Abstract
Black tea is a highly popular beverage, and its pigments, polymerized catechins such as theaflavins (TFs), are attracting attention due to their beneficial health effects. In this study, to test the inhibitory activities of TFs on the intestinal absorption of cholesterol, we investigated their effects on phosphatidylcholine (PC) vesicles in the absence or presence of a bile salt. (−)-Epicatechin gallate, (−)-epigallocatechin gallate, and TFs formed insoluble complexes with PC vesicles. Galloylated TFs such as TF2A, TF2B, and TF3 precipitated far more than other polyphenols. The subsequent addition of taurocholate redispersed the polyphenol-PC complexes, except that a large amount of TF2A remained insoluble. After incubation with taurocholate-PC micelles, TF2A elevated the turbidity of the micelle solution, providing red sediments. The TF2A-specific effect was dependent on the PC concentration. These results suggest that TF2A interacts with PC and aggregates in a specific manner different from catechins and other TFs.
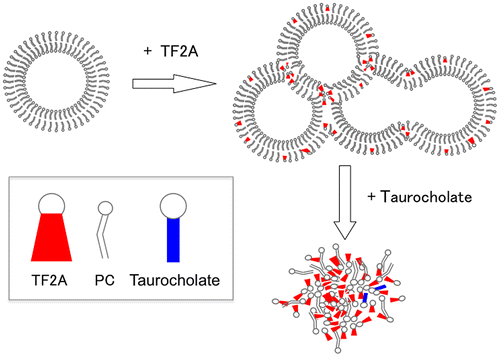
Specific interaction of TF2A with PC vesicles and the effects of subsequent addition of taurocholate.

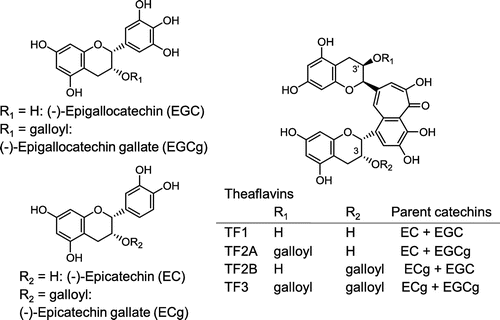
Theaflavins (TFs), the characteristic orange or orange-red pigments in black tea leaves and their exudates, contribute to the astringency of various kinds of brewed tea. TFs are produced during the fermentation of Camellia sinensis leaves, in which endogenous polyphenol oxidase and peroxidase are involved [Citation1,2]. Condensation of two oxidized catechins and decarboxylation afford TF production [Citation3,4]. Condensation of different pairs of catechins, one with a dihydroxylated B-ring (catechol-type) and the other with a trihydroxylated B-ring (pyrogallol-type), results in the formation of four kinds of TFs, theaflavin (TF1), theaflavin-3-O-gallate (TF2A), theaflavin-3′-O-gallate (TF2B), and theaflavin-3,3′-di-O-gallate (TF3) (Figure ). Although the concentration of TFs in black tea is fairly low, about 8–20 mg/100 mL of tea brewed from a teabag [Citation5], TFs have recently attracted attention due to their various bioactivities, such as antioxidant activity against LDL oxidation [Citation6], radical-scavenging activity [Citation7], anticancer activity [Citation8,9], antidiabetic effects [Citation10], and inhibitory activity against bone loss in models of osteoporosis [Citation11]. In addition, it has been reported that black tea intake lowers blood total and/or LDL cholesterol [Citation12]. Meta-analyses have also shown that black tea consumption effectively lowers serum LDL cholesterol, especially in hypercholesterolemia subjects [Citation13]. Some in vitro and in vivo studies have suggested that TFs decrease the micellar solubility of cholesterol [Citation14,15], probably resulting in the acceleration of steroid excretion in feces [Citation16]. However, the mechanisms of TF-induced decrease in micellar solubilization of cholesterol are still unclear.
