed on December 2012.
Amounts of α-, β-, γ-, δ-Tocs and -Toc-3s and γ-Ory in the rice bran extracts were measured following the methods of Chen and BergmanCitation37) and Sookwong et al.,Citation10) using 2-propanol as an extraction solvent. In the present work, 2-propanol was similarly used to prepare the seven rice bran extracts 1–7. Details of the preparation of extracts 1–7 were described in a previous report.Citation44)
Measurements of the concentrations of 4 Tocs and 4 Toc-3s and γ-Ory contained in rice bran extracts 2–7 were performed using a HPLC-MS/MS technique and UV–vis spectrophotometry, respectively. Details of the measurements were described in a previous study.Citation10,44) The same rice bran extracts 2–7 were used to measure 1O2-quenching rates ( and
) and SOAC values in the present work. The concentrations of 4 Tocs, 4 Toc-3s, and γ-Ory reported in our previous work are summarized in Table S1.Citation44) The preparation of Okka Modoshi (extract 1) and measurements of the concentrations of 4 Tocs, 4 Toc-3s, and γ-Ory were similarly performed. The results obtained for extract 1 together with those reported for extracts 2–7 are listed in Table S1.
UV-HPLC measurement of the concentrations of carotenoids contained in rice bran extracts 1–7
Concentrations of carotenoids in rice bran extracts 1–7 were determined using an UV-HPLC technique.Citation45,46) Details of the UV-HPLC measurements are as follows. Rice bran extract (100 mg) was mixed with 0.5 mL of ethanolic pyrogallol (6%, w/v), 0.5 mL of water, and 0.1 mL of 1.8 M KOH aqueous solution. The mixture was saponified at 70 °C for 60 min. 3.0 mL of n-hexane was added to the mixture, followed by vortexing for 5 min. After centrifugation at 1,000 g for 10 min at 4 °C, the upper hexane layer was collected. The hexane extraction was repeated, and the hexane layers were combined and concentrated in a stream of nitrogen gas. The dried hexane extract was redissolved in 100 μL of methanol/tert-butyl methyl ether (2:3, v/v). An aliquot (40 μL) of the solution was subjected to UV-HPLC measurement.Citation45,46) Separation was performed at 35 °C using a silica column (4.6 × 250 mm, 5 μm, YMC, Kyoto, Japan). Carotenoids were detected with a UV–visible light detector at 463 nm, eluting with a binary gradient consisting of the following HPLC solvents: A, methanol/tert-butyl methyl ether/water (83:15:2, v/v/v; containing 3.9 mM ammonium acetate) and B, methanol/tert-butyl methyl ether/water (8:90:2, v/v/v; containing 2.6 mM ammonium acetate). The gradient profile was as follows: 0–12 min, 10–45% B linear; 12–24 min, 45–100% B linear; 24–30 min 100% B. Typical chromatograms of standard carotenoids (A) and a rice bran extract (Nipponbare (3)) (B) are shown in Fig. , panels (A) and (B), respectively. These chromatograms indicated that three carotenoids (lutein, β-carotene, and zeaxanthin) were present in the rice bran extract (Nipponbare (3)). Lutein, β-carotene, and zeaxanthin concentrations in the rice bran extract were calculated using calibration curves of carotenoid standards. The concentrations for each sample were determined three times, and the average values are summarized in Table . These three carotenoids (lutein, β-carotene, and zeaxanthin) were similarly found in the other rice bran extracts.
Fig. 1. Measurement of the amounts of carotenoids contained in Nipponbare.
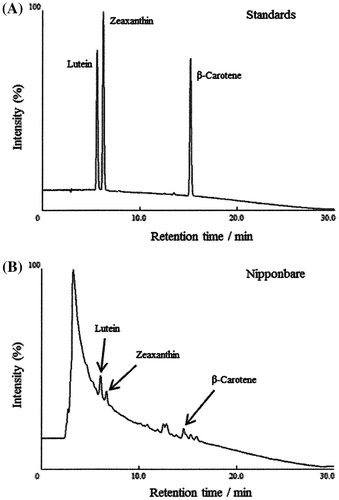
Table 2. Amounts of three carotenoids contained in rice bran extracts 1–7.
Measurements of the rate constants (kQ (S))
Measurements of the rate constants (kQ (S)) were performed in ethanol, using a Shimadzu UV–vis spectrophotometer (UV-1800), equipped with a six-channel cell positioner and an electron-temperature control unit (CPS-240A). All measurements were performed at 35.0 ± 0.5 °C. Experimental errors in the rate constants ( and
) were estimated to be approximately 7% in ethanol solution. Details of the measurements are described in our previous work.Citation19–23)
Analyses of the second-order rate constants ( and
and  ) and SOAC values
) and SOAC values
As reported in previous studies,Citation19,47,48) the and
values for the reaction of 1O2 with an AO were determined by Equations (Equation3
(3) ) and (Equation4
(4) ), respectively.
