Abstract
Oxidative stress may cause a wide variety of free radical reactions to produce deleterious modifications in membranes, proteins, enzymes, and DNA. Reactive Oxygen Species (ROS) generated by myeloperoxidase (MPO) can induce lipid peroxidation and also play an important role in the generation of reactive chlorinating and brominating species. As the universal biomarkers, chemical, and immunochemical approach on oxidatively modified and halogenated tyrosines has been carried out. As amido-type adduct biomarkers, chemical, and immunochemical evaluation of hexanoyl- and propanoyl-lysines, hexanoyl- and propanoyl-dopamines and phospholipids were prepared and developed for application of evaluation of novel antioxidative functional food factors. We have also involved in application of oxidatively modified DNAs such as 8-hydroxy- and 8-halogenated deoxyguanosines as the useful biomarkers for age-related diseases using both in vitro and in vivo systems. Application of these oxidative stress biomarkers for novel type of functional food development and recent approach for development of novel evaluation systems are also discussed.
Graphical abstract
Establishment of oxidative stress biomarkers for evaluation on protective role of evidence-based functional foods are the most important approach both in academic and industrial fields.
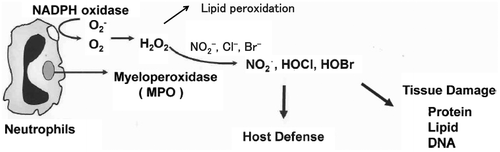
ROS generated by phagocytic white blood cells are critical to host defense because they kill invading pathogens [Citation1], however, they are also potentially dangerous because they may damage tissues at sites of inflammation. The heme enzyme myeloperoxidase (MPO), synthesized and secreted by neutrophils and monocytic cells, is an important source of ROS. It uses hydrogen peroxide (H2O2) generated by the phagocyte NADPH oxidase and induces lipid peroxidation [Citation2] and also converts halogen ion to produce potent cytotoxin hypochlorous acid (HOCl) and/or hypobromous acid (HOBr) [Citation3]. These active halogenating species modify various biomolecules such as nucleic acids, proteins, phospholipids, and other biomolecules.
Oxidative stress and inflammation reaction
Inflammation, to a greater or lesser degree, is a frequent event in the human body. Among the immune cells, neutrophils are some of the most important cells in first defense against a microbial invasion, and has granules containing peroxidases, such as MPO. One of these reactive species is known as superoxide radical (), which is released as the respiratory burst and is converted to H2O2 by spontaneous disproportionation reactions. MPO is a heme protein secreted by phagocytic white blood cells. Its physiological function is to utilize the H2O2 produced by membrane-associated NADPH oxidase to induce lipid peroxidation at a site of inflammation [Citation2]. MPO can also catalyze the formation of hypohalous acids such as HOCl and/or HOBr, using H2O2 and Br−/Cl− [Citation3]. The ROS and reactive halogenating species modify various biomolecules such as nucleic acids, proteins, and lipids (Figure ). By use of MPO knockout mice, it has already been shown that these peroxidases have an important role in the generation of reactive chlorinating and brominating species in vivo [Citation4]. From this background, our research group started chemical and immunochemical research on modification of the protein by the reactive intermediates causes protein tyrosine halogenation.
Modified tyrosine by oxidative stress as biomarkers
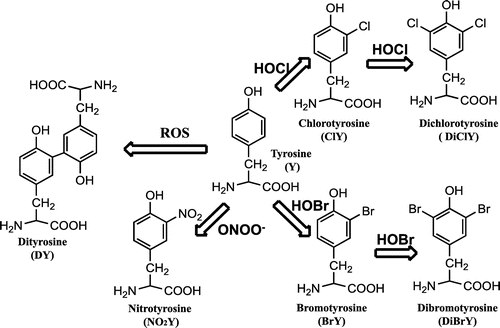
Neutrophils are some of the most important cells for the first defense system against a microbial invasion, and has granules containing peroxidases such as MPO. Lipid peroxidation was induced by ROS generated fromMPO reaction through NADPH oxidation activation. And also MPO catalyze the formation of HOCl and/or HOBr using H2O2 and Br−/Cl−. Neutrophil MPO can catalyze dityrosine (DY) formation in the presence of H2O2. DY can also be generated by metal-catalyzed oxidation [Citation5], suggesting that the DY may become one of universal protein oxidation markers. We have immunochemically detected DY in lipofuscin of pyramidal neuron of aged human brains [Citation6], and the atherosclerotic lesion of apoE knockout mice [Citation7]. The active halogenating species can modify proteins. The modification of protein by the reactive intermediates causes protein tyrosine halogenation forming chlorotyrosine (ClY) or bromotyrosine (BrY).
During halogenation, the incorporation of two halogens into one tyrosine moiety also occurs and, as a consequence, 3,5-dichlorotyrosine (DiClY) and/or 3,5-dibromotyrosine (DiBrY) are generated (Figure ). From this background, we decided to prepare monoclonal antibodies specific to the oxidatively modified tyrosines, and two novel monoclonal antibodies obtained from the immunized mice reacted with DiBrY and DiClY [Citation8]. As one of example for the antibody in an immunohistochemical study, lipopolysaccharide (LPS) was intraperitoneally administered to mice, and livers were removed. Positive staining of LPS-treated mouse liver tissues by both the anti-dihalotyrosine antibody and anti-myeloperoxidase antibody were estimated, suggesting that inflammatory tissue damage induces the formation of dihalotyrosine in vivo.
