Abstract
Bacterial cells possess a signal transduction system that differs from those described in higher organisms, including human cells. These so-called two-component signal transduction systems (TCSs) consist of a sensor (histidine kinase, HK) and a response regulator, and are involved in cellular functions, such as virulence, drug resistance, biofilm formation, cell wall synthesis, cell division. They are conserved in bacteria across all species. Although TCSs are often studied and characterized individually, they are assumed to interact with each other and form signal transduction networks within the cell. In this review, I focus on the formation of TCS networks via connectors. I also explore the possibility of using TCS inhibitors, especially HK inhibitors, as alternative antimicrobial agents.
Bacterial signal transduction and drug discovery.
Bacterial two-component signal transduction systems (TCS) play a major role in sensing environmental stresses and transducing the information inside cells to effect changes in gene expression. TCSs are composed of a histidine kinase (HK) sensor, residing in the inner membrane, and a response regulator (RR), located in the cytoplasm. In Escherichia coli, 29 HKs and 32 RRs were found by complete genome sequencing.Citation1) The HK sensor responds to individual environmental stresses by autophosphorylating itself, transferring the phosphoryl group at its histidine residue to the conserved aspartate residue of its cognate RR. Consequently, phosphorylated RRs regulate some genes involved in adaptation to environmental condition.Citation2)
In addition to each TCS responding to an individual environmental stress, complex regulatory networks between TCSs are assumed to exist, allowing bacteria to cope with a variety of environmental conditions.Citation3) The mRNA profiles of 36 TCS deletion mutants were examined by DNA microarray analysis.Citation4) Results indicated the existence of a network of functional interactions, or signal transductions, between different TCSs. These complex regulatory networks contribute to the fine-tuning of the adaptive abilities of the cell, which enhances survival.
In Chapter I, the signal transduction cascade between the EvgS (HK)/EvgA (RR) and PhoQ (HK)/PhoP (RR) TCSs are examined, and used as an example of the complex regulatory networks that exist in bacterial cells. Then, in Chapter II, I describe how drug discovery targeting such TCSs could provide alternatives to conventional antibiotics.
I. Bacterial signal transduction networks
I.i. PhoQ(HK)/PhoP(RR) TCS
The PhoQ/PhoP regulatory system monitors the availability of extracellular Mg2+ in Salmonella typhimurium,Citation5,6) playing a fundamental physiological role in the response to Mg2+ starvation. PhoQ (Mg2+ sensor) phosphorylates PhoP, with the phosphorylated PhoP activating the transcription of some 30 genes.Citation7,8) The PhoQ/PhoP TCS is also present in E. coli,Citation9,10) Shigella flexneri, Yersinia enterocolitica, and Yersinia pestis.Citation11)
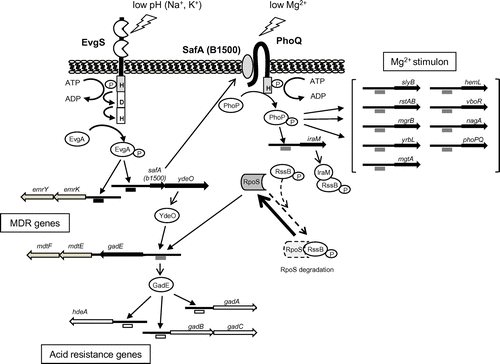
The E. coli PhoQ/PhoP system was identified as a promising model for studying ligand-induced signal transduction. First, extracellular Mg2+-responsive genes and promoters in E. coli, were confirmed to investigate their regulation by PhoQ/PhoP.Citation12) As a result, Mg2+-responsive promoters of phoPQ, mgtA, and mgrB were found. Expression of these genes was induced by Mg2+ limitation, and was dependent on PhoP and PhoQ. The transcription start sites were also determined by S1 nuclease analysis, and a (T/G)GTTTA (PhoP box) direct repeat was identified in the corresponding promoter regions of each of these genes.
To further investigate the PhoQ/PhoP regulatory network, we analyzed the genome-wide transcription profile of E. coli strain W3110 in the presence or absence of 30 mM MgCl2 (LB medium) using a DNA microarray.Citation13) The dependency of the cells on the PhoQ/PhoP TCS under Mg2+ starvation was also examined using E. coli mutants lacking PhoQ or PhoP. Consequently, 26 genes that contained PhoP box-like sequences, with (T/G)GTTTA direct repeats within 500 bp (upstream) of the initiation codon, were found. S1 nuclease assays of the 26 promoters verified six new Mg2+ stimulon genes, hemL, nagA, rstAB, slyB, vboR, and yrbL, in addition to the phoPQ, mgrB, and mgtA genes reported previously.Citation12) Gel shift and DNaseI footprinting assays confirmed that all of the genes were directly regulated by PhoP (Fig. ).