(3)
(4)
where SBlank and SAO are the slopes of the first-order plots (i.e. ln (absorbance) vs. t plots) of the disappearance of DPBF in the absence and presence of the AO, respectively (see Fig. (C)), and
are the half-lives of DPBF in the absence and presence of AO, respectively, and kd is the rate of natural deactivation of 1O2 in ethanol (kd = 8.3 × 104 s−1).Citation49) Equations (Equation3
(3) ) and (Equation4
(4) ) indicate that the kQAO (S) and kQAO (t1/2) values can be obtained from SBlank/SAO and
vs. [AO] plots (see Fig. (D) and (E)), respectively.Citation19,21)
Fig. 2. Measurement of the second-order rate constant (kQ) for the reaction of Wataribune with singlet oxygen.
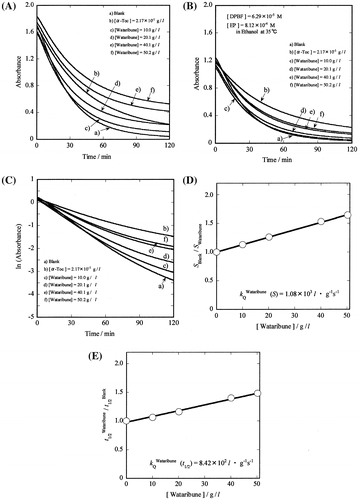
As shown in Fig. (C), ln (absorbance) vs. t plots indicated that the decay of DPBF for Wataribune (extract 2) followed first-order kinetics at ~5 < t < ~60 min. Consequently, analysis of the decay curve was performed for this time region. This was an important condition for obtaining accurate rate constants ( and
) for AOs.Citation19)
As proposed in a previous work,Citation19) the relative SOAC value for an AO was defined as Equation (Equation2(2) ). Equation (Equation2
(2) ) indicates that the SOAC value corresponds to the ratio (
) of the quenching rate of singlet oxygen by the AO (
) to that of α-Toc (
). According to Equation (Equation2
(2) ), the SOAC value was determined by measurement of the half-life of DPBF. In the case of rice bran extracts 1–7, the unit g/L was used for the concentrations of the extracts, and the unit for
and
values was L g−1 s−1. The SOAC values for extracts 1–7 were also calculated using g/L as the unit of the concentration of AO in Equation (Equation2
(2) ).
Results
1O2-quenching rates and SOAC values for rice bran extracts 1–7 in ethanol solution
Measurements of ,
and SOAC values were performed for extracts 1–7 in ethanol.Citation19–21) Fig. (A) shows an example of the reaction between DPBF and EP in the absence and presence of Wataribune (extract 2) in ethanol. As carotenoids are included in extract 2 (Table ), baseline corrections were performed using the UV–vis absorbance at 411 nm of extract 2 (Supplementary Fig. S1).Citation20,23) The corrected decay curves are shown in Fig. (B). SBlank and SWataribune values were calculated from the slopes of ln (absorbance) vs. t plots (see Fig. (C)). SBlank/SWataribune and
vs. [Wataribune (by g/L unit)] plots are shown in Fig. , panels (D) and (E), respectively. Both the SBlank/SWataribune and
vs. [Wataribune] plots show similar slopes, i.e. similar rate constants (see Table ). Furthermore, the relative SOAC value (1.94 × 10−3) (given on a weight basis (g/L)) was determined, using Equation (Equation2
(2) ).
Similar measurements were performed for extracts 1 and 3–7 in ethanol, and the ,
, and SOAC values obtained are summarized in Table . Considerable agreement between the ratios of
to
and the SOAC values was obtained for the extracts 1–7, as expected from Equation (Equation2
(2) ). These results indicate that accurate analyses of the rate constants (
) and SOAC values were performed for rice bran extracts 1–7, although the SOAC values were 1–2 orders of magnitude smaller than for vegetables (such as carrot and tomato), which include high concentrations of carotenoids.Citation20,23)
As listed in Table , the values decrease in the order of extract 1 ≥ 2 ~ 3 ≥ 4 ~ 5 ~ 6 > 7. The
value (1.15 × 103 L g1 s−1) of Okka Modoshi (1) with the highest activity was only 1.33 times higher than that (8.62 × 102) of Moritawase (7) which had the lowest activity.
