Abstract
The C-3-OH, C-4 carbonyl oxygen and hydrogenation of C2=C3 bond on the C-ring of 2R,3R-dihydromyricetin (DMY) proved to be not necessary for the antibacterial activity against Staphylococcus aureus. DMY significantly decreased the intracellular ATP of S. aureus cells but had few effects on pHin, proline oxidation, succinate dehydrogenase activity or malate dehydrogenase activity.
Food-borne diseases are still an important and pressing issue facing the food industry and consumers worldwide. Staphylococcus aureus, a pathogenic Gram-positive bacterium, is one of the most common causes of food-borne diseases. To date, various chemical preservatives have been used to inhibit the growth of pathogenic bacteria in food. However, the growing negative awareness of consumers on synthetic preservatives coupled with the increasing demand for organic foods and natural products have promoted the development of natural antimicrobials as safer alternative preservatives.
Previously, we have demonstrated that 2R,3R-dihydromyricetin (DMY, 1, Figure ), a flavonoid isolated from Cedrus deodara pine needles, showed excellent inhibitory activity against S. aureus [Citation1]. As for the structure-activity relationship of flavonoids, there has been comparatively little work on the contribution of C-ring towards antibacterial activity. Also, whether the γ-pyrone structure at the C-ring of DMY is crucial to the antibacterial activity remains elusive. On the other hand, investigations on the antibacterial mechanism of DMY were mainly focused on common mode of action involving cytoplasmic membrane disruption. More importantly, it has been reported that the antibacterial activity of one certain flavonoid such as quercetin was attributed to multiple mechanisms [Citation2]. Thus, in this study, the influence of the γ-pyrone structure at the C-ring of DMY on the antibacterial activity was evaluated, and then the antibacterial effect of DMY on the cellular functions of S. aureus was further investigated.
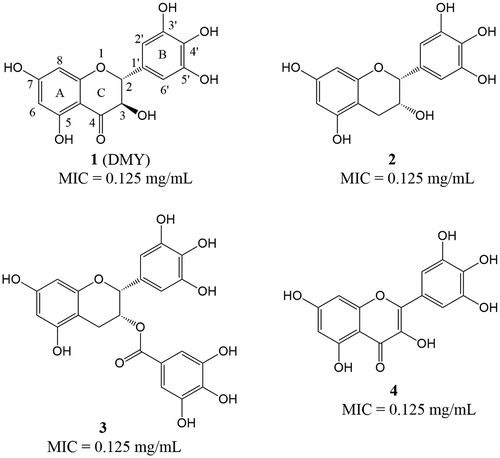
Figure 1. Structures of compounds 1-4 and their minimum inhibitory concentration (MIC) values against S. aureus ATCC 6538.
Staphylococcus aureus ATCC 6538 was purchased from the China Medical Culture Collection Center (Beijing, China). 2R,3R-Dihydromyricetin (HPLC purity ≥ 98%) was obtained from pine needles of Cedrus deodara according to our previous report [Citation3]. Flavonoids (HPLC purity ≥ 98%) including epigallocatechin (2), epigallocatechin-3-gallate (3) and myricetin (4) were purchased from Sigma Aldrich Co. (St. Louis, MO, USA).
The minimum inhibitory concentration (MIC) values of compounds 1-4 against S. aureus ATCC 6538 were determined by broth microdilution method as previously reported [Citation1]. As shown in Figure , all the test compounds exhibited potent inhibitory activity against S. aureus with a comparable MIC value of 0.125 mg/mL. The results showed that elimination of the carbonyl group at position 4 of DMY did not affect its activity when compared with 2, suggesting that the carbonyl group at position 4 on the C-ring was not essential. Similarly, the antibacterial activity of 2 was comparable to that of 3 with a substitution of gallic acid moiety, which might indicate that the C-3-OH on the C-ring of DMY was not important. However, this rationale should be further proven by comparing the MIC value of DMY with that of the analog whose structure differing only in without hydroxyl group at position 3 on the C-ring. Moreover, although a previous study has reported that the hydrogenation of C2=C3 bond reduces the antifungal activity of 5,7-dihydroxyflavonoids [Citation4], we found that the MIC value of DMY was comparable to that of 4 with C2=C3 bond. Considering that compound 4 bearing C2=C3 bond possesses a nearly planar skeleton structure, it could be speculated that the stereoscopic configuration of DMY probably did not affect its antibacterial activity. However, it is necessary to compare the antibacterial activities of the stereo isomers to evaluate the stereoscopic effect of DMY, because the stereospecific inhibition activity of flavanonols have been reported in a previous study [Citation5]. From the results herein, it is reasonable to propose that the presence of the C-3-OH, C-4 carbonyl oxygen and hydrogenation of C2=C3 bond on the C-ring of DMY were not essential for the antibacterial activity against S. aureus.