I.ii. EvgS (HK)/EvgA (RR) TCS
The EvgS/EvgA TCS is well-characterized in E. coli, and has a high degree of sequence similarity to the BvgS/BvgA TCS of Bordetella pertussis.Citation14) EvgS is a HK hybrid sensor, and is composed of an N-terminal periplasmic region and a C-terminal cytoplasmic region that is divided into four domains: linker, transmitter, receiver, and Hpt.Citation15) EvgS is an autophosphorylating kinase that responds to environmental stimuli and modulates EvgA by phosphorylation.Citation16,17) The periplasmic domain of EvgS is involved in signal recognition, ultimately transducing the signal into a transcriptional network via a phosphorylation cascade. We attempted to identify signals involved in the activation of EvgS and found that a high concentration of alkali metals (Na+, K+), in addition to low pH, was essential for activation.Citation18)
To analyze the genes that are regulated by EvgS/EvgA, a spontaneous E. coli mutant (evgS1), which has a constitutively active EvgS, was used. Initial results showed that transcription of emrKY, a multi-drug efflux operon, was positively regulated by the EvgS/EvgA TCS.Citation19) We then examined transcriptional levels of multi-drug efflux genes in the EvgS1 constitutive mutant using an E. coli DNA microarray.Citation20) Five genes and operons, including emrKY, mdtEF (yhiUV), acrAB, mdfA, and tolC, showed increased expression in the presence of the EvgS1 mutant. This was supported by the fact that the EvgS1 mutant, which lacked the major multi-drug efflux pump gene acrA, became multi-drug resistant (MDR).Citation20)
In addition to the upregulation of multi-drug efflux genes, activation of EvgS also upregulated several acid resistance-related genes to cause acid resistance in exponential grown cells.Citation17,21) Activation of the EvgS/EvgA TCS initiates a transcriptional cascade of acid resistance genes that encode the regulators EvgA and YdeO, along with the central regulator of acid resistance genes, GadE (Fig. ).Citation17,21) Interestingly, the EvgS/EvgA-regulated multi-drug efflux genes mdtE and mdtF form an operon with gadE. These results indicated that by activation of the EvgS/EvgA TCS, the MDR phenotype as well as acid resistance occur concurrently through a regulatory network containing multiple genes (Fig. ).
I.iii. Signal transduction cascade between EvgS/EvgA and PhoQ/PhoP
In the afore-mentioned microarray analysis of the evgS1 mutant,Citation20) 225 open-reading frames (ORFs) showed significant increases in transcription. To identify TCSs that interact with EvgS/EvgA, we selected genes that were upregulated by other TCSs,Citation4) and matched them with the genes that were upregulated in response to the activation of the EvgS sensor.Citation22) Expression of 13 of the 27 PhoQ/PhoP upregulated genes was enhanced in the evgS1 mutant, clearly indicating interaction between the EvgS/EvgA and PhoQ/PhoP TCSs. Of these 13 genes, nine (hemL, mgtA, phoP, phoQ, rstA, rstB, slyB, yrbL, and mgrB) were previously reported as belonging to the PhoQ-/PhoP-dependent Mg2+ stimulon (Fig. ).Citation13)
The promoter regions of 13 genes were analyzed by S1 mapping. Enhanced expression of all 13 genes in the evgS1 mutant was confirmed, validating the microarray data.Citation20) Therefore, this EvgS-/EvgA-enhanced gene expression was clearly PhoQ-/PhoP-dependent. This regulatory network between the two TCSs was also initiated by overproduction of the EvgA regulator. These results demonstrated the existence of signal transduction from the EvgS/EvgA TCS to the PhoQ/PhoP TCS via a connector.Citation23)
I.iv. Bacterial signal transduction network via connectors
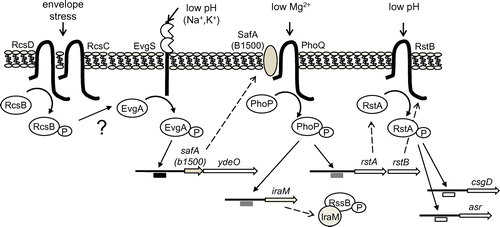
A small protein (65aa, B1500) that connects the EvgS/EvgA and PhoQ/PhoP TCSs was first found using a shotgun screening approach.Citation24) Expression of b1500 is directly regulated by the EvgS/EvgA TCS. B1500 is localized in the inner membrane, and bacterial two-hybrid data showed that it formed a complex with the sensor PhoQ. Because B1500 connected the signal transduction between the EvgS/EvgA and PhoQ/PhoP TCSs by directly interacting with PhoQ, thus, activating the PhoQ/PhoP TCS, it was named sensor-associating factor A (SafA).Citation17,24) PmrD also connected the signal transduction between PhoQ/PhoP and PmrB/PmrA TCS in Salmonella.Citation25) However, while, PmrD resides in the cytoplasm and interacts with the RR PmrA, SafA is located in the inner membrane and interacts with the sensor PhoQ at the periplasm.
