Abstract
Carnosine (β-Ala-l-His), an imidazole dipeptide, is known to have many functions. Recently, we demonstrated in a double-blind randomized controlled trial that carnosine is capable of preserving cognitive function in elderly people. In the current study, we assessed the ability of carnosine to activate the brain, and we tried to clarify the molecular mechanisms behind this activation. Our results demonstrate that carnosine permeates the blood brain barrier and activates glial cells within the brain, causing them to secrete neurotrophins, including BDNF and NGF. These results point to a novel mechanism of carnosine-induced neuronal activation. Our results suggest that carnosine should be recognized as a functional food factor that helps achieve anti-brain aging.
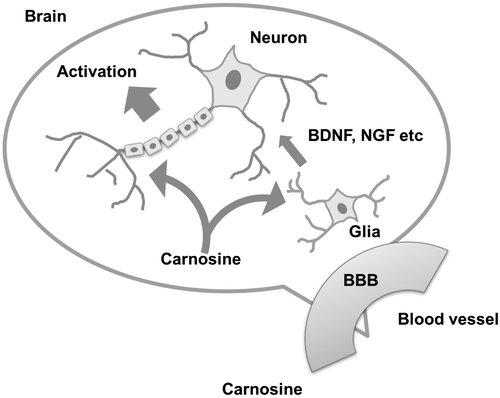
Graphical abstract
Schematic diagram of carnosine function in the brain.
Keywords:
Chicken breast meat contains large amounts of imidazole dipeptide (IDP) (about 1% of chicken breast meat), of which carnosine (β-alanyl-L-histidine) and its derivative anserine (β-alanyl-1-methyl-L-hisitidine) are two types. IDP is known to have an anti-fatigue effect [Citation1], to induce anti-depressant-like activity in rats [Citation2], and to enhance memory function in elderly people [Citation3]. Among IDPs, much research has focused on establishing the many functions of carnosine [Citation4–7]. For example, carnosine has been shown to prevent various diseases, including diabetes, neurodegenerative diseases, metabolic syndrome, and cancer [Citation8]. Furthermore, recent studies have suggested that carnosine is potentially beneficial in the treatment of Alzheimer’s disease [Citation9–11].
In the present study, we focused on the ability of carnosine to activate the brain. Although previous studies have revealed that carnosine activates brain function, the molecular mechanisms by which it does so remain unclear. Here, we tried to clarify the molecular basis for carnosine-induced activation of brain function.
Materials and methods
Cell culture and reagents
We used a human primary glioblastoma cell line, U-87 MG (ATCC, Manassas, VA, USA), and a human neuroblastoma cell line, SH-SY5Y (ATCC). U-87 MG cells were cultured in Eagle’s Minimum Essential Medium with Earle’s Balanced Salts (MEM-E; DS Pharma Biomedical, Osaka, Japan) with 10% fetal bovine serum (FBS; Life Technologies, Gaithersburg, MD, USA), 1% non-essential amino acid (DS Pharma Biomedical), and 1 mM sodium pyruvate (DS Pharma Biomedical). SH-SY5Y cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS. Both cell lines were cultured at 37 °C in 5% CO2.
Evaluation of BBB permeability
The blood brain barrier (BBB) permeability of carnosine (Wako, Osaka, Japan) was determined using a BBB kit (MBT-24H, PharmaCo-Cell, Nagasaki, Japan) according to the manufacturer’s instructions. Brain side and blood side was separated by membrane filter (pore size: 3.0 μm) embedded with Macaca irus-derived endothelial cells, Wistar rat-derived pericyte and astrocyte, which was constructed by using Millicell cell culture insert plate. This culture system was incubated for 3 days and test samples were added to the blood side in culture insert. Caffeine (Wako) was used as a positive control. Sodium fluoride (NaF, Wako) was used as a negative control. Permeated carnosine was detected using high performance liquid chromatography (SPD 10Vap; Shimadzu, Kyoto, Japan) equipped with a TSKgel ODS-80Ts column (4.6 × 150 mm, Tosoh Bioscience, Tokyo, Japan) eluted with 100 mM 1-pentansulfonic acid/200 mM ammonium dihydrogen phosphate/4% acetonitrile (pH was adjusted to 2.0) at a flow rate of 0.8 mL/min. Apparent permeability (Papp) was calculated using the following equation: Papp = (dQ/dT)/(A × C0), where dQ/dT is the cumulative amount in the receiver compartment vs. time, A is the surface of the filter, and C0 is the initial concentration of the tracer in the luminal compartments.