In the small intestine, the mixed micellization of fat and cholesterol with secreted bile is necessary for their digestion and absorption [Citation17]. Bile consists mainly of phosphatidylcholine (PC) and bile salts [Citation18]. Green tea catechins, in particular (−)-epicatechin gallate (ECg) and (−)-epigallocatechin gallate (EGCg), bind strongly to PC-containing small vesicles [Citation19–21] and change the membrane structure [Citation19]. Due to such properties, they can also change micelle structures and are effective in decreasing the micellar solubility of cholesterol [Citation22]. Indeed, studies have shown significant decreases in serum cholesterol by the administration of green tea beverages or extracts [Citation23,24]. Attempts have subsequently been made to clarify the molecular mechanisms through which EGCg decreases the micellar solubilization of cholesterol. Kobayashi et al. [Citation25] found that EGCg exclusively eliminates PC from micelles via its specific interaction with PC rather than with cholesterol. On the other hand, Ogawa et al. [Citation26] suggested that the regiospecific interaction between EGCg and taurocholic acid lowers the solubility of PC and cholesterol in micellar solutions. Experiments using mixed micelles containing PC, bile salts, and cholesterol may make it difficult to analyze the molecular interactions related to the decrease in cholesterol micellar solubility owing to multiple confounding combinations.
We therefore investigated the effects of ECg, EGCg, and TFs not only on small PC vesicles but also on simple bile salt-PC micelles in the absence of cholesterol, focusing on their interaction with PC and taurocholic acid. We found that TF2A interacted with PC more specifically than other polyphenols, resulting in the formation of a bile salt-resistant precipitate. Since it is known that the interaction of (−)-epicatechin (EC) and (−)-epigallocatechin (EGC) with PC is quite weak compared to ECg and EGCg [Citation19,20], we did not investigate the effects of EC and EGC in this study.
Materials and methods
Materials
(−)-Epicatechin gallate (ECg) and (−)-epigallocatechin gallate (EGCg) were kindly provided by Mitsui Norin (Shizuoka, Japan). Phosphatidylcholine (PC) from egg yolk, phosphatidylethanolamine (PE) from egg yolk, phosphatidylserine (PS) from bovine brain, and sodium taurocholate were obtained from Sigma-Aldrich (St Louis, MO, USA). Purified TFs were prepared as described previously [Citation27]. All other reagents used were of analytical grade.
Preparation of small PC vesicles and bile salt-PC micelles
Egg PC was dissolved in a small amount of chloroform. When either egg PE or bovine PS was incorporated into PC micelles, chloroform-methanol (2:1 mixture) was used to dissolve and mix all phospholipids. The solution was put in a round-bottomed flask and dried with a vacuum pump. For small PC vesicles, the thin film of egg PC was suspended in 15 mM Na-phosphate buffer, pH 6.5, containing 132 mM NaCl [Citation15,25,26], and then it was sonicated in a cup-horn equipped with an ultrasonic disruptor (UD-211, Tomy Seiko, Tokyo, Japan) for 10 min at 50% of the duty cycle with 80% of output level. The suspension was passed through a 22G syringe needle of 150 mm length at least five times. Both sonication and passing through the syringe were repeated four times until the clarity of the suspension increased. Then the solution was centrifuged at 20,000 × g for 15 min to remove potential remaining vesicular aggregates. The supernatant was filtered through a 0.2 μm filter (GD/X PVDF, Whatman, Florham Park, NJ, USA), and the PC concentration in the filtrate was determined with a test kit from Wako Pure Chemical (Osaka, Japan) and adjusted to 3 mM. For preparation of micelles, the thin film of egg PC on the inner surface of the flask was suspended in buffer containing 6.6 mM Na-taurocholate [Citation15,25,26]. The suspension was sonicated for 10 min at 30% of the duty cycle with 50% of output level. The sonication step was repeated three times. The final PC concentration was adjusted to 3 mM. Both the small PC vesicles and bile salt-PC micelle solutions were stabilized for 20 h at 37 °C before use for the experiment.
Incubation of small PC vesicles with tea polyphenols followed by addition of bile salts
An aliquot (20 μL) of respective polyphenol solutions was mixed with 600 μL of the small PC vesicle solution. After the mixture was incubated for 30 min at 37 °C, 62 μL of 15 mM Na-phosphate buffer, pH 6.5, containing 132 mM NaCl in the absence or presence of 66 mM Na-taurocholate was added and further incubated for 30 min at 37 °C, where the final concentrations of PC and taurocholate were 2.6 and 6.6 mM, respectively. Then, the mixture was centrifuged at 5000 × g for 15 min at 25 °C. The supernatant (Sup) and precipitate (Ppt) fractions were used for RP-HPLC analyses as described below.