Figure 2. Tyrosine Modification by ROS, halogenating species (HOCl and HOBr), and peroxynitrite (ONOO−).
We also carried out the research on correlation of activation of MPO and increase in modificated tyrosines during the process of inflammation in the skin tissue, and it is found to be more likely to be modified, as it is located at the outer layer of a body that is exposed to UV radiation on a daily basis [Citation9]. To investigate the influence of the oxidatively modified tyrosine on UV-exposed skin, we detected the nitrotyrosine (NO2Y) or halogenated tyrosine, and DY in photo-aged model mice [Citation10]. This result suggests that the modified tyrosine was produced during the process of inflammation by UV irradiation using a low dose of UV irradiation to which we are exposed in daily life, and it is suggested that UV exposure in daily life may induce the production of modified tyrosine and skin aging. We also investigated the possible connection between inflammation and protein denaturation, which might lead to skin aging by human study, and we focused on halogenated tyrosine as a denatured substance produced during the inflammation process. Immunohistochemical analysis indicated that both inflammatory cells and halogenated tyrosines increased with aging in both photoexposed and photoprotected skin. Elastic van Gieson (EVG) staining confirmed that the localization of halogenated tyrosine was close to the sites at which protein was denatured [Citation11].
Biomarkers for lipid-derived modification of lysine
Lipid peroxidation generates various oxidation products. Among them, aldehydes have high reactivity against biomolecules such as proteins and nucleotides [Citation12]. The aldehyde-modified biomolecules have been immunechemically identified in various tissues (Figure ). On the other hand, we have also found the formation of novel adducts having amide as the linkage between oxidized lipid and lysine. One of adducts, hexanoyl-lysine (HEL) derived from n-6 polyunsatsurated fatty acids (PUFA) such as linoleic acid and arachidonic acid (AA) have been detected in atherosclerotic plaques using polyclonal and monoclonal antibodies to HEL [Citation13,14]. Several amide-type adducts, azelayl-lysine (AZL), succinyl-lysine (SUL), glutaroyl-lysine (GLL), and propanoyl-lysine (PRL) have also been identified recently [Citation15–17].
Figure 3. Polyunsaturated fatty acid (PUFA) and generation of adducts between lipid-derived products and protein.
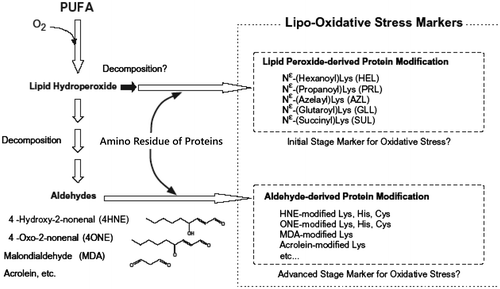
Because these adducts are probably generated from oxidation products of n-3 and/or n-6 PUFA with lysine residues, the simultaneous determination of all adducts may be a useful fingerprint to make clear what kinds of lipid are oxidized in vivo (Figure ). Since SUL, AZL, and GLL have carboxyl terminal moiety (COOH) derived from parent PUFA, these should be esterified with cholesterols and phospholipids in vivo. To immunochemically detect the carboxyalkylamide-type adducts in a tissue, the scission of lipid ester bond has to be done by phospholipase A2 or alkaline hydrolysis of ester bond [Citation17]. On the other hand, alkylamide-type adducts, HEL and PRL, do not need such pre-treatments for detection, therefore, we focused on the monoclonal antibodies to HEL and PRL first.
Figure 4. Classification of amides, HEL, and PRL are markers for n − 6 and n − 3 PUFA oxidation, respectively.
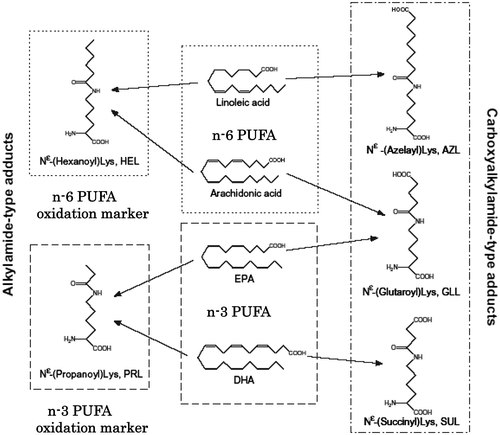
The HEL has been gradually used as one of the important biomarkers because a monoclonal antibody specific to HEL has been prepared [Citation18] and the antibody to HEL and an ELISA kit are commercially available. On the other hand, PRL is a novel biomarker derived from n − 3 PUFA such as docosahexaenoic acid (DHA), and development of simple and accurate methods to measure PRL has been anticipated. To improve this situation, we have prepared monoclonal antibody to PRL. The serum from PRL-KLH immunized mice showed not only strong reactivity to PRL-BSA but also weak reactivity against HEL-BSA, however, the obtained antibody specifically recognizes PRL but not other amide adducts such as HEL, SUL, and GLL. The monoclonal antibody against PRL to evaluate the generation of PRL both in vitro and in vivo [Citation19]. We have also observed the positive staining of PRL but not HEL in hippocampus of old rat brain (manuscript in preparation). This possibly means that n-3 PUFA are specifically oxidized in brain during aging. Immunohistochemical detection using anti-PRL antibody suggested that propanoylation was generated in the hippocampus area and temporal lobe in the brain of Alzheimer’s disease (AD) patients. Our recent data suggests that PRL has the capacity of not only the useful biomarker of AD but also trigger of pathological degradation to lead to AD etiology although the detailed examination is undergoing.
