Abstract
A fungus J2 producing laccase with high yield was screened in soils and identified as Abortiporus biennis. The production of laccase was induced by 0.1 mM Cu2+, 0.1 mM tannic acid, and 0.5 M ethanol. The laccase from Abortiporus biennis J2 was purified to electrophoretic homogeneity by a couple of steps. The N-terminal amino acid sequence of the enzyme was AIGPTADLNISNADI. The properties of the purified laccase were investigated. The result showed the laccase from Abortiporus biennis J2 is a thermo and pH stable enzyme. The laccase activity was inhibited by Hg2+, Cd2+, Fe2+, Ag+, Cu2+, and Zn2+, while promoted by Mg2+, Mn2+ at 10 mM level. Purified laccase was used to the clarification of litchi juice. After treatment with this laccase, the phenolic content of litchi juice had been found to be greatly reduced along with an increase in the clarity of the juice. The result indicated the potential of this laccase for application in juice procession.
Laccase (benzenediol: oxygen oxidoreductase, EC 1.10.3.2) is one of the important ligninolytic enzymes playing a key role in efficient degradation of lignin in nature. Laccase is widely distributed in the higher plants, some insects, a few bacteria, and fungi.Citation1,2) The white rot fungi are the main laccase producers. This enzyme catalyzes the oxidation of aromatic compounds besides lignin in the presence of oxygen as an electron acceptor. Due to a broad range of substrates, laccase has great potential in various applications including pulp delignification, degradation of different recalcitrant compounds, dye decolorization, soil bioremediation, sewage treatment, and juice clarification.Citation3–8) In view of the broad biotechnological applications of laccases, there is an increasing need to purify and identify different sources of laccases having diverse properties. Up to date, over 100 laccases have been isolated from fungal cultures and their biochemical properties as well as their catalytic characteristics have been investigated.Citation9) However, most of them are common laccases with low level of enzymatic activities and mesophilic thermo or pH stability. This hinders their large-scale commercial and industrial use in most application fields. Therefore, searches for new sources of laccases with better properties such as high level of enzymatic activity and more stability are still desirable.Citation10,11)
Litchi (Litchi chinensis Sonn.) is one of the important commercial fruits, which is widely planted in South China. Due to the delicious taste and abundant nutrition, litchi is accepted by consumers all over the world.Citation12) In addition to being consumed directly as fresh fruit, litchi is also processed into juice, canned fruit and dried fruit. There are abundant phenolic compounds in litchi juice, which are the main factors involved in turbidity, formation of haze or sediments, affecting final product quality, and storage stability of litchi juice.Citation13,14) The main purpose of the juice clarification is to reduce the amount of phenolic compounds and decrease the turbidity of the product. Due to their ability to oxidize a wide range of phenolic substrates, laccases had been used successfully for the clarification of juices, beer, and tea.Citation15–17) The activity and stability of laccases was able to significantly affect the efficiency of juice clarification.Citation18)
The present study described production, induction, and purification of a novel laccase from Abortiporus biennis J2. The characterization of the purified laccase was investigated, including molecular weight, the effect of pH, temperature, heavy metal ions on enzyme activity, thermo, and pH stability. In addition, the potential of the purified laccase in the clarification of litchi juice was evaluated.
Materials and methods
Chemicals
2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) were purchased from Sigma–Aldrich (St. Louis, MO, USA), Sephadex G-100 and Sepharose Fast Flow were provided by GE Healthcare (USA). All other chemicals were of analytical grade.
Medium and culture conditions
The basal laccase production medium (per liter): wheat bran extracts 100 mL, yeast extract 1.5 g, glucose 1.0 g, NH4Cl 0.5 g, trace element solution 1 mL. Wheat bran extracts were prepared according to the following methods: 4.5 g wheat bran was boiled in 100 mL for 15 min, and the mixture was filtered by gauze. The filtrate was wheat bran extracts and it was added to the medium. The composition of trace solution was as follows (per liter): KH2PO4 2 g, CaCl2. 2H2O 0.1 g, KCl 0.5 g, MgSO4.7H2O 0.5 g, CuSO4.5H2O 0.25 g. The medium was adjusted to pH 5.0 and autoclaved at 115 °C for 30 min. The producing laccase fungus from slant was inoculated into 250-mL Erlenmeyer flask containing 100 mL laccase production medium. Then, the fungus was cultivated at 30 °C on a rotary shaker at 180 r/min for 6–12 days.
Isolation, screening, and identification of laccase-producing microorganism
Soil samples containing rotted wood pieces were collected from forests of Guangzhou, China. Soil samples were diluted with sterile water, and then, an aliquot of the supernatant was spread on potato dextrose agar (PDA) plate containing a laccase indicator (0.01% guaiacol). Plates were incubated at 30 °C until reddish-brown halos of laccase-producing fungi formed. The size of the reddish-brown halo around the fungal colony in agar plate was measured to identify laccase secreting ability of the strains. Positive colonies were inoculated on PDA with and without indicators to confirm the presence of laccase. The isolated strain with the largest reddish-brown halos was selected for further research. Identification of the strain was conducted on the basis of morphological characterization and subsequently confirmed by internal transcribed spacer (ITS) rDNA sequence analysis, and the sequence was submitted to GenBank with the accession number.