Measurement of the value was similarly performed for γ-Ory in ethanol. The
value (2.69 × 103 L g1 s−1) obtained for γ-Ory was 177 times smaller than that (4.76 × 105) of α-Toc (see Table ). The concentrations (1400–1970 mg/100 g) of γ-Ory in extracts 1–7 were similar, and were more than an order of magnitude greater than the total concentrations of Tocs and Toc-3s (112–152 mg/100 g), as listed in Table S1. This result suggests that γ-Ory also contributes to the quenching of 1O2 in rice bran extracts 1–7.
Amounts of eight vitamin E homologues, three carotenoids, and γ-oryzanol in rice bran extracts 1–7
Measurements of the concentrations of 4 Tocs, 4 Toc-3s, 3 carotenoids, and γ-Ory were performed, in order to compare the (Obsd.) values observed for rice bran extracts 1–7 with the
(Calcd.) values calculated using the rate constant (
) and concentration ([AO-i]) of each AO-i included in the extracts.
As described in the Materials and Methods section, rice bran extracts generally include 4 Tocs, 4 Toc-3s, and γ-Ory. In fact, the UV–vis absorption spectrum of Koshihikari (6) shows a maximum at λmax = 328 nm, indicating that γ-Ory is included in Koshihikari (6) (see Fig. S1).Citation44) The amounts of the 4 Tocs and 4 Toc-3s and γ-Ory in extracts 1–7 were determined using a HPLC-MS/MS technique and UV–vis spectrophotometry, respectively, as reported in a previous study,Citation44) and are listed in Table S1.
It has been reported that carotenoids are contained in rice bran extracts.Citation39,41) For example, the UV–vis absorption spectrum of Koshihikari (6) shows a broad absorption in the wavelength region of 400–500 nm (see Fig. S1), suggesting that carotenoids are included in Koshihikari (6).Citation19–23) However, to the best of our knowledge, the particular carotenoids and their amounts in rice bran extracts have not been reported. In the present study, at least three kinds of carotenoids (lutein, β-carotene, and zeaxanthin) were found in extracts 1–7, and the amounts (in mg/100 g) of 3 carotenoids were determined using an UV-HPLC technique (see Table ). The amounts of the 3 carotenoids increased in the order of zeaxanthin < β-carotene < lutein, and was independent of the type of rice bran extract. On the other hand, the amount of total carotenoids in extracts 1–7 varied significantly from 1.082 mg/100 g in Koshihikari (6) to 0.143 in Milyang 23 (4), as listed in Table . The amount of total carotenoids in Koshihikari (6) was 7.57 times greater than the amount in Milyang 23 (4).
Discussion
Comparison of observed and calculated 1O2-quenching activities for rice bran extracts 1–7
It is well known that various AOs coexist not only in foods and plants, but also in human tissues. The (Obsd.) (L g−1 s−1) values for rice bran extracts 1–7 were measured by the SBlank/SExtract vs. [Extract (g/L)] plot, using Equation (Equation5
(5) ).
(5)
(6)
As reported in a previous study,Citation44) 4 Tocs, 4 Toc-3s, and γ-Ory (abbreviated as AO-i) were found in extracts 1–7 (see Table S1). The concentrations of three carotenoids (lutein, β-carotene, and zeaxanthin) were determined in the present study (Table ). Furthermore, polyphenols are also found in rice bran,Citation41,43) although the concentrations of these polyphenols have not been reported.
Recently, measurements of 1O2-quenching rate constants () of AOs (4 Tocs, 4 Toc-3s, and 3 carotenoids) were performed in ethanol solution.Citation21,22) The
value for γ-Ory in ethanol was measured in the present work. The
values of these AOs are listed in Table .
values of α-, β-, γ-, and δ-Toc-3s were in good agreement with those (
) of the corresponding α-, β-, γ-, and δ-Tocs. The
values of the 3 carotenoids (lutein, β-carotene, and zeaxanthin) were similar, and were approximately two orders of magnitude larger than the values of Tocs and Toc-3s.Citation21)
Table 3. Comparison between observed and calculated 1O2-quenching rates ( (Obsd.) and
(Obsd.) and  for Koshihikari (6) in ethanol solution.
for Koshihikari (6) in ethanol solution.
Consequently, the (Calcd.) values for rice bran extracts 1–7 were calculated using Equation (Equation6
(6) ), i.e. {Σ kQAO-i (S) [AO-i]/105}, where [AO-i] is the concentration (in mg/100 g) of 4 Tocs, 4 Toc-3s, 3 carotenoids, and γ-Ory in extracts 1–7 (see Table S1 and Table ). The
(Calcd.) values obtained for rice bran extracts 1–7 are listed in Tables and .
