Abstract
We compared the effects of two major isoflavones, daidzein and genistein, on lipid metabolism in rats. Daidzein (150 mg/kg diet), genistein (150 mg/kg diet), daidzein and genistein (1:1, 300 mg/kg diet), or control diets were fed to 4 groups of 6-week-old ovariectomized (Ovx) and non-Ovx Sprague Dawley rats for 4 weeks. Dietary daidzein, but not genistein, reduced serum and hepatic total cholesterol levels significantly relative to that by the control group, regardless of whether the rats had undergone ovariectomy. Genistein did not exhibit any physiological effects on lipid levels, but did affect genes involved in cholesterol metabolism. These results indicate that daidzein and genistein may influence lipid regulation via differing modes of action.
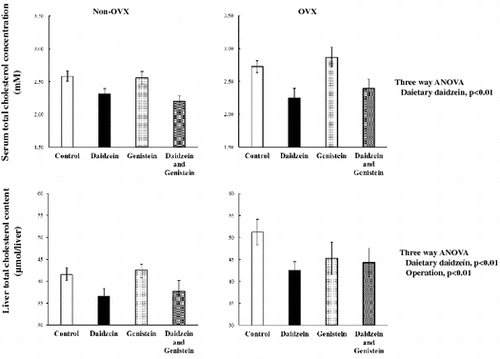
Dietary daidzein, but not genistein, reduced serum and hepatic total cholesterol levels significantly relative to that by the control group, regardless of whether the rats had undergone ovaritectmy.
Hypercholesterolemia is associated with cardiovascular disease. Animal and human nutrition studies have shown that soy intake lowers blood lipid levels and reduces atherosclerosis.Citation1–3) The United States FDA has reported that a diet containing 25 g of soy per day may reduce the risk of coronary heart disease, and associated risks.Citation4,5) However, the soy components responsible for these effects and their underlying mechanisms of action are still unclear.
Dietary soy contains proteins, lipids, dietary fibers, and bioactive compounds including soy isoflavones. Daidzein and genistein classes of phytoestrogens are the predominant isoflavones in the soy. Daidzein is further metabolized into equal by intestinal enterobacteria. In fact, daidzein and genistein, are the major isoflavones, and their metabolites have been detected in the blood and urine of humans and animals.Citation6,7) IsoflavonesCitation8–11) or soy proteinCitation12–14) containing intact isoflavones have hypocholesterolemic effects in animal models. Soy protein containing isoflavones also affect hepatic lipid metabolism in experimental animals.Citation15,16) In a previous study of Sprague Dawley (SD) rats, we found that an AIN76 diet containing isoflavone-rich fermented soybean extract (FSBE) exhibited hypocholesterolemic effects in female rats with or without ovariectomy; we observed no such effect in male rats.Citation17) Lee et al. reported that female Golden Syrian hamsters fed a diet containing pure synthetic daidzein, genistein, or glycitein (0.9 mmol/kg diet) presented significantly lower total plasma and non-HDL cholesterol than control animals.Citation18) Balmir et al. reported that male SD rats and hamsters fed a diet containing isoflavone-rich soy protein isolate presented decreased total cholesterol and LDL levels. On feeding casein with supplemental isoflavones to the hamster group, the same authors found decreased levels of total and LDL cholesterol. These results indicate that isoflavones alone may lower cholesterol.Citation19)
Isoflavones are estrogen receptor agonists, and genistein is more potent than daidzein.Citation20) However, after digestion, enterobacteria convert daidzein into equol, which exhibits stronger estrogenic effects than genistein.Citation21,22) The present study investigated the soy extract components, daidzein and genistein, to confirm that they are responsible for the hypocholesterolemic effect on Ovx and non-Ovx female rats. In the previous study, we have found female-specific hypocholesterolemic effect in the rats fed the diet containing 155 mg/kg diet of genistein, 127 mg/kg diet of daidzein, and 18 mg/kg diet of glycitein.Citation17) Thus, we examined the hypocholesterolemic effect of dietary daidzein and genistein at a concentration of 150 mg/kg diet in Ovx and non-Ovx female rats. We also investigated gene expression of the key regulators of cholesterol and bile acid metabolism in the liver and small intestinal mucosa (upper and lower).