Incubation of bile salt-PC micelle solution with tea polyphenols
Solutions of catechins and TFs were individually prepared at 31 × final concentrations using 20% ethanol/pure water. Ten microliters of each polyphenol sample were put in a well of a 96-well microplate. After 300 μL of the bile salt-PC micelle solution or the taurocholate-containing buffer was added into the well, the microplate was incubated at 37 °C on a thermoshaker (PST-60HL plus, Biosan, Riga, Latvia). Absorbance at 595 nm (A595) of the mixture was measured using a microplate reader (Model 550, Bio-Rad Laboratories, Hercules, CA, USA). The turbidity was expressed as the difference of A595 values between the polyphenol-micelle mixture and the polyphenol in taurocholate-containing buffer solution.
After a 60-min incubation at 37 °C, 80 μL of the mixture was diluted with 1 mL of 50% ethanol/25 mM citric acid, and this sample was used to determine the total concentration of polyphenol by RP-HPLC analysis. An aliquot (150 μL) of the remaining mixture was centrifuged at 3000 × g for 10 min at 25 °C. The Sup and Ppt were also diluted with 50% ethanol/25 mM citric acid and analyzed by RP-HPLC. RP-HPLC was carried out using a Hitachi HPLC system (L-2130 pump, L-2400 UV detector) equipped with a SUS line filter (GL Science, Tokyo, Japan) and a Phenomenex SynergiTM 4 μm Polar-RP 80Å (4.6 × 150 mm) column (Shimadzu GLC, Tokyo, Japan). Forty microliters of the sample were injected. Catechins were eluted using an aqueous 20% MeCN solution containing 0.05% phosphoric acid at a flow rate of 1 mL/min, and were detected at 280 nm. For TF1, TF2A, TF2B, and TF3, aqueous 34% MeCN solutions containing 0.05% phosphoric acid were used as eluents at a flow rate of 1 mL/min, and each retention time (tR) was identified using the TF standards, which were obtained as described previously [Citation27].
The remaining Sup, which was still not diluted, was used to determine PC and taurocholic acid concentrations by RP-HPLC. For PC analysis, an aliquot (60 μL) of the Sup was injected to a Cosmosil Cholester (4.6 × 150 mm) column (Nacalai Tesque, Kyoto, Japan), and the PC molecules were eluted at tR from 3.5 to 15 min with methanol at a flow rate of 1 mL/min and detected at 220 nm. For analysis of taurocholic acid, an aliquot (50 μL) of the Sup was injected to a Phenomenex SynergiTM 4 μm Polar-RP 80Å (4.6 × 150 mm) column, and the bile acid was eluted at tR of 6.8 min with 25 mM NaH2PO4/40% MeCN at a flow rate of 1 mL/min and detected at 220 nm. The analysis of chromatograms was performed with the data processing software Chromato-PRO (Run Time Corporation, Tokyo, Japan).
Statistical analysis
Statistical analyses were performed using the StatPlus®:mac LE2009 software.
Results and discussion
TF2A forms specific precipitates resistant against bile salts
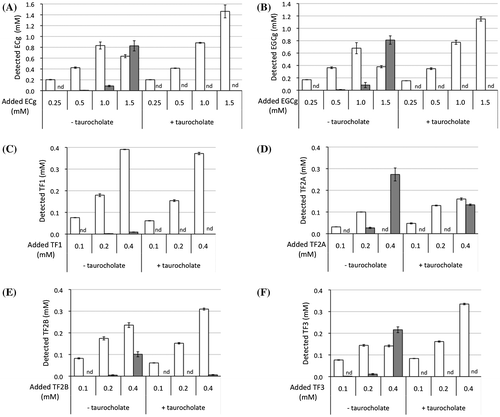
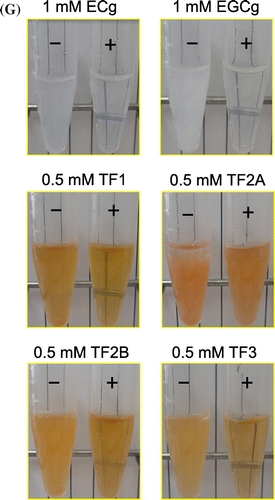
While incubating small PC vesicles prepared in the absence of taurocholate with ECg, EGCg, or four kinds of TFs for 30 min at 37 °C, most samples, except for TF1, became cloudy and the polyphenol and PC concentrations in the Sup significantly decreased (Figures and ). Incubation of PC vesicles with 1 mM TF1 provided orange sediments, and TF1 in the Sup also decreased to 0.4 mM (data not shown). A comparison of the dose-dependent effects of these polyphenols suggested that TF2A most strongly interacted with PC. Although ECg and EGCg have already been reported to bind to the surface of PC vesicles [Citation19–21], there is little information on the interaction of TFs with simple liposomal PC membranes despite their anticancer, anticholesterol, and hypolipidemic effects observed in experiments