Abstract
Obesity is a key factor in metabolic syndrome. The study of metabolic syndrome focuses on the anti-weight gain properties of physiological mechanisms and food components. Abnormal energy metabolism is a major risk factor of metabolic syndrome. Chronic inflammation is a feature of obesity; cytokines from hypertrophied adipocytes cause inflammation in both adipose tissue and blood vessels, resulting in symptoms of metabolic syndrome. Tumor necrosis factor-α causes insulin resistance in adipocytes and regression of brown adipocytes, resulting in abnormal energy metabolism. Functional foods can serve as a strategy for prevention and treatment of obesity linked with metabolic processes in white and brown adipose tissues. Diet-induced thermogenesis caused by certain food components stimulates burning of stored fat within adipose tissues. A mechanistic understanding of dietary thermogenesis via the sympathetic nerve system will prove valuable for the development of precise strategies for the practical prevention of metabolic syndrome.
Graphical abstract
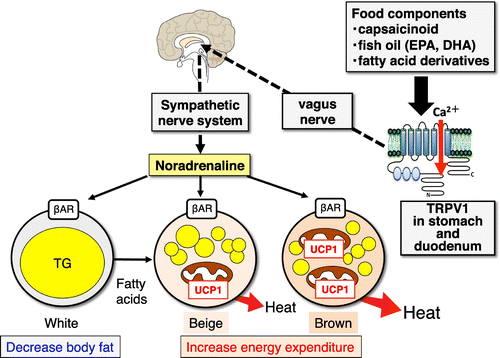
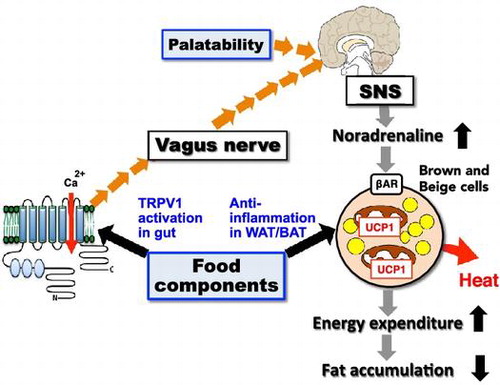
Anti-obesity effect of food components via activation of the TRPV1-SNS-BAT axis and anti-inflammation in WAT/BAT. Additionally, food intake is followed by sensory stimulation of palatability, resulting in the activation of the SNS-BAT axis.
Up to 2.1 billion people worldwide are classified as overweight or obese, which can result in the onset of lifestyle-related diseases such as metabolic syndrome [Citation1]. Being overweight is considered a risk factor for many illnesses. Extreme cases result in what is known as “obesity disease,” which often requires medical treatment, as well as exercise and dietary therapies. Using functional foods, which are known to increase wellness, strategies can be implemented in which individual eating habits are monitored closely to determine the potential of these foods as prevention or remedy for lifestyle-related diseases or metabolic syndrome. I have investigated in detail the potential of this stance as a treatment for obesity or lifestyle-related diseases. This review introduces nutritional biochemistry research regarding regulatory factors of metabolic syndrome, with a focus on the role of adipose tissue.
Influence of pungent ingredient intake on obesity and lipid metabolism
Obesity is a key factor of metabolic syndrome and has been an issue of social and economic importance in medicine in Japan. I began researching the relationship between obesity and food composition in the early 1980s, when the prevalence of weight gain in Europe and America was becoming a social concern. I focused on the anti-weight-gain properties of food ingredients. What first caught my attention was the pungent spice ingredients in a typical meal, especially capsaicin, a pungent constituent of the red pepper. A previous study by our research group has reported that capsaicin inhibited fat accumulation and decreased blood triglyceride levels when a high-fat diet was used to induce weight gain [Citation2]. The mechanism of this pungent ingredient activated the sympathetic nervous system (SNS); it was clearly shown that this depended on a rise in energy metabolism via moderate adrenaline secretion from the adrenal gland [Citation3]. It was also shown that other pungent ingredients in spices such as ginger and pepper have the same mechanism of action [Citation4]. I emphasized the new concept of pungent principle-induced thermogenesis in 1987 [Citation5]. Currently, the anti-weight-gain properties of pungent ingredients are being utilized for development of functional foods.