Materials and methods
Animals and diets
We purchased female Sprague-Dawley rats (Japan SLC, Hamamatsu, Japan; 6 weeks old). The experimental protocol was approved by the Laboratory Animal Care Committee of Ehime University, and the rats were maintained in accordance with the Guidance for the Care and Use of Laboratory Animals of Ehime University. All animals were housed individually in a room controlled by a 12-h light-dark cycler (dark phase: 15:00–3:00) at constant temperature (23 ± 1 °C) and humidity (55–65%). Animals were fed regular tap water and an isoflavone-free diet based on AIN-93G during the acclimatization and recovery period.
Experiment 1
After 7 d of acclimatization, the female rats were divided into 4 experimental groups with similar mean body weights (156 g) composed of 8 rats. Each group was given free access to the control diet (C diet) or a diet containing 150 mg daidzein, 150 mg genistein, or 300 mg mixture of daidzein and genistein per kg diet (D, G, or D + G diet) for 4 weeks. The C diet comprised (in g/kg): casein (New Zealand Dairy Board, Wellington, New Zealand), 200; cellulose (Danisco Japan., Inc., Tokyo, Japan), 50; soybean oil (J-oil Mills., Inc., Tokyo, Japan), 70; AIN-93 mineral mixture, 35; AIN-93 vitamin mixture, 10; l-cystine (Nacalai tesque, Kyoto, Japan), 3; sucrose (Nippon Beet Sugar Manufacturing, Tokyo, Japan), 100; and α-corn starch (Sanwa Starch, Nara, Japan), 532. The two major isoflavones, daidzein and genistein, were purchased from the LC laboratories (MA, USA). The additions of daidzein and genistein were performed at the expense of α-corn starch. During the experimental period, the body weight and amount of food consumed by weight were recorded each morning, and the respective diet was replenished. The rats were killed by decapitation, and a blood sample corresponding to a non-fasting state was collected from the neck at 20:00 h on the last day of the experimental period, identically to the condition in the previous study.Citation17) Subsequently, the serum was separated and extracted by centrifugation at 1,500 × g for 10 min at 4 °C, and stored at –50 °C until analysis. The liver was removed immediately after blood collection, weighed, and stored at –50 °C until analysis. Similarly, the small intestine (upper and lower) was excised immediately. After washing with ice-cold saline, the mucosa was scraped out and homogenized in Sepasol-RNA I Super G (Nacalai tesque, Kyoto, Japan), kept at room temperature for 5 min, and stored at –80 °C until further analysis. The perirenal, mesentery, and ovary fat tissues were removed and weighed: the sum of the weights is considered as the total white fat weight. During the final 3 d of the experimental period, feces were collected from each rat and freeze-dried, weighed, and stored in a desiccator until analysis.
Experiment 2
After 2 d of acclimatization, 35 rats were selected for the bilateral ovariectomy operation under sodium pentobarbital anesthesia (Kyoritsu Pharmaceutical Co., Ltd., Tokyo, Japan, 30 mg/kg of body weight, intraperitoneal injection). During this period, the operated rats were fed the control diet with casein. After 5 d recovery period, the Ovx rats were divided into 4 groups with a mean weight of 144 g (n = 8), and each group was given free access to the C diet or D, G, or D + G diets and water for 4 weeks. The present study did not have a sham-operated group, because serum and hepatic cholesterol concentrations of sham-operated female rats were not appreciably different from those of intact female rats in our previous study.Citation17) At the end of the experimental period, the rats were decapitated, as described in experiment 1.
Lipid analysis
Total serum cholesterol and triglyceride concentrations were measured by absorbance using commercially available colorimetric cholesterol and triglyceride reagents (Cholesterol E-test Wako and TG E-test Wako, respectively; Wako Pure Chemical Industries Co., Osaka, Japan).
