Abstract
A co-cultivation study of two fungal strains showed that Aspergillus ustus could inhibit Aspergillus repens growth. The bioactive compound responsible for the observed activity was purified and identified as a sesterterpene, ophiobolin K. Ophiobolin K exhibited marked inhibition against both fungi and bacteria, especially A. repens, A. glaucus and gram-positive bacteria including Bacillus subtilis, Staphylococcus aureus, and Micrococcus luteus.
We previously reported that phenylahistin (PLH), an anti-mitotic metabolite produced by the fungus Aspergillus ustus, could be converted to its dehydro derivative (ΔPLH) by enzymes present in cell-free extracts of the actinomycete Streptomyces albulus, a producer of the dehydro cyclic dipeptide (CDP), albonoursin [Citation1]. We showed that dehydrophenylahistin (ΔPLH) exhibited 1000-fold greater anti-mitotic activity in the sea urchin eggs development bioassay than PLH. We speculated that co-cultivation of A. ustus and S. albulus might result in the production of ΔPLH. The result showed that the interaction of two CDP producing strains led to the production of ΔPLH, indicating that co-cultivation of CDP-producing organisms can be a more efficient means of producing bioactive CDPs than single-strain approaches.
Based on this finding, we speculated that co-cultivation of two CDP-producing fungal strains, A. ustus and A. repens [Citation2], may result in the production of metabolites with improved bioactivity compared with those obtained from single culture. Interestingly, co-cultivation resulted in the potent inhibition of A. repens growth, leading us to investigate the bioactive compounds produced by A. ustus that could inhibit A. repens growth.
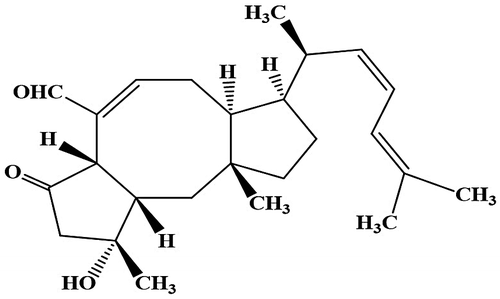
In the present study, an antifungal compound produced by A. ustus was identified via a spore germination assay using A. repens as the test fungi (Supplemental materials). Mycelial cultures of A. ustus [Citation3] on Petri dishes (90 × 15 mm, n = 54) were separated and extracted with EtOAc. The EtOAc layer was concentrated under vacuum, which was subjected to reverse-phase chromatography using an Oasis® HLB cartridge (Oasis HLB 60 μm, 6 cc, 500 mg) with methanol (MeOH) stepwise elution to provide three fractions (60% MeOH, 80% MeOH and 100% MeOH). An aliquot of each fraction was evaluated for antifungal activity. The most active fraction (80% MeOH fraction, 29.9 mg) was further fractionated by preparative HPLC (Inertsil® ODS-3, 5 μm, 20 mm × 250 mm; (GL Sciences, Japan)) using an isocratic elution of 80% CH3CN at a flow rate of 10 mL/min. The eluates were monitored using UV absorbance at 240 nm. An aliquot of each fractions was tested for antifungal activity. During purification, the purity and content of active compound were determined by analytical HPLC (Inertsil® ODS-3, 5 μm, 4.6 mm × 250 mm; (GL Sciences, Japan)) using an isocratic elution of 80% CH3CN at a flow rate of 0.53 mL/min at a wavelength of 240 nm. The antifungal compound was obtained as a white powder (2.07 mg). The active compound was identified as ophiobolin K (Figure ) based on a combination of spectroscopic analyses (NMR, UV, HRESIMS, optical rotation) (Supplemental materials) and by comparison with published analytical data [Citation4–6].
The antimicrobial activities of ophiobolin K were evaluated by a spore germination assay and broth microdilution assay (Supplemental materials) against six fungal strains and seven bacterial strains, respectively. Antifungal activity of ophiobolin K is summarized in Table . Ophiobolin K showed inhibitory activities against all of the fungal strains. Interestingly, the growth inhibitory activity against A. repens and A. glaucus was remarkably high, with MIC value of 0.78 μg/mL for both strains, while relatively low antifungal activity was observed against A. oryzae, A. flavus, A. niger and P. aurantiogriseum with MICs ranging from 25 to 50 μg/mL. The potency of ophiobolin K against A. repens and A. glaucus was found to be approximately 100 times greater than that observed against A. oryzae, A. flavus, A. niger and Penicillium aurantiogriseum. Furthermore, ophiobolin K was a more potent inhibitor of A. repens and A. glaucus growth than the positive control cycloheximide (MIC of 1.0 and 16 μg/mL, respectively). Dannaoui et al. [Citation7] reported that the standard antifungal agents, amphotericin B and itraconazole exhibited antifungal activity against the Aspergillus spp., A. flavus, A. niger and A. terreus with MIC values ranging from 0.12 to 2.0 μg/mL. These values are comparable to previously reported MICs for ketoconazole against Aspergillus spp. ranging from 0.5 to 2.0 μg/mL [Citation8]. Based on the MIC values less than 1.0 μg/mL, ophiobolin K can be considered a highly potent growth inhibitor against A. repens and A. glaucus, with bioactivity close to that of standard antifungal agents. The strains used in this study, A. glaucus and A. repens, were isolated from the final product of dried bonito (Katsuobushi). It seems likely that ophiobolin K may show specific activity against the fungi from dried bonito. This is certainly an interesting issue for further research.
Table 1. Antifungal activity of ophiobolin K.
The antibacterial activity of ophiobolin K against gram-positive and gram-negative bacteria was also investigated. The MICs of ophiobolin K and positive control (chloramphenicol) are given in Table . Ophiobolin K exhibited antibacterial activity against gram-positive and gram-negative bacteria except for P. aeruginosa. Interestingly, ophiobolin K showed potent activity against gram-positive bacteria (Bacillus subtilis, Staphylococcus