Abstract
Coffee polyphenols (CPPs), including chlorogenic acid, exert various physiological activities. The purpose of this study was to investigate the effects of CPPs on skin properties and microcirculatory function in humans. In this double-blind, placebo-controlled study, 49 female subjects with mildly xerotic skin received either a test beverage containing CPPs (270 mg/100 mL/day) or a placebo beverage for 8 weeks. The ingestion of CPPs significantly lowered the clinical scores for skin dryness, decreased transepidermal water loss, skin surface pH, and increased stratum corneum hydration and the responsiveness of skin blood flow during local warming. Moreover, the amounts of free fatty acids and lactic acid in the stratum corneum significantly increased after the ingestion of CPPs. These results suggest that an 8-week intake of CPPs improve skin permeability barrier function and hydration, with a concomitant improvement in microcirculatory function, leading to efficacy in the alleviation of mildly xerotic skin.
Eight-Week intake of coffee polyphenols improves skin hydration and permeability barrier function, with a concomitant improvement of microcirculatory function.
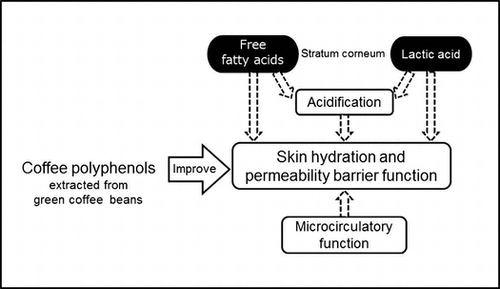
Skin, the largest organ covering the entire body, plays a crucial role in the defense against the invasion of pathogens and harmful agents from the external environment and in the maintenance of internal homeostasis. The stratum corneum is the outermost layer of the skin and is responsible for the prevention of water loss and the retention of water content.Citation1) The stratum corneum is composed of corneocytes embedded in highly ordered lipid lamellae, consisting of ceramides (CERs), free fatty acids (FFAs), and cholesterol.Citation2) In addition, natural moisturizing factors (NMFs), which include organic acids, free amino acids, and inorganic ions, exist within the corneocytes.Citation3) Dry skin (xerosis), which is characterized by roughness and the scaling, flaking, and cracking of the skin, has been reported to be caused by a decrease in the water-holding capacity of the stratum corneum, which results from a reduction in lipids and NMFs,Citation4,5) and is observed frequently even in healthy individuals. Thus, it is important to treat dry skin conditions to prevent serious skin diseases and to maintain good health.
The external environment, including temperature, humidity, ultraviolet radiation, and chemicals, affects the barrier function and hydration of the skin. Additionally, skin properties have been suggested to be influenced by internal physiological conditions, mental stress, and dietary components.Citation6,7) The cutaneous microcirculation system plays a key role in the supply of nutrients, hormones, and oxygen and in the elimination of waste. Nomura et al. recently reported that the skin permeability barrier function, defined by transepidermal water loss (TEWL), was associated with the responsiveness of skin blood flow (SkBF) and was determined by SkBF changes during local warming of the skin, in healthy adult subjects.Citation8)
Coffee is a popular drink worldwide; many studies on the effects of coffee on human health have been conducted.Citation9) The protective effects of coffee have been reported against many diseases, such as diabetes mellitus, hypertension, and Alzheimer’s disease.Citation10−12) The efficacy of coffee is partly attributable to coffee polyphenols (CPPs), including caffeoylquinic acids (CQAs; particularly 5-CQA, also called chlorogenic acid), dicaffeoylquinic acids (diCQAs), and feruloylquinic acids (FQAs). Green (unroasted) coffee beans are rich in CPPs, which are destructed and transformed during roasting.Citation13,14) In addition, various biological functions of CPPs, such as anti-oxidative, anti-inflammatory and neuroprotective actions, and regulatory effects on glucose and lipid metabolism, have been reported.Citation15−20) As CPPs have been reported to improve peripheral endothelial function,Citation21,22) we speculated that they acted on blood vessels in the skin and regulated cutaneous microcirculation. In addition, some studies have demonstrated that CPPs were effective in the prevention of skin inflammation and pigmentation caused by ultraviolet radiation.Citation23−25) However, little is known about the effects of CPPs on skin properties, including permeability barrier function and stratum corneum hydration. To clarify the effects of CPPs in green coffee beans on skin properties and microcirculatory function, we conducted a double-blind, placebo-controlled, randomized study in healthy females with mildly xerotic skin.
Materials and methods
Test beverages. The CPPs were prepared from green coffee beans (Robusta coffee, Coffea canephora) by hot water extraction. Caffeine was removed from the samples by adsorption onto activated carbon. CPPs included the following nine compounds: 5-caffeoylquinic acid (5-CQA), 3-caffeoylquinic acid (3-CQA), 4-caffeoylquinic acid (4-CQA), 3,4-dicaffeoylquinic acid (3,4-diCQA), 3,5-dicaffeoylquinic acid (3,5-diCQA), 4,5-dicaffeoylquinic acid (4,5-diCQA), 3-feruloylquinic acid (3-FQA), 4-feruloylquinic acid (4-FQA), and 5-feruloylquinic acid (5-FQA); the nomenclature is based on the IUPAC numbering systems. The quantity of CQAs and FQAs in the CPPs were determined by high-performance liquid chromatography by using a Cadenza CD C18 column (4.6 mm i.d. × 150 mm; Intact, Kyoto, Japan) and a Prominence Inert LC system (Shimadzu, Kyoto, Japan). 5-CQA (Sigma-Aldrich, Poole, UK) was used to generate a calibration curve. For this study, 270 mg CPPs (as sum of CQAs and FQAs) were included in a 100-mL beverage. The beverage was fruit-flavored and seasoned with acidulants and sweeteners such as citric acid and sucralose. For the placebo, a 100-mL beverage was prepared that was composed of the same acidulants, sweeteners, and flavor, but did not contain CPPs.