Basic research on the improvement of lipid metabolism in the liver and its application to food science
The key factors in the development of metabolic syndrome are abnormalities in lipid and energy metabolism; for example, fatty liver disease shows symptoms of these defects as an initial pathological change. Lipid and energy metabolism are mainly regulated by nuclear receptors affecting gene transcription. These receptors are characterized by ligand-dependent transcription. Nuclear receptors are historically considered to be the target molecules for drug and functional food developments. Liver fatty acid synthesis genes are regulated by sterol regulatory element-binding protein 1 (SREBP-1), which requires the liver X receptor (LXR)-binding site in the promoter of SREBP-1 to activate transcription. Thus, we searched for food components that affect the fatty acid synthesis systems of the liver. We found that certain spice ingredients act as inhibitors of LXRα, which down-regulates transcription of genes involved in fatty acid synthesis [Citation6,7]. Fatty acid levels in the liver are regulated by peroxisome proliferator-activated receptor alpha (PPARα), which regulates the expression of genes in the β-oxidizing system. Therefore, we screened for food components that affect the fatty acid oxidation systems of the liver. It was found that the fatty acid-derived compounds 9- and 13-oxo-octadecadienoic acids— found in tomato— act as agonists of PPARα, suggesting these compounds have the potential to improve obesity-induced dyslipidemia and hepatic steatosis [Citation8]. It was recently reported that tomato juice intake for 8 weeks lowered high baseline serum triglyceride levels in middle-aged women [Citation9]. These findings serve as the foundation for developing functional foods.
Research on functional control of adipocytes
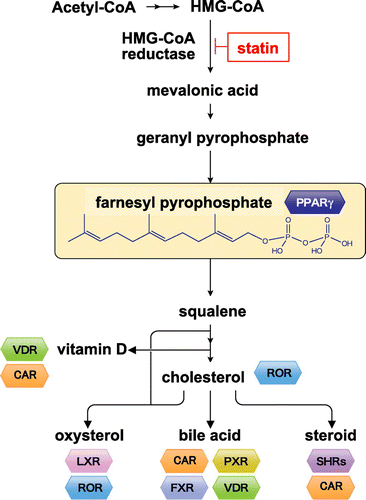
Obesity induces the development and hypertrophy of white adipocytes, resulting in insulin resistance, inflammation, and abnormal secretion of malignant adipocytokines. Adipocytokines such as leptin, adiponectin, TNF-α, plasminogen activator inhibitor type 1, and MCP-1 play crucial roles in the pathogenesis of insulin resistance and metabolic syndrome [Citation10]. Fat accumulation on visceral depots up-regulates expression of malignant adipocytokines such as TNF-α. Our group initially focused on the nuclear receptor PPARγ, which is a master regulator for the differentiation and functional regulation of white adipocytes, and searched for its associated endogenous and exogenous factors [Citation11,12]. We found that farnesyl pyrophosphate, a component of the mevalonate pathway in adipocytes, is an endogenous factor in the regulation of differentiation and function of adipocytes (Figure ) [Citation13]. We also built an evaluation system using dual-luciferase reporter assays for identifying exogenous factors. We used this system to identify many food ingredients such as auraptene from citrus [Citation14], isoprenoids from herbs [Citation15], and β-cryptoxanthin from tangerine and papaya [Citation16]. Animal experiments revealed that these food factors normalize blood glucose and triglyceride levels and reduce liver fat content.
Figure 1. Metabolites of the isoprenoid biosynthesis pathway and nuclear receptors. The isoprenoid pathway (also known as the mevalonate pathway) produces many isoprenoid-based molecules that can serve as ligands for numerous nuclear receptors. Isoprenoid FPP (farnesyl pyrophosphate) may serve as an endogenous ligand for PPARγ. VDR: vitamin D receptor, CAR: constitutive androstane receptor, ROR: RAR-related orphan receptor, LXR: liver X receptor, PXR: pregnane X receptor, FXR: farnesoid X receptor, SHRs: steroid hormone receptors.