Table 4. Comparison between observed and calculated 1O2-quenching rates ( (Obsd.) and
(Obsd.) and  (Calcd.) (See Text)) for rice bran extracts 1–7 in ethanol, ratio of the rate constants (
(Calcd.) (See Text)) for rice bran extracts 1–7 in ethanol, ratio of the rate constants ( (Obsd.)/
(Obsd.)/ (Calcd.)), and contributions of (i) 4 Tocs and 4 Toc-3s, (ii) 3 Cars, and (iii) γ-Ory.
(Calcd.)), and contributions of (i) 4 Tocs and 4 Toc-3s, (ii) 3 Cars, and (iii) γ-Ory.
As shown in Equation (Equation6(6) ), the total
(Calcd.) value can be expressed as the sum of (i)
[Toc-i (& Toc-3-i)]/105, (ii)
[Car-i]/105, and (iii)
[γ-Ory]/105 values calculated by considering the individual contributions from (i) 4 Tocs and 4 Toc-3s, (ii) 3 carotenoids, and (iii) γ-Ory, respectively.
For example, (i) [Toc-i (& Toc-3-i)]/105, (ii)
[Car-i]/105, and (iii)
[γ-Ory]/105 values calculated for Koshihikari (6) were 5.11 × 102, 3.38 × 102 and 3.82 × 101 L g−1 s−1, respectively (see Table ), and the total
(Calcd.) value was 8.87 × 102 L g−1 s−1. The contributions of (i) 4 Tocs and 4 Toc-3s, (ii) 3 carotenoids, and (iii) γ-Ory were 57.6, 38.1, and 4.3%, respectively, indicating that the three different kinds of AOs used in the calculation contribute to the 1O2-quenching activity of Koshihikari (6).
Similar calculations were performed for extracts 1–5 and 7, as listed in Table . The ratios ( (Obsd.)/
(Calcd.)) for γ-rich Koshihikari (5), Koshihikari (6), and Moritawase (7) were 1.05, 1.03, and 1.05, respectively. The result indicates that the total 1O2-quenching activities of extracts 5, 6, and 7 may be reasonably simulated by considering the contribution of 8 vitamin E homologues, 3 carotenoids, and γ-oryzanol contained in rice bran extracts 5, 6, and 7. The contribution of other AOs (such as polyphenols) appears to be negligible.
Furthermore, this result suggests that the interactions between the 8 vitamin E homologues, 3 carotenoids, and γ-Ory and their interactions with the many other molecules contained in the rice bran extracts are weak and negligible in solution. It is interesting that the total 1O2-quenching rates ( (Obsd.)) of rice bran extracts 1–7 that include many types of biological molecules (such as carbohydrates, lipids, proteins, and nucleic acids) may be explained by simply considering the sum of the contributions of the contained AOs.
On the other hand, as listed in Table , (Obsd.) values observed for Okka Modoshi (1), Wataribune (2), Nipponbare (3), and Milynag 23 (4) were 1.78, 1.49, 1.21, and 1.46 times larger than the corresponding (
(Calcd.)) values calculated using the concentrations of 4 Tocs, 4 Toc-3s, 3 carotenoids, and γ-Ory. Other AOs (such as polyphenols and/or other carotenoids) in extracts 1–4 may contribute to the
(Obsd.) values. In fact, UV chromatograms of Nipponbare (3) showed several peaks (Fig. (B)) probably due to other carotenoids. However, details of these contributing compounds are not clear at present.
Comparison of the contributions of (i) 4 tocopherols and 4 tocotrienols, (ii) 3 carotenoids, and (iii) γ-Oryzanol to the 1O2-quenching activity of rice bran extracts 1–7
As listed in Table , the total 1O2-quenching activities of 4 Tocs and 4 Toc-3s (i.e. the values of { [Toc-i (& Toc-3-i)]/105}) were calculated for extracts 1–7. The values varied from 4.97 × 102 L g−1 s−1 for Moritawase (7) to 6.10 × 102 for Nipponbare (3), showing that the latter was only 1.23 times greater than the former. The ratios of {
[Toc-i (& Toc-3-i)]/105} to
(Obsd.) were 43.8% to 59.6% for extracts 1–7. This result indicates that the
(Obsd.) values observed for extracts 1–7 may not be accurately reproduced considering only the contributions of 4 Tocs and 4 Toc-3s.