We then investigated how SafA interacts with PhoQ.Citation26) Bacterial two-hybrid and reporter assays revealed that the C-terminal region (aa 41–65) of SafA-activated PhoQ at the periplasm. Adding synthetic SafA (aa 41–65) peptide to the cell culture also activated the PhoQ/PhoP TCS. Direct interaction between the C-terminal region of SafA and the sensor domain of PhoQ was observed by means of surface plasmon resonance and nuclear magnetic resonance (NMR) spectroscopy.
The SafA-activated PhoQ/PhoP system is also involved in the acid resistance induced by the EvgS/EvgA system.Citation27) We clarified the following mechanism of how SafA induces the acid resistance via PhoQ/PhoP TCS. EvgS/EvgA activation resulted in the accumulation of cellular RpoS in exponential-phase cells in a SafA-, PhoQ-, and PhoP-dependent manner.Citation27) This RpoS accumulation was caused by another connector, IraM (107 aa), regulated by PhoQ/PhoP.Citation28) Thus, the connector SafA produced by activation of the EvgS/EvgA TCS activates the PhoQ/PhoP TCS to induce another connector, IraM, which binds and inactivates the RR RssB.Citation27) As a result, RpoS is not degraded by the ClpXP protease and accumulates within the cell to regulate acid resistance gene (Fig. ). Indeed, in EvgS-activated exponential phase cells (unpublished result), we found the expression of 25 of the 65 previously reported RpoS upregulated genes.Citation29)
Finally, we have proposed a complex network of acid resistance gene regulation, initiated by the EvgS/EvgA system (Fig. ). The EvgA-YdeO-GadE cascade of acid resistance gene regulation is induced by EvgS/EvgA, and a branched pathway operating via SafA-PhoQ/PhoP-IraM-RssB-RpoS is also involved in the acid resistance phenotype.
The PhoQ/PhoP system also controls the acid-responsive RstB/RstA TCS by directly binding of PhoP to the promoter of the rstAB operon,Citation13,30) adding another branch to the network. A RR, RcsB, part of the RcsC/RcsD/RcsB system, also participates in this network (Fig. ). RcsB is essential for survival during extreme acid challenge during stationary phase, and regulates transcription of acid resistance genes by forming a heterodimer with GadE.Citation31) Furthermore, acid resistance in the exponential phase is regulated by RcsB, which acts on promoters that are regulated by the EvgS/EvgA TCS, suggesting a signal transduction between RcsC/RcsD/RcsB and EvgS/EvgA.Citation32)
II. Drug discovery targeting TCSs
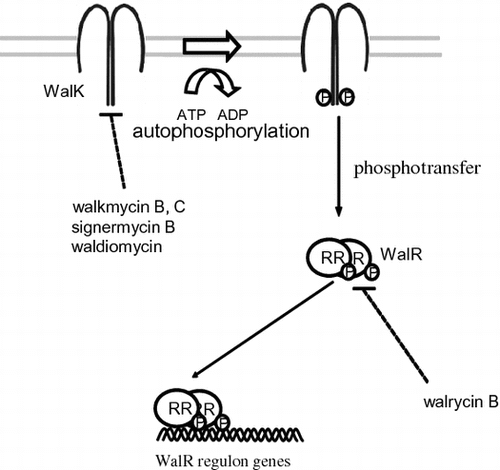
TCSs are attractive targets for alternative antibiotics because although many HK and RR genes are present in bacterial genomes, none have been identified in mammalian genomes.Citation33) In addition, some TCSs are essential for bacterial viability, such as CckA/CtrA in Caulobacter crescentus, MtrB/MtrA in Mycobacterium tuberculosis, HP166/HP165 in Helicobacter pylori, and WalK/WalR (aka YycG/YycF) in Bacillus subtilis, Enterococcus faecalis, Streptococcus pneumoniae, and Staphylococcus aureus. WalK/WalR has previously been considered as a novel target for antibacterial agents against MDR bacteria, including methicillin-resistant S. aureus (MRSA) and vancomycin-resistant E. faecalis (VRE). Here, we introduce novel drug discovery systems to isolate inhibitors of the WalK/WalR TCS, an essential TCS for bacterial growth, in an attempt to develop a new class of antibacterial agents.