Chronic inflammation in obese white adipose tissue and metabolic syndrome
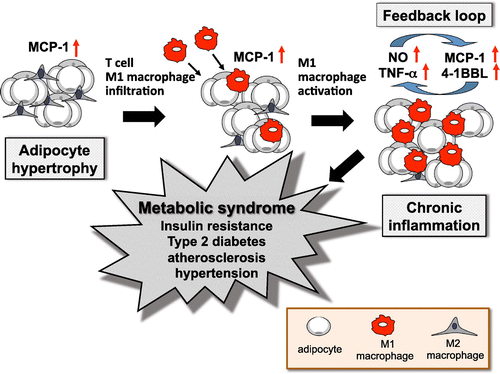
It has become accepted in recent years that chronic inflammation is a feature of obesity (Figure ) [Citation17–19]. The development of symptoms of metabolic syndrome, such as a lipid metabolism abnormality, abnormal glucose tolerance, and insulin resistance, have been found to depend on an inflammatory reaction by macrophages (Mϕ) found in obese white adipose tissue (WAT) [Citation20]. Mϕ are recruited by MCP-1 from hypertrophied adipocytes, resulting in their accumulation of WAT [Citation19,21]. Over time, concentration of anti-inflammatory constitutive M2ϕ decreases and that of inflammatory M1ϕ increases; these stimulate one another to cause abnormality of adipose cells, leading to chronic inflammation and obesity disease. Secretion of inflammatory factors such as TNF-α [Citation20] and 4-1BBL (also known as CD137L) [Citation22] causes inflammation not only in the adipose tissue, but also in the blood vessels and various internal organs, resulting in symptoms of metabolic syndrome. TNF-α in particular causes insulin resistance in adipocytes and muscle cells by impairing stimulation of the insulin-receptor signaling cascade by insulin. The factor 4-1BBL, a member of the TNF superfamily expressed on monocytes/macrophages, acts as an inflammatory signal to modulate proliferation and further cytokine secretion [Citation23]. Understanding the factors controlling macrophages may be beneficial in preventing obesity-related inflammation. If the phenotype and/or proliferation of the macrophage in such adipose tissue could be controlled, a new remedy could be developed for treating or preventing lifestyle-related diseases such as obesity and metabolic syndrome.
Figure 2. Inflammation of adipose tissue and symptoms of metabolic syndrome. Metabolic syndrome has been found to depend on an inflammatory reaction by the M1 macrophages (Mϕ) that exists in obese white adipose tissue (WAT). M1 Mϕ infiltrate into obese WAT, where they are recruited by MCP-1 from hypertrophied adipocytes, resulting in additional accumulation of M1 Mϕ and an increase of inflammatory cytokines in WAT. These form a positive feedback loop to cause hypertrophy of adipose cells, leading to mutual and chronic inflammation.
Food component-mediated control of inflammatory reactions due to metabolic syndrome in WAT
Macrophage-mediated inflammation can promote pathological changes in WAT. Classically activated M1ϕ express high levels of pro-inflammatory mediators, including MCP-1, which may act as a chemokine. Activation of M2ϕ mediates anti-inflammatory effects by enhancing the expression of genes such as IL-10. The regulation of M1ϕ/M2ϕ activity is a possible target for the amelioration of inflammation in WAT. Taurine, found in abundance in fish and shellfish, was found to reduce symptoms of obesity and metabolic syndrome while providing further insight into the regulation of M1ϕ and M2ϕ. This shows potential as a treatment to attenuate chronic inflammation in WAT and reduce obesity-related insulin resistance [Citation24]. We also found that xanthoangelol (XA) and 4-hydroxyderrcin, extracted from Angelica keiskei, suppress obesity-induced inflammatory responses by inhibiting c-Jun N-terminal kinase phosphorylation, nuclear factor-κB, and activator protein 1 in a co-culture system composed of macrophages and adipocytes [Citation25]. In mice fed with a high-fat diet, XA reduced inflammation in WAT. Piceatannol, a natural analog of resveratrol, is a phytochemical found in passion fruit seeds that was also found to significantly suppress production of nitric oxide and inflammatory cytokines (such as TNF-α and IL-6) in cell culture models of obese WAT [Citation26] . These results suggest that anti-inflammatory compounds from food materials show great potential as factors to suppress obesity-induced inflammation and inflammation-induced adipocyte dysfunction.