Laccase activity assays
Laccase activity was determined spectrophotometrically by using ABTS as the substrate. The reaction mixture contained 0.5 mL of 1 mM ABTS, 2.4 mL of 20 mM sodium acetate buffer (pH 5.0) and 0.1 mL of diluted enzyme sample. Enzyme assay was performed by measuring the absorbance increase at 420 nm (ε = 36,000 M−1 cm−1). One unit of laccase activity was defined as the amount of enzyme that oxidized 1 μmol of ABTS per min.Citation19)
Induction of laccase
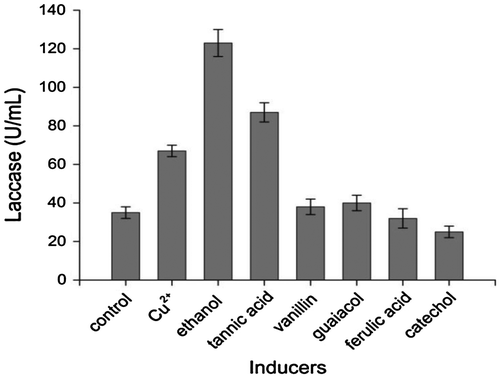
The laccase-producing fungus was inoculated in basal laccase production medium with different inducers including 0.1 mM CuSO4, tannic acid, vanillin, guaiacol, ferulic acid, catechol, and 0.5 M ethanol (final concentration), respectively. The fungus was incubated at 30 °C on a rotary shaker at 180 r/min for 6 days. The laccase activity in culture broth was measured and compared with the control in the absence of inducers.
Purification of laccase
After 6 days of fungal cultivation when laccase activity reached its maximum, the supernatant was collected by centrifugation at 8000g for 10 min at 4 °C for the removal of mycelia. Ammonium sulfate was added to supernatant up to 40% saturation. After standing for 4 h, the solution was centrifuged at 12,000×g for 20 min at 4 °C, Meanwhile, the supernatant was increased to 90% saturation by the addition of ammonium sulfate. Storing the solution overnight at 4 °C, the precipitate obtained by centrifugation was dissolved in sodium acetate buffer (20 mM, pH 5.0) and dialyzed against the same buffer. The dialyzed sample was loaded onto a DEAE-Sepharose Fast Flow anion-exchange column (1.6 cm × 25 cm) equilibrated with sodium acetate buffer (20 mM, pH 5.0). Proteins were eluted initially with the same buffer at a flow rate of 2 mL/min and then with a linear gradient salt solution (0.1–0.5 M NaCl). The collected fractions (1 mL/tube) were subjected to laccase assay and protein measurement (A280). Laccase active fractions were pooled, dialyzed, concentrated by polyethylene glycol and applied for further purification on Sephadex G-100 column (1.6 cm × 40 cm). The loaded proteins were eluted with sodium acetate buffer (20 mM, pH 5.0) at a flow rate of 0.5 mL/min. Fractions (0.5 mL/tube) were measured for laccase activity and protein concentration. The fractions containing high activity laccase were then pooled, concentrated by freeze-drying for further research.
Gel electrophoresis
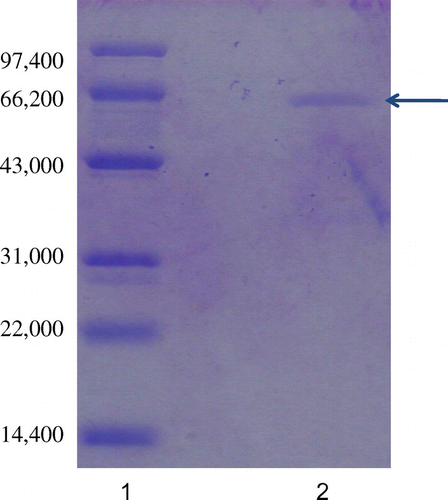
The purity and molecular mass of the purified laccase was determined by SDS-PAGE using a 5% stacking gel and a 12% resolving gel; This was followed by staining with Coomassie brilliant blue R-250 and compared with molecular weight markers. Phosphorylase B (97,400), bovine serum albumin (66,200), actin (43,000), carbonic anhydrase (31,000), growth hormone (22,000), and lysozyme (14,400) were used as molecular weight markers.
N-terminal amino acid sequencing
The purified laccase on SDS-PAGE gel was electroblotted on to a polyvinylidene fluoride membrane and then stained. The stained protein portion was excised and the N-terminal amino acid sequence of purified laccase was determined by the automated Edman degradation method using ABI Procise 491 HT protein sequencer (Applied Biosystems, CS, USA).