II.i. WalK (YycG; HK)/WalR (YycF; RR) TCS
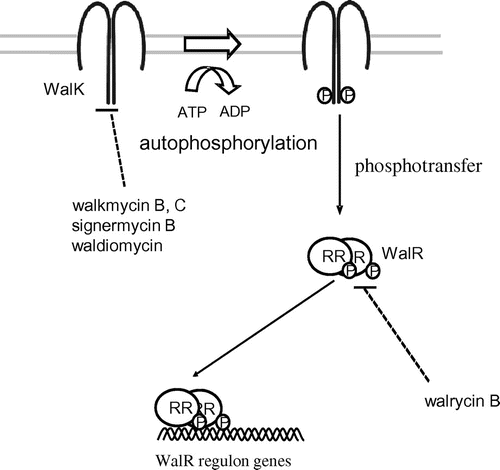
WalK is a membrane-linked HK that contains a conserved catalytic (ATP-binding) domain and dimerization domain in the cytoplasmic C-terminal region.Citation34) WalK cytoplasmic domains have been purified, and results of in vitro studies show that they are autophosphorylated prior to phosphorylating their cognate RR, WalR.Citation35) In vivo, using a bacterial two-hybrid system, we showed interactions between WalK/WalK, as well as WalR/WalR.Citation36) In addition, a solution-state NMR study described dimerization of the homodimeric core domain of EnvZ, which belongs to the same Pho subfamily of HKs as WalK.Citation37) Dimerization appears to be essential for WalK autophosphorylation. In fact, a new antibiotic signermycin B inhibited autophosphorylation as well as WalK dimerization by binding to the WalK dimerization domain, thereby hindering WalK/WalR signal transduction involved in cell growth and division.Citation38)
WalR is a typical RR, with an N-terminal receiver domain and a C-terminal DNA-binding domain. The receiver domain contains a conserved aspartic acid residue that serves as the phosphorylation site. The C-terminal domains of WalR from B. subtilis and S. aureus are similar to that of the PhoB RR from E. coli, and contain a winged helix-turn-helix motif for DNA binding. Both WalRs form a dimer to bind to a DNA sequence consisting of two hexanucleotide direct repeats separated by five nucleotides.Citation34,39,40)
In B. subtilis, the WalK/WalR TCS activates expression of yocH, yvcE, and lytE, which encode autolysins, and ydjM, which is predicted to encode a cell wall-associated protein.Citation34,41,42) In addition, WalK/WalR negatively regulates the expression of yoeB and yjeA, which encode proteins that modulate autolysin activity.Citation34,41) In S. aureus, the regulation of nine cell wall metabolism genes is WalK-/WalR-dependent.Citation34,43) These studies have revealed that the WalK/WalR TCS functions as a master regulatory system for cell wall metabolism.
II.ii. Walkmycin
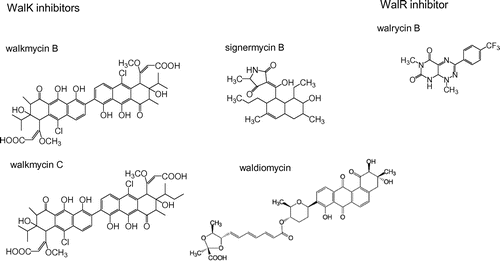
After screening Streptomyces sp. culture broths using a differential growth assay,Citation44) walkmycin A, B, and C, which were produced by strain MK632-100F11, were identified as WalK inhibitors.Citation45) The chemical structures of walkmycin B and C were determined to match that of di-anthracenone (C44H44Cl2O14) (Fig. ). The minimum inhibitory concentrations (MICs) of walkmycin B against B. subtilis and S. aureus were 0.39 and 0.20 μg/ml, and the half maximal inhibitory concentration (IC50) measurements against WalK were 1.6 and 5.7 μM, respectively. Surface plasmon resonance analysis revealed an equilibrium dissociation constant, KD, of 7.63 μM at the higher affinity site of B. subtilis WalK. These results suggest that walkmycin B inhibits WalK autophosphorylation by binding to the WalK cytoplasmic domain, which has a bactericidal effect.