Total liver lipid was extracted from 1 g liver with 25 mL of chloroform:methanol (2:1) and gravimetrically measured by the method of Folch et al.Citation23) Liver cholesterol (total, free, and esterified) and triglyceride concentrations were enzymatically determined with commercial diagnostic kits (Cholesterol E-test Wako, Free-Cholesterol E-test Wako, TG E-test Wako, respectively; Wako Pure Chemical Industries Co., Osaka, Japan.) by taking 5 mL sample from the adjusted 25 mL liver lipid extract. For the cholesterol test, 200 μL of this extract was evaporated, and the residue was mixed with 200 μL of propane-2-ol containing triton X 100 (9:1 v/v). From this mixture, 100 μL was taken and mixed with 900 μL coloring solution and incubated at 37 °C for 30 min. After which, 400 μL chloroform was added, the solution was vortexed and centrifuged at 1160 × g for 5 min at 4 °C, and the upper layer was decanted for cholesterol profiling. Cholesterol esters were quantitated by subtracting free cholesterol from total cholesterol.
Fecal bile acid was extracted from 0.05 g feces with 5 mL of chloroform:methanol (1:1) at 70 °C for 60 h. The bile acid level was enzymatically analyzed by the 3-β-hydroxysteroid dehydrogenase assay method of Sheltaway and Losowsky, using taurocholic acid as the standard.Citation24)
Total fecal lipids were extracted from 0.5 g feces with 20 mL of chloroform:methanol (2:1) and gravimetrically measured, as described by Folch et al.Citation23)
RNA isolation and cDNA synthesis
Total RNA was isolated from the liver and small intestinal mucosa (upper and lower) as per the method described by Chomczynski and Sacchi.Citation25) mRNA was isolated from total RNA using Oligotex-dT30 (Takara Bio, Inc., Shiga, Japan) to synthesize cDNA using reverse transcriptase (Reverse Transcriptase XL [AMV] and RT-PCR, 5 U/μL, Takara Bio Inc., Shiga, Japan) a thermal cycler (ABI GeneAmp2400; PerkinElmer, Inc., Waltham, USA). mRNA expression was determined by quantitative PCR using the StepOnePlus real-time PCR system (Applied Biosystems, Carlsbad, California, USA). The primer sets of cholesterol 7α-hydroxylase (CYP7A1), sterol 12-alpha-hydroxylase (CYP8B1), sterol 27hydroxylase (CYP27), low-density lipoprotein receptor (LDLR), hydroxymethylglutaryl-coenzyme reductase (HMG-CoA-R), apolipoprotein-B (Apo-B), liver X receptor (LXR), farnesoid X receptor (FXR), acyl-CoA:cholesterol acyl transferase (ACAT), ileal sodium-dependent bile acid transporter (IBAT), microsomal triglyceride transfer protein (MTP), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and cyclophilin (Table ) were used with the THUNDERBIRD SYBR qPCR mix (TOYOBO, Osaka, Japan). The basic amplification program was set to 50 cycles: denaturizing at 95 °C for 15 s, annealing and primer extension at 60 °C for 1 min. Fluorescence was recorded at 530 nm during extension. Relative mRNA expression was calculated using the crossing point of each target gene, with the GAPDH or cyclophilin gene as the reference, and the corresponding real-time PCR efficiency of respective primer sets as per the method previously reported.Citation26)
Table 1. The primers used for reverse transcription-polymerase chain reaction (RT-PCR) analysis.
Statistical analysis
All data are presented as the mean ± standard error (SEM). The main effects and associations between daidzein administration (dietary daidzein), genistein administration (dietary genistein), and the operation (Ovx) were assessed by three-way analysis of variance (ANOVA). If the ANOVA result indicated a significant interaction, we proceeded to post hoc tests. Comparisons between each group were made using an unpaired Student’s t-test with Bonferroni corrections. All statistical tests were performed using the IBM SPSS Statistics software package (SPSS Japan Inc., an IBM company). Statistical tests with p < 0.05 were considered significant.
Results
Growth parameters, serum lipid concentration, and fat tissue weight
The final body weight, body weight gain, and food intake in rats fed the D diet were significantly lower than those in rats fed the C diet only in the Ovx group; a significant three-way interaction was observed (Table ). Dietary daidzein significantly decreased serum cholesterol and triglyceride concentrations in the female rats, regardless of whether they had undergone ovariectomy, although Ovx significantly increased serum triglyceride concentrations (Table ). Furthermore, Ovx significantly decreased the ovary fat weight, and increased the perirenal fat weight and total white fat weight, while dietary daidzein significantly reduced the perirenal fat weight and the total white fat weight (Table ). The mesentery fat weight in rats fed D diets was significantly lower than that in rats fed C diets only in the Ovx group; a significant interaction between dietary daidzein and the operation was observed (Table ).