Subjects. A total of 150 healthy women (25–40 years of age, BMI 18.5–25.0 kg/m2) with mildly xerotic skin who felt stress in their daily living, were recruited for the study. The exclusion criteria were: drinking seven or more cups of coffee per week, any allergies, skin or vascular diseases, pregnancy, breastfeeding, and smoking.
Study design. The study was carried out during November 2014 and February 2015 at the Ebisu Skin Research Center of Inforward Inc, Tokyo, Japan. The present study was approved by the Ethical Committees of Shinkohkai Medical Corporation and Kao Corporation, and was conducted under the Declaration of Helsinki. The participants received an adequate explanation of the study and provided written informed consent. All 150 of the recruited participants were evaluated for skin conditions as described below and then 54 subjects with a relatively high dryness score and a low skin hydration of the lower cheek were selected for the study. The study was based on a double-blind, placebo-controlled, randomized design. The subjects were divided into two groups: the placebo group ingested the placebo beverage that did not contain CPPs and the CPP group ingested the CPP beverage (270 mg CPPs/100 mL) per day for 8 weeks. There were no significant differences in age, dryness scores, or skin hydration values between the two groups. The subjects consumed one bottle of their assigned beverage (CPP or placebo) after their evening meal before bedtime. All subjects were instructed not to alter lifestyle factors, such as coffee consumption, dietary supplementation, or cosmetic use, throughout the study period. Forty-nine of the 54 subjects (placebo group, n = 26; CPP group, n = 23) completed the study.
Assessment and measurements of skin properties. The assessment and measurements of skin were performed before ingestion and at 4 and 8 weeks after the start of ingestion. After washing the skin areas to be examined, the subjects were allowed to acclimatize for 15 min in a conditioned chamber (20 ± 2 °C temperature, 40 ± 5% relative humidity). The skin dryness at two sites of the lower cheeks and at 12 sites of the hands (fingers and backs of the hands) was evaluated by using a grading scale for skin roughness and scaling (0, Normal; 1, Slight; 2, Mild; 3, Moderate; 4, Severe) by dermatologists. TEWL, stratum corneum hydration based on the capacitance measurement in a dielectric medium, and skin surface pH of the lower cheek and the back of the hand were measured by using a Tewameter TM300, Corneometer CM825, and a Skin-pH-meter PH905 (Courage + Khazaka Electronic GmbH, Koln, Germany), respectively.
Analysis of lipids in the stratum corneum. Samples of tape-stripped stratum corneum were obtained before ingestion and at 8 weeks after the start of ingestion. Three consecutive tape-strippings were performed on the skin of the lower cheek using an acryl film tape (25 × 30 mm; Teraoka Seisakusho, Tokyo, Japan). Lipids in the stratum corneum were determined as previously described.Citation26) Briefly, the tapes were sonicated for 10 min in chloroform/methanol/2-propanol (10:45:45, vol/vol/vol), and this lipid solution was then analyzed by reversed-phase LC-MS consisting of an L-column ODS (2.1 mm i.d. × 150 mm; Chemicals Evaluation and Research Institute, Tokyo, Japan) and an Agilent 1100 Series LC/MSD SL system equipped with a multi-ion source (Agilent Technologies, Palo Alto, CA, USA). Each lipid was detected by selected ion monitoring. For this study, FFAs, CERs, cholesterol, and cholesterol sulfate were detected. FFAs include straight-chain, branched-chain, and mono-unsaturated FFAs with a carbon chain length of 20–30 (C20-C30). CERs comprise 12 subclasses, denoted as CER [NDS], [NS], [NH], [NP], [ADS], [AS], [AH], [AP], [EODS], [EOS], [EOH], [EOP],Citation26) which contain ultra-long-chain FAs (C32-C54). To quantify the soluble proteins, the tapes were incubated at 60 °C for 150 min in a buffer composed of 0.1 M NaOH and 1% sodium dodecyl sulfate and the resulting solutions were measured by using a BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA).
Analysis of organic acids and free amino acids in the stratum corneum. Five consecutive tape-strippings were performed on the skin of the lower cheek using an acryl film tape (25 × 50 mm; Teraoka Seisakusho). The tapes were immersed in methanol with 0.1% formic acid and sonicated for 10 min after the addition of an equivalent amount of 0.1% aqueous formic acid solution. The extracts were dried in a nitrogen stream and were then dissolved in 0.1% aqueous formic acid solution. Each sample solution was analyzed by reversed-phase LC/ESI-MS consisting of an L-column ODS (2.1 mm i.d. × 150 mm; Chemicals Evaluation and Research Institute) and an Agilent 1100 Series LC/MSD SL system (Agilent Technologies) to determine lactic acid, pyrrolidone carboxylic acid, and urocanic acid. Lactic acid (Kanto Kagaku, Tokyo, Japan), 2-pyrrolidone-5-carboxylic acid (Tokyo Kasei, Tokyo, Japan), and trans-urocanic acid (Tokyo Kasei) were used to generate calibration curves. The chromatographic separation conditions were: mobile phase, 0.1% aqueous formic acid solution; flow rate, 0.2 mL/min; and column temperature, 40 °C. The mass spectrometry parameters were as follows: flow of heated dry nitrogen gas, 13.0 L/min; nebulizer gas pressure, 30 psi; heater temperature of nitrogen gas, 350 °C; capillary voltage, 1500 V; and fragmenter voltages, 100 V and 120 V. Each organic acid was detected by selected ion monitoring as m/z 89.0 ([M-H]-) for lactic acid, m/z 128.0 ([M-H]−) and m/z 130.0 ([M + H]+) for pyrrolidone carboxylic acid, and m/z 137.0 ([M-H]−) and m/z 139.0 ([M + H]+) for urocanic acid. Furthermore, each sample solution was subjected to an ion exchange column #2622SC (Hitachi, Tokyo, Japan) and an amino acid analyzer L-8800 (Hitachi) to measure free amino acids (Asp, Thr, Ser, Glu, Gly, Ala, Val, Ile, Leu, Tyr, Phe, Lys, and Arg). An amino acid mixture standard solution (Wako, Osaka, Japan) was used to generate a calibration curve. To quantify the soluble proteins, the tapes were incubated at 25 °C for 15 min in a buffer composed of 0.1 M Tris-HCl (pH 8.0) and 0.5% Triton X-100 and the resulting solutions were measured by using a BCA protein assay kit (Thermo Fisher Scientific).