TCSs often regulate gene clusters that contribute to pathogenicity, and thus, they are frequently proposed as potential drug targets for attenuating the virulence of pathogens.Citation46) We evaluated the effects of walkmycin C on two major virulence factors of Streptococcus mutans: biofilm formation and acid tolerance.Citation47) When walkmycin C was used at sub-MIC levels, the cells formed abnormal biofilms, and an increase in acid sensitivity was observed. These results show that walkmycin C represses two virulence-associated phenotypes of S. mutans, indicating the possibility of developing HK inhibitors into anti-virulence drugs.
II.iii. Signermycin B
While, using the differential growth assay to screen for WalK inhibitors from natural sources,Citation44,45) significant bactericidal activity was detected in broth cultures of Streptomyces sp. strain MK851-mF8. Isolation and characterization of the active compound revealed that inhibitory activity was derived from a novel antibiotic, which we named signermycin B.Citation38) The structure of signermycin B, consisting of a tetramic acid moiety and decalin ring, was determined by mass spectrometry, NMR, and X-ray crystallography (Fig. ).
Signermycin B exhibited antimicrobial activity against Gram-positive bacteria that contain the WalK/WalR TCS, including MRSA and VRE, with MIC values ranging from 3.13 μg/ml to 6.25 μg/ml. The IC50s of signermycin B against WalK in these organisms ranged from 37 to 62 μM. However, signermycin B was ineffective against all tested Gram-negative bacteria that did not contain TCSs that control essential genes. Signermycin B also repressed expression of the WalR regulon, thereby inhibiting cell division. These phenotypes were consistent with those of strains missing the WalK/WalR system
Furthermore, we examined the binding affinity of signermycin B to the dimerization and ATP-binding domains of WalK using surface plasmon resonance. Signermycin B bound to the dimerization domain but not ATP-binding domain of WalK. Several other HK inhibitors were identified, but most caused protein aggregation and disrupted the membrane integrity of bateria.Citation48–50) Signermycin B is the first example of an antibiotic that binds to the WalK dimerization domain to inhibit autophosphorylation.
II.iv. Waldiomycin
The discovery of walkmycin and signermycin B, targeting WalK, suggested that the screening method can effectively isolate WalK inhibitors produced by Streptomyces sp. Therefore, we continued to screen metabolites from Streptomyces sp., focusing on the discovery of a new antibiotic. We identified a novel WalK inhibitor from a soil sample collected at Shiogama, Miyagi, Japan, designated waldiomycin. Waldiomycin belongs to the family of angucycline antibiotics, and is structurally related to dioxamycin (Fig. ).Citation51) Waldiomycin inhibits WalK from S. aureus and B. subtilis at IC50s of 8.8 and 10.2 μM, respectively, and shows antibacterial activity against MRSA and B. subtilis, with MICs ranging from 4 to 8 μg/ml.Citation51,52)
This compound also inhibited various class I HKs other than WalK and directly bound to the conserved H-box and X-region, which are the two conserved regions in the dimerization domain of the EnvZ HK.Citation53) These results suggested that waldiomycin inhibited the phosphorylation of the conserved histidine in the H-box by binding adjacent to the phosphorylation site.
II.v. Walrycin
We developed a high-throughput screening system for WalR inhibitors,Citation44,54,55) and found a novel inhibitor targeting the WalR response regulator, walrycin B (Fig. ).Citation55) Walrycin B has MICs of 0.39 μg/ml for B. subtilis and 1.56 μg/ml for MRSA, but not effective against E. coli which lacks the WalK/WalR TCS. Overproduction of WalR in either B. subtilis or S. aureus correspondingly reduces bacterial sensitivity to walrycin B. Furthermore, treatment with walrycin B leads to lowered expression of WalR regulon genes and killed cells by inhibiting cell division, leading to phenotypes consistent with those of cells starved for the WalK/WalR TCS. Indeed, B. subtilis cells form extremely long aseptate filaments and S. aureus cells form large aggregates in the presence of walrycin B.
III. Conclusions
The studies reviewed here, describe signal transduction cascades between TCSs in E. coli and, together, strongly suggest the existence of a TCS network. We have found that the small membrane protein SafA connects the signal transduction between the EvgS/EvgA and PhoQ/PhoP systems by directly interacting with PhoQ, thus, activating the PhoQ/PhoP TCS. Activation of PhoQ/PhoP enhanced the acid resistance phenotype conferred by the transcriptional cascade of acid resistance genes induced by EvgS/EvgA (Figs. and ).
For drug discovery, bacterial signal transduction pathways are considered as important as the signal transduction pathways in animal cells. In particular, the WalK/WalR TCS is an attractive target for antibacterial agents against MRSA and VRE. We have developed a novel drug discovery system to isolate inhibitors of the WalK/WalR system in an attempt to develop a new class of antibacterial agents (walkmycin B and C, signermycin B, waldiomycin, and walrycin B) (Figs. and ).