using animals and cultured cells [Citation15,28–30]. Since galloyl groups seem to contribute to the membrane-binding properties of catechins through enhancing hydrophobic interactions [Citation19–21], it is not surprising that higher amounts of mono- and di-gallated TFs other than TF1 precipitated after incubation with 3 mM PC vesicles (Figures (C–F) and (C–F)). Comparison of effects of three gallated TFs suggests that the 3-position, but not the number, of galloyl group in TF2A and TF3 is required for a strong interaction with PC. However, these precipitates, except for TF2A, drastically diminished after the subsequent addition of taurocholate, releasing almost all polyphenols (Figure ) as well as PC (Figure ) into the Sup fraction. In the case of TF2A at 0.4 mM, TF2A and PC partially precipitated in the presence of taurocholate (Figures (D) and (D)). This suggests the existence of TF2A-PC complexes resistant to the detergent action of taurocholate, to which a highly specific interaction between TF2A and PC contributes (Scheme ). Even at concentrations lower than 0.2 mM, TF2A might induce rearrangement of PC vesicles, probably being incorporated to the TF2A-PC complexes below the limit of detection. A slight amount of TF2B remained in the Ppt fraction after the addition of taurocholate (Figure (E)), meanwhile PC was not precipitated (Figure (E)). In contrast to the case with simple PC vesicles, both TF3 and PC were completely solubilized by addition of taurocholate (Figures (F) and (F)). What roles the mono-galloyl group at 3-position of TF2A plays in the specific interaction with PC in the presence of taurocholate are still unclear.
Figure 2. Distribution of tea polyphenols (A: ECg, B: EGCg, C: TF1, D: TF2A, E: TF2B, and F: TF3) in the Sup (open bars) and Ppt (closed bars) fractions after a 30-min incubation with PC vesicles at 37 °C (left), and their redispersion by subsequent addition of taurocholate and a 30-min incubation at 37 °C (right). Data are means ± SD (n = 3) (nd: not detected). Total concentrations of polyphenols in the Sup and Ppt fractions were lower than those added into samples due to the instability of polyphenols during incubation at 37 °C for 60 min. Images of the PC vesicle solutions treated with respective polyphenols in the absence (–) or presence (+) of taurocholate are shown (G).
Figure 3. Precipitation of PC after incubation with tea polyphenols (A: ECg, B: EGCg, C: TF1, D: TF2A, E: TF2B, and F: TF3) at 37 °C (left), and their redispersion by subsequent addition of taurocholate (right). Data are means ± SD (n = 3). *Significantly lower than control (p < 0.05).
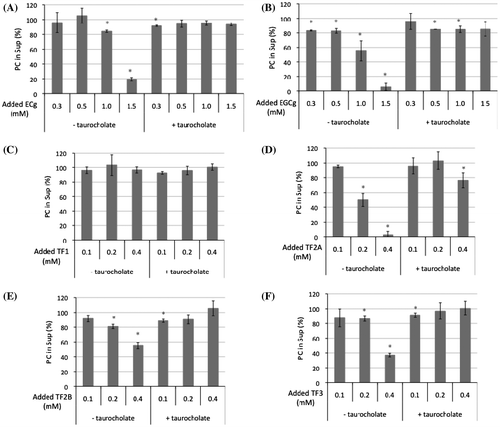
Figure 4. Effects of tea polyphenols on taurocholate-PC micelles. (A) Changes in turbidity of the micelle solution during incubation with tea polyphenols at 37 °C. Data for ECg (open circles), EGCg (closed circles), TF1 (open triangles), TF2A (closed triangles), TF2B (open squares), TF3 (closed squares), and control (x marks) are means ± SD (n = 3). (B) Distribution of tea polyphenols in the Sup (open bars) and Ppt (closed bars) fractions after a 60-min incubation (nd: not detected). Data are means ± SD (n = 3).
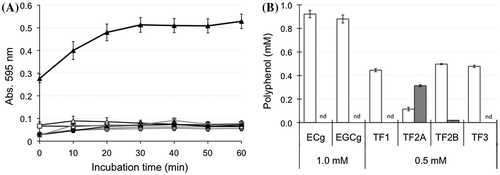
Figure 5. Effects of phospholipid composition on the interaction between TF2A and bile salt-PC micelles. (A) Changes in turbidity of the micelle solution during incubation with 0.5 mM TF2A at 37 °C. Data for PC micelles (open circles), PC:PE (closed circles), and PC:PS (open triangles) are means ± SD (n = 3). (B) Distribution of TF2A in the Sup (open bars) and Ppt (closed bars) fractions after a 60-min incubation. Data are means ± SD (n = 3). *Significantly different from PC (p < 0.05).