Chemical approaches are also beneficial for estimation of identification and quantification of these biomarkers, therefore, we decided to develop the methods for simultaneous quantitation of PRL and HEL in human urine using liquid chromatography tandem mass spectrometry (LC/MS/MS) without derivatization. This method was applied for an isotope dilution method [Citation20], because it needs less than 100 μl of urine to determine the amounts of both adducts, and the level of urinary HEL and PRL were found to be correlated with the other oxidative stress markers, 8-hydroxy-2′-deoxyguanosine (8-OHdG), DY, and isoprostanes, and the increase in excretion of HEL and PRL into urine of diabetic patients was also confirmed compared to healthy subjects (Figure ). This result suggests that PRL may be good marker for n-3 PUFA-derived oxidative stress in vivo.
Biomarkers for lipid hydroperoxide modification of dopamine and phospholipids
Parkinson disease (PD) is a neurodegenerative disorder characterized by a dramatic loss of dopaminergic neurons in the substantia nigra and the subsequent deficiency of dopamine in the brain areas [Citation21]. Until now, very little is known about why and how the PD neurodegenerative process begins and progresses; however, an increasing body of evidence suggests that oxidative stress, mitochondrial dysfunction, and impairment of the ubiquitin-proteasome system may be involved in the pathogenesis of PD [Citation22]. Recent studies indicate that there are high levels of basal oxidative stress in the substantia nigra pars compacta in the normal brain, and this is increased in PD [Citation23].
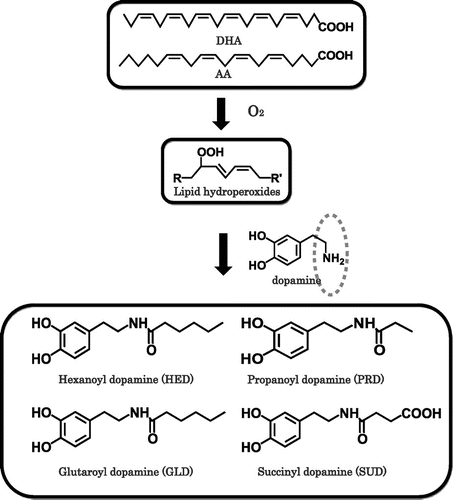
Oxidative stress in the brain easily leads to the lipid peroxidation reaction because of a high concentration of polyunsaturated fatty acids, such as DHA (C22:6/n − 3) and AA (C18:4/n − 6), which are present in the brain [Citation24]. PUFA are located almost exclusively in the sn2 position of the phosphoglycerides found in the neural cell membranes. The beneficial physiological effects of DHA and AA have been frequently reported [Citation25], however, PUFA are highly unsaturated, thus making them particularly susceptible to peroxidation. During the lipid peroxidation reaction of DHA, DHA hydroperoxides are generated as primary products and they were found to act as a potential inducer of neuronal cell death through a mitochondrial dysfunction-mediated pathway. Subsequent decomposition leads to the formation of reactive mediates, including aldehydes which can covalently modify biomolecules [Citation26].
We chemically synthesized four dopamine-modified adducts derived from DHA and AA. We were particularly interested in the formation of the dopamine adducts by chemical reactions and in vivo experiments, as well as the cytotoxicity evaluation using neuronal cells. All four dopamine adducts were detected upon incubation of dopamine with fatty acid hydroperoxides and an in vivo experiment using rat brain tissue (Figure ). Furthermore, we focused on an AA-derived adduct, hexanoyl dopamine (HED), which induced severe cytotoxicity in human dopaminergic neuroblastoma SH-SY5Y cells compared with other adducts. The HED-induced cell death was found to include apoptosis that might be mediated by ROS and mitochondrial abnormality in SH-SY5Y cells. In addition, we found that the presence of monoamine transporters in the cells was essential for the HED-induced cytotoxicity, suggesting the specificity of the cytotoxicity to the cells. Taken together, the DHA- and AA-derived dopamine adducts may be useful biomarkers of the dopamine deficiency, and the formation of these adducts may indicate a novel mechanism responsible for the pathogenesis in Parkinson disease [Citation27].
Figure 5. Mechanism of formation of dopamine adducts Propanoyl dopamine (PRD) and succinyl dopamine (SUD) were derived from n − 3 PUFA such as DHA, and hexanoyl dopamine (HED) and Glutaroyl dopamine (GLD) were derived from n − 6 PUFA such as AA.