Chronic inflammation hinders brown adipose tissue function
In addition to classical brown adipocytes in brown adipose tissue (BAT), a new type of brown-like adipocytes (beige cells) in WAT has recently been rediscovered. These thermogenic cells are recognized as key regulators of homeostasis in energy metabolism and of pathogenesis in obesity-related diseases (Figure ) [Citation27]. Little is known regarding the mechanisms that regulate the emergence or regression of these adipocytes in obese adipose tissue. Chronic inflammation results in metabolic disorders in obese WAT [Citation28–30]. We have previously shown that activated Mϕ suppress the development of beige cells in cell culture models of obese WAT and obese WAT of mice [Citation31,32]. Depletion of M1ϕ using clodronate liposomes eliminated suppression of beige cells and significantly reduced TNF-α expression in WAT. Interestingly, combination treatment of clodronate liposomes and cold exposure resulted in changes in the expression levels of specific marker genes for beige cells simultaneously with some metabolic benefits such as cold resistance, body weight reduction, and blood glucose normalization in obese mice. These results suggest that a reduction of inflammation could enhance energy expenditure in obese WAT.
Figure 3. Differentiation scheme of white/brown adipocyte and beige cells. Recently, considerable research has been done on the original stem cell containing UCP-1. Mouse interscapular tissues are rich in brown adipocytes containing UCP-1. This is a classical brown adipocyte, which is generated from the same stem cells as muscle tissues. The brown-like white adipocyte, called the beige cell or brite cell, is reported to be found in white adipose tissue. Recent studies have indicated that beige adipocytes have thermogenic activity comparable to that of classical brown adipocytes and contribute significantly to the regulation of body fat content.
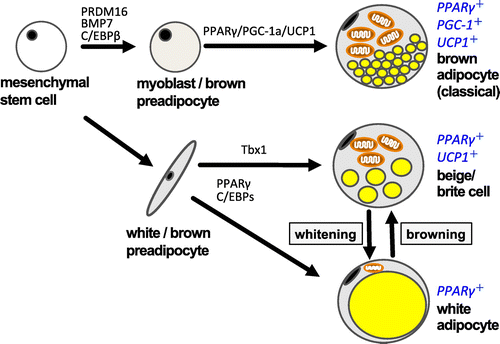
Furthermore, we showed that the extracellular signal-related kinase (ERK) is an important factor in suppression of UCP-1 transcriptional activation during the interaction between white adipocytes and activated macrophages in obese WAT [Citation32]. We also showed that IL-1β expression was increased in the adipose tissues of obese mice and in cell culture models of obese WAT [Citation33]. In addition, IL-1β suppressed β-adrenergic receptors— which stimulate regulation of UCP-1 expression— via the ERK pathway in adipocytes. These findings suggest that UCP-1 transcriptional suppression in beige cells may be associated with obese and diabetic conditions. Anti-inflammatory agents or food factors in obese WAT could be more than just insulin sensitizers; they could serve to increase energy expenditure to improve obesity-related metabolic disorders.
Thermogenesis evoked by food components in WAT and BAT
Enhancing energy metabolism in WAT and BAT is a potential strategy in combating obesity [Citation27,34]. Humans are born with the capacity for thermogenesis; BAT consists of classical brown adipocytes to regulate body temperature in infants and children. Additionally, it has recently been discovered that beige cells act as regulators of energy homeostasis in adult humans. Humans experience an increase in body temperature during food intake. This phenomenon is called diet-induced thermogenesis (DIT). DIT causes an increase in heat production dependent on energy metabolism, as well as an increase in nutrient digestion and absorption. The former effect is due to sensory stimulation by non-energetic food components such as palatability, smell, and taste. Recently, it was found that DIT depends on the function of BAT through the transient receptor potential vanilloid 1 (TRPV-1) channel and the SNS in humans [Citation35]. For example, capsinoids, a non-pungent component of hot peppers, activate mouse [Citation36] and human [Citation37] BAT, increasing energy expenditure. This suggests that the activation of TRP by certain food components can be an effective means of maintenance and activation of BAT.