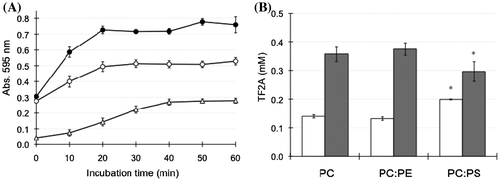
Figure 6. Effects of PC concentration on the interaction between TF2A and bile salt-PC micelles. (A) PC-dependent elevation of turbidity of micelle solutions incubated with 0.5 mM TF2A for 60 min at 37 °C. Data are means ± SD (n = 3). (B) Distribution of TF2A in the Sup (open bars) and Ppt (closed bars) fractions after a 60-min incubation. Data are means ± SD (n = 3).
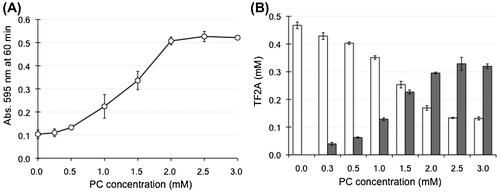
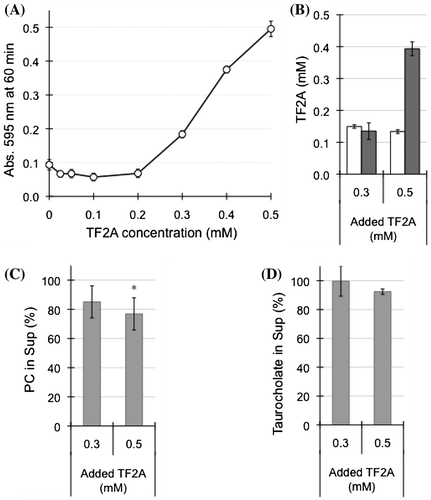
Figure 7. Effects of TF2A concentration on the interaction between TF2A and bile salt-PC micelles. (A) TF2A-dependent elevation of turbidity of micelle solutions containing 3 mM PC incubated with various concentrations of TF2A for 60 min at 37 °C. Data are means ± SD (n = 3). (B) Distribution of TF2A in the Sup (open bars) and Ppt (closed bars) fractions after a 60-min incubation. Data are means ± SD (n = 3). (C) Residual PC in the Sup fraction (% of total PC in the micelle solution) after a 60-min incubation with 0.3 and 0.5 mM TF2A. Data are means ± SD (n = 3). (D) Residual taurocholate in the Sup fraction (% of total taurocholate in the micelle solution) after a 60-min incubation with 0.3 and 0.5 mM TF2A. Data are means ± SD (n = 3). *Significantly lower than control (p < 0.05).
TF2A induces an increase in the turbidity of bile salt-PC micelle solutions
Reflecting the detergent action of taurocholate, the clarity of PC vesicle solutions in the absence of any polyphenols increased (data not shown). When taurocholate-PC micelle solutions were incubated with 1 mM of catechins (ECg and EGCg) or 0.5 mM of TFs (TF1, TF2A, TF2B, and TF3), only TF2A significantly increased the turbidity of the sample solution (Figure (A)). The turbidity increased with incubation time and reached a plateau at about 30 min (Figure (A)). After a 60-min incubation, an orange-red precipitate was obtained by centrifugation of the TF2A-treated micelle solution, and TF2A proved to be in the insoluble fraction (Figure (B)). Meanwhile, ECg, EGCg, and other TFs did not increase the turbidity of the micelle solutions, though a slight amount of TF2B was detected in a minor precipitate (Figure (B)).
To compare micelles with different compositions of phospholipids, 10% of PC was replaced with PE or PS. As a result, we observed that the polyphenols used in the present study, except for TF2A, did not provide precipitates in micelle solutions (data not shown). Immediately after the addition of TF2A, the micelle solution of PC and PE (PC:PE) became more distinctively turbid than that of only PC (Figure (A)). However, the amounts of TF2A present in both Sup and Ppt fractions were not significantly different between PC and PC:PE samples (Figure (B)). These results suggest that despite similar amounts of TF2A in each micelle, the structural patterns of these two kinds of micelles rearranged by TF2A are distinguishable from each other. In contrast, PS-incorporated micelles (PC:PS) suppressed both the TF2A-induced elevation of turbidity (Figure (A)) and the amount of TF2A in the precipitate (Figure (B)). This is probably due to electrostatic repulsion between TF2A and the micelle surface with a negative charge present in the head-group of PS [Citation20].