Table 2. Effect of daidzein and genistein on body weight, body weight gain, and food intake and serum lipid concentration.Table Footnotea
Table 3. Effect of daidzein and genistein on total white fat (ovary, mesentery, perirenal fat).Table Footnotea
Liver lipid concentration
Along with lowering serum lipid levels, daidzein also decreased hepatic lipid concentrations (Table ). Total liver wet weight and phospholipid content in rats fed the D diet was significantly lower than that in rats fed the C diet only in the Ovx group; a significant interaction between dietary daidzein and the operation was observed (Table ). Ovx resulted in a significant increase in the total liver lipid content, hepatic triglyceride content, and hepatic total and esterified cholesterol contents, and a nonsignificant increase in free cholesterol content (main effect, p = 0.05). Dietary daidzein significantly decreased hepatic triglycerides content, and total and free cholesterol contents, regardless of whether the rats had undergone ovariectomy (Table ). On the other hand, genistein decreased the hepatic free cholesterol and increased the esterified cholesterol, regardless of ovariectomy status (Table ). Daidzein did not exhibit any apparent effect on liver esterified cholesterol concentration.
Table 4. Effect of daidzein and genistein on liver wet weight, total lipids, cholesterol and triglyceride concentration; fecal bile and lipid excretion.Table Footnotea
Fecal bile acid and lipid excretion
Dietary daidzein significantly increased the fecal bile acid excretion in both Ovx and non-Ovx rats. In contrast, dietary genistein significantly increased the fecal bile acid in Ovx rats only with operational effect (Table ). The amount of fecal lipid excretion was significantly higher in non-Ovx rats, whereas, the amount was significantly lower in the Ovx rats fed with dietary daidzein; we observed a three-way interaction (Table ).
Lipid metabolism and transportation-mediated gene expression in the liver and small intestine
The present study confirmed that dietary daidzein significantly reduces serum and hepatic lipid levels in both Ovx and non-Ovx female rats. To determine the underlying mechanism of the cholesterol lowering effect of daidzein, we investigated the expression of genes responsible for cholesterol metabolism in the liver and small intestine. We found that daidzein intake was not associated with any changes in the mRNA expression, except for the expression of ApoB mRNA. Daidzein intake decreased ApoB expression in the liver, regardless of whether the rats had undergone ovariectomy (Table ). However, genistein influenced some of the genes involved in cholesterol regulation. Dietary genistein intake tended to increase the ACAT2 mRNA expression (main effect, p = 0.05) and CYP7A1 mRNA expression (main effect, p = 0.07), and significantly upregulated the FXR mRNA expression in the liver (Table ). Dietary genistein significantly suppressed MTP mRNA expression in the upper intestine of Ovx rats; a significant interaction between dietary genistein intake and Ovx was observed (Table ). Genistein intake exhibited an operational effect by upregulating lower small intestinal FXR gene expression in non-Ovx rats (Table ).
Table 5. Effect of daidzein and genistein on the mRNA expression for lipid metabolism genes in the liver.Table Footnotea,Table Footnotef
Table 6. Effect of daidzein and genistein on the mRNA expression for lipid metabolism genes in the small intestinal mucosa.Table Footnotea,Table Footnoteg
All hepatic genes were relatively highly expressed in Ovx rats (Table ), whereas the expression of upper and lower small intestinal genes were relatively low in Ovx rats, except for the upper small intestinal FXR (Table ). Significant interactions were observed between dietary genistein and the operation in the upper small intestinal MTP and lower small intestinal MTP and FXR gene expression (Table ).