Effect of pH and temperature on laccase activity and stability
The effect of pH on laccase activity was studied in different buffers (20 mM) at a pH ranging from 2.0 to 10.0 at 30 °C. The pH stability of the enzyme was estimated by pre-incubating in different buffers (20 mM) at different pH range from 2 to 10 at 30 °C for 24 h and the residual activities were determined. The buffer systems used were sodium acetate buffer for pH 2.0–6.0, sodium phosphate buffer for pH 6.0–7.0 and Tris–HCl buffer for pH 8.0–10.0. To investigate the effect of temperature on laccase activity, this laccase was conducted in 20 mM sodium acetate buffer (pH 5.0) under different temperatures from 20 to 70 °C. The thermostability of the laccase was determined by preincubating the enzyme in 20 mM sodium acetate buffer (pH 5.0) at various temperatures (20–70 °C) for 6 h, and residual activities were measured at intervals as described above.
Effect of heavy metal ions on laccase activity
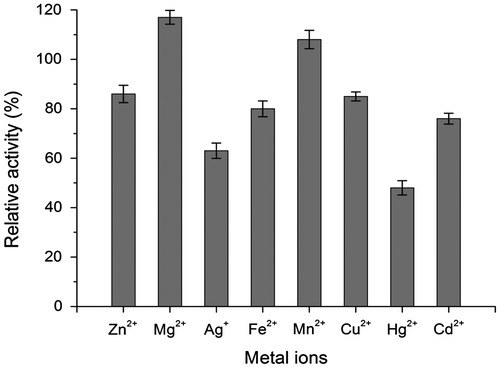
Effect of different heavy metal ions (Ag+, Cd2+, Cu2+, Hg2+, Fe2+, Zn2+, Mg2+, Mn2+) on the laccase activity was measured by incubating laccase with each metal ions in 20 mM sodium acetate buffer (pH 5.0) at 30 °C for 15 min. The final concentration of metal ions was 10 mM. The residual activity of laccase was determined as described above. Laccase activity assayed in the absence of any metal ion was taken as 100%.
The clarification of litchi juice by purified laccase
Litchi fruits (feizixiao) were purchased from a local fruit market in Guangzhou, China and stored at 4 °C until use. Juice was extracted from fresh litchi using a juice extractor and the extracted pulp was filtered in muslin cloth to obtain the raw litchi juice. The purified laccase (0–25 U/mL litchi juice) was mixed with the raw fruit juices and incubated at room temperature for 1 h. Then, the reaction was terminated at 80 °C for 1 min. The laccase-free and laccase-treated juices were centrifuged at 4000 rpm for 5 min and used for the quantitative analyses including total phenolic content, color, and clarity of juices. The total phenolic content in the juice before and after enzyme treatment was determined by the method of Folin–Ciocalteu.Citation12) The total phenolic content in juices of enzyme-free treatment (control) was taken as 100%. The clarity and color of the juice, before and after treatment with laccase, were determined by measuring the absorbance at 650 and 420 nm using UV visible spectrophotometer, respectively.
Results
Isolation and identification of laccase-producing fungi
Isolation and preliminary screening for laccase production were carried out at the PDA medium containing guaiacol as indicators. The isolate with the biggest reddish brown oxidation halo was accepted as the best laccase-producing isolate and therefore was selected for the present work. The ITS rDNA region of strain J2 was amplified, sequenced, and submitted to GenBank (Accession Number: KJ094473). The obtained sequence was compared with those in the NCBI Nucleotide Sequence Database by using the BLAST algorithm. According to ITS rDNA gene sequence and morphological characteristics, strain J2 was identified as Abortiporus biennis. The time course of laccase production by Abortiporus biennis J2 was monitored in basal laccase production medium. As shown in Fig. , laccase activity was detected after 4 days of incubation and the maximum laccase activity was measured on 6th day, which reached 37 U/mL.
Fig. 1. The time course of laccase secretion by Abortiporus biennis J2 in basal laccase production medium at 30 °C on a rotary shaker at 150 rpm.
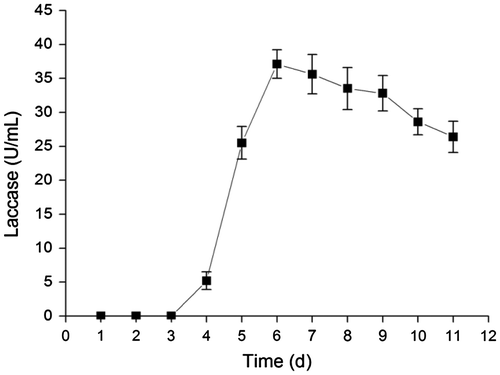
Figure showed that effect of inducers on laccase production by Abortiporus biennis. 0.5 M ethanol, 0.1 mM Cu2+ and tannic acid can effectively promoted laccase production of Abortiporus biennis with comparison to control culture. The ethanol was the most effective inducer in all tested chemicals. Addition of 0.5 M ethanol in culture increased 2.5 times laccase activity than control culture.