Evaluation of the responsiveness of SkBF during local warming. The evaluation of the responsiveness of SkBF during local warming was performed before ingestion and at 8 weeks after the start of ingestion. The evaluation and data analysis were conducted by the method described by Nomura et al.Citation8) with minor modification. The subjects were allowed to acclimatize for 15 min in a room at 24 °C and the SkBF of the forearm was then measured by using a laser doppler blood flow (LDF) probe (OP type, ADVANCE, Tokyo, Japan) and an LDF meter (ALF21, ADVANCE). A circular probe (diameter, 3 cm) equipped with a temperature control stimulator (TS-8000, Physio-Tech, Tokyo, Japan) was used for local warming. The combination probe consisted of an LDF and a heater probe was fixed to the skin of the forearm. To measure the mean arterial pressure from the middle finger, a Finometer MIDI (Finapres Medical Systems, Amsterdam, the Netherlands) was used. Initially, the probe temperature was maintained at 33 °C, followed by confirming stable SkBF signals, the probe temperature was elevated by 7 °C at a rate of 0.1 °C/s, maintained for 4 min, and then lowered at the same rate. The SkBF signals, mean arterial pressure and skin temperature were recorded continuously on a PowerLab (ADInstruments, Otago, New Zealand) device by using a LabChart (ADInstruments).
The regional SkBF, expressed as cutaneous vascular conductance (CVC, mL/min/100 g tissue/mm Hg), was calculated by the division of SkBF (mL/min/100 g tissue) by the mean arterial pressure (mm Hg). The baseline refers to the mean blood flow 1 min before warming and the initial peak refers to the mean blood flow 30 s at the first peak, with the baseline as a reference. The SlopeMAX% was calculated based on the maximum change in blood flow per min from the start of warming until the initial peak as a percentage of the initial peak (100%). Four subjects, who could not be evaluated owing to technical reasons, were excluded.
Analysis of blood components. The blood collection was performed before ingestion and at 8 weeks after the start of ingestion. The blood samples were obtained after 3 h of fasting and analyzed by LSI Medience Inc, Tokyo, Japan.
Statistical analysis. SPSS statistics version 23 (IBM, Armonk, NY, USA) was used for all statistical analyses. The results were presented as the means ± S.E. A value of p < 0.05 was considered statistically significant. The values at the initial measurement and at 4 and 8 weeks after ingestion were assessed by using Dunnett’s test or a paired t-test. Two-way repeated measures ANOVA or Student’s t-test were used to analyze the differences between two groups.
Results
Dryness score and skin properties
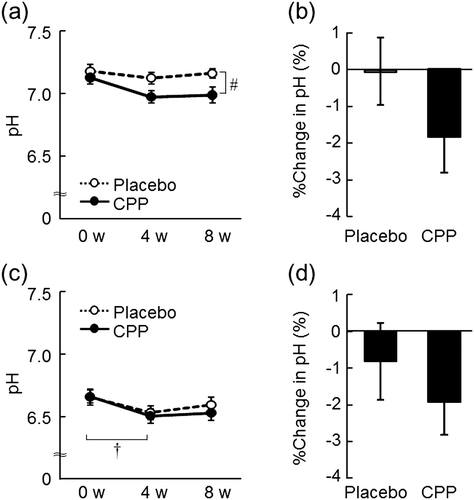
The dryness scores of both the lower cheeks and the hands significantly improved at 8 weeks after the ingestion of CPPs (p = 0.006, Fig. (a); and p = 0.010, Fig. (b)), but not in the placebo group. As measured in the lower cheek, TEWL, an indicator of skin permeability barrier function, significantly decreased after 4 and 8 weeks of CPP ingestion (p = 0.010 and p < 0.001, respectively), and there was a significant time-by-group interaction between the placebo and CPP groups (p = 0.001, Fig. (a)). The change in TEWL of the lower cheek between 0 and 8 weeks was 21% larger in the CPP group (p < 0.001, Fig. (b)). TEWL of the back of the hand significantly decreased after eight weeks of CPP ingestion (p < 0.001), but there was no significant change in the placebo group (Fig. (c)). The change in TEWL of the back of the hand over 8 weeks was 18% larger in the CPP group (p = 0.034, Fig. (d)). In the lower cheek, the hydration of the stratum corneum, as measured by the capacitance, significantly increased after 4 and 8 weeks of CPP ingestion (both p < 0.001), and there was a significant time-by-group interaction between the groups (p < 0.001, Fig. (a)). The change in the hydration of the lower cheek was 46% larger in the CPP group (p < 0.001, Fig. (b)). There were no significant differences in the capacitance values of the back of the hand during the test period (Fig. (c)); however, the change in capacitance value after ingestion was 13% larger in the CPP group (p = 0.044, Fig. (d)). The skin surface pH of the lower cheek decreased in the CPP group, which was determined as a significant effect by ANOVA (p = 0.016, Fig. (a)). The surface pH of the back of the hand significantly decreased after 4 weeks of CPP ingestion (p = 0.030), but there was no significant difference in the placebo group (Fig. (c)). The changes in the pH of both sites between the groups were not statistically significant (Fig. (b) and (d)).