We previously showed that fish oil intake enhances energy expenditure and prevents fat accumulation and its related metabolic disorders by activation of UCP-1 in BAT [Citation38]. Recently, it was found that the activation of UCP-1 depends on the activation of the SNS via gastrointestinal TRPV-1 in WAT and BAT. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in fish oil stimulate the vagus nerve through TRPV-1 in the gut, activating the SNS via the hypothalamus, which then activates BAT through β-adrenergic receptors on brown adipocytes and beige cells (Figure ) [Citation39]. This novel function of EPA and DHA is a focus in the development of functional foods. Some other food components enhance BAT function through SNS and adrenergic receptors. For example, oleuropein from olive oil and polymethoxyflavone from black ginger (Kaempferia parviflora) are polyphenols, which result in a decrease in body weight and enhance whole-body energy consumption concurrently with UCP-1 expression in BAT [Citation40–42]. The dietary compounds responsible for BAT and UCP-1 activation are reviewed in greater detail elsewhere [Citation43].
Perspectives
Obesity is defined as an excessive accumulation of adipose tissue. It has been found that physiological differences in the regions where adipose tissue develops are closely associated with the pathogenesis of disease, and that the development of visceral adipose tissues connecting with the portal vein— such as mesenteric fat— leads to diabetes, hypertension, and arteriosclerotic diseases [Citation44]. These adipose tissues can be responsible for the development of metabolic syndrome. Therefore, future research into obesity and metabolic syndrome should focus on clarifying the mechanism underlying the novel regulation of metabolism— specifically in WAT and BAT— that lead to the development of diseases. Moreover, future studies should focus on analyzing food components and phytochemicals associated with the development of diseases and the metabolic properties of organs and tissues controlling energy storage and expenditure—such as adipose tissues— especially WAT containing beige cells. These results may then be used to create practical applications of such components. Metabolomics, which is defined as the comprehensive analysis of metabolites in a biological specimen, is an emerging technology that holds promise to inform the practice of precision food science and nutrition [Citation45–47]. Further longitudinal studies on human subjects are necessary to confirm the above ideas.
Disclosure statement
The author declares no conflict of interest.
Funding
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sport, Science and Technology of Japan [grant numbers 22228001, 16H02551, and 16K14927]; the Research Project on Development of Agricultural Products and Foods with Health-promoting benefits (NARO) Japan [grant number 2013-A-10].
Acknowledgements
I am most grateful to Drs. Nobuyuki Takahashi, Yuriko Oi-Kano, Shizuka Hirai, Tsuyoshi Goto, Tomoya Sakamoto, Haruya Takahashi, and Minji Kim as a confidential coworker. I would further like to express my appreciation to the all members of our research group, especially Ms. Sayoko Shinoto for secretarial assistance. Finally, I would like to express my thanks to the Japan Society for Bioscience, Biotechnology and Biochemistry for the JSBBA Award 2016.
Notes
This review was written in response to the author’s receipt of the JSBBA Award in 2016.
References
- Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781.10.1016/S0140-6736(14)60460-8
- Kawada T, Hagihara K, Iwai K. Effects of capsaicin on lipid metabolism in rats fed a high fat diet. J Nutr. 1986;116:1272–1278.
- Kawada T, Watanabe T, Takaishi T, et al. Capsaicin-induced beta-adrenergic action on energy metabolism in rats: influence of capsaicin on oxygen consumption, the respiratory quotient, and substrate utilization. Proc Soc Exp Biol Med. 1986;183:250–256.10.3181/00379727-183-42414
- Kawada T, Sakabe S-I, Watanabe T, et al. Some pungent principles of spices cause the adrenal medulla to secrete catecholamine in anesthetized rats. Exp Biol Med. 1988;188:229–233.10.3181/00379727-188-2-RC1
- Kawada T. Effect of food intake on diet-induced thermogenesis: its inductive signals and mechanism. Nippon Nōgeikagaku Kaishi. 1987;61:1462–1465.10.1271/nogeikagaku1924.61.1462
- Uemura T, Goto T, Kang M-S, et al. Diosgenin, the main Aglycon of fenugreek, inhibits LXRα activity in HepG2 cells and decreases plasma and hepatic triglycerides in obese. Diabetic Mice J Nutr. 2011;141:17–23.