Purification of laccase secreted by Abortiporus biennis
The extracellular laccase secreted by Abortiporus biennis J2 was purified by ammonium sulfate precipitation, ion-exchange chromatography on DEAE-Sepharose Fast Flow and gel filtration on a Sephadex 100 column. The steps used for purification of this enzyme in 500 mL culture broth are summarized in Table . The purified enzyme showed 5.8-fold increase in the activity with a final recovery yield of 46.3%. The purity of laccase was confirmed by the presence of a single band on SDS-PAGE and its molecular weight was approximately 66,000 (Fig. ).
Table 1. Purification of Laccase from Abortiporus biennis J2.
Analysis of the N-terminal amino acid sequence
The N-terminal amino acid sequence of Abortiporus biennis laccase is AIGPTADLNISNADI, which has the highest similarity to Coriolopsis trogii laccase (AMJ39539.1) and considerable homology to those of other fungal laccases. A comparison of N-terminal amino acid sequence of Abortiporus biennis laccase with other fungal laccases was shown in Table .
Table 2. Comparison of the N-terminal amino acid sequences of Abortiporus biennis laccase with other fungal laccases.
Effect of temperature and pH on laccase activity and stability
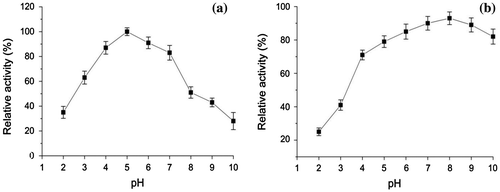
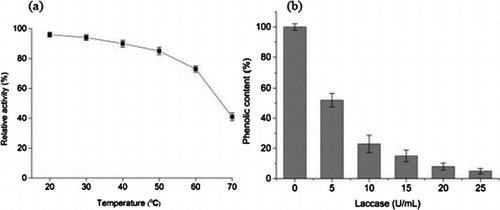
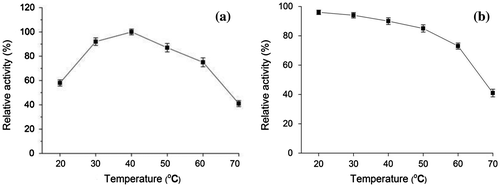
The effect of temperature on the purified laccase activity was determined at different temperatures ranging from 20 to 70 °C (Fig. (a)). The optimum temperature for maximum laccase activity is 40 °C. The enzymatic activity decreased to 75%, 41% at 60 °C and 70 °C, respectively. The thermal stability of the purified laccase was determined upon pre-incubation at a temperature range of 20–70 °C for 6 h (Fig. (b)). The laccase was almost completely active (over 90%) at 20–40 °C. The enzymatic activity maintained 85% of the initial activity after 6 h of incubation at 50 °C. The remaining activity decreased to 73% of original activity at 60 °C for 6 h.
Fig. 4. Effect of temperature on laccase activity (a) and stability of purified laccase (b) Relative activity on laccase activity was defined as the percentage of activity measured with respect to the maximum enzyme activity. Original enzyme activity on stability of laccase was defined as 100%.
Effect of pH on laccase activity was investigated in the pH range of 2–10 at 30 °C when ABTS was used as substrate. The relative activity of purified laccase remained above 85% in a pH range of 4.0–7.0 with the maximum laccase activity at 5.0 (Fig. (a)). At pH levels of 3 and 8, the relative activity decreased to 59% and 51%, respectively. As seen in Fig. (b), the purified laccase was stable in a pH range of 6.0–10.0 remaining above 80% of original activity after 24 h. The relative activity of laccase sharply decreased to 37% at pH 4.0, but remained 90 and 83% of original activity at pH 9.0 and 10.0, respectively.
Effect of heavy metal ions on laccase activity
Heavy metal ions are common environmental pollutants and can affect the stability of the extracellular enzymes from microorganisms. Therefore, the effect of heavy metal ions on laccase activity was assessed at 10 mM concentration. As shown in Fig. , the enzyme activity was stimulated by Mg2+, Mn2+, whereas inhibited by Cu2+, Hg2+, Cd2+, Fe2+, Ag+, and Zn2+. The metal ion Hg2+ had the strongest inhibition activity on the enzyme.