Differentiation of SH-SY5Y cells
As described elsewhere [Citation12,13], SH-SY5Y cells were cultured in DMEM medium supplemented with 10% FBS for 1 day, and cells were then cultured in DMEM medium supplemented with 10% FBS and 10 mM retinoic acid (Wako) for 5 days. After washing the cells with DMEM medium, cells were treated with 100 ng/mL of brain-derived neurotrophic factor (BDNF, Sigma, St Louis, MO, USA) and cultured in serum-free DMEM medium for an additional 6 days.
Quantitative reverse transcriptase polymerase chain reaction
After U-87 MG cells were treated with carnosine, total RNA was prepared using TRIzol reagent (Life Technologies, CA, USA) and purified using the SV total RNA isolation system (Promega, Madison, WI, USA). cDNA was prepared using the ReverTra Ace qPCR RT kit (Toyobo, Osaka, Japan) according to the manufacturers’ instructions [Citation14,15]. Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was performed using THUNDERBIRD SYBR qPCR Mix (Toyobo, Osaka. Japan) and CFX96 Touch Deep Well Real-time PCR System (Bio-Rad Laboratories, Hercules, CA, USA). PCR amplification began with a 180-s denaturation step at 95 °C followed by 39 cycles of denaturation at 95 °C for 10 s and annealing and extension at 55 °C for 30 s. Samples were normalized to the corresponding β-actin level. The following PCR primers were used: β-actin forward primer, 5′-TGGCACCCAGCACAATGAA-3′ and reverse primer, 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′; BDNF forward primer, 5′-GTTGGGAGCCTGAAATAGTG-3′ and reverse primer, 5′-AGGATGCTGGTCCAAGTGGTG-3′; GDNF forward primer, 5′-CCAACCCAGAGAATTCCAGA-3′ and reverse primer, 5′-TTTCATAGCCCAGACCCAAG-3′; NGF forward primer, 5′-CATCATCCCATCCCATCTTC-3′ and reverse primer, 5′-TTCACCTCTCCCAACACCATC-3′; NTF4 forward primer, 5′-AAGGCTGATAACGCTGAGGAAG-3′ and reverse primer, 5′-AATGCCCGCACATAGGACTG-3′.
BDNF ELISA
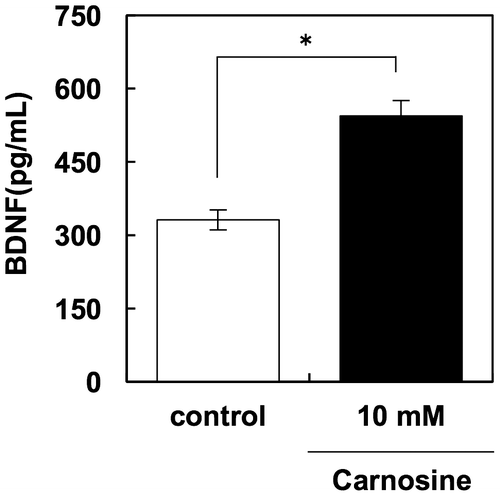
Cells were seeded at a density of 1 × 105 cells/well in a 12 well plate and were cultured for 24 h. After adding 10 mM carnosine, supernatant of U87 MG cells were collected, and the concentration of BDNF in the supernatant was determined using the BDNF Emax Immunoassay system (Promega) according to the manufacturer’s instructions.