Discussion
Dietary daidzein, but not genistein, significantly reduced serum cholesterol in rats, regardless of ovariectomy status (Table ). Our previous experiments on isoflavone-rich FSBE showed that FSBE decreased serum cholesterol in Ovx and non-Ovx rats, but not in males.Citation17) Dietary daidzein decreased the food intake only in Ovx rats (Table ). We reported previously that the serum cholesterol concentration of rats pair-fed a diet containing FSBE was not significantly different from that in rats fed the control diet.Citation17) The decreased food intake in rats fed the D diet might not simply be associated with the hypocholesterolemic effect. Relative to daidzein, genistein reportedly has greater affinity for the estrogen receptors and stronger inhibitory effect on the activity of tyrosine kinase, which affects the regulatory capacity of lipids.Citation20,27) Takahashi et al. showed that genistein was more effective than daidzein at lowering serum triglycerides when administered to SD male rats at dietary doses of 1 or 2 g/kg.Citation28) However, concentrations of genistein in these literal data might be much higher than the nutritional levels used in the present study. Firstly, these could be approximately 5 or 10-fold higher than that of the AIN-93G-based diet that casein were replaced by soy protein isolate, which used as isoflavones source in many nutritional reports, as the sole protein source.Citation29) Secondly, these are much higher than the value estimated from Japanese consumption of soy isoflavones, which is estimated at very high level compared to other countries. For example, for daidzein, mean intake estimated from food-frequency questionnaires and dietary records was 18.3 mg/d and for genistein, that was 31.4 mg/d.Citation30) The daily total isoflavones to total nutrient intake (426.9 g/d) is recalculated as 43 and 74 mg/kg nutrient.
The hypocholesterolemic effect might be exhibited by the metabolite of daidzein, i.e. equol. Equol has higher binding affinity for ERα and antioxidant activity than genistein and daidzein.Citation21,31) In female rats fed a diet containing FSBE, the serum equol concentration was several fold higher than that of genistein both in Ovx and non-Ovx rats.Citation32) Furthermore, serum equol concentration was more than threefold higher than that of daidzein in non-Ovx rats fed a diet containing 150 mg/kg daidzein.Citation33) Unpublished results of experiments on Ovx and non-Ovx rats showed that the serum equol concentration was more than 2000 nmol/L in rats fed a diet containing daidzein, regardless of whether the rats had undergone ovariectomy. Thus, the stronger estrogenic and antioxidative activities and higher concentrations could be responsible for the physiological effects of equol compared to genistein in rats. In addition, dietary equol administration also reportedly reduced plasma total cholesterol in Ovx rats,Citation34) although the dose of equol seems to be too high (400 mg/kg diet) to explain the contribution of equol to hypocholesterolemic effect of daidzein in the present study.
The reduction of serum cholesterol is associated with stimulation of bile acid excretion and bile acid synthesis in the liver, catalyzed by the cholesterol 7-α hydroxylase (CYP7A1), the rate-limiting enzyme in the classical bile acid synthesis pathway.Citation35,36) In the present study, however, daidzein increased fecal bile excretion in both Ovx and non-Ovx rats but did not affect the hepatic mRNA for cytochrome P450 enzymes (CYP7A1, CYP27, and CYP8B1) and their promoter, FXR (Table ). Increased levels of bile acids decrease mRNA expression of HMG-CoA-R, the rate-limiting enzyme in cholesterol biosynthesis, and increase mRNA expression of LDLR.Citation36,37) However, daidzein did not affect the mRNA expression of HMG-CoA-R and LDLR either in the present study (Table ). Our previous study showed that dietary FSBE increased the hepatic LDLR gene expression in non-Ovx rats but not Ovx rats.Citation17) Other functional components of FSBE might be associated with an increased expression of LDLR mRNA. It is not clear whether increased hepatic LDLR gene expression contributed to hypocholesterolemic effect of daidzein. As such, further studies will be needed. The increased fecal bile excretion can also be correlated with the lower reabsorption rate of bile salts from the intestine back into the bloodstream for hepatic uptake, mediated by the IBAT. Ross-Viola et al. found that the IBAT mRNA levels were reduced by 55% in both liver and intestinal samples by feeding mice isoflavone at high and low doses.Citation16) In the present study, however, daidzein did not change the IBAT mRNA expression in the small intestine. In addition, we reported previously that dietary FSBE did not affect the fecal bile acid excretion, despite decreasing serum cholesterol concentration.Citation17) In the present study also, there was no significant correlation between the serum total cholesterol and fecal bile excretion (data not shown), suggesting that an increase in the fecal bile excretion caused by daidzein may not contribute to the decrease in cholesterol synthesis and clearance of serum cholesterol. As we did not analyze the fecal steroids of all feeding period (only 3 days), we could not fully discuss about the fecal steroids balance. Further studies are needed to determine whether the fecal bile excretion caused by daidzein contribute to the decreases in serum and hepatic cholesterol by examining the time-dependent changes in the fecal steroid levels, and the activity and gene expressions of enzymes and transporters involved in the regulation of whole body cholesterol balance.