Application of purified laccase in the clarification of litchi juice
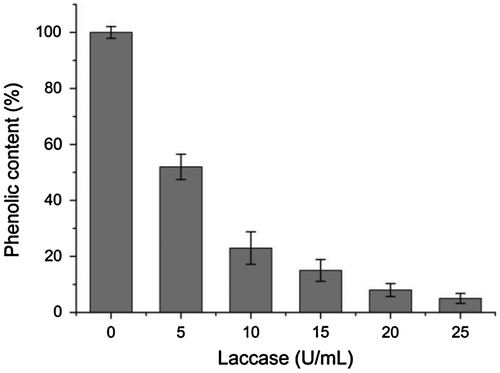
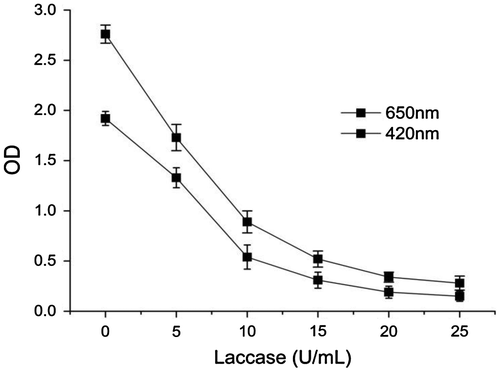
The effectiveness of purified laccase in litchi juice treatment was investigated. The clarity and total phenolic content of juice after enzyme treatment were compared with the untreated sample. The phenolic compounds present in the raw fruit juices are responsible for haze formation and browning during storage. The change of phenolic content in litchi juice after various concentration laccase treatment were shown in Fig. . The phenolic content in clarified juice was significantly reduced to 5% after 25 U/mL laccase treatment. The final phenolic contents in litchi juices decreased with increase in enzyme concentration. Laccase can catalyze the oxidation of juice phenolic compounds, which can lead to brown and cloud juice, characterized by high absorbance values at 420 and 650 nm, respectively. As shown in Fig. , absorbance at 420 and 650 nm in litchi juice decreased significantly after enzyme treatment. The result indicated that clarity of litchi juice was improved after enzymatic treatment, compared to untreated sample. This result showed that purified laccase from Abortiporus biennis J2 has good ability to clarify the litchi juice, then improve clarity, stability, and quality of litchi juice. This laccase, therefore, has excellent potential to be used as processing aids for the litchi juice clarification.
Discussion
The production of laccase was difficult in culture broth. The yield of laccases by most fungi was too low for the purpose of application. The maximum laccase production of Trichoderma harzianum WL1 reached 4.36U/mL at an incubation period of 4 days.Citation20) The laccase activity of Paraconiothyrium variabile was 65U/L in submerged culture on Day 9.Citation21) A basidiomycete, Trametes sp. 420 produced laccases at 6.81–7.87 U/mL.Citation22) The activity of the laccase secreted by Abortiuporus biennis was higher than these reported laccase-producing fungi. The production of laccase from Abortiuporus biennis could be effectively promoted by Cu2+, tannic acid and ethanol at low concentration. The ethanol was an effective inducer of Abortiuporus biennis laccase, which greatly increased laccase activity in culture broth. Generally, inducers play a key role in production of laccase by fungi. It was reported that the secretion of laccase from fungi was induced by a variety of chemicals such as phenol compound, ethanol and metals.Citation23–25) Cu2+ has been reported to regulate the expression of fungal laccase gene at the transcription level.Citation26,27) Induction of laccase activity by phenolic compounds has been reported in many genera of fungi. The presence of lignin in the culture medium increased laccase production in Cyathus Bulleri.Citation28) The induction of laccase production has been reported in Marasmius quercophilus by ferulic acid, gallic acid, catechol, and veratric acid in Trametes pubescens and Phlebia radiate.Citation29–31)
The molecular mass of laccase from Abortiporus biennis J2 accords with the range of molecular weights for most of the fungal laccases reported (50,000–90,000). Laccases from Trametes versicolor (60,000),Citation11) Odontotermes formosanus (65,000),Citation32) Perenniporia tephropora (63,000),Citation33) L. lividus (64,800)Citation34), and Dichomitus squalens (66,000)Citation35) have a molecular weight very close to that of Abortiporus biennis J2 laccase. On the other hand, Leptosphaerulina chartarum laccase has a small molecular weight of 38,000.Citation36) The N-terminal amino acid sequence of Abortiporus biennis laccase was AIGPTADLNISNADI, which showed considerable homology to those of other fungal laccases.
The optimal temperature for purified enzyme was similar to Lentinula edodes or Rigidoporus lignosus at 40 °C.Citation37,38) In general, the fungal laccases are completely inactivated at 60 °C temperature for 6 h. These results indicated that the laccase from Abortiporus biennis J2 is a thermostable enzyme. The optimal pH for the laccase activity from Abortiporus biennis J2 was acidic, just like other fungal laccases including Leptosphaerulina chartarum,Citation36) Agaricus blazeiCitation39) or Panus tigrinus.Citation40) The purified laccase was stable in a broad pH range of 6.0–10.0. This may be a useful property for various industrial and biotechnological applications. The purified laccase was more stable in an alkaline pH, which was different from the laccases from Daedalea quercinaCitation41) or Pycnoporus sanguineus.Citation42)
This purified laccase was activated by Mg2+, Mn2+, whereas inhibited by Cu2+, Hg2+, Cd2+, Fe2+, Ag+, and Zn2+.Although the activity of typical fungal laccases is generally promoted by low level of Cu2+, high concentration (over 0.5 mM) Cu2+ can inhibit laccase activity.Citation43) The laccases from Odontotermes formosanus and Pycnoporus. sp was strongly inhibited by Fe2+.Citation32,44) By contrast, the stimulatory effect of Fe2+ on the Streptomyces psammoticus laccase has been reported.Citation45) The effect of metal ions on laccase activity from different microorganisms was different.