Quantitative evaluation of neurite growth
SH-SY5Y cells were fixed with 4% paraformaldehyde for 30 min and then blocked with a blocking buffer (1 × PBS, 1% bovine serum albumin, 5% FBS, 0.2% Triron X-100) for 1 h. Next, cells were stained with a Milli-Mark FluoroPan Neuronal Marker (Merk Millipore, Billerica, MA, USA) at room temperature for 2 h. Neurite growth was quantitatively determined using a Neurite Outgrowth Assay kit (Merk Millipore), according to the manufacturer’s instructions [Citation16].
Statistical analysis
All results are expressed as mean ± standard deviation. Statistical differences were determined by two-sided Student’s t-tests, with a P-value of less than 0.05 considered as statistically significant.
Results
BBB permeability of carnosine and subsequent neuronal activation
Firstly, we evaluated the BBB permeability of IDP using a BBB kit. Caffeine and NaF were used as a positive and a negative control, respectively. As shown in Figure , carnosine and anserine efficiently permeated the BBB, as evidenced by the increased apparent permeability coefficient, Papp. These results suggested that carnosine and anserine can permeate the BBB and enter into the brain.
Figure 1. BBB permeability of IDP and activation of neuronal cells by carnosine. (A) BBB permeability of IDP. BBB permeability of IDP was assessed using a BBB kit and estimated by the resulting permeability coefficient (Papp). (B) Activation of SH-SY5Y cells by carnosine. Carnosine was added to SH-SY5Y cells, and neurite (arrow) growth was observed by fluorescence microscopy (BZ-X700, Keyence, Osaka, Japan).
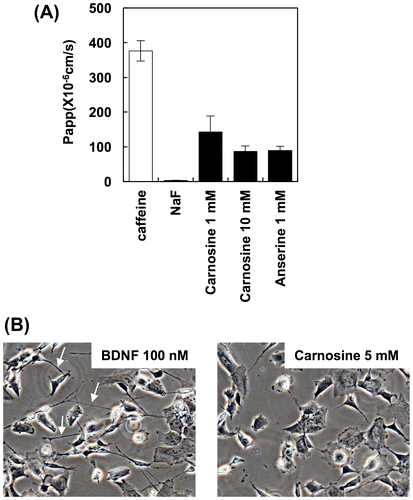
Next, we evaluated the ability of permeated carnosine to activate neuronal cells. As shown in Figure (B), carnosine treatment did not activate neurite growth in SH-SY5Y cells. Neurites indicated by arrow (Figure (A)) can not be observed in SH-SY5Y cells treated with carnosine (Figure (B)). These results suggest that permeated carnosine activates neuronal cells by different mechanisms.
Carnosine induces gene expression of neurotrophin in U-87 MG cells
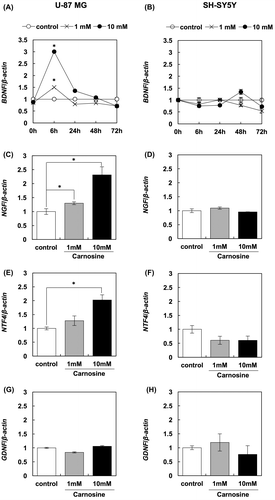
In order to investigate another possible mechanism of carnosine-dependent activation of neuronal cells, we evaluated changes in the expression of neurotrophin genes in glial and neuronal cells in response to carnosine treatment. Our results clearly show that carnosine treatment increased the expression of BDNF, NGF, and NTF4 in U-87 MG cells, but not in SH-SY5Y cells (Figure ). Furthermore, this enhancement of BDNF expression was confirmed in protein levels using a BDNF ELISA kit. These results show that 10 mM carnosine significantly increased the production of BDNF in U-87 MG cells (Figure ). Taken together, these results suggest that carnosine activates neuronal cells through increased production of neurotrophins in glial cells.