Another potential mechanism of reducing serum and hepatic cholesterol would involve the decreasing ApoB mRNA expression in the liver.Citation38) The reduced ApoB secretion would reduce the assembly and secretion of VLDL in the liver, and ultimately lower the LDL cholesterol level. Clarkson et al. also showed that an increased serum LDL-cholesterol concentration in Ovx rats was due to increased VLDL assembly and secretion caused by increased lipoprotein lipase activity.Citation39) In the present study, daidzein significantly decreased ApoB mRNA expression (Table ), which may be linked to decreased serum cholesterol levels in both Ovx and non-Ovx female rats. However, we do not have evidence to support the hypothesis that ApoB is the main factor in reducing the serum and hepatic cholesterol levels.
On the other hand, genistein did not show any lowering effect on serum and hepatic lipid levels (Tables and ). However, genistein significantly decreased hepatic free cholesterol, and significantly increased esterified cholesterol, in both Ovx and non-Ovx female rats (Table ). These effects could be explained by an increased trend of hepatic ACAT2 gene expression in both Ovx and non-Ovx female rats (main effect, p = 0.05; Table ). ACAT-2 is a major cholesterol esterification enzyme and may control the amount of hepatic-free cholesterol available for secretion into bile acid pool or into the VLDL formation.Citation40,41) Dietary genistein significantly increased fecal bile acid excretion (Table ). Consistent with an increased fecal bile acid excretion, an increased trend by genistein intake was observed in hepatic CYP7A1 gene expression (main effect, p = 0.07; Table ). It may be due to the acceleration of the bile acid excretion and bile acid synthesis induced by genistein intake. However, dietary genistein did not alter serum and hepatic total cholesterol levels (Tables and ). Therefore, we speculated that the balance of steroids could be maintained by the acceleration in de novo synthesis of cholesterol. This speculation does not necessarily contradict the result that HMG-CoA-R gene expression was not changed by dietary genistein in this study, because regulation of this enzyme is achieved by not only transcriptional control, but also control of phosphorylation.Citation42) We consider that FXR gene expression might be up-regulated in response to an increase in bile acid levels.Citation43) The accelerating effect of genistein on bile acid synthesis, caused by the up-regulation of CYP7A1 gene expression, might surpass FXR responses.
In conclusion, dietary daidzein, but not genistein, reduced the serum and hepatic total cholesterol levels of Ovx and non-Ovx female rats. The decrease in serum cholesterol by daidzein could be explained by the decreasing effect of ApoB mRNA expression. However, we found no evidence to support this hypothesis. Genistein, on the other hand, significantly did not show any lowering effect on serum and hepatic lipid levels. The hypocholesterolemic effect might be exhibited by the metabolite of daidzein, equol, which has strong estrogenic and antioxidative activities and high bioavailability. Further investigation at the molecular level is required to understand the effects of dietary daidzein and equol on cholesterol regulation.
Author contribution
KB conducted the experiments, analyzed the results, and wrote the manuscript with the aids by SA. MF and TK contributed to analysis and interpretation of data, and assisted in the preparation of the manuscript. All the authors reviewed the results and approved the final version of the manuscript.
Funding
This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology, Japan, under a Monbukagakusho scholarship.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgement
We also thank the members of the Fujitani and Kishida groups for their helpful contributions in dissection.
References
- Lissin LW, Cooke JP. Phytoestrogens and cardiovascular health. J Am Coll Cardiol. 2000;35:1403–1410.10.1016/S0735-1097(00)00590-8
- Setchell KD. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy phytoestrogens. Am J Clin Nutr. 1998;68(6 suppl):1333S–1348S.