- Moriwaki S, Murakami H, Takahashi N, et al. Yamogenin in fenugreek inhibits lipid accumulation through the suppression of gene expression in fatty acid synthesis in hepatocytes. Biosci Biotechnol Biochem. 2014;78:1231–1236.10.1080/09168451.2014.915736
- Kim YI, Hirai S, Goto T, et al. Potent PPARα activator derived from tomato juice, 13-oxo-9,11-octadecadienoic acid, decreases plasma and hepatic triglyceride in obese diabetic mice. PLoS ONE 7: e31317. DOI:10.1371/journal.pone.0031317.
- Hirose A, Terauchi M, Tamura M, et al. Tomato juice intake increases resting energy expenditure and improves hypertriglyceridemia in middle-aged women: an open-label, single-arm study. Nutr J. 2015;14:34. DOI:10.1186/s12937-015-0021-4.
- Matsuzawa Y. The metabolic syndrome and adipocytokines. FEBS Lett. 2006;580:2917–2921.10.1016/j.febslet.2006.04.028
- Goto T, Takahashi N, Hirai S, et al. Various terpenoids derived from herbal and dietary plants function as PPAR modulators and regulate carbohydrate and lipid metabolism. PPAR Res. 2010;2010:483958.
- Goto T, Kim YI, Takahashi N, et al. Natural compounds regulate energy metabolism by the modulating the activity of lipid-sensing nuclear receptors. Mol Nutr Food Res. 2013;57:20–33.10.1002/mnfr.v57.1
- Goto T, Nagai H, Egawa K, et al. Farnesyl pyrophosphate regulates adipocyte functions as an endogenous PPARgamma agonist. Biochem J. 2011;438:111–119.10.1042/BJ20101939
- Takahashi N, Senda M, Lin S, et al. Auraptene regulates gene expression involved in lipid metabolism through PPAR-alpha activation in diabetic obese mice. Mol Nutr Food Res. 2011;58:1791–1797.10.1002/mnfr.v55.12
- Goto T, Kim YI, Funakoshi K, et al. Farnesol, an isoprenoid, improves metabolic abnormalities in mice via both PPARα-dependent and -independent pathways. Am J Physiol – Endocrinol Metab. 2011;301:E1022–1032.
- Goto T, Kawada T. β-Cryptoxanthin directly and indirectly improves abnormalities of glucose and lipid metabolism through PPARγ and leptin in obese diabetic mice. Under submission.
- Yu R, Park JS, Kawada T, et al. Alteration of a macrophages inflammatory protein-related protein-2 (MRP-2) response by high fat and cholesterol diet in mice. Life Sci. 2002;70:2535–2545.10.1016/S0024-3205(02)01526-6
- Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830.10.1172/JCI200319451
- Yu R, Kim CS, Kwon BS, et al. Mesenteric adipose tissue-derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obesity. 2006;14:1353–1362.10.1038/oby.2006.153
- Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934.10.1038/nri2449
- Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505.10.1172/JCI26498
- Kim CS, Tu TH, Kawada T, et al. The immune signaling molecule 4-1BB stimulation reduces adiposity, insulin resistance, and hepatosteatosis in obese mice. Endocrinol. 2010;151:4725–4735.10.1210/en.2010-0346
- Le NH, Kim CS, Tu TH, et al. Blockade of 4-1BB and 4-1BBL interaction reduces obesity-induced skeletal muscle inflammation. Mediators Inflamm. 2012;2012:972629. DOI:10.1155/2012/972629.
- Lin S, Hirai S, Yamaguchi Y, et al. Taurine improves obesity-induced inflammatory responses and modulates the unbalanced phenotype of adipose tissue macrophages. Mol Nutr Food Res. 2013;57:2155–2165.10.1002/mnfr.v57.12
- Li Y, Goto T, Ikutani R, et al. Xanthoangelol and 4-hydroxyderrcin suppress obesity-induced inflammatory responses. Obesity. 2016;24:2351–2360.10.1002/oby.21611
- Yamamoto T, Li Y, Hanafusa Y, et al. Piceatannol exhibits anti-inflammatory effects on macrophages interacting with adipocytes. Food Sci Nutr. 2016;5:76–85.