Disclosure statement
No potential conflict of interest was reported by the author.
Acknowledgments
I am grateful to Professors Emeritus Tohru Komano (Kyoto University), the late Manjiro Noda (Kindai University), and Masayori Inouye (UMDNJ) for their continuous guidance and encouragement. I would also like to thank Professors Michio Himeno, Hiroshi Sakai, Junji Morita, Makoto Kawamukai, and Norihiko Misawa, and Drs. Yoko Eguchi, Toshihide Okajima and Masayuki Igarashi for their encouragement and collaboration. I would further like to express our appreciation to all of the members of our research group.
Notes
This review was written in response to the author’s receipt of the JSBBA Award in 2017.
Abbreviations: HK, histidine kinase; RR, response regulator; TCS, two-component signal transduction system; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus faecalis; MIC, minimum inhibitory concentration; IC50, half maximal inhibitory concentration; MDR, multi-drug resistant; NMR, nuclear magnetic resonance; ORFs, open reading frames.
References
- Mizuno T. Compilation of all genes encoding two-component phosphotransfer signal transducers in the genome of Escherichia coli. DNA Res. 1997;4:161–168.10.1093/dnares/4.2.161
- Gao R, Stock AM. Biological insights from structures of two-component proteins. Ann Rev Microbiol. 2009;63:133–154.10.1146/annurev.micro.091208.073214
- Eguchi Y, Utsumi R. Introduction to bacterial signal transduction networks. In: Utsumi R, editor. Bacterial signal transduction: networks and drug targets. New York (NY): Springer; 2008. p. 1–6.
- Oshima T, Aiba H, Masuda Y, et al. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol Microbiol. 2002;46:281–291.10.1046/j.1365-2958.2002.03170.x
- Vescovi EG, Soncini FC, Groisman EA. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174.10.1016/S0092-8674(00)81003-X
- Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001;183:1835–1842.10.1128/JB.183.6.1835-1842.2001
- Soncini FC, Vescovi EG, Groisman EA. Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J Bacteriol. 1995;177:4364–4371.10.1128/jb.177.15.4364-4371.1995
- Soncini FC, Vescovi EG, Solomon F, et al. Molecular basis of the magnesium deprivation response in Salmonella typhimurium: identification of PhoP-regulated genes. J Bacteriol. 1996;178:5092–5099.10.1128/jb.178.17.5092-5099.1996
- Groisman EA, Heffron F, Solomon F. Molecular genetic analysis of the Escherichia coli phoP locus. J Bacteriol. 1992;174:486–491.10.1128/jb.174.2.486-491.1992
- Kasahara M, Nakata A, Shinagawa H. Molecular analysis of the Escherichia coli phoP-phoQ operon. J Bacteriol. 1992;174:492–498.10.1128/jb.174.2.492-498.1992
- Groisman EA, Heffron F. Regulation of Salmonella virulence by two-component regulatory systems. In: Hoch JA, Silhavy TJ, editors. Two-component signal transduction. Washington (DC): ASM Press; 1995. p. 319–332.10.1128/9781555818319
- Kato A, Tanabe H, Utsumi R. Molecular characterization of the PhoP-PhoQ two-component system in Escherichia coli K-12: identification of extracellular Mg2+-responsive promoters. J Bacteriol. 1999;181:5516–5520.