Clarification is an important step used in fruit juices industry processing. Clear and stable juice is obtained by clarification.Citation46) Juice clarification is categorized into two types: enzyme clarification and nonenzyme clarification. Enzyme clarification has a number of advantages over the physical–chemical treatment. Enzymes used in food processing have historically been considered non-toxic.Citation47) Laccases catalyze the oxidation of juice phenols to o-quinones, which are highly reactive compounds responsible for the formation of haze and sediment in fruit juice. Thus, laccase can be used to clarify fruit juices.Citation18,48,49) Treatment with laccase removed phenolic compounds of pomegranate juice with high efficiency. The results showed a removal of 40% of total phenolic compounds after laccase treatment.Citation50) Giovanelli and RavasiniCitation51) obtained a stable and clear apple juice by applying laccase in combination with membrane filtration without the other addition. Laccase treatment has also been found to be more effective for color and flavor stability compared to conventional treatments, such as the addition of ascorbic acid and sulphites.Citation52)
Conclusion
In this present study, the laccase-producing microorganism was isolated from soil and identified as Abortiporus biennis J2 on the basis of physiological, morphological and biochemical characteristics. The laccase was purified to homogeneity using a couple of steps with 5.8 purification fold. The molecular weight of this enzyme was determined to be approximate 66,000 with SDS-PAGE. The N-terminal amino acid sequence of the enzyme was AIGPTADLNISNADI. The purified laccase was found to have maximum activity at 40 °C and at pH 5.0. In the experiments for stability of pH and temperature, the isolated laccase showed thermo-stability, specific activity over wide range of pH and certain resistance to some heavy metals. The laccase was able to significantly reduce the phenol content of litchi juice and improve the juice clarity and stability. The laccase from Abortiporus biennis J2 showed it can play a potential role in the application of juice clarification.
Author contributions
L.Y. designed the study. J.Y.Y. and S.B.K conducted the experiments. R.Y. analyzed the data. L.Y. and Y.Q.G. wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Science and Technology Planning Project [grant number 2015A020212033] of Guangdong Province, China; University Student Renovation Training Project from South China Normal University.
Notes
Abbreviations: ABTS, 2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate); PDA, potato dextrose agar; ITS, internal transcribed spacer; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
References
- Shankar S, Shikha. Laccase production and enzymatic modification of lignin by a novel Peniophora sp. Appl Biochem Biotechnol. 2012;166:1082–1094.10.1007/s12010-011-9496-4
- Liu L, Lin Z, Zheng T, et al. Fermentation optimization and characterization of the laccase from Pleurotus ostreatus strain 10969. Enzyme Microb Technol. 2009;44:426–433.10.1016/j.enzmictec.2009.02.008
- Couto SR, Herrera JLT. Industrial and biotechnological applications of laccases: a review. Biotechnol Adv. 2006;24:500–513.10.1016/j.biotechadv.2006.04.003
- Que Y, Sun S, Xu L, et al. High-level coproduction, purification and characterisation of laccase and exopolysaccharides by Coriolus versicolor. Food Chem. 2014;159:208–213.10.1016/j.foodchem.2014.03.063
- Cañas AI, Camarero S. Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes. Biotechnol Adv. 2010;28:694–705.
- Giardina P, Sannia G. Laccases: old enzymes with a promising future. Cell Mol Life Sci. 2015;72:855–856.10.1007/s00018-014-1821-y
- Hildén K, Hakala TK, Maijala P, et al. Novel thermotolerant laccases produced by the white-rot fungus Physisporinus rivulosus. Appl Microbiol Biot. 2007;77:301–309.10.1007/s00253-007-1155-x
- Bezerra TMDS, Bassan JC, Santos VTDO, et al. Covalent immobilization of laccase in green coconut fiber and use in clarification of apple juice. Process Biochem. 2014;50:417–423.
- Rivera-Hoyos CM, Morales-Álvarez ED, Poutou-Piñales RA, et al. Fungal laccases. Fungal Biol Rev. 2013;27:67–82.