Fig. 1. The effects of CPPs on the dryness score of the lower cheeks (a) and the hands (b). The values are the means ± S.E. of the placebo group (n = 26) and the CPP group (n = 23). †† p < 0.01 vs. Week 0 (0 w) (Dunnett’s test).
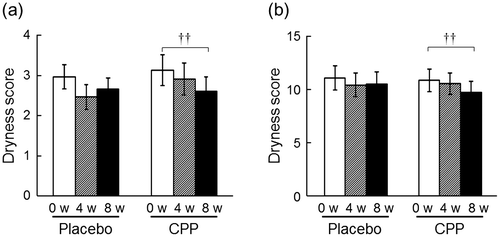
Fig. 2. The effects of CPPs on TEWL of the lower cheek (a) and the back of the hand (c). The percentage change in TEWL of the lower cheek (b) and back of the hand (d) after 8 weeks of ingestion. The values are the means ± S.E. of the placebo group (n = 26) and the CPP group (n = 23). †† p < 0.01, ††† p < 0.001 vs. 0 w (Dunnett’s test). ## p < 0.01 vs. the placebo group (two-way repeated measures ANOVA). * p < 0.05, *** p < 0.001 vs. the placebo group (Student’s t-test).
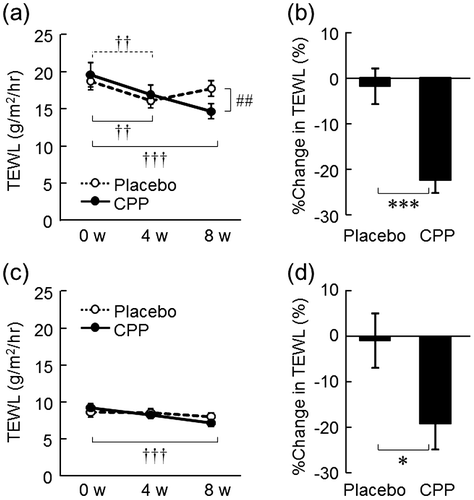
Fig. 3. The effects of CPPs on skin hydration of the lower cheek (a) and the back of the hand (c). The percentage change in capacitance of the lower cheek (b) and the back of the hand (d) after 8 weeks of ingestion. The values are the means ± S.E. of the placebo group (n = 26) and the CPP group (n = 23). ††† p < 0.001 vs. 0 w (Dunnett’s test). ### p < 0.001 vs. the placebo group (two-way repeated measures ANOVA). * p < 0.05, *** p < 0.001 vs. the placebo group (Student’s t-test).
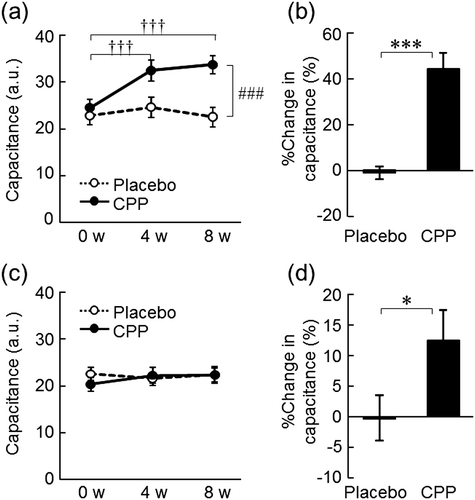
Fig. 4. The effects of CPPs on pH of the lower cheek (a) and the back of the hand (c). The percentage change in pH of the lower cheek (b) and the back of the hand (d) after 8 weeks of ingestion. The values are the means ± S.E. of the placebo group (n = 26) and the CPP group (n = 23). † p < 0.05 vs. 0 w (Dunnett’s test). # p < 0.05 vs. the placebo group (two-way repeated measures ANOVA).
Stratum corneum components
The level of total FFAs (C20-C30) in the stratum corneum of the lower cheek was significantly increased after 8 weeks of CPP ingestion (p < 0.001), and there was a significant time-by-group interaction between the placebo and CPP groups (p = 0.037, Fig. (a)). In the CPP group only, the amounts of straight-chain FAs (C21, C22, C23, C24, C25, C26, and C30) and mono-unsaturated FAs significantly increased at week 8 compared with that at week 0 (Table ). The levels of CERs, cholesterol, and cholesterol sulfate were not significantly different. In the CPP group, the level of lactic acid in the stratum corneum of the lower cheek significantly increased after 8 weeks (p = 0.016), but there was no significant change in the placebo group (Fig. (b)); however, no significant effect was detected by ANOVA. The levels of pyrrolidone carboxylic acid, urocanic acid, and free amino acids were not significantly changed in either group (Table ).
Fig. 5. The effects of CPPs on FFAs (total amount of C20-C30 FFAs) (a) and lactic acid (b) in the stratum corneum of the lower cheek. The values are the means ± S.E. of the placebo group (n = 26) and the CPP group (n = 23). † p < 0.05, ††† p < 0.001 vs. 0 w (paired t-test). # p < 0.05 vs. the placebo group (two-way repeated measures ANOVA). FFAs; free fatty acids.
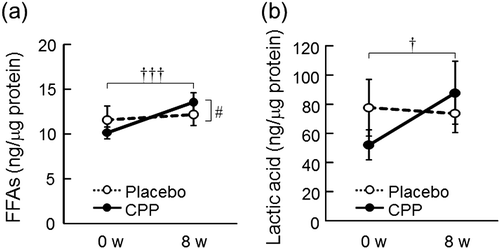
Table 1. The effects of CPPs on components in the stratum corneum of the lower cheek.