In addition, full understanding of the antibacterial mechanism of DMY was also necessary and important for its potential application in food industry. The level of intracellular ATP is an important parameter for assessing the available energy in a microorganism, which could be a potential target of antimicrobial agents. The intracellular ATP concentration of S. aureus was measured according to the previous reported method with minor modifications [Citation6]. The intracellular ATP concentration in the untreated S. aureus cells was detected to be 71.27 ± 3.65 nmol/L, whereas the addition of DMY at a concentration of 1 × MIC significantly decreased (p < 0.01) the intracellular ATP concentration from 71.27 ± 3.65 to 11.50 ± 6.25 nmol/L (Figure (A)). Obviously, a further reduction was observed along with increasing the concentration of DMY but not in a dose-dependent manner. The results were consistent with a recent study related to the effect of syringic acid on the intracellular ATP concentration of Cronobacter sakazakii [Citation6]. Previous studies have suggested that reduction in intracellular ATP might be due to decreased rate of ATP synthesis and/or increased rate of ATP hydrolysis inside the cells [Citation7] Moreover, depletion of internal ATP pool was demonstrated to be relevant to a change of the membrane potential [Citation8] Hence, the results herein were consistent with our previous findings about the membrane hyperpolarization effect of DMY on S. aureus [Citation1].
Figure 2. Effect of 2R, 3R-dihydromyricetin on the intracellular ATP concentration (A) and intracellular pH (B) of S. aureus ATCC 6538. Bars represent the standard deviation (n = 3). *p < 0.01.
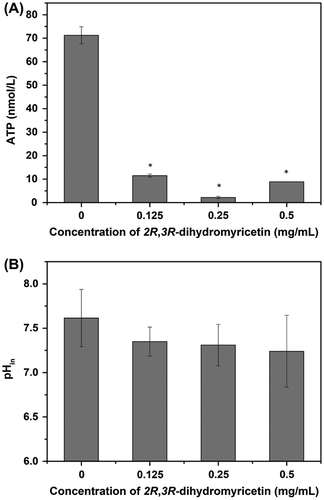
The depletion of intracellular ATP and hyperpolarization of cell membrane in the presence of DMY implied an ion gradient variation across the cell membrane. Consequently, the pHin of S. aureus cells after treatment with DMY was determined as the previous reported method [Citation6]. The pHin of normal S. aureus cells was found to be 7.61 ± 0.32 (Figure (B)). The addition of DMY caused a slight decrease in pHin of S. aureus, while no significant difference (p > 0.05) was observed between treated and untreated cells. Considering that membrane hyperpolarization primarily occurred due to the pH change or diffusion of ions, specifically K+ [Citation9], it could be speculated that the hyperpolarization effect of DMY on the membrane of S. aureus was predominantly achieved by increasing movement of other ions except for hydrogen ions.
In view of the significant role of succinate dehydrogenase (SDH) and malate dehydrogenase (MDH) in the biosynthesis of ATP, their activities were determined to explore whether the reduction of intracellular ATP was caused by the decreased ATP synthesis [Citation10]. However, there were minor changes in the SDH and MDH activities of DMY treated S. aureus cells, and no significant difference (p > 0.05) was observed even at high concentration (Figure S3). Therefore, it was indicated that DMY treatment nearly did not affect the bioenergy synthesis pathway of S. aureus via inhibiting SDH activity or MDH activity. Combined with these results, it was suggested that the reduction of intracellular ATP of S. aureus after treatment with DMY was more likely due to the increased rate of ATP hydrolysis and/or leakage of ATP with damaged cell membrane.