To investigate whether the precipitation of TF2A was dependent on PC concentration, micelle solutions of various PC concentrations were prepared and incubated with 0.5 mM TF2A. During the incubation of TF2A in a buffer containing only taurocholate (0 mM PC), no change in the state of sample solution was observed. As the PC concentration increased, the turbidity of the micelle solution was elevated accompanied by an increase of TF2A in the Ppt fractions (Figure (A and B)). Such effects were saturated at higher than 2 mM PC, even though TF2A still remained in the Sup fraction at around 0.15 mM (Figure (B)). On the other hand, the addition of various concentrations of TF2A into micelle solutions containing 3 mM PC elevated the solution turbidity as TF2A increased (Figure (A)). However, the dose-response showed a sigmoidal curve with a potent critical point at 0.2 mM (Figure (A)). TF2A in the Sup fraction remained around at 0.15 mM in samples containing both 0.3 and 0.5 mM TF2A (Figure (B)), confirming that TF2A concentrations higher than 0.2 mM are required for its aggregation in bile salt-PC micelle solutions. The critical dose of TF2A required for precipitation in the presence of both PC and taurocholate as shown in Figure (A) is similar to that shown in Figure (D). Concentrations of PC and taurocholic acid in the Sup fractions were partially reduced by the addition of TF2A (Figure (C and D)), but the effects did not significantly depend on TF2A concentration. These results suggest that the TF2A-PC complex formed by TF2A at around 0.2 mM in the presence of taurocholate forms a core to attract free TF2A molecules for non-covalent self-assembly (Scheme ).
Although the PC and taurocholate contents in the final precipitate were low, the TF2A-specific interaction with PC might be related to the inhibition of micellar solubilization of cholesterol [Citation14,15]. In addition, a single oral administration of TFs elevates cremasteric blood flow in rats, and the magnitude of this effect is in the order of TF2B >> TF2A >> TF1 = TF3 [Citation31]. The mechanism underlying this phenomenon remains unclear. However, the TF2A aggregation in the bile-like mixture observed in this study may be one reason for the difference in activity between TF2A and TF2B. Once TF2A has formed insoluble complexes with bile, it would be difficult to exert its biological activity through the epithelium in the gastrointestinal tract.
The 8–20 mg of total TFs in a 100-mL tea brewed from a teabag [Citation5] are estimated to be at a concentration of 0.11–0.27 mM using the molecular weight of TF mono-gallate. Their low bioavailability [Citation32] suggests that they are bioactive in the mouth and gastrointestinal tract. Since TF2A accounts for average 25% of TFs in the black tea leaves [Citation33,34], the concentrations of TF2A we used in this study may be somewhat higher than those in the intestine of human drinking black tea. However, it is possible that TF2A at lower concentration perturbs the emulsification of lipids and cholesterol in the intestine by specific interaction with PC from either foods or bile acid. Furthermore, it is expected that TFs and other tea polyphenols coexisting in a regular cup of tea at even lower concentrations additively or synergistically strengthen their PC-binding activities, given that the interactions of ECg and EGCg with liposomal PC membranes are remarkably enhanced in the presence of EC [Citation20]. In contrast, the possibility cannot be denied that the interaction of TFs with PC is canceled by their binding to some carbohydrates [Citation35] and proteins [Citation36,37] in the intestinal tract. Further investigation into the effects of other food ingredients on TF2A should be undertaken.
Conclusion
In conclusion, TF2A specifically interacted with PC and with taurocholate-PC micelles resulting in an insoluble complex involving PC and taurocholate. This may be related to the ability of TF2A to lower the micellar solubility of cholesterol [Citation15]. Since micelles prepared with cholesterol as well as other bile salts have altered lipid membrane fluidity and rigidity [Citation38,39], the pattern of TF2A-induced rearrangement of micelles and the resulting structure would be different from that observed in this study. Electron microscopic analyses by Vermeer et al. [Citation14] confirmed that TF2A at 0.4 mM, but not three other TFs, affords precipitates consisting of multilamellar vesicles, though they used vesicles consisting of mono-olein, oleic acid, lyso-phosphatidylcholine, cholesterol, and a bile salt mix. Thus, it is possible that such a drastic reconstruction of bile salt-PC micelles occurs during incubation with TF2A. Further investigations using mimetic models of bile and intestinal micelles are necessary to clarify the inhibitory effects of TF2A and/or black tea on cholesterol absorption.