Responsiveness of SkBF during local warming
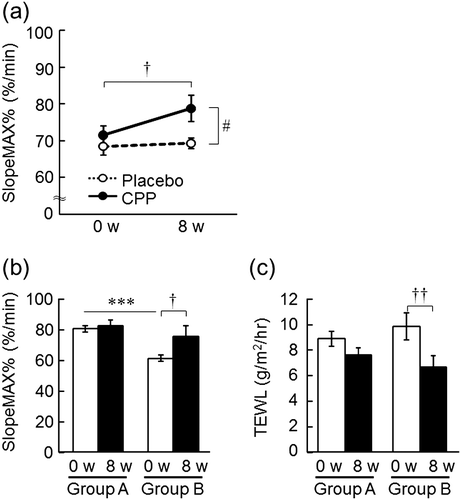
The SlopeMAX%, which is the relative maximum change in SkBF of the forearm during local warming, significantly increased after 8 weeks compared with 0 weeks in the CPP group (p = 0.033), but there was no change in the placebo group, and a significant effect of the group was detected by ANOVA (p = 0.044, Fig. (a)). Moreover, the CPP group was divided into two subgroups according to the initial value of the SlopeMAX%: group A comprised 12 subjects whose initial value of the SlopeMAX% was higher than the average of all subjects (SlopeMAX% = 70.2%/min) and group B comprised 9 subjects whose initial value of the SlopeMAX% was lower than the average of all subjects. The initial value of the SlopeMAX% in group B was significantly lower than that in group A (p < 0.001), and significantly increased after 8 weeks (p = 0.044, Fig. (b)) with a concomitant decrease in TEWL of the back of the hand (p = 0.002, Fig. (c)).
Fig. 6. The effects of CPPs on the responsiveness of SkBF during local warming (a). The effects of the difference of initial responsiveness of SkBF on the efficacy of CPPs: SlopeMAX% of the forearm (b) and TEWL of the back of the hand (c). The values are the means ± S.E. of the placebo group (n = 24) and the CPP group (n = 21) (a); Group A (n = 12) and Group B (n = 9) (b, c). † p < 0.05, †† p < 0.01 vs. 0 w (paired t-test). # p < 0.05 vs. the placebo group (two-way repeated measures ANOVA). *** p < 0.001 vs. Group A (Student’s t-test). SkBF; skin blood flow.
Blood components
Although there were significant changes in aspartate aminotransferase (AST), γ-glutamyltransferase (γ-GT), phospholipids, hematocrit, mean cell volume (MCV), and mean cell hemoglobin concentration (MCHC) after the ingestion of CPPs, these values were within the normal range (Table ).
Table 2. The effects of CPPs on components in the blood.
Discussion
This study presented the first demonstration that the ingestion of CPPs improved skin permeability barrier function and stratum corneum hydration in humans. Increased levels of FFAs and lactic acid in the stratum corneum may lead to the improvement of skin properties, such as increases in water capacity of the stratum corneum and decreases in skin surface pH and TEWL. Moreover, the cutaneous microcirculation was improved by the ingestion of CPPs. The effects of CPPs on the microcirculation and skin permeability barrier function were higher in subjects who showed weakened responsiveness of SkBF, which suggested that there was a relationship between improvement of the microcirculation and skin properties. In addition to several human studies on the use of CPPs,Citation27−29) no ingestion-related serious adverse events occurred in this study and the variables of the blood did not deviate from the standard values, which supported the efficacy and the safety of oral intake of CPPs (270 mg/day) for an eight-week period.
The improving effects of CPPs on skin properties, including stratum corneum hydration and skin permeability barrier function, were clearly observed in the lower cheek (Figs. ). The stratum corneum hydration of the lower cheek was negatively correlated with the dryness score (n = 150, R = −0.33, p < 0.001, by Spearman’s rank correlation coefficient, data not shown), and was also known to be lower than that of any other location on the face in healthy women,Citation30) which suggested that the lower cheek was highly susceptible to dry conditions. As we recruited subjects with mildly xerotic skin in the lower cheek for this study, this may have led to the clear improvements observed in the lower cheek.
Recent studies have proposed that the acidification of the stratum corneum was important in skin barrier homeostasis.Citation31,32) As the skin surface pH increases with age, it has been suggested that the preservation of an acidic pH was effective for the suppression of senile xerosis.Citation33) The skin pH of patients with atopic dermatitis, one of the most common skin diseases presenting with xerosis and barrier dysfunction, is higher than that of healthy individuals.Citation34) Additionally, the affected sites showed higher pH values than non-affected sites of atopic dermatitis patients.Citation34,35) The increased pH caused by a decrease in filaggrin expression or stress affects various physiological responses, such as the excessive activity of serine proteases, in particular kallikrein 5 and 7, and the inhibition of the activity of lipid processing enzymes, including β-glucocerebrosidase, result in the skin dysfunction.Citation36) In this study, significant acidification caused by CPPs was detected particularly in the lower cheek (Fig. (a)). Collectively, these facts suggested that the reduction of the skin pH by the ingestion of CPPs may contribute to the improvement of skin properties, including permeability barrier function and stratum corneum hydration.
FFAs in the stratum corneum are generated by secretory phospholipase A2 and ceramidase and are involved in the regulation of skin pH and permeability barrier function.Citation37−39) In patients with atopic dermatitisCitation40) and psoriasis,Citation41) and in Netherton syndrome model mice that over-express elastase-2,Citation42) FFAs levels are decreased. In addition, the level of long-chain FFAs (C > 23), which show a negative correlation with TEWL,Citation43) are decreased in the stratum corneum of patients with atopic dermatitis and Netherton syndrome; these changes are considered to lead to aberrant barrier homeostasis through the alteration of lipid layer organization.Citation43,44) Long-chain FFAs are important for stratum corneum integrity and cutaneous permeability barrier function, as supported by a previous report that investigated the structural assembly of stratum corneum model membranes based on a CER with long-chain FFAs (C22 and C26).Citation45) It was also demonstrated that the absence of functional elongation of very long-chain fatty acids-4 (ELOVL4) reduced the level of very long-chain FFAs (C ≥ 28) and impaired the skin barrier.Citation46) In our study, the analysis of lipid components in the stratum corneum indicated that CPPs increased the amount of long-chain FFAs (total amount of FFAs (C20-C30) and straight-chain FAs (C21, C22, C23, C24, C25, C26, and C30)) (Fig. (a), Table ). Taken together, these facts suggested that an increase in the FFA levels by the ingestion of CPPs induced a reduction in TEWL and skin pH. In contrast to the FFAs, no significant changes were observed in the amounts of CERs, cholesterol, and cholesterol sulfate in the stratum corneum, suggesting a smaller contribution of these lipids to the effects of CPPs.