Figure 2. Carnosine augments the expression of neurotrophin genes. The effects of carnosine on expression of BDNF in U-87 MG cells (A) and SH-SY5Y cells (B) were evaluated by qRT-PCR. The effects of carnosine on the expression of NGF (C and D), NTF4 (E and F) and GDNF (G and H) in U-87 MG cells and SH-SY5Y cells, respectively, were also evaluated by qRT-PCR.
Supernatant of U-87 MG cells treated with carnosine induces neurite outgrowth in SH-SY5Y cells
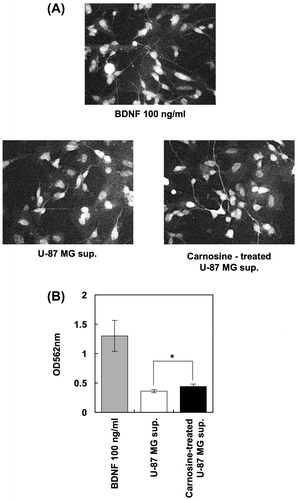
Because carnosine induced the production of neurotrophins in glial cells but not neuronal cells, we next evaluated whether the supernatant of U-87 MG cells treated with carnosine activated and induced neurite growth in SY-SY5Y cells. We found that the carnosine-treated U-87 MG supernatant induced neurite growth in SH-SY5Y cells (Figure ). These results suggest that carnosine activates and induces neurite growth in neuronal cells by stimulating the production of neurotrophins from glial cells.
Figure 4. Carnosine promotes neurite outgrowth in neuronal cells via glial cells. After U-87 MG cells were treated with 5 mM carnosine for 24 h, supernatant was collected and added to SH-SY5Y cells. BDNF (100 ng/mL) was used as a positive control. Cells were stained with Milli-Mark FluoroPan Neuronal Marker (A). Neurite length was determined using a Neurite Outgrowth Assay kit (B).
Discussion
Previously, we posited that carnosine activates brain function through an indirect mechanism [Citation11,17]. Specifically, we reported that carnosine activates intestinal epithelial cells and causes them to secrete various secretory factors that reach the brain and activate brain function. This mechanism was suggested by the observation that supernatant of Caco-2 cells treated with carnosine induced neurite growth in SH-SY5Y cells. Further studies have revealed that expression of several neurotrophins including BDNF, NGF, NT-4, and GDNF were augmented in Caco-2 cells treated with carnosine, and that BDNF participates in neurite growth in SH-SY5Y cells [Citation11]. Thus, we tested the expression of these neurotrophins in SH-SY5Y cells treated with carnosine. Results suggest that ingested carnosine augments the gene expression of neurotrophins, including BDNF, in the intestine and that these neurotrophins are carried by the blood stream to the brain and activate brain function.
In the present study, we put forth another molecular mechanism of carnosine activation of brain function. Our results demonstrate that carnosine can permeate the BBB and reach the brain, where it can go on to activate glial cells and cause them to secrete various neurotrophins, including BDNF and NGF. These glial cell-derived neurotrophins activate neuronal cells in an indirect manner in response to carnosine (Figure ).
Previously, we used DNA microarray analysis to identify several secretory factors produced by glial cells in response to carnosine (data not shown). In future studies, we hope to assess whether these secretory factors activate neuronal cells and to clarify the signaling network of these factors.
We previously demonstrated that carnosine and anserine supplementation is capable of preserving cognitive function in elderly people using a double-blind randomized controlled trial [Citation3]. Therefore, carnosine is expected to be recognized as a useful functional food factor. In the future, we hope to fully determine the detailed molecular mechanisms of carnosine-induced activation of brain function, and we would like to validate the possibility that carnosine should be accepted as a functional food factor that contributes to anti-brain aging.