On the other hand, the values of { [Car-i]/105} calculated for 3 carotenoids varied remarkably from 4.50 × 101 L g−1 s−1 for Milyang (4) to 3.38 × 102 for Koshihikari (6). The latter is 7.51 times as large as the former. In fact, as listed in Table , the total content of the 3 carotenoids varied notably from 0.143 mg/100 g for Milyang (4) to 1.082 mg/100 g for Koshihikari (6), with the latter 7.57 times greater than the former. As listed in Table , carotenoids are found in Koshihikari (6). The total content (1.082 mg/100 g) of the 3 carotenoids in Koshihikari (6) was two orders of magnitude smaller than that (118.7 mg/100 g) of the 4 Tocs and 4 Toc-3s (Table S1). However, the
values of the carotenoids were generally two orders of magnitude larger than the
values of Tocs and Toc-3s (see Table ), which suggests a significant contribution of carotenoids to the
value of Koshihikari (6).Citation20–22) The ratio ({
[Car-i]/105}/
(Obsd.)) for Koshihikari (6) was 36.9%, indicating a considerable contribution of the 3 carotenoids to the 1O2-quenching activity. Similarly, {
[Car-i]/105} values were calculated for extracts 1–5 and 7. The results showed that the 3 carotenoids contribute to the 1O2-quenching activity of rice bran extracts, although the ratios ({
[Car-i]/105}/
(Obsd.)) for extracts 1–7 varied remarkably from 4.6 to 36.9% (see Table ).
As described in the previous section, the value of γ-Ory in ethanol was 2.69 × 103 L g−1 s−1, which was two orders of magnitude smaller than the values (1.65 × 105–5.27 × 105 L g−1 s−1) of the 4 Tocs and 4 Toc-3s (see Table ). However, the concentrations of γ-Ory found in rice bran extracts 1–7 were more than one order of magnitude greater than the concentrations of total Tocs and Toc-3s (see Table S1), which suggests a contribution of γ-Ory to the
(Obsd.) value. The {
[γ-Ory]/105} values for γ-Ory were calculated for extracts 1–7 (Table ). The values of the ratio ({
[γ-Ory]/105}/
(Obsd.)) were 3.5 to 6.1%. This result indicates that γ-Ory also contributes to the 1O2-quenching of rice bran extracts 1–7, although the degree of the contribution is small.
Comparison between 1O2-quenching activities of rice bran extracts 1–7 and many food extracts
Recently, and SOAC values were measured for 24 kinds of food extracts (16 vegetable, 7 fruit, and palm oil extracts).Citation20,22,23) The
values obtained for these food extracts varied from 3.18 × 104 L g1s−1 for tomato to 1.55 × 102 for green melon in ethanol/chloroform/D2O (50:50:1, v/v/v) solution. Vegetable extracts (such as tomato, carrot, and red paprika), which include high concentrations of carotenoids, showed large
values (i.e. large relative SOAC values).Citation23) The
value for crude palm oil extract was 7.32 × 103 L g1 s−1.Citation22)
The values of rice bran extracts 1–7 varied from 1.15 × 103 L g1 s−1 for extract 1 to 8.62 × 102 L g1 s−1 for extract 7 in ethanol. The
values obtained for AOs in ethanol were found to correlate linearly with the
values (correlation coefficient = 0.97) in ethanol/chloroform/D2O solution, showing the ratio (
(in ethanol)/
(in ethanol/chloroform/D2O)) of 1.72 ± 0.04.Citation21,22) As expected, the values obtained for rice bran extracts 1–7 were smaller than those obtained for vegetable extracts that include high concentrations of carotenoids.
Measurements of the concentrations of 4 Tocs and 4 Toc-3s included in various rice bran extracts were performed by Sookwong et al.Citation10). They reported that high concentrations of Toc-3s are included in rice bran extracts 1–7 and the concentrations of 4 Tocs and 4 Toc-3s included vary remarkably, depending on the kinds of rice brans. In fact, as listed in Table S1, Okka Modoshi (1) and Nipponbare (3) contain high total concentrations of α-Toc and α-Toc-3 (72.7 and 84.9 mg/100 g), respectively. On the other hand, high concentrations of γ-Toc and γ-Toc-3 were included in Milyang-23 (4) and γ-Rich Koshihikari (5). The total concentrations of α-Toc and α-Toc-3 (20.8 and 12.9 mg/100 g) in extracts 4 and 5 are 5.93 and 9.34 times smaller than the corresponding those of γ-Toc and γ-Toc-3 (123.5 and 128.2 mg/100 g), respectively.
Similarly, the amount of total carotenoids in extracts 1–7 varied significantly from 1.082 mg/100 g in Koshihikari (6) to 0.143 in Milyang 23 (4), as listed in Table . The amount of total carotenoids in Koshihikari (6) was 7.57 times greater than the amount in Milyang 23 (4). Consequently, we may expect that 7 kinds of rice bran extracts 1–7 show different the kQ (S) and SOAC values (that is, 1O2-quenching activity). However, to our regret, the observed difference in the kQ (S) values was less than 1.33 times (see Table ), against expectation.