The analysis of organic acids indicated that the content of lactic acid in the stratum corneum was increased at week 8 compared with week 0 only in the CPP group (Fig. (b)). Previous studies have reported low lactic acid levels in atopic dermatitis patients,Citation47) and that the topical application of lactic acid was effective for the improvement of xerosis.Citation48) In healthy individuals, the levels of lactic acid in the stratum corneum are positively correlated with skin hydration and negatively correlated with skin surface pH.Citation49) Lactic acid is one of the major components of NMFs, which participate in the maintenance of the water content of the stratum corneum.Citation50) A recent study has demonstrated that lactic acid, especially in the form of potassium lactate, increased the water-holding capacity of the stratum corneum via an increase in the interaction between water molecules and keratin, the main structural protein in the epidermis.Citation51) Accordingly, the skin hydration and acidification effects of CPPs were suggested to be caused, at least in part, by an increase in lactic acid content in the stratum corneum. Lactic acid in the stratum corneum is mainly derived from sweat glands. Because 5-CQA, a major component of CPPs, has an inhibitory effect on acetylcholinesterase, which hydrolyzes acetylcholine, a neurotransmitter responsible for increased sweating,Citation52) it is likely that CPPs increase the level of lactic acid in the stratum corneum via an increase in sweating, which subsequently improved the skin properties.
The responsiveness of SkBF during local warming was evaluated in the forearm, because it is a stable area that can be easily measured. The SlopeMAX%, defined as the relative maximum change in SkBF to local warming, was increased by 8 weeks of CPP ingestion (Fig. ), suggesting that CPPs improve the responsiveness of SkBF against external environmental changes. The subgroup analysis based on the initial values of the SlopeMAX% showed that the skin permeability barrier function was improved significantly by CPP ingestion with a concomitant improvement in the responsiveness of SkBF in subjects with lower initial SlopeMAX% values (group B), which was suggestive of a relationship between skin properties and microcirculation. In support of that relationship, a previous study showed that the skin barrier function was impaired in patients with chronic venous insufficiency caused by permanent venous and capillary hypertension.Citation53) In addition, patients with diabetes mellitus have a delayed internal temperature threshold for active cutaneous vasodilation during whole body heatingCitation54) and the hydration state of their skin is reduced, similar to senile xerosis, with decreased sebaceous gland activity.Citation55) The cutaneous microcirculation is controlled by various physiological systems, including autonomic nerves. Nomura et al. reported a close relationship between cutaneous microcirculatory function, autonomic nerve activity, and skin barrier function; that is, the responsiveness of SkBF during local warming was lower, sympathetic activity was higher, and parasympathetic activity was lower in individuals with a decreased skin permeability barrier function.Citation8) On the other hand, Suzuki et al. demonstrated that 5-CQA reduced oxidative stress and increased nitric oxide bioavailability, leading to an improvement in endothelium-dependent vasodilation.Citation56) Therefore, the effect of CPPs on endothelial function was considered to contribute to the improvement of the cutaneous microcirculation. Caffeine’s effect on microvascular function which has been reported,Citation57) may not be a key contributor to the efficacy in this study because the test beverage was decaffeinated. In addition, the other components of green coffee beans (including saccharides, organic acids, and proteins) or some of its metabolites,Citation58) may also be responsible for the observed effects. Further studies are needed to elucidate the detailed mechanisms of CPPs to improve skin properties and microcirculation.
In summary, the eight-week intake of CPPs extracted from green coffee beans simultaneously improves epidermal permeability barrier function, stratum corneum hydration, and microcirculatory function, leading to an efficacy in the treatment of mildly xerotic skin. Our study suggests that CPPs are promising and safe materials to maintain esthetic and healthy skin, in addition to providing health benefits, such as anti-hypertension and anti-obesity effects.
Author contributions
Satoko Fukagawa, Satoshi Haramizu, and Takatoshi Murase designed the study. Satoko Fukagawa analyzed the data and wrote the manuscript. Takatoshi Murase supervised writing the manuscript. Shun Sasaoka, Yuka Yasuda, and Hisashi Tsujimura participated in the analysis of components in the stratum corneum. All authors reviewed and approved this paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
The authors thank Dr. Kayoko Numano at Queen’s Square Dermatology/Allergology, Dr. Seiji Kawana, Professor Emeritus of Dermatology, Nippon Medical School, and Dr. Chiaki Ishizaki at Dermatology of Sangenjaya first Hospital, for clinical assessment of skin dryness. The authors also thank Satoshi Sugawara and Akane Suma at Skin Care Laboratories, Kao Corporation, for providing the test beverages.
References
- Elias PM. Defensive functions of the stratum corneum: integrative aspects. In: Elias PM, Feingold KR, editors. Skin barrier. New York (NY): Taylor & Francis; 2005. p. 5–14.10.1201/b14173
- Feingold KR. The outer frontier: the importance of lipid metabolism in the skin. J Lipid Res. 2009;50:S417–S422.
- Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004;17:43–48.10.1111/dth.2004.17.issue-s1
- Rawlings AV, Matts PJ. Stratum corneum moisturization at the molecular level: an update in relation to the dry skin cycle. J Invest Dermatol. 2005;124(6):1099–1110.10.1111/j.1523-1747.2005.23726.x
- Hashizume H. Skin aging and dry skin. J Dermatol. 2004;31(8):603–609.10.1111/jde.2004.31.issue-8
- Denda M, Tsuchiya T, Elias PM, et al. Stress alters cutaneous permeability barrier homeostasis. Am J Physiol Regulatory Integrative Comp Physiol. 2000;278(2):R367–R372.
- Boelsma E, van de Vijver LP, Goldbohm RA, et al. Human skin condition and its associations with nutrient concentrations in serum and diet. Am J Clin Nutr. 2003;77(2):348–355.
- Nomura T, Yoshida-Amano Y, Yoshida K, et al. Relationships between transepidermal water loss, cutaneous microcirculatory function and autonomic nervous activity. Int J Cosmet Sci. 2016;39(3):275–283. DOI:10.1111/ics.12373
- Butt MS, Sultan MT. Coffee and its consumption: benefits and risks. Crit Rev Food Sci Nutr. 2011;51(4):363–373.10.1080/10408390903586412
- Akash MSH, Rehman K, Chen S. Effects of coffee on type 2 diabetes mellitus. Nutrition. 2014;30(7–8):755–763.10.1016/j.nut.2013.11.020
- Jee SH, He J, Whelton PK, et al. The effect of chronic coffee drinking on blood pressure: A meta-analysis of controlled clinical trials. Hypertension. 1999;33(2):869–873.
- Eskelinen MH, Ngandu T, Tuomilehto J, et al. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimer’s Dis. 2009;16(1):85–91.10.3233/JAD-2009-0920
- Clifford MN. Chlorogenic acids and other cinnamates: nature, occurrence and dietary burden. J Sci Food Agric. 1999;79(3):362–372.10.1002/(ISSN)1097-0010
- Moon JK, Yoo HS, Shibamoto T. Role of roasting conditions in the level of chlorogenic acid content in coffee beans: correlation with coffee acidity. J Agric Food Chem. 2009;57(12):5365–5369.10.1021/jf900012b
- Zhang LY, Cosma G, Gardner H, et al. Effect of chlorogenic acid on hydroxyl radical. Mol Cell Biochem. 2003;247(1–2):205–210.10.1023/A:1024103428348
- McCarty MF. A chlorogenic acid-induced increase in GLP-1 production may mediate the impact of heavy coffee consumption on diabetes risk. Med Hypotheses. 2005;64(4):848–853.10.1016/j.mehy.2004.03.037
- Cho AS, Jeon SM, Kim MJ, et al. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol. 2010;48(3):937–943.10.1016/j.fct.2010.01.003
- Murase T, Misawa K, Minegishi Y, et al. Coffee polyphenols suppress diet-induced body fat accumulation by downregulating SREBP-1c and related molecules in C57BL/6 J mice. Am J Physiol Endocrinol Metab. 2011;300(1):E122–E133.10.1152/ajpendo.00441.2010
- dos Santos MD, Almeida MC, Lopes NP, et al. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol Pharm Bull. 2006;29(11):2236–2240.10.1248/bpb.29.2236
- Li Y, Shi W, Li Y, et al. Neuroprotective effects of chlorogenic acid against apoptosis of PC12 cells induced by methylmercury. Environ Toxicol Pharmacol. 2008;26(1):13–21.10.1016/j.etap.2007.12.008
- Ochiai R, Sugiura Y, Shioya Y, et al. Coffee polyphenols improve peripheral endothelial function after glucose loading in healthy male adults. Nutr Res. 2014;34(2):155–159.10.1016/j.nutres.2013.11.001
- Ochiai R, Sugiura Y, Otsuka K, et al. Coffee bean polyphenols ameliorate postprandial endothelial dysfunction in healthy male adults. Int J Food Sci Nutr. 2015;66(3):350–354.10.3109/09637486.2015.1007453
- Fukushima Y, Takahashi Y, Hori Y, et al. Skin photoprotection and consumption of coffee and polyphenols in healthy middle-aged Japanese females. Int J Dermatol. 2015;54(4):410–418.10.1111/ijd.2015.54.issue-4
- Kang NJ, Lee KW, Shin BJ, et al. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis. 2009;30(2):321–330.
- Chiang HM, Lin TJ, Chiu CY, et al. Coffea arabica extract and its constituents prevent photoaging by suppressing MMPs expression and MAP kinase pathway. Food Chem Toxicol. 2011;49(1):309–318.10.1016/j.fct.2010.10.034
- Ohno Y, Nakamichi S, Ohkuni A, et al. Essential role of the cytochrome P450 CYP4F22 in the production of acylceramide, the key lipid for skin permeability barrier formation. Proc Natl Acad Sci USA. 2015;112(25):7707–7712.10.1073/pnas.1503491112
- Kozuma K, Tsuchiya S, Kohori J, et al. Antihypertensive effect of green coffee bean extract on mildly hypertensive subjects. Hypertens Res. 2005;28(9):711–718.10.1291/hypres.28.711
- Watanabe T, Arai Y, Mitsui Y, et al. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin Exp Hypertens. 2006;28(5):439–449.10.1080/10641960600798655
- Onakpoya I, Terry R, Ernst E. The use of green coffee extract as a weight loss supplement: a systematic review and meta-analysis of randomised clinical trials. Gastroenterol Res Pract. 2011;2011:382852.