Phospholipids such as phosphatidylethanolamine and phosphatidylcholine play crucial roles in the biological system to maintain the cellular environmental condition [Citation28]. Despite that, oxidative stress targets these phospholipids containing PUFA and accompanies the oxidatively modified phospholipids. Our recent studies suggested that oxidatively modified phospholipids have the relationship with inflammation and might induce the atherosclerosis formation by uptake of oxidized LDL through scavenger receptor as ligands. Red blood cells, which have been studied the bilayer model, are also modified by oxidative stress because hemoglobin can mediate and produce the reactive oxygen species, which leads to lipid peroxidation of biomembrane. In these oxidation processes of biomolecules, hexanoylation against phosphatidylethanolamine and phosphatidylserine, which has the primary amine and is the target of this modification, generates the oxidized membrane such as erythrocyte ghosts [Citation29]. This unique structure of phosphatidylethanolamine and phosphatidylserine is important for application as the useful biomarker to evaluate the oxidation of biomembrane in vivo using liquid chromatography tandem mass spectrometry and monoclonal antibody [Citation30].
Biomarkers for oxidative modification of DNA
There are many indications that oxidative damage can occur in DNA during the peroxidative breakdown of the membrane polyunsaturated fatty acids, in particular, oxidation of 2′-deoxyguanosine (dG) to 8-OHdG by hydroxy radical. We have been succeeded in obtaining specific monoclonal antibody to 8-OHdG, and developed a new enzyme-linked immunosorbent assay (ELISA) method in quantitating 8-OHdG by competitive inhibition [Citation31]. By application of monoclonal antibody specific to 8-OHdG, excretion of 8-OHdG has been observed in urines of lung cancer patients with radiotherapy, chemotherapy, and response to treatment [Citation32]. High level of 8-OHdG has been detected in normal human epidermis after a single dose of ultraviolet radiation [Citation33]. We also expected that other endogenous lipid peroxidation products react with DNA and form exocyclic DNA adducts [Citation34]. Investigation of the in vivo formation of 7-(2-oxo-heptyl)-substituted 1,Nε-etheno-2′-deoxyguanosine (Oxo-heptyl-εdG), one of the major products from the reaction of 13-hydroperoxyoctadecadienoic acid (13-HPODE) with dG has been carried out [Citation35]. The monoclonal antibody specific to Oxo-heptyl-εdG was prepared using a chemically synthesized conjugate of Oxo-heptyl-εdG and carrier protein as immunogen, and the characterization showed that the obtained antibody (mAb6A3) is specific to the Oxo-heptyl-εdG moiety. Using the novel antibody, the presence of the Oxo-heptyl-εdG adduct in vivo was immunohistochemically revealed in the liver of rats fed a choline-deficient, l-amino acid-defined diet, an endogenous carcinogenesis model, for 3 days [Citation35]. In addition, the Oxo-heptyl-εdG formation was confirmed in DNAs treated with peroxidized linoleic acid, AA and γ-linolenic acid, respectively, suggesting that the hydroperoxides of n-6 PUFA could be the potential sources of Oxo-heptyl-εdG formation in vivo. Recently, we found that dG is also the major target by lipid peroxidation products [Citation36]. Our result suggests the first evidence that the n-6 lipid hydroperoxide mediated DNA adduct, may be a potential biomarker for the early phase of carcinogenesis and other age-related disease. DNA is considered an important target of modification due to the potential for inducing gene mutation, and various modifications of DNA bases by MPO-derived species have been described [Citation37].
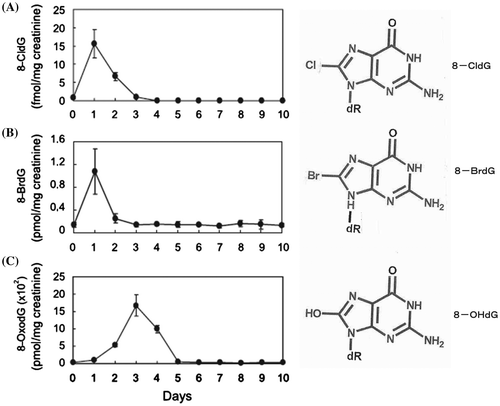
Although chlorination of dG has been reported [Citation38], there are limited data on dG halogenation, and we decided to carry out the research on DNA halogenation, especially biological activity of the brominated DNA. In order to find the potential role of DNA damage by leukocyte-derived ROS generated by MPO, we started our project to investigate the formation of 8-halogenated 2′-deoxyguanosines (8-halo-dGs) during inflammatory events. 8-bromo-2′-deoxyguanosine (8-BrdG) and 8-chloro-2′-deoxyguanosine (8-CldG) were generated by treatment of MPO with hydrogen peroxide at physiological concentrations of Cl− and Br−. The formation of 8-halo-dGs with other oxidative stress biomarkers in LPS-treated rats was assessed by LC/MS/MS and immunohistochemistry using a novel monoclonal antibody (mAb8B3) to 8-BrdG-conjugated keyhole limpet hemocyanin. The antibody recognized both 8-BrdG and 8-CldG. In the liver of LPS-treated rats, immunostaining for 8-halo-dGs, halogenated tyrosines, and MPO were increased at 8 h, while those of 8-OHdG and NO2Y were increased at 24 h. Urinary excretion of both 8-CldG and 8-BrdG were also observed earlier than those of 8-OHdG (Figure ). Moreover, levels of the 8-halo-dGs in urine from human diabetic patients were eight times higher than in healthy subjects (N = 10, healthy and diabetic, p < 0.0001), while there was a moderate difference in 8-OHdG between the two groups (p < 0.001). Interestingly, positive mAb8B3 antibody staining was observed in liver tissue from hepatocellular carcinoma patients, but not in liver tissue from human cirrhosis patients. These data suggest that 8-halo-dGs may be potential biomarkers of early inflammation [Citation39].