- Minagawa S, Ogasawara H, Kato A, et al. Identification and molecular characterization of the Mg2+ stimulon of Escherichia coli. J Bacteriol. 2003;185:3696–3702.10.1128/JB.185.13.3696-3702.2003
- Utsumi R, Katayama S, Taniguchi M, et al. Newly identified genes involved in the signal transduction of Esherichia coli K-12. Gene. 1994;140:73–77.10.1016/0378-1119(94)90733-1
- Perraud AL, Weiss V, Gross R. Signaling pathways in two-component phosphorelay systems. Trends Microbiol. 1999;7:115–120.10.1016/S0966-842X(99)01458-4
- Utsumi R, Kawamoto K, Yamazaki K, et al. Characterization of the signal transduction via EvgS and EvgA in Escherichia coli. J Gen Appl Microbiol. 1996;42:155–162.10.2323/jgam.42.155
- Itou J, Eguchi Y, Utsumi R. Molecular mechanism of transcriptional cascade initiated by the EvgS/EvgA system in Escherichia coli K-12. Biosci Biotechnol Biochem. 2009;73:870–878.10.1271/bbb.80795
- Eguchi Y, Utsumi R. Alkali metals in addition to acidic pH activate the EvgS histidine kinase sensor in Escherichia coli. J Bacteriol. 2014;196:3140–3149.10.1128/JB.01742-14
- Kato A, Ohnishi H, Yamamoto K, et al. Transcription of emrKY is regulated by the EvgA-EvgS two-component system in Escherichia coli K-12. Biosci Biotechnol Biochem. 2000;64:1203–1209.10.1271/bbb.64.1203
- Eguchi Y, Oshima T, Mori H, et al. Transcriptional regulation of drug efflux genes by EvgAS, a two-component system in Escherichia coli. Microbiology. 2003;149:2819–2828.10.1099/mic.0.26460-0
- Masuda N, Church GM. Regulation network of acid resistance genes in Escherichia coli. Mol Microbiol. 2003;48:699–712.10.1046/j.1365-2958.2003.03477.x
- Eguchi Y, Okada T, Minagawa S, et al. Signal transduction cascade between EvgA/EvgS and PhoP/PhoQ two-component systems of Escherichia coli. J Bacteriol. 2004;186:3006–3014.10.1128/JB.186.10.3006-3014.2004
- Mitrophanov AY, Groisman EA. Signal integration in bacterial two-component regulatory systems. Genes Dev. 2008;22:2601–2611.10.1101/gad.1700308
- Eguchi Y, Itou J, Yamane M, et al. B1500, a small membrane protein,connects the two-component systems EvgS/EvgA and PhoQ/PhoP in Escherichia coli. Proc Natl Acad Sci USA. 2007;104:18712–18717.
- Kato A, Groisman EA. Connecting two-component regulatory systems by a protein that protects a response regulator from dephosphorylation by its cognate sensor. Genes Dev. 2004;18:2302–2313.10.1101/gad.1230804
- Eguchi Y, Ishii E, Yamane M, et al. The connector SafA interacts with the multi-sensing domain of PhoQ in Escherichia coli. Mol Microbiol. 2012;85:299–313.10.1111/mmi.2012.85.issue-2
- Eguchi Y, Ishii E, Hata K, et al. Regulation of acid resistance by connectors of two-component signal transduction systems in Escherichia coli. J Bacteriol. 2011;193:1222–1228.10.1128/JB.01124-10
- Bougdour A, Cunning C, Baptiste PJ, et al. Multiple pathways for regulation of sigmaS (RpoS) stability in Escherichia coli via the action of multiple anti-adaptors. Mol Microbiol. 2008;68:298–313.10.1111/mmi.2008.68.issue-2
- Loewen PC, Hu B, Strutinsky J, et al. Regulation in the RpoS regulon of Escherichia coli. Can J Microbiol. 1998;44:707–717.10.1139/cjm-44-8-707
- Ogasawara H, Hasegawa A, Kanda E, et al. Genomic SELEX search for target promoters under the control of the PhoQP-RstBA signal relay cascade. J Bacteriol. 2007;189:4791–4799.10.1128/JB.00319-07
- Castanie-Cornet MP, Cam K, Bastiat B, et al. Acid stress response in Escherichia coli: mechanism of regulation of gadA transcription by RcsB and GadE. Nucleic Acids Res. 2010;38:3546–3554.10.1093/nar/gkq097
- Johnson MD, Burton NA, Gutierrez B, et al. RcsB Is required for inducible acid Resistance in Escherichia coli and acts at gadE-dependent and -independent promoters. J Bacteriol. 2011;193:3653–3656.10.1128/JB.05040-11
- Watanabe T, Okada A, Gotoh Y, et al. Inhibitors targeting two-component signal transduction. In: Utsumi R, editor. Bacterial signal transduction: networks and drug targets. New York (NY): Springer; 2008. p. 229–236.10.1007/978-0-387-78885-2
- Dubrac S, Bisicchia P, Devine KM, et al. A matter of life and death: cell wall homeostasis and the WalKR (YycGF) essential signal transduction pathway. Mol Microbiol. 2008;70:1307–1322.10.1111/mmi.2008.70.issue-6
- Yamamoto K, Kitayama T, Minagawa S, et al. Antibacterial agents that inhibit histidine protein kinase YycG of Bacillus subtilis. Biosci Biotechnol Biochem. 2001;65:2306–2310.10.1271/bbb.65.2306
- Watanabe T, Hashimoto Y, Umemoto Y, et al. Molecular characterization of the essential response regulator protein YycF in Bacillus subtilis. J Mol Microbiol Biotechnol. 2003;6:155–163.
- Tomomori C, Tanaka T, Dutta R, et al. Solution structure of the homodimeric core domain of Escherichia coli histidine kinase EnvZ. Nat Struct Biol. 1999;6:729–734.