Previously, it has been suggested that phenolics could regulate cellular redox response through the proline-linked pentose phosphate pathway in bacterial model system [Citation11]. If DMY behaves as proline analogs, the addition of proline could overcome the inhibition of DMY with aromatic ring structure, therefore implying that the site of action of DMY could be proline dehydrogenase. The proline growth response assay was carried out as previously reported [Citation11]. However, our results showed that the inhibitory activity of DMY was not influenced by the addition of 0.5, 1 or 5 mM proline, regardless of the tested dose of DMY was 1 or 2 mg/disc (data not shown). So, the results indicated that DMY did not inhibit S. aureus by inhibition of proline oxidation via proline dehydrogenase.
In summary, we have demonstrated that the C-3-OH, C-4 carbonyl oxygen and hydrogenation of C2=C3 bond on the C-ring of DMY were not essential in eliciting the antibacterial activity against S. aureus. Additionally, studies of the cellular metabolism revealed that DMY significantly decreased the intracellular ATP in S. aureus cells but had few effects on pHin, proline oxidation, SDH activity or MDH activity. The results verified our previous proposal that DMY principally exerted antibacterial activity against S. aureus by damaging the cell membrane. However, it remains unclear which chemical group is the crucial structural moiety of DMY for the antibacterial activity. Moreover, the direct target molecules of DMY against S. aureus that result in the reduction of intracellular ATP need to be further investigated.
Authors contribution
H.G. and Y.-P.W. conceived and designed the experiments; Y.-P.W., J.-R.B. and D.-D.B. performed the experiments; Y.-P.W., K.Z and H.G. analyzed the data; Y.-N.H., K.X. and Y.R. contributed reagents and materials; Y.-P.W. and H.G. wrote the paper. All authors read and approved the final manuscript.
Supplemental data
Supplemental data for this article can be accessed https://doi.org/10.1080/09168451.2017.1413324.
Funding
This work was supported by the program for National Natural Science Foundation of China [grant number 31571936].
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary_data.docx
Download MS Word (145.2 KB)References
- Wu YP, Bai JR, Zhong K, et al. A dual antibacterial mechanism involved in membrane disruption and DNA binding of 2R,3R-dihydromyricetin from pine needles of Cedrus deodara against Staphylococcus aureus. Food Chem. 2017;218:463–470.10.1016/j.foodchem.2016.07.090
- Cushnie TPT, Lamb AJ. Recent advances in understanding the antibacterial properties of flavonoids. Int J Antimicrob Agents. 2011;38:99–107.10.1016/j.ijantimicag.2011.02.014
- Liang X, Wu YP, Qiu JH, et al. A potent antibrowning agent from pine needles of cedrus deodara: 2R,3R-Dihydromyricetin. J Food Sci. 2014;79:C1643–C1648.10.1111/jfds.2014.79.issue-9
- Yang S, Zhou J, Li D, et al. The structure-antifungal activity relationship of 5,7-dihydroxyflavonoids against Penicillium italicum. Food Chem. 2017;224:26–31.10.1016/j.foodchem.2016.12.001
- Jiang WJ, Ishiuchi K, Furukawa M, et al. Stereospecific inhibition of nitric oxide production in macrophage cells by flavanonols: synthesis and the structure-activity relationship. Bioorg Med Chem. 2015;23:6922–6929.10.1016/j.bmc.2015.09.042
- Shi C, Sun Y, Zheng Z, et al. Antimicrobial activity of syringic acid against Cronobacter sakazakii and its effect on cell membrane. Food Chem. 2016;197:100–106.10.1016/j.foodchem.2015.10.100
- Burt S. Essential oils: their antibacterial properties and potential applications in foods – a review. Int J Food Microbiol. 2004;94:223–253.10.1016/j.ijfoodmicro.2004.03.022
- Ultee A, Kets EP, Smid EJ. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 1999;65:4606–4610.
- Bot C, Prodan C. Probing the membrane potential of living cells by dielectric spectroscopy. Eur Biophys J. 2009;38:1049–1059.10.1007/s00249-009-0507-0
- Yao X, Zhu X, Pan S, et al. Antimicrobial activity of nobiletin and tangeretin against Pseudomonas. Food Chem. 2012;132:1883–1890.10.1016/j.foodchem.2011.12.021
- Ranilla LG, Apostolidis E, Shetty K. Antimicrobial activity of an Amazon medicinal plant (Chancapiedra) (Phyllanthus niruri L.) against Helicobacter pylori and lactic acid bacteria. Phytother Res. 2012;26:791–799.10.1002/ptr.v26.6