Figure 6. Urinary excretion of 8-CldG, 8-BrdG, and 8-OHdG of LPS-treated rats. In order to measure LPS-induced change of modified dGs, urine of rats was diluted 5X by water. The fraction containing 8-modified dG for the samples was collected by HPLC, and quantification of 8-modified dG (A: 8-CldG, B: 8-BrdG, and C: 8-OHdG) in fractions was performed by LC-MS/MS using internal standards.
Recently, the effects of taurine, the most abundant free amino acid in the leukocyte cytosol have been examined, on the hypohalous acid-dependent formation of 8-CldG and 8-BrdG [Citation40]. The reaction of taurine with HOBr yielded taurine bromamine, which is the most stable among other bromamines of amino acids. Taurine also enhanced the bromination of only dG among the four 2′-deoxynucleosides, whereas it inhibited the 8-CldG formation. The specificity of taurine for the enhanced formation of halogenated dG is completely different from that of nicotine, an enhancer of chlorination. The amount of dibrominated taurine (taurine dibromamine) closely correlated with the formation of 8-BrdG, suggesting that taurine dibromamine might be a plausible mediator for the dG bromination in vivo.
Application of oxidative stress biomarkers for functional food research
In order to develop a simple and convenient method for detection and quantification of oxidative stress biomarkers, we immobilized antibodies and antigens on the surface of an azopolymer coated glass slide (antibody array) to establish “on-chip ELISAs.” The sensitivity and accuracy of the on-chip ELISA were similar to a conventional ELISA using a polystyrene plate. Using the assay system, we proved that representative oxidative stress biomarkers such as 8-OHdG, HEL, and PRL could be simultaneously measured from μL of urine. Now, we are sure that this new concept antibody array has promising applications in proteomic studies, and could be used to examine biomarkers to investigate functional foods [Citation41].
Recently, our research group has been involved in the role of Coprinus comatus, an edible mushroom containing a high amount of ergothioneine (EGT) [Citation42], for protection from oxidative damage caused during inflammation process, and found that EGT inhibited the UV-B-induced inflammatory responses and DNA halogenation, suggesting that EGT is a promising anti-inflammatory agent from mushrooms. EGT rich Coprinus extract inhibited both MPO activity and HOBr-induced DNA damage by measuring 8-BrdG formation in vivo in addition to inhibition of UV-B-induced inflammatory responses. The HOBr scavenging effect of EGT was also found to be higher than those of ascorbic acid and glutathione [Citation43].
Advances in understanding the neurodegenerative pathologies are creating new opportunities for the development of neuroprotective therapies, such as antioxidant food factors, lifestyle modification and drugs. However, the biomarker by which to determine the effect of the agent on neurodegeneration is limited. Because we found the important role of HED at early stage of inflammation, we tried to establish an analytical system using for the detection of HED and its toxicity to the neuroblstoma cell line, SH-SY5Y cells, therefore, we decided to develop an application to investigate the neuroprotective effect of antioxidant foods. By assessing the protective effect of antioxidant food factors on HED formation, sesamie lignans, especially, sesaminol-6-catechol (SMLC) showed the most significant inhibitive effect on the in vitro HED formation [Citation44]. We also recently found that SMLC markedly prevented HED-induced ROS generation and cell death in SH-SY5Y cells. In addition, sesaminol catechol (SMLC) was detected in the brain of rats fed with SMLC-contained foods, suggesting that it could cross brain–blood barrier (BBB) and exhibit the neuroprotective effect in vivo. SMLC is one of the main metabolites produced by p-450 during injestion of sesame lignans, in particular, sesaminol glucosides [Citation45].
Recently, we have been involved in investigation of the effect and the mechanism of astaxanthin (AST) on ROS-mediated apoptosis in dopaminergic SH-SY5Y cells. The treatment with DHA hydroperoxide (DHA-OOH), ROS-inducing neurotoxin, led to a significant decrease in viable dopaminergic SH-SY5Y cells by MTT assay, whereas a significant protection was shown while the cells were pretreated with AST.
HEL was found as a sensitive biomarker of oxidative stress in muscle during exercise and applied for evaluation of feeding effects of plant flavonoids, especially lemon flavonoid suppressed oxidative modification of rat skeletal muscle by exercise [Citation46]. HEL modification of carnitine palmitoyltransferase, I was also found to be increased by exercise, while AST-containing diet prevented this increase [Citation47].