Summary
In recent years, ROS have attracted much attention. For example, LOO and 1O2 react with many different biological targets including lipids,Citation6) proteins,Citation7) and DNA,Citation8,9) and induce the degradation of biological systems. Many kinds of naturally occuring AOs are included in these systems, and may function as efficient LOO
scavengers and/or 1O2 quenchers. Recently, the SOAC assay method to assess 1O2-quenching activity of carotenoids and phenolic AOs contained in foods and plants was proposed.Citation19–23) However, examples of the application of the SOAC assay method have been limited to vegetable and fruit extracts. In the present work, measurements of 1O2-quenching rates (kQ (S)), i.e. relative SOAC values, were performed for seven kinds of rice bran extracts in ethanol. The concentrations (in mg/100 g) of 4 Tocs and 4 Toc-3s, 3 carotenoids, and γ-Ory found in the extracts were determined using HPLC-MS/MS, UV-HPLC, and UV–vis absorption spectroscopy, respectively. Furthermore, comparisons of the kQ (S) (Obsd.) values for the rice bran extracts with the sum of the product {
[AO-i]} of the
values reported for each AO contained in the extracts and the [AO-i] values were performed. From these results, it has been ascertained that the SOAC method is applicable to general food extracts to evaluate their 1O2-quenching activity.
Author contribution
K. Mukai designed research and wrote the manuscript. E. Ishikawa, T. Abe, and A. Ouchi performed experiment and analyzed the data. K. Murata prepared rice bran samples. T. Miyazawa supervised the research. S. Nagaoka and K. Nakagawa helped to draft the manuscript. K. Nakagawa provided valuable advice and analyzed the data.
Supplemental material
The supplemental material for this paper is available at http://10.1080/09168451.2015.1069701.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Grant-in-Aid for Challenging Exploratory Research [grant 24658123] from the Japan Society for the Promotion of Science and from the Skylark Food Science Institute.
Supporting_Information__Table_S1_and_Figure_S1_.docx
Download MS Word (71.8 KB)Acknowledgments
We are very grateful to Ms. Kyoka Takahashi of Ehime University for the measurement of the kQ value of γ-oryzanol. We are also very grateful to Prof. Hisako Sato and Ms. Miwa Ochi of Ehime University for their kind help in the preparation of the rice bran extracts.
References
- Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Radical Biol. Med. 1993;14:303–311.10.1016/0891-5849(93)90027-R
- Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001;49:4619–4626.10.1021/jf010586o
- Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004;52:4026–4037.10.1021/jf049696w
- Omata Y, Saito Y, Yoshida Y, Niki E. Simple assessment of radical scavenging capacity of beverages. J. Agric. Food Chem. 2008;56:3386–3390.10.1021/jf703771v
- Takashima M, Horie M, Shichiri M, Hagihara Y, Yoshida Y, Niki, E. Assessment of antioxidant capacity for scavenging free radicals in vitro: a rational basis and practical application. Free Radical Biol. Med. 2012;52:1242–1252.10.1016/j.freeradbiomed.2012.01.010
- Girotti AW. Photodynamic lipid peroxidation in biological systems. Photochem. Photobiol. 1990;51:497–509.10.1111/php.1990.51.issue-4
- Davies MJ, Truscott RJW. Photo-oxidation of proteins and its role in cataractogenesis. J. Photochem. Photobiol. B: Biol 2001;63:114–125.10.1016/S1011-1344(01)00208-1
- Devasagayam TPA, Steenken S, Obendorf MSW, Schulz WA, Sies H. Formation of 8-hydroxy(deoxy)guanosine and generation of strand breaks at guanine residues in DNA by singlet oxygen. Biochemistry. 1991;30:6283–6289.10.1021/bi00239a029
- Piette J. New trends in photobiology: Biological consequences associated with DNA oxidation mediated by singlet oxygen. J. Photochem. Photobiol. B: Biol. 1991;11:241–260.10.1016/1011-1344(91)80030-L
- Sookwong P, Nakagawa K, Murata K, Kojima Y, Miyazawa T. Quantitation of tocotrienol and tocopherol in various rice brans. J. Agric. Food Chem. 2007;55:461–466.10.1021/jf0621572
- Mangels AR, Holden JM, Beecher GR, Forman MR, Lanza E. Carotenoid content of fruits and vegetables: an evaluation of analytic data. J. Am. Diet. Assoc. 1993;93:284–296.10.1016/0002-8223(93)91553-3