- Guo LQ, Lin SX, Zheng XB, et al. Production, purification and characterization of a thermostable laccase from a tropical white-rot fungus. World J Microbiol Biotechnol. 2011;27:731–735.10.1007/s11274-010-0502-8
- Zhu Y, Zhang H, Cao M, et al. Production of a thermostable metal-tolerant laccase from Trametes versicolor and its application in dye decolorization. Biotechnol Bioprocess Eng. 2011;16:1027–1035.10.1007/s12257-011-0129-0
- Zhang R, Zeng Q, Deng Y, et al. Phenolic profiles and antioxidant activity of litchi pulp of different cultivars cultivated in Southern China. Food Chem. 2013;136:1169–1176.10.1016/j.foodchem.2012.09.085
- Jiang G, Lin S, Wen L, et al. Identification of a novel phenolic compound in litchi (Litchi chinensis Sonn.) pericarp and bioactivity evaluation. Food Chem. 2013;136:563–568.10.1016/j.foodchem.2012.08.089
- Patel AK, Singhania RR, Pandey A. Novel enzymatic processes applied to the food industry. Curr Opin Food Sci. 2016;7:64–72.10.1016/j.cofs.2015.12.002
- Dhillon GS, Kaur S, Brar SK, et al. Flocculation and haze removal from crude beer using in-house produced laccase from Trametes versicolor cultured on brewer’s spent grain. J Agric Food Chem. 2012;60:7895–7904.10.1021/jf301747z
- Murugesan GS, Angayarkanni J, Swaminathan K. Effect of tea fungal enzymes on the quality of black tea. Food Chem. 2003;79:411–417.
- Minussi RC, Rossi M, Bologna L, et al. Phenols removal in musts: Strategy for wine stabilization by laccase. J Mol Catal B: Enzym. 2007;45:102–107.10.1016/j.molcatb.2006.12.004
- Lettera V, Pezzella C, Cicatiello P, et al. Efficient immobilization of a fungal laccase and its exploitation in fruit juice clarification. Food Chem. 2016;196:1272–1278.10.1016/j.foodchem.2015.10.074
- Sharma D, Goel G, Sud A, et al. A novel laccase from newly isolated Cotylidia pannosa and its application in decolorization of synthetic dyes. Biocatal Agric Biotechnol. 2015;4:661–666.
- Sadhasivam S, Savitha S, Swaminathan K, et al. Production, purification and characterization of mid-redox potential laccase from a newly isolated Trichoderma harzianum WL1. Process Biochem. 2008;43:736–742.10.1016/j.procbio.2008.02.017
- Forootanfar H, Faramarzi MA, Shahverdi AR, et al. Purification and biochemical characterization of extracellular laccase from the ascomycete Paraconiothyrium variabile. Bioresour Technol. 2011;102:1808–1814.10.1016/j.biortech.2010.09.043
- Tong P, Hong Y, Xiao Y, et al. High production of laccase by a new basidiomycete, Trametes sp. Biotechnol Lett. 2007;29:295–301.10.1007/s10529-006-9241-1
- Kuhar F, Papinutti L. Optimization of laccase production by two strains of Ganoderma lucidum using phenolic and metallic inducers. Rev Argent Microbiol. 2014;46:144–149.
- Bertrand B, Martínez-Morales F, Tinoco R, et al. Induction of laccases in Trametes versicolor by aqueous wood extracts. World J Microbiol Biotechnol. 2014;30:135–142.10.1007/s11274-013-1420-3
- Lee IY, Jung KH, Lee CH, et al. Enhanced production of laccase in Trametes vesicolor by the addition of ethanol. Biotechnol Lett. 1999;21:965–968.
- Saparrat M, Balatti PA, Martínez MJ, et al. Differential regulation of laccase gene expression in Coriolopsis rigida LPSC No. 232. Fungal Biol-UK. 2010;114:999–1006.
- Piscitelli A, Giardina P, Lettera V, et al. Induction and transcriptional regulation of laccases in fungi. Cur Genomics. 2011;12:104–112.10.2174/138920211795564331
- Vasdev K, Kuhad R. Induction of laccase production in Cyathus bulleri under shaking and static culture conditions. Folia Microbiol. 1994;39:326–330.10.1007/BF02814322
- Zucca P, Rescigno A, Olianas A, et al. Induction, purification, and characterization of a laccase isozyme from Pleurotus sajor-caju and the potential in decolorization of textile dyes. J Mol Catal B: Enzym. 2011;68:216–222.10.1016/j.molcatb.2010.11.008
- Lorenzo M, Moldes D, Sanromán MÁ. Effect of heavy metals on the production of several laccase isoenzymes by Trametes versicolor and on their ability to decolourise dyes. Chemosphere. 2006;63:912–917.10.1016/j.chemosphere.2005.09.046
- Farnet AM, Criquet S, Cigna M, et al. Purification of a laccase from Marasmius quercophilus induced with ferulic acid: reactivity towards natural and xenobiotic aromatic compounds. Enzyme Microb Technol. 2004;34:549–554.10.1016/j.enzmictec.2003.11.021
- Zhou Y, Deng T, Pan C, et al. Purification of a laccase from fungus combs in the nest of Odontotermes formosanus. Process Biochem. 2010;45:1052–1056.10.1016/j.procbio.2010.03.012
- Ben Younes S, Mechichi T, Sayadi S, et al. Purification and characterization of the laccase secreted by the white rot fungus Perenniporia tephropora and its role in the decolourization of synthetic dyes. J Appl Microbiol. 2007;102:1033–1042.