Moreover, AST pretreatment was found to significantly inhibit apoptosis, mitochondrial abnormalities and intracellular ROS generation occurred in DHA-OOH-treated cells. The neuroprotective effect of AST is suggested to be dependent upon its antioxidant potential and mitochondria protection; therefore, it is suggested that supplementation of AST may be an effective treatment for oxidative stress-associated neurodegeneration.
Our research group has also developed an in vitro evaluating system for the functionalities of food through optical sensing techniques using the innate immune response of HL-60 cells by the simultaneous measurement of chemiluminescence and fluorescence indicating generation and Ca2+ of neutrophils stimulated by agonists, respectively [Citation48]. Using differentiated HL60 cells as neutrophil-like cells, the f-MLP was used as agonist, and it was found that AST have strong anti-inflammation activity [Citation49]. More recently, AST was found to inhibit the
production of (superoxide flashes) and calcium release from mitochondria, and activated apoptosis signal pathway, and also protected cells from apoptosis in SH-SY5Y cells. Now, our research group focused on the novel mechanism behind neuroprotective activity of phytochemicals in aging and age-associated neurodegenerative disorders, and also behind dual functions of phytochemicals in neuronal and cancer cells [Citation50]. We are now trying to apply this system for an easy-to-use in vivo oxidative stress measurement system using only one drop of human blood. We expect that this system would be effective in identifying the type of foods and supplements that can be develop “Order-made functional foods” suited to reduce individual oxidative stress in your particular physical constitution.
Conclusion
ROS generated by phagocytic white blood cells are critical to host defense, however, they are also potentially dangerous because they may damage tissues at sites of inflammation. MPO secreted by phagocytic white blood cells utilize the H2O2 produced by membrane-associated NADPH oxidase to induce lipid peroxidation, and also catalyze the formation reactive halogens using hydrogen peroxide and Br−/Cl−. As the universal biomarkers, chemical and immunochemical approach on oxidatively modified and halogenated tyrosines generated during the process of inflammation process has been carried out. We also succeeded to develop various amido-type adduct biomarkers [Citation51], and developed for application of evaluation of novel antioxidative functional food factors. We have also started our new project to investigate a novel biomarker of oxidative DNA damage other than 8-OHdG. Of course, research efforts on metabolic pathways of dietary antioxidants in the digestive tracts are also required. Although our approach started just recently, they help us to understand the relationship between antioxidative activity and disease prevention including dementia caused by oxidative brain damage. Now, we expect to establish simple and reliable systems for development of “Evidence-based functional foods” by collaboration of both academic and industry sites stress biomarkers.
Disclosure statement
No potential conflict of interest was reported by the author.
Acknowledgments
I’d like to express my sincere thank collaborators both in academic and industrial fields, especially, Professor Yoji Kato (University of Hyogo) for his important contribution during in this study.
References
- Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44.10.1016/S0002-9343(00)00481-2
- Zhang R, Brennan ML, Shen Z, et al. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at a site of inflammation. J Biol Chem. 2002;277:46116–46122.10.1074/jbc.M209124200
- Senthilmohan R, Kettle AJ. Bromination and chlorination reaction of myeloperoxidase at physical concentrations of bromide and chloride. Arch Biochem Biophys. 2006;445:235–244.10.1016/j.abb.2005.07.005
- Brennan ML, Anderson MM, Shih DM, et al. Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest. 2001;107:419–430.10.1172/JCI8797
- Kato Y, Kitamoto N, Kawai Y, et al. The hydrogen peroxide/copper ion system, but not other metal-catalyzed oxidation systems, produces protein-bound dityrosine. Free Radical Biol Med. 2001;31:624–632.10.1016/S0891-5849(01)00623-2
- Kato Y, Maruyama W, Naoi M, et al. Immunohistochemical detection of dityrosine in lipofuscin pigments in the aged human brain. FEBS Lett. 1998;439:231–234.10.1016/S0014-5793(98)01372-6
- Kato Y, Wu X, Naito M, et al. Immunochemical detection of protein dityrosine in atherosclerotic lesion of apo-E-deficient mice using a novel monoclonal antibody. Biochem Biophys Res Commun. 2000;275:11–15.10.1006/bbrc.2000.3265
- Kato Y, Kawai Y, Morinaga H, et al. Immunogenicity of a brominated protein and successive establishment of a monoclonal antibody to dihalogenated tyrosine. Free Radical Biol Med. 2005;38:24–31.10.1016/j.freeradbiomed.2004.09.013
- Ishitsuka Y, Masunaga T, Koide C, et al. Repeated irradiation with suberythermal ultraviolet B reduces the number of epidermal Langerhans cells. Arch Dermatol Res. 2003;295:155.10.1007/s00403-003-0397-4
- Ishitsuka Y, Maniwa F, Koide C, et al. Detection of modified tyrosines as an inflammation marker in a photo-aged skin model. Photochem Photobiol. 2007;83(3):698–705.10.1562/2006-07-24-RA-978
- Ishitsuka Y, Maniwa F, Koide C, et al. Clinical and experimental dermatology, increased halogenated tyrosine levels are useful markers of human skin ageing reflecting denatured proteins by the past skin inflammation. Clin Exp Dermatol. 2012;37:252–258.