- Messina MJ. Legumes and soybeans: overview of their nutritional profiles and health effects. Am J Clin Nutr. 1999;70(3 suppl):439S–450S.
- F.l.h.c.s.p.a.c.h. Food and Drug Administration, HHS Final Rule. Fed Regist. 1999;64:57700–57733.
- Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995;333(5):276–282.10.1056/NEJM199508033330502
- Cederroth CR, Nef S. Soy, phytoestrogens and metabolism: A review. Mol Cell Endocrinol. 2009;304:30–42.10.1016/j.mce.2009.02.027
- Gu L, House SE, Prior RL, et al. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J Nutr. 2006;136(5):1215–1221.
- Legette LL, Lee WH, Martin BR, et al. Genistein, a phytoestrogen, improves total cholesterol, and Synergy, a prebiotic, improves calcium utilization, but there were no synergistic effects. Menopause. 2011;18(8):923–931.10.1097/gme.0b013e3182116e81
- Cassidy A, Bingham S, Setchell K. Biological effects of isoflavones in young women: importance of the chemical composition of soyabean products. Br J Nutr. 1995;74:587–601.10.1079/BJN19950160
- Kirk EA, Sutherland P, Wang SA, et al. Dietary isoflavones reduce plasma cholesterol and atherosclerosis in C57BL/6 mice but not LDL receptor-deficient mice. J Nutr. 1998;128:954–959.
- Pilšáková L, Riečanský I, Jagla F. The physiological actions of isoflavone phytoestrogens. Physiol Res. 2010;59:651–664.
- Anthony MS, Clarkson TB, Bullock BC, et al. Soy protein versus soy phytoestrogens in the prevention of diet-induced coronary artery atherosclerosis of male cynomolgus monkeys. Arteriosler Thromb Vasc Biol. 1997;17:2524–2531.10.1161/01.ATV.17.11.2524
- Kritchevsky D. Dietary protein, cholesterol and atherosclerosis: a review of the early history. J Nutr. 1995;125:589S–593S.
- Lucas EA, Khalil DA, Daggy BP, et al. Ethanol-extracted soy protein isolate does not modulate serum cholesterol in golden Syrian hamsters: a model of postmenopausal hypercholesterolemia. J Nutr. 2001;131:211–214.
- Takahashi Y, Ide T. Effects of soy protein in isoflavone on hepatic fatty acid synthesis and oxidation and mRNA expression of uncoupling proteins and peroxisome proliferator-activated receptor γ in adipose tissues of rats. J Nutr Biochem. 2008;19:682–693.10.1016/j.jnutbio.2007.09.003
- Ross-Viola JS, Mezei O, Welsh J, et al. Effects of low- and high- isoflavone soy protein-based diets on cholesterol metabolism and enterohepatic bile salt recirculation in mice. FASEB J. 2006;20:A854.
- Kishida T, Mizushige T, Nagamoto M, et al. Lowering effect of an isoflavone-rich fermented soybean extract on the serum cholesterol concentration in female rats with or without ovariectomy but not in male rats. Biosci Biotechnol Biochem. 2006;70(7):1547–1556.10.1271/bbb.50008
- Lee SO, Renouf M, Ye Z, et al. Isoflavone glycitein diminished plasma cholesterol in female golden syrian hamsters. J Agric Food Chem. 2007;55:11063–11067.10.1021/jf070972r
- Balmir F, Staack R, Jeffrey E, et al. An extract of soy flour influences serum cholesterol and thyroid hormones in rats and hamsters. J Nutr. 1996;126(12):3046–3053.
- Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139(10):4252–4263.10.1210/endo.139.10.6216
- Yuan JP, Wang JH, Liu X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora–implications for health. Mol Nutr Food Res. 2007;51:765–781.10.1002/(ISSN)1613-4133
- Ricketts ML, Moore DD, Banz WJ, et al. Molecular mechanisms of action of the soy isoflavones includes activation of promiscuous nuclear receptors. A review. J Nutr Biochem. 2005;16(6):321–330.10.1016/j.jnutbio.2004.11.008
- Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226(1):497–509.