Author contribution
A.Narai-Kanayama designed the study, and wrote the initial draft of the manuscript. K. Saruwatari, N. Mori, and A. Narai-Kanayama contributed to analysis and interpretation of data, and assisted in the preparation of the manuscript. T. Nakayama has contributed to data collection and interpretation, and critically reviewed the manuscript. All authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by a Grant from the Cross-Ministerial Strategic Innovation Promotion Program (SIP), Urgent Project for Development and Diffusion of Innovative Technology towards Realization of the Aggressive Agriculture, Forestry, and Fisheries.
Acknowledgement
We thank Prof. Ikuo Ikeda of Tohoku University for his kind advice for how to prepare bile salt-PC micelle solutions.
References
- Subramanian N, Venkatesh P, Ganguli S, et al. Role of polyphenol oxidase and peroxidase in the generation of black tea theaflavins. J Agric Food Chem. 1999;47:2571–2578.
- Verloop AJW, Vincken JP, Gruppen H. Peroxidase can perform the hydroxylation step in the “oxidative cascade” during oxidation of tea catechins. J Agric Food Chem. 2016;64:8002–8009.
- Tanaka T, Inoue K, Betsumiya Y, et al. Two types of oxidative dimerization of the black tea polyphenol theaflavin. J Agric Food Chem. 2001;49:5785–5789.
- Tanaka T, Mine C, Inoue K, et al. Synthesis of theaflavin from epicatechin and epigallocatechin by plant homogenates and role of epicatechin quinone in the synthesis and degradation of theaflavin. J Agric Food Chem. 2002;50:2142–2148.
- Henning SM, Fajardo-Lira C, Lee HW, et al. Catechin content of 18 teas and a green tea extract supplement correlates with the antioxidant capacity. Nutr Cancer. 2003;45:226–235.
- Leung LK, Su Y, Chen R, et al. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J Nutr. 2001;131:2248–2251.
- Yang Z, Tu Y, Xia H, et al. Suppression of free-radicals and protection against H2O2-induced oxidative damage in HPF-1 cell by oxidized phenolic compounds present in black tea. Food Chem. 2007;105:1349–1356.
- Kobalka AJ, Keck RW, Jankun J. Synergistic anticancer activity of biologicals from green and black tea on DU 145 human prostate cancer cells. Cent Eur J Immunol. 2015;40:1–4.
- GAO Y, RANKIN GO, TU Y, et al. Theaflavin-3, 3′-digallate decreases human ovarian carcinoma OVCAR-3 cell-induced angiogenesis via Akt and Notch-1 pathways, not via MAPK pathways. Int J Oncol. 2016;48:281–292.
- Matsui T, Tanaka T, Tamura S, et al. α-glucosidase inhibitory profile of catechins and theaflavins. J Agric Food Chem. 2007;55:99–105.
- Nishikawa K, Iwamoto Y, Kobayashi Y, et al. DNA methyltransferase 3a regulates osteoclast differentiation by coupling to an S-adenosylmethionine–producing metabolic pathway. Nat Med. 2015;21:281–287.
- Davies MJ, Judd JT, Baer DJ, et al. Black tea consumption reduces total and LDL cholesterol in mildly hypercholesterolemic adults. J Nutr. 2003;133:3298S–3302S.
- Zhao Y, Asimi S, Wu K, et al. Black tea consumption and serum cholesterol concentration: Systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2015;34:612–619.
- Vermeer MA, Mulder TP, Molhuizen HO. Theaflavins from black tea, especially theaflavin-3-gallate, reduce the incorporation of cholesterol into mixed micelles. J Agric Food Chem. 2008;56:12031–12036.
- Ikeda I, Yamahira T, Kato M, et al. Black-tea polyphenols decrease micellar solubility of cholesterol in vitro and intestinal absorption of cholesterol in rats. J Agric Food Chem. 2010;58:8591–8595.
- Miyata Y, Tanaka T, Tamaya K, et al. Cholesterol-lowering effect of black tea polyphenols, theaflavins, theasinensin a and thearubigins, in rats fed high fat diet. Food Sci Technol Res. 2011;17:585–588.
- Woollett LA, Wang Y, Buckley DD, et al. Micellar solubilisation of cholesterol is essential for absorption in humans. Gut. 2006;55:197–204.