- Holden JM, Eldridge AL, Beecher GR, et al. Carotenoid content of U.S. foods: an update of the database. J. Food Compos. Anal. 1999;12:169–196.10.1006/jfca.1999.0827
- Aizawa K, Inakuma T. Quantitation of carotenoids in commonly consumed vegetables in Japan. Food Sci. Technol. Res. 2007;13:247–252.10.3136/fstr.13.247
- Tuberoso CIG, Kowalczyk A, Sarritzu E, Cabras P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007;103:1494–1501.10.1016/j.foodchem.2006.08.014
- Hu J-N, Zhu X-M, Adhikari P, Li D, Kim I-H, Lee K-T. Determination of tocopherol contents in refined edible oils using an HPLC method. J. Food Sci. Nutr. 2009;14:260–264.10.3746/jfn.2009.14.3.260
- Foote CS, Denny RW. Chemistry of singlet oxygen VII. Quenching by β-carotene. J. Am. Chem. Soc. 1968;90:6233–6235.10.1021/ja01024a061
- Sies H, Stahl W, Sundquist AR. Antioxidant functions of vitamins. Vitamin E and C, β-carotene, and other carotenoids. Ann. N.Y. Acad. Sci. 1992;669:7–20.10.1111/nyas.1992.669.issue-1
- Di Mascio P, Sundquist AR, Devasagayam TPA, Sies H. Assay of lycopene and other carotenoids as singlet oxygen quenchers. Methods Enzymol. 1992;213:429–438.10.1016/0076-6879(92)13144-M
- Ouchi A, Aizawa K, Iwasaki Y, Inakuma T, Terao J, Nagaoka S, et al. Kinetic study of the quenching reaction of singlet oxygen by carotenoids and food extracts in solution. Development of a singlet oxygen absorption capacity (SOAC) assay method. J. Agric. Food Chem. 2010;58:9967–9978.10.1021/jf101947a
- Aizawa K, Iwasaki Y, Ouchi A, Inakuma T, Nagaoka S, Terao J, et al. Development of singlet oxygen absorption capacity (SOAC) assay method. 2. Measurements of the SOAC values for carotenoids and food extracts. J. Agric. Food Chem. 2011;59:3717–3729.10.1021/jf104955a
- Mukai K, Ouchi A, Takahashi S, Aizawa K, Inakuma T, Terao J, et al. Development of singlet oxygen absorption capacity (SOAC) assay method. 3. Measurements of the SOAC values for phenolic antioxidants. J. Agric. Food Chem. 2012;60:7905–7916.10.1021/jf302021r
- Mukai K, Ishikawa E, Ouchi A, Nagaoka S, Suzuki T, Izumisawa K, et al. Kinetic study of the quenching reaction of singlet oxygen by α-, β-, γ-, δ-tocotrienols and palm oil and soybean extracts in solution. Biosci. Biotechnol. Biochem. 2014;78:2089–2101.10.1080/09168451.2014.943653
- Iwasaki Y, Takahashi S, Aizawa K, Mukai K. Development of singlet oxygen absorption capacity (SOAC) assay method. 4. Measurements of the SOAC values for vegetable and fruit extracts. Biosci. Biotechnol. Biochem. 2015;79:280–291.10.1080/09168451.2014.972329
- Niki E. Antioxidant action of tocotrienols. In: Tan B, Watson R, Preedy V, editors. Tocotrienols: Vitamin E beyond tocopherols. 2nd ed, Chapter 17. Boca Raton (FL): CRC/AOCS Press; 2012. P. 233–240.
- Parker RA, Pearce BC, Clark RW, Gordon DA, Wright JJK. Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem. 1993;268:11230–11238.
- Black TM, Wang P, Maeda N, Coleman RA. Palm tocotrienols protect ApoE +/- mice from diet-induced atheroma formation. J. Nutr. 2000;130:2420–2426.
- Qureshi AA, Sami SA, Salser WA, Khan FA. Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hypercholesterolemic humans. Atherosclerosis. 2002;161:199–207.10.1016/S0021-9150(01)00619-0
- Sakai M, Okabe M, Tachibana H, Yamada K. Apoptosis induction by γ-tocotrienol in human hepatoma Hep3B cells. J. Nutr. Biochem. 2006;17:672–676.10.1016/j.jnutbio.2005.11.001
- Eitsuka T, Nakagawa K, Miyazawa T. Down-regulation of telomerase activity in DLD-1 human colorectal adenocarcinoma cells by tocotrienol. Biochem. Biophys. Res. Commun. 2006;348:170–175.10.1016/j.bbrc.2006.07.029
- Nesaretnam K, Meganathan P. Tocotrienols: inflammation and cancer. Ann. N.Y. Acad. Sci. 2011;1229:18–22.10.1111/j.1749-6632.2011.06088.x
- Shibata A, Nakagawa K, Kawakami Y, Tsuzuki T, Miyazawa T. Suppression of γ-tocotrienol on UVB induced inflammation in HaCaT keratinocytes and HR-1 hairless mice via inflammatory mediators multiple signaling. J. Agric. Food Chem. 2010;58:7013–7020.10.1021/jf100691g
- Nakagawa K, Shibata A, Yamashita S, Tsuzuki T, Kariya J, Oikawa S, et al. In vivo angiogenesis is suppressed by unsaturated vitamin E, tocotrienol. J. Nutr. 2007;137:1938–1943.