- Sheltawy MJ, Losowsky MS. Determination of faecal bile acids by an enzymic method. Clin Chim Acta. 1975;64:127–132.10.1016/0009-8981(75)90194-1
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159.10.1016/0003-2697(87)90021-2
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:2002–2007.
- Geahlen RL, Koonchanok NM, McLaughlin JL, et al. Inhibition of protein-tyrosine kinase activity by flavanoids and related compounds. J Nat Prod. 1989;52(5):982–986.10.1021/np50065a011
- Takahashi Y, Odbayar TO, Ide TA. A comparative analysis of genistein and daidzein in affecting lipid metabolism in rat liver. J Clin Biochem Nutr. 2009;44(3):223–230.10.3164/jcbn.08-211
- Su Y, Shankar K, Simmen RC. Early soy exposure via maternal diet regulates rat mammary epithelial differentiation by paracrine signaling from stromal adipocytes. J Nutr. 2009;139(5):945–951.10.3945/jn.108.103820
- Yamamoto S, Sobue T, Sasaki S, et al. Validity and reproducibility of a self-administered food-frequency questionnaire to assess isoflavone intake in a Japanese population in comparison with dietary records and blood and urine isoflavones. J Nutr. 2001;131(10):2741–2747.
- Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol – a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132(12):3577–3584.
- Kishida T, Mizushige T, Ohtsu Y, et al. Dietary soy isoflavone-aglycone lowers food intake in female rats with and without ovariectomy. Obesity (Silver Spring). 2008;16(2):290–297.10.1038/oby.2007.68
- Fujitani M, Mizushige T, Bhattarai K, et al. Dynamics of appetite-mediated gene expression in daidzein-fed female rats in the meal-feeding method. Biosci Biotechnol Biochem. 2015;79(8):1342–1349.10.1080/09168451.2015.1025034
- Rachon D, Vortherms T, Seidlova-Wuttke D, et al. Effects of dietary equol on body weight gain, intra-abdominal fat accumulation, plasma lipids, and glucose tolerance in ovariectomized Sprague-Dawley rats. Menopause. 2007;14(5):925–932.10.1097/gme.0b013e31802d979b
- Lu TT, Makishima M, Repa JJ, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515.10.1016/S1097-2765(00)00050-2
- Pandak WM, Schwarz C, Hylemon PB, et al. Effects of CYP7A1 overexpression on cholesterol and bile acid homeostasis. Am J Physiol Gastrointest Liver Physiol. 2001;281(4):G878–G889.
- Bjorkhem I, Andersson U, Sudjama-Sugiaman E, et al. Studies on the link between HMG-CoA reductase and cholesterol 7 alpha-hydroxylase in lymph-fistula rats: evidence for both transcriptional and post-transcriptional mechanisms for down-regulation of the two enzymes by bile acids. J Lipid Res. 1993;34(9):1497–1503.
- Kang SK, Davis RA. Cholesterol and hepatic lipoprotein assembly and secretion. Biochim Biophys Acta. 2000;1529(1–3):223–230.10.1016/S1388-1981(00)00151-7
- Clarkson TB, Adams MR, Kaplan JR, et al. Psychosocial and reproductive influences on plasma lipids, lipoproteins and atherosclerosis in nonhuman primates. J Lipids Res. 1984;25:1629–1634.
- Lee RG, Shah R, Sawyer JK, et al. ACAT2 contributes cholesteryl esters to newly secreted VLDL, whereas LCAT adds cholesteryl esters to LDL in mice. J Lipid Res. 2005;46(6):1205–1212.10.1194/jlr.M500018-JLR200
- Thompson GR, Naoumova RP, Watts GF. Role of cholesterol in regulating apolipoprotein B secretion by the liver. J Lipid Res. 1996;37(3):439–447.
- Istvan ES, Palnitkar M, Buchanan SK, et al. Crystal structure of the catalytic portion of human HMG-CoA reductase: insights into regulation of activity and catalysis. EMBO J. 2000;19(5):819–830.10.1093/emboj/19.5.819
- Xu G, Pan LX, Li H, et al. Regulation of the farnesoid X receptor (FXR) by bile acid flux in rabbits. J Biol Chem. 2002;277(52):50491–50496.10.1074/jbc.M209176200