- Kajimura S, Saito M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol. 2014;76:225–249.10.1146/annurev-physiol-021113-170252
- Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830.10.1172/JCI200319451
- Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25:2062–2068.10.1161/01.ATV.0000183883.72263.13
- van den Berg SM, van Dam AD, Rensen PC, et al. Immune modulation of brown(ing) adipose tissue in obesity. Endocr Rev. 2016 Nov 16:er20161066.10.1210/er.2016-1066
- Sakamoto T, Takahashi N, Sawaragi Y, et al. Inflammation induced by RAW macrophages suppresses UCP1 mRNA induction via ERK activation in 10T1/2 adipocytes. Am J Physiol Cell Physiol. 2013;304:C729–C738.10.1152/ajpcell.00312.2012
- Sakamoto T, Nitta T, Maruno K, et al. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 expression in mice. Am J Physiol Endocrinol Metab. 2016;310:E676–E687.
- Goto T, Naknukool S, Yoshitake R, et al. Proinflammatory cytokine interleukin-1beta suppresses cold- induced thermogenesis in adipocytes. Cytokine. 2016;77:107–114.10.1016/j.cyto.2015.11.001
- Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531.10.2337/db09-0530
- Saito M. Capsaicin and related food ingredients reducing body fat through the activation of TRP and brown fat thermogenesis. Adv Food Nutr Res. 2015;76:1–28. DOI:10.1016/bs.afnr.2015.07.002.
- Masuda Y, Haramizu S, Oki K, et al. Upregulation of uncoupling proteins by oral administration of capsiate, a nonpungent capsaicin analog. J Appl Physiol. 2003;95:2408–2415.10.1152/japplphysiol.00828.2002
- Yoneshiro T, Aita S, Kawai Y, et al. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am J Clin Nutr. 2012;95:845–850.10.3945/ajcn.111.018606
- Kawada T, Kayahashi S, Hida Y, et al. Fish (Bonito) oil supplementation enhances the expression of uncoupling protein in brown adipose tissue of rat. J Agric Food Chem 1998;46:1225–1227. 10.1021/jf9711000
- Kim MJ, Goto T, Yu R, et al. Fish oil intake induces UCP1 upregulation in brown and white adipose tissue via the 2 sympathetic nervous system. Sci. Rep. 2015;5:18013.
- Yoshino S, Kim M, Awa R, et al. Kaempferia parviflora extract increases energy consumption through activation of BAT in mice. Food Sci Nutr. 2014;634–637.10.1002/fsn3.2014.2.issue-6
- Oi-Kano Y, Iwasaki Y, Nakamura T, et al. Oleuropein aglycone enhances UCP1 expression in brown adipose tissue in high-fat-diet-induced obese rats by activating β-adrenergic signaling. J Nutr Biochem. 2016;40:209–218.
- Matsushita M, Yoneshiro T, Aita S, et al. Kaempferia parviflora extract increases whole-body energy expenditure in humans: roles of brown adipose tissue. J Nutr Sci Vitaminol. 2015;61:79–83.10.3177/jnsv.61.79
- Sakamoto T, Takahashi N, Goto T, et al. Dierary factors evoke thermogenesis in adipose tissues. Obesity Res Clin Pract. 2014;8:e533–e539.10.1016/j.orcp.2013.12.002
- Funahashi T, Matsuzawa Y. Metabolic syndrome: clinical concept and molecular basis. Ann Med. 2007;39:482–494.10.1080/07853890701491026
- Takahashi H, Goto T, Yamazaki Y, et al. Metabolomics reveal 1-palmitoyl lysophosphatidylcholine production by peroxisome proliferator-activated receptor α. J Lipid Res. 2015;56:254–265.10.1194/jlr.M052464
- Brennan L. Metabolomics in nutrition research – a powerful window into nutritional metabolism. Essays Biochem. 2016;60:451–458.10.1042/EBC20160029
- Cheung W, Keski-Rahkonen P, Assi N, et al. A metabolomic study of biomarkers of meat and fish intake. Am J Clin Nutr. 2017:600–608. DOI:10.3945/ajcn.116.146639.