- Watanabe T, Igarashi M, Okajima T, et al. Isolation and characterization of signermycin B, an antibiotic that targets the dimerization domain of histidine kinase WalK. Antimicrob Agents Chemother. 2012;56:3657–3663.10.1128/AAC.06467-11
- Okajima T, Doi A, Okada A, et al. Response regulator YycF essential for bacterial growth: X-ray crystal structure of the DNA-binding domain and its PhoB-like DNA recognition motif. FEBS Lett. 2008;582:3434–3438.10.1016/j.febslet.2008.09.007
- Doi A, Okajima T, Gotoh Y, et al. X-ray crystal structure of the dna-binding domain of response regulator WalR essential to the cell viability of Staphylococcus aureus and interaction with target DNA. Biosci Biotechnol Biochem. 2010;74:1901–1907.10.1271/bbb.100307
- Bisicchia P, Noone D, Lioliou E, et al. The essential YycFG two-component system controls cell wall metabolism in Bacillus subtilis. Mol Microbiol. 2007;65:180–200.10.1111/mmi.2007.65.issue-1
- Howell A, Dubrac S, Andersen KK, et al. Genes controlled by the essential YycG/YycF two-component system of Bacillus subtilis revealed through a novel hybrid regulator approach. Mol Microbiol. 2003;49:1639–1655.10.1046/j.1365-2958.2003.03661.x
- Dubrac S, Boneca IG, Poupel O, et al. New insights into the WalK/WalR (YycG/YycF) essential signal transduction pathway reveal a major role in controlling cell wall metabolism and biofilm formation in Stapylococcus aureus. J Bacteriol. 2007;189:8257–8269.10.1128/JB.00645-07
- Okada A, Gotoh Y, Watanabe T, et al. Targeting two-component signal transduction: a novel drug discovery system. Methods Enzymol. 2007;422:386–395.10.1016/S0076-6879(06)22019-6
- Okada A, Igarashi M, Okajima T, et al. Walkmycin B targets WalK (YycG), a histidine kinase essential for bacterial cell growth. J Antibiot. 2010;63:89–94.10.1038/ja.2009.128
- Gotoh Y, Eguchi Y, Watanabe T, et al. Two-component signal transduction as potential drug targets in pathogenic bacteria. Curr Opin Microbiol. 2010;13:232–239.10.1016/j.mib.2010.01.008
- Eguchi Y, Kubo N, Matsunaga H, et al. Development of an antivirulence drug against streptococcus mutans: repression of biofilm formation, acid tolerance, and competence by a histidine kinase inhibitor, walkmycin C. Antimicrob Agents Chemother. 2011;55:1475–1484.10.1128/AAC.01646-10
- Hilliard JJ, Goldschmidt RM, Licata L, et al. Multiple mechanisms of action for inhibitors of histidine protein kinases from bacterial two-component systems. Antimicrob Agents Chemother. 1999;43:1693–1699.
- Macielag MJ, Goldschmidt R. Inhibitors of bacterial two-component signaling systems. Expert Opin Invest Drugs. 2000;9:2351–2369.10.1517/13543784.9.10.2351
- Stephenson K, Hoch JA. Developing inhibitors to selectively target two-component and phosphorelay signal transduction systems of pathogenic microorganisms. Curr Med Chem. 2004;11:765–773.10.2174/0929867043455765
- Igarashi M, Watanabe T, Hashida T, et al. Waldiomycin, a novel WalK-histidine kinase inhibitor from Streptomyces sp. MK844-mF10. J Antibiot. 2013;66:459–464.10.1038/ja.2013.33
- Fakhruzzaman M, Inukai Y, Yanagida Y, et al. Study on in vivo effects of bacterial histidine kinase inhibitor, Waldiomycin, in Bacillus subtilis and Staphylococcus aureus. J Gen Appl Microbiol. 2015;61:177–184.10.2323/jgam.61.177
- Eguchi Y, Okajima T, Tochio N, et al. Angucycline antibiotic waldiomycin recognizes common structural motif conserved in bacterial histidine kinases. J Antibiot. 2017;70:251–258.10.1038/ja.2016.151
- Furuta E, Yamamoto K, Tatebe D, et al. Targeting protein homodimerization: a novel drug discovery system. FEBS Lett. 2005;579:2065–2070.10.1016/j.febslet.2005.02.056
- Gotoh Y, Doi A, Furuta E, et al. Novel antibacterial compounds specifically targeting the essential WalR response regulator. J Antibiot. 2010;63:127–134.10.1038/ja.2010.4