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biol Med. 1991;11:81–128.10.1016/0891-5849(91)90192-6
- Kato Y, Mori Y, Makino Y, et al. Formation of N(epsilon)-(hexanonyl)lysine in protein exposed to lipid hydroperoxide. A plausible marker for lipid hydroperoxide-derived protein modification. J Biol Chem. 1999;274:20406–20414.10.1074/jbc.274.29.20406
- Fukuchi Y, Miura Y, Nabeno Y, et al. Immunohistochemical detection of oxidative stress biomarkers, dityrosine and N(epsilon)-(hexanoyl)lysine, and C-reactive protein in rabbit atherosclerotic lesions. J Atheroscler Thromb. 2008;15:185–192.10.5551/jat.E543
- Kawai Y, Kato Y, Fujii H, et al. Immunochemical detection of a novel lysine adduct using an antibody to linoleic acid hydroperoxide-modified protein. J Lipid Res. 2003;44:1124–1131.10.1194/jlr.M200442-JLR200
- Kawai Y, Fujii H, Kato Y, et al. Esterified lipid hydroperoxide-derived modification of protein: formation of a carboxyalkylamide-type lysine adduct in human atherosclerotic lesions. Biochem Biophys Res Commun. 2004;313:271–276.10.1016/j.bbrc.2003.11.123
- Kawai Y, Fujii H, Okada M, et al. Formation of N(epsilon)-(succinyl)lysine in vivo: a novel marker for docosahexaenoic acid-derived protein modification. J Lipid Res. 2006;47:1386–1398.10.1194/jlr.M600091-JLR200
- Kato Y, Miyake Y, Yamamoto K, et al. Preparation of a monoclonal antibody to N(epsilon)-(hexanonyl)lysine: application to the evaluation of protective effects of flavonoid supplementation against exercise-induced oxidative stress in rat skeletal muscle. Biochem Biophys Res Commun. 2000;274:389–393.10.1006/bbrc.2000.3150
- Hisaka S, Kato Y, Kitamoto N, et al. Chemical and immunochemical identification of propanoylllysine derived from oxidized n-3 polyunsaturated fatty acid. Free Radical Biol Med. 2009;46:1463–1471.10.1016/j.freeradbiomed.2009.02.030
- Kato Y, Yoshida A, Naito M, et al. Identification and quantification of N(epsilon)-(hexanoyl)lysine in human urine by liquid chromatography/tandem mass spectrometry. Free Radical Biol Med. 2004;37:1864–1874.10.1016/j.freeradbiomed.2004.09.007
- Galvan A, Wichmann T. Pathophysiology of Parkinsonism. Clin Neurophysiol. 2008;119:1459–1474.10.1016/j.clinph.2008.03.017
- Schapira AH. Cause of neuronal death in Parkinson’s disease. Adv Neurol. 2001;86:155–162.
- Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53:S26–S38.10.1002/(ISSN)1531-8249
- Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290.10.1007/BF02536034
- Hadders-Algra M. Prenatal long-chain polyunsaturated fatty acid status: the importance of a balanced intake of docosahexanoic acid and arachidonic acid. J Perinat Med. 2008;36:101–109.
- Liu XB, Shibata T, Hisaka S, et al. DHA hydroperoxides as a potential inducer of neuronal cell death: a mitochondrial dysfunction-mediated pathway. J Clin Biochem Nutr. 2008;43(1):26–33.10.3164/jcbn.2008040
- Liu XB, Yamada N, Maruyama W, et al. Formation of dopamine adducts derived from brain polyunsaturated fatty acids: mechanism for Parkinson’s disease. J Biol Chem. 2008;283(50):34887–34895.10.1074/jbc.M805682200
- Tsuji K, Kawai Y, Kato Y, et al. Formation of Ne -(hexanoyl)ethanolamine, a novel phosphatidylethanolamine adduct, during the oxidation of erythrocyte membrane and low-density lipoprotein. Biochem Biophys Res Commun. 2003;306:706–711.10.1016/S0006-291X(03)01038-6
- Linderkamp O, Friederichs E, Boehler T, et al. Age dependency of red blood cell deformability and density: studies in transient erythroblastopenia of chilidhood. Br J Haematol. 1993;83:125–129.10.1111/j.1365-2141.1993.tb04642.x
- Hisaka S, Yamada N, Naito K, et al. The immunological and chemical detection of N-(hexanoyl) phosphatidylethanolamine and N-(hexanoyl)- phosphatidylserine in an oxidative model induced by carbon tetrachloride. Biochem Biophys Res Commun. 2010;393:631–636.10.1016/j.bbrc.2010.02.043
- Toyokuni S, Tanaka T, Hattori Y, et al. Quantitative Immuunohistochemical determination of 8-hydroxy-2′-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab Invest. 1997;76(3):365–374.