- Alvaro D, Cantafora A, Attili AF, et al. Relationships between bile salts hydrophilicity and phospholipid composition in bile of various animal species. Comp Biochem Physiol B. 1986;83:551–554.
- Kajiya K, Kumazawa S, Nakayama T. Steric effects on interaction of tea catechins with lipid bilayers. Biosci Biotechnol Biochem. 2001;65:2638–2643.
- Kajiya K, Kumazawa S, Nakayama T. Effects of external factors on the interaction of tea catechins with lipid bilayers. Biosci Biotechnol Biochem. 2002;66:2330–2335.
- Uekusa Y, Kamihira M, Nakayama T. Dynamic behavior of tea catechins interacting with lipid membranes as determined by NMR spectroscopy. J Agric Food Chem. 2007;55:9986–9992.
- Ikeda I, Imasato Y, Sasaki E, et al. Tea catechins decrease micellar solubility and intestinal absorption of cholesterol in rats. Biochim Biophys Acta. 1992;1127:141–146.
- Kim A, Chiu A, Barone MK, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011;111:1720–1729.
- Zheng XX, Xu YL, Li SH, et al. Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta-analysis of 14 randomized controlled trials. Am J Clin Nutr. 2011;94:601–610.
- Kobayashi M, Nishizawa M, Inoue N, et al. Epigallocatechin gallate decreases the micellar solubility of cholesterol via specific interaction with phosphatidylcholine. J Agric Food Chem. 2014;62:2881–2890.
- Ogawa K, Hirose S, Nagaoka S, et al. Interaction between tea polyphenols and bile acid inhibits micellar cholesterol solubility. J Agric Food Chem. 2016;64:204–209.
- Matsuura K, Usui Y, Kan T, et al. Structural specificity of electric potentials in the coulometric-array analysis of catechins and theaflavins. J Clin Biochem Nutr. 2014;55:103–109.
- Kaur S, Greaves P, Cooke DN, et al. Breast cancer prevention by green tea catechins and black tea theaflavins in the C3(1) SV40 T,t antigen transgenic mouse model is accompanied by increased apoptosis and a decrease in oxidative DNA adducts. J Agric Food Chem. 2007;55:3378–3385.
- Adhikary A, Mohanty S, Lahiry L, et al. Theaflavins retard human breast cancer cell migration by inhibiting NF-kappaB via p53-ROS cross-talk. FEBS Lett. 2010;584:7–14.
- Lin CL, Huang HC, Lin JK. Theaflavins attenuate hepatic lipid accumulation through activating AMPK in human HepG2 cells. J Lipid Res. 2007;48:2334–2343.
- Saito A, Nakazato R, Suhara Y, et al. The impact of theaflavins on systemic-and microcirculation alterations: The murine and randomized feasibility trials. J Nutr Biochem. 2016;32:107–114.
- Mulder TP, van Platerink CJ, Wijnand Schuyl PJ, et al. Analysis of theaflavins in biological fluids using liquid chromatography-electrospray mass spectrometry. J Chromatogr B Biomed Sci Appl. 2001;760:271–279.
- Wright LP, Mphangwe NIK, Nyirenda HE, et al. Analysis of the theaflavin composition in black tea (Camellia sinensis) for predicting the quality of tea produced in Central and Southern Africa. J Sci Food Agric. 2002;82:517–525.
- Sakamoto A, Inoue H, Nakagawa M. Content of chemical constituents of 12 kinds of black tea. Nippon Shokuhin Kagaku Kogaku Kaishi. 2012;59:326–330.
- Hara Y, Honda M. The inhibition of α-amylase by tea polyphenols. Agric Biol Chem. 1990;54:1939–1945.
- Yamazaki T, Sagisaka M, Ikeda R, et al. The human bitter taste receptor hTAS2R39 is the primary receptor for the bitterness of theaflavins. Biosci Biotechnol Biochem. 2014;78:1753–1756.
- Lei S, Xu D, Saeeduddin M, et al. Characterization of molecular structures of theaflavins and the interactions with bovine serum albumin. J Food Sci Technol. 2017;54:3421–3432.
- Eckhardt ERM, Moschett A, Renooij W, et al. Asymmetric distribution of phosphatidylcholine and sphingomyelin between micellar and vesicular phases: potential implications for canalicular bile formation. J Lipid Res. 1999;40:2022–2033.
- Njauw CW, Cheng CY, Ivanov VA, et al. Molecular interactions between lecithin and bile salts/acids in oils and their effects on reverse micellization. Langmuir. 2013;29:3879–3888.