- Shibata A, Nakagawa K, Sookwong P, Tsuduki T, Tomita S, Shirakawa H, et al. Tocotrienol inhibits secretion of angiogenic factors from human colorectal adenocarcinoma cells by suppressing hypoxia-inducible factor-1α. J. Nutr. 2008;138:2136–2142.10.3945/jn.108.093237
- Khanna S, Roy S, Slivka A, Craft TKS, Chaki S, Rink C, et al. Neuroprotective properties of the natural vitamin E α-tocotrienol. Stroke. 2005;36:2258–2264.
- Sookwong P, Nakagawa K, Yamaguchi Y, Miyazawa T, Kato S, Kimura F, et al. Tocotrienol distribution in foods: estimation of daily tocotrienol intake of Japanese population. J. Agric. Food Chem. 2010;58:3350–3355.10.1021/jf903663k
- Sambanthamurthi R, Sundram K, Tan Y-A. Chemistry and biochemistry of palm oil. Prog. Lipid Res. 2000;39:507–558.10.1016/S0163-7827(00)00015-1
- Chen M-H, Bergman CJ. A rapid procedure for analysing rice bran tocopherol, tocotrienol and γ-oryzanol contents. J. Food Compos. Anal. 2005;18:319–331.10.1016/j.jfca.2003.09.016
- Xu Z, Godber JS. Purification and identification of components of γ-oryzanol in rice bran oil. J. Agric. Food Chem. 1999;47:2724–2728.10.1021/jf981175j
- Stöggl W, Huck C, Wongyai S, Scherz H, Bonn G. Simultaneous determination of carotenoids, tocopherols, and γ-oryzanol in crude rice bran oil by liquid chromatography coupled to diode array and mass spectrometric detection employing silica C30 stationary phases. J. Sep. Sci. 2005;28:1712–1718.10.1002/(ISSN)1615-9314
- Abdul-Hamid A, Raja Sulaiman RR, Osman A, Saari N. Preliminary study of the chemical composition of rice milling fractions stabilized by microwave heating. J. Food Comp. Anal. 2007;20:627–637.10.1016/j.jfca.2007.01.005
- Nakornriab M, Sriseadka T, Wongpornchai S. Quantification of carotenoid and flavonoid components in brans of some Thai black rice cultivars using supercritical fluid extraction and high-performance liquid chromatography-mass spectrometry. J. Food Lipids. 2008;15:488–503.10.1111/(ISSN)1745-4522
- Oka T, Fujimoto M, Nagasaka R, Ushio HH, Hori M, Ozaki H. Cycloartenyl ferulate, a component of rice bran oil-derived γ-oryzanol, attenuates mast cell degranulation. Phytomedicine. 2010;17:152–156.10.1016/j.phymed.2009.05.013
- Ajitha MJ, Mohanlal S, Suresh CH, Jayalekshmy A. DPPH radical scavenging activity of tricin and its conjugates isolated from “Njavara” rice bran: a density functional theory study. J. Agric. Food Chem. 2012;60:3693–3699.10.1021/jf204826e
- Mukai K, Ouchi A, Abe T, Murata K, Nakagawa K, Miyazawa T. Kinetic study of the scavenging reaction of the aroxyl radical by seven kinds of rice bran extracts in ethanol solution. Development of an aroxyl radical absorption capacity (ARAC) assay method. J. Agric. Food Chem. 2014;62:11901–11909.10.1021/jf503996z
- Nakagawa K, Kiko T, Hatade K, Asai A, Kimura F, Sookwong P, et al. Development of a high-performance liquid chromatography-based assay for carotenoids in human red blood cells: application to clinical studies. Anal. Biochem. 2008;381:129–134.10.1016/j.ab.2008.06.038
- Nakagawa K, Kiko T, Hatade K, Sookwong P, Arai H, Miyazawa T. Antioxidant effect of lutein towards phospholipid hydroperoxidation in human erythrocytes. Br. J. Nutr. 2009;102:1280–1284.10.1017/S0007114509990316
- Young RH, Wehrly K, Martin RL. Solvent effects in dye-sensitized photooxidation reactions. J. Am. Chem. Soc. 1971;93:5774–5779.10.1021/ja00751a031
- Thomas MJ, Foote CS. Chemistry of singlet oxygen XXVI. Photooxygenation of phenols. Photochem. Photobiol. 1978;27:683–693.10.1111/php.1978.27.issue-6
- Wilkinson F, Helman WP, Ross AB. Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expanded and revised compilation. J. Phys. Chem. Ref. Data. 1995;24:663–1021.10.1063/1.555965