Author contributions
S. Y., M. S. and T. M. conceived and designed the study. S. Y and K. K. performed experiments. The draft manuscript was written by Y. K., and T. H. and T. F. reviewed and revised the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
The authors thank Hiroko Hagiwara (Cell Inovator, Fukuoka, Japan) for performing data analysis that contributed to the study design.
References
- Maemura H, Goto K, Yoshioka T, et al. Effects of carnosine and anserine supplementation on relatively high Intensity endurance performance. Int J Sport Health Sci. 2006;4:86–94.
- Tomonaga S, Yamane H, Onitsuka E, et al. Carnosine-induced antidepressant-like activity in rats. Pharmacol Biochem Behav. 2008;89:627–632.10.1016/j.pbb.2008.02.021
- Hisatsune T, Kaneko J, Kurashige H, et al. Effect of anserine/carnosine supplementation on verbal episodic memory in elderly. J Alzheimers Dis. 2015;50:149–159.10.3233/JAD-150767
- Davey CL. The effects of carnosine and anserine on glycolytic reactions in skeletal muscle. Arc Biochem Biophys. 1960;89:296–302.10.1016/0003-9861(60)90058-8
- Chasovnikova LV, Formazyuk VE, Sergienko VI, et al. The antioxidative properties of carnosine and other drugs. Biochem Int. 1990;20:1097–1103.
- Johnson P, Aldstadt J. Effects of carnosine and anserine on muscle and non-muscle phosphorylases. Comp Biochem Physiol, B. 1984;78:331–333.10.1016/0305-0491(84)90039-7
- Batrukova MA, Rubtsov AM. Histidine-containing dipeptides as endogenous regulators of the activity of sarcoplasmic reticulum Ca-release channels. Biochim Biophys Acta. 1997;1324:142–150.10.1016/S0005-2736(96)00216-7
- Budzeń S, Rymaszewska J. The biological role of carnosine and its possible applications in medicine. Adv Clin Exp Med. 2013;22:739–744.
- Corona C, Frazzini V, Silvestri E, et al. Effects of dietary supplementation of carnosine on mitochondrial dysfunction, amyloid pathology, and cognitive deficits in 3xTg-AD mice. PLoS ONE. 2011;6:e17971.10.1371/journal.pone.0017971
- Herculano B, Tamura M, Ohba A, et al. β-alanyl-L-histidine rescues cognitive deficits caused by feeding a high fat diet in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2013;33:983–997.
- Kadooka K, Fujii K, Matsumoto T, et al. Mechanisms and consequences of carnosine-induced activation of intestinal epithelial cells. J Funct Foods. 2015;13:32–37.10.1016/j.jff.2014.12.024
- Encinas M, Iglesias M, Liu Y, et al. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem. 2000;75:991–1003.
- Jämsä A, Hasslund K, Cowburn RF, et al. The retinoic acid and brain-derived neurotrophic factor differentiated SH-SY5Y cell line as a model for Alzheimer’s disease-like tau phosphorylation. Biochem Biophys Res Commun. 2004;319:993–1000.10.1016/j.bbrc.2004.05.075
- Yamashita S, Ogawa K, Ikei T, et al. Biochem Biophys Res Commun. 2012;417:630–634.10.1016/j.bbrc.2011.12.021
- Yamashita S, Ogawa K, Ikei T, et al. FOXO3a potentiates hTERT gene expression by activating c-MYC and extends the replicative life-span of human fibroblast. PLoS ONE. 2014;9:e101864.10.1371/journal.pone.0101864
- Udono M, Kadooka K, Yamashita S, et al. Quantitative analysis of cellular senescence phenotypes using an imaging cytometer. Methods. 2012;56:383–388.10.1016/j.ymeth.2012.02.012
- Fujii K, Abe K, Kadooka K, et al. Carnosine activates the CREB pathway in Caco-2 cells. Cytotechnology. 2017;69:523–527.10.1007/s10616-017-0089-0