- Erhola M, Toyokuni S, Okada K, et al. Biomarker evidence of DNA oxidation in lung cancer patients: association of urinary 8-hydroxy-2′-deoxyguanosine excretion with radiotherapy, chemotherapy and response to treatment. FEBS Lett. 1997;409:287–291.10.1016/S0014-5793(97)00523-1
- Ahmed NU, Ueda M, Nikaido O, et al. High level of 8-hydroxy-2′-deoxyguanosine appear in normal human epidermis after single dose of ultraviolet radiation. British J Dermantol. 1999;140:226–231.10.1111/j.1365-2133.1999.02653.x
- Chung F-L, Chen H-JC, Nath RG. Lipid peroxidation as a potential endogenous source for the formation of exocyclic DNA adducts. Carcinogenesis. 1996;17(10):2105–2111.10.1093/carcin/17.10.2105
- Kawai Y, Kato Y, Nakae D, et al. Immunohistochemical detection of a substituted 1, N2-ethenodeoxyguanosine adduct by ω-6 polyunsaturated fatty acid hydroperoxides in the liver of rats fed a choline-deficient, L-amino acid-defined diet. Carcinogenesis. 2002;23:485–489.
- Kawai Y, Uchida K, Osawa T. 2′-Deoxycytidine in free nucleosides and double-stranded DNA as the major target by lipid peroxidation products. Free Radical Biol Med. 2004;36:529–541.10.1016/j.freeradbiomed.2003.12.006
- Henderson JP, Byun J, Williams MV, et al. Bromination of deoxycytidine by eosinophil peroxidase: a mechanism for mutagenesis by oxidative damage of nucleotide precursors. Proc Nat Acad Sci USA. 2001;98:1631–1636.10.1073/pnas.98.4.1631
- Masuda M, Suzuki T, Friesen M, et al. Chlorination of guanosine and other nucleosides by hypochlorous acid and myeloperoxidase of activated human neutrophils. J Biol Chem. 2001;276:40486–40496.
- Asahi T, Kondo H, Masuda M, et al. Chemical and immunochemical detection of 8-halogenated deoxyguanosines at early stage inflammation. J Biol Chem. 2010;285:9282–9291.10.1074/jbc.M109.054213
- Asahi T, Nakamura Y, Kato Y, et al. Specific role of taurine in the 8-brominated-2′-deoxyguanosine formation. Arch Biochem Biophys. 2015;586:45–50.10.1016/j.abb.2015.10.002
- Hoshino F, Watanabe O, Wu X, et al. Low-cost and easy-to-use “on-chip ELISA” for developing health-promoting foods. In: Kato Y, editor. Lipid hydroperoxidederived modification of biomolecules. New York (NY): Springer; 2014. p. 151–161.10.1007/978-94-007-7920-4
- Ito T, Kato M, Tsuchida H, et al. Ergothioneine as an anti-oxidative/anti-inflammatory component in several edible mushrooms. Food Sci Technol Res. 2011;17(2):103–110.10.3136/fstr.17.103
- Asahi T, Wu X, Shimoda H, et al. A mushroom-derived amino acid, ergothioneine, is a potential inhibitor of inflammation-related DNA halogenation. Biosci Biotechnol Biochem. 2016;80(2):313–317.10.1080/09168451.2015.1083396
- Liu X, Yamada N, Osawa T. Assessing the neuroprotective effect of antioxidative food factors by application of lipid-derived dopamine modification adducts. Lipidomics Vol.2: Methods and Protocols. In: Armstrong D, editor. Methods in molecular biology. Vol. 580. London: Humana Press; 2009. p. 143–152.
- Miyake Y, Fukumoto S, Okada M, et al. Antioxidative catechol lignans converted from sesaminol triglucoside by culturing with Aspergillus. J Agric Food Chem. 2005;53(1):22–27.10.1021/jf048743 h
- Kato Y, Miyake Y, Yamamoto K, et al. Preparation of monoclonal antibody to Nε-(Hexanonyl)lysine: application to the evaluation of oxidative modification of rat skeletal muscle by exercise with flavonoid supplimentation. Biochem Biophys Res Commun. 2000;274:389–393.10.1006/bbrc.2000.3150
- Aoi W, Naito Y, Takanami Y, et al. Astaxanthin improves muscle lipid metabolism in exercise via inhibitory effect of oxidative CPT I modification. Biochem Biophys Res Commun. 2008;366:892–897.10.1016/j.bbrc.2007.12.019
- Kazumura K, Sato Y, Satozono H, et al. Simultaneous monitoring of superoxides and intracellular calcium ions in neutrophils by chemiluminescence and fluorescence: Evaluation of action mechanisms of bioactive compounds in foods. J Pharm Biomed Anal. 2013;84:90–96.10.1016/j.jpba.2013.05.048
- Kazumura K, Sato Y, Mochizuki M, et al. A study on health promoting power in astaxanthin by using dual-mode observation of innate immune responses in neutrophils, ACN2015; May 16; Yokohama; 2015.
- Wu YQ, Shamoto-Nagai M, Maruyama W, et al. Phytochemicals prevent mitochondrial membrane permeabilization in apoptosis induced by PK11195: A novel cellular mechanism underlying the neuroprotective anti-aging function of bioactive dietary compounds. J Neural Transm. 2017;124:89–98.10.1007/s00702-016-1624-4
- Kato Y, Osawa T. Detection of lipid-lysine amide-type adduct as a marker of PUFA oxidation and its applications. Arch Biochem Biophys. 2010;501:182–187.10.1016/j.abb.2010.06.010