- Kobayashi H, Tagami H. Distinct locational differences observable in biophysical functions of the facial skin: with special emphasis on the poor functional properties of the stratum corneum of the perioral region. Int J Cos Sci. 2004;26(2):91–101.10.1111/ics.2004.26.issue-2
- Hachem JP, Crumrine D, Fluhr J, et al. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121(2):345–353.10.1046/j.1523-1747.2003.12365.x
- Ali SM, Yosipovitch G. Skin pH: from basic science to basic skin care. Acta Derm Venereol. 2013;93(3):261–267.10.2340/00015555-1531
- Choi EH, Man MQ, Xu P, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127(12):2847–2856.10.1038/sj.jid.5700913
- Eberlein-König B, Schäfer T, Huss-Marp J, et al. Skin surface pH, stratum corneum hydration, trans-epidermal water loss and skin roughness related to atopic eczema and skin dryness in a population of primary school children. Acta Derm Venereol. 2000;80(3):188–191.10.1080/000155500750042943
- Seidenari S, Giusti G. Objective assessment of the skin of children affected by atopic dermatitis: a study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Derm Venereol. 1995;75(6):429–433.
- Lee HJ, Lee SH. Epidermal permeability barrier defects and barrier repair therapy in atopic dermatitis. Allergy Asthma Immunol Res. 2014;6(4):276–287.10.4168/aair.2014.6.4.276
- Fluhr JW, Kao J, Ahn SK, et al. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 2001;117(1):44–51.10.1046/j.0022-202x.2001.01399.x
- Chan A, Mauro T. Acidification in the epidermis and the role of secretory phospholipases. Dermatoendocrinol. 2011;3(2):84–90.10.4161/derm.3.2.15140
- Houben E, Hachem JP, De Paepe K, et al. Epidermal ceramidase activity regulates epidermal desquamation via stratum corneum acidification. Skin Pharmacol Physiol. 2008;21(2):111–118.10.1159/000114872
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound ω-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119(1):166–173.10.1046/j.1523-1747.2002.01833.x
- Motta S, Sesana S, Ghidoni R, et al. Content of the different lipid classes in psoriatic scale. Arch Dermatol Res. 1995;287(7):691–694.10.1007/BF00371745
- Bonnart C, Deraison C, Lacroix M, et al. Elastase 2 is expressed in human and mouse epidermis and impairs skin barrier function in Netherton syndrome through filaggrin and lipid misprocessing. J Clin Invest. 2010;120(3):871–882.10.1172/JCI41440
- van Smeden J, Janssens M, Kaye EC, et al. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp Dermatol. 2014;23(1):45–52.10.1111/exd.12293
- van Smeden J, Janssens M, Boiten WA, et al. Intercellular skin barrier lipid composition and organization in netherton syndrome patients. J Invest Dermatol. 2014;134(5):1238–1245.10.1038/jid.2013.517
- Schroeter A, Kiselev MA, Hauss T, et al. Evidence of free fatty acid interdigitation in stratum corneum model membranes based on ceramide [AP] by deuterium labelling. Biochim Biophys Acta. 2009;1788(10):2194–2203.10.1016/j.bbamem.2009.07.024
- Vasireddy V, Uchida Y, Salem N Jr, et al. Loss of functional ELOVL4 depletes very long-chain fatty acids (> or =C28) and the unique omega-O-acylceramides in skin leading to neonatal death. Hum Mol Genet. 2007;16(5):471–482.10.1093/hmg/ddl480
- Sugawara T, Kikuchi K, Tagami H, et al. Decreased lactate and potassium levels in natural moisturizing factor from the stratum corneum of mild atopic dermatitis patients are involved with the reduced hydration state. J Dermatol Sci. 2012;66(2):154–159.10.1016/j.jdermsci.2012.02.011
- Dahl MV, Dahl AC. 12% lactate lotion for the treatment of xerosis. A double-blind clinical evaluation. Arch Dermatol. 1983;119(1):17–30.
- Nakagawa N, Sakai S, Matsumoto M, et al. Relationship between NMF (lactate and potassium) content and the physical properties of the stratum corneum in healthy subjects. J Invest Dermatol. 2004;122(3):755–763.10.1111/j.0022-202X.2004.22317.x
- Jokura Y, Ishikawa S, Tokuda H, et al. Molecular analysis of elastic properties of the stratum corneum by solid-state 13C-nuclear magnetic resonance spectroscopy. J Invest Dermatol. 1995;104(5):806–812.10.1111/1523-1747.ep12607005
- Nakagawa N, Naito S, Yakumaru M, et al. Hydrating effect of potassium lactate is caused by increasing the interaction between water molecules and the serine residue of the stratum corneum protein. Exp Dermatol. 2011;20(10):826–831.10.1111/exd.2011.20.issue-10
- Kwon SH, Lee HK, Kim JA, et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur J Pharmacol. 2010;649(1–3):210–217.10.1016/j.ejphar.2010.09.001
- Angelova-Fischer I, Wuthe D, Zillikens D, et al. Noninvasive bioengineering assessment of the skin barrier function in patients with chronic venous insufficiency. Br J Dermatol. 2010;162(5):1071–1075.10.1111/bjd.2010.162.issue-5
- Wick DE, Roberts SK, Basu A, et al. Delayed threshold for active cutaneous vasodilation in patients with type 2 diabetes mellitus. J Appl Physiol. 2006;100(2):637–641.10.1152/japplphysiol.00943.2005
- Sakai S, Kikuchi K, Satoh J, et al. Functional properties of the stratum corneum in patients with diabetes mellitus: similarities to senile xerosis. Br J Dermatol. 2005;153(2):319–323.10.1111/bjd.2005.153.issue-2
- Suzuki A, Yamamoto N, Jokura H, et al. Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats. J Hypertens. 2006;24(6):1065–1073.10.1097/01.hjh.0000226196.67052.c0
- Noguchi K, Matsuzaki T, Sakanashi M, et al. Effect of caffeine contained in a cup of coffee on microvascular function in healthy subjects. J Pharmacol Sci. 2015;127(2):217–222.10.1016/j.jphs.2015.01.003
- Del Rio D, Stalmach A, Calani L, et al. Bioavailability of coffee chlorogenic acids and green tea flavan-3-ols. Nutrients. 2010;2(8):820–833.10.3390/nu2080820