- Sahay R, Yadav K. Purification and characterization of laccase secreted by L. lividus. Appl Biochem Biotech. 2009;157:311–320.
- Périé FH, Reddy GVB, Blackburn NJ, et al. Purification and characterization of laccases from the white-rot basidiomycete Dichomitus squalens. Arch Biochem Biophys. 1998;353:349–355.10.1006/abbi.1998.0625
- Sajben-Nagy E, Manczinger L, Škrbić B, et al. Characterization of an extracellular laccase of Leptosphaerulina chartarum. World J Microbiol Biotechnol. 2014;30:2449–2458.10.1007/s11274-014-1670-8
- Nagai M, Sakamoto Y, Nakade K, et al. Purification of a novel extracellular laccase from solid-state culture of the edible mushroom Lentinula edodes. Mycoscience. 2009;50:308–312.10.1007/S10267-008-0478-5
- Cambria M, Cambria A, Ragusa S, et al. Production, purification, and properties of an extracellular laccase from Rigidoporus lignosus. Protein Expres Purif. 2000;18:141–147.10.1006/prep.1999.1126
- Ullrich R, Huong L, Dung N, et al. Laccase from the medicinal mushroom Agaricus blazei: production, purification and characterization. Appl Microbiol Biotechnol. 2005;67:357–363.10.1007/s00253-004-1861-6
- Quaratino D, Federici F, Petruccioli M, et al. Production, purification and partial characterisation of a novel laccase from the white-rot fungus Panus tigrinus CBS 577.79. Antonie van Leeuwenhoek. 2007;91:57–69.
- Baldrian P. Purification and characterization of laccase from the white-rot fungus Daedalea quercina and decolorization of synthetic dyes by the enzyme. Appl Microbiol Biotechnol. 2004;63:560–563.10.1007/s00253-003-1434-0
- Lu L, Zhao M, Zhang BB, et al. Purification and characterization of laccase from Pycnoporus sanguineus and decolorization of an anthraquinone dye by the enzyme. Appl Microbiol Biotechnol. 2007;74:1232–1239.10.1007/s00253-006-0767-x
- Wu YR, Luo ZH, Chow RKK, et al. Purification and characterization of an extracellular laccase from the anthracene-degrading fungus Fusarium solani MAS2. Bioresour Technol. 2010;101:9772–9777.10.1016/j.biortech.2010.07.091
- Wang ZX, Cai YJ, Liao XR, et al. Purification and characterization of two thermostable laccases with high cold adapted characteristics from Pycnoporus sp. SYBC-L1. Process Biochem. 2010;45:1720–1729.10.1016/j.procbio.2010.07.011
- Niladevi KN, Jacob N, Prema P. Evidence for a halotolerant-alkaline laccase in Streptomyces psammoticus: Purification and characterization. Process Biochem. 2008;43:654–660.10.1016/j.procbio.2008.02.002
- Oszmiański J, Wojdyło A. Effects of various clarification treatments on phenolic compounds and color of apple juice. Eur Food Res Technol. 2007;224:755–762.10.1007/s00217-006-0370-5
- Sharma HP, Patel H, Sharma S. Enzymatic extraction and clarification of juice from various fruits – a review. Crit Rev Food Sci. 2016. Doi:10.1080/10408398.2014.977434
- Stanescu MD, Gavrilas S, Ludwig R, et al. Preparation of immobilized Trametes pubescens laccase on a cryogel-type polymeric carrier and application of the biocatalyst to apple juice phenolic compounds oxidation. Eur Food Res Technol. 2012;234:655–662.10.1007/s00217-012-1676-0
- Gassara-Chatti F, Brar SK, Ajila CM, et al. Encapsulation of ligninolytic enzymes and its application in clarification of juice. Food Chem. 2013;137:18–24.10.1016/j.foodchem.2012.09.083
- Neifar M, Ellouze-Ghorbel R, Kamoun A, et al. Effective clarification of pomegranate juice using laccase treatment optimized by response surface methodology followed by ultrafiltration. J Food Process Eng. 2011;34:1199–1219.10.1111/jfpe.2011.34.issue-4
- Giovanelli G, Ravasini G, et al. Apple juice stabilization by combined enzyme-membrane filtration process. LWT - Food Sci Technol. 1993;26:1–7.10.1006/fstl.1993.1001
- Upadhyay P, Shrivastava R. Agrawal PKBioprospecting and biotechnological applications of fungal laccase. Biotech. 2016;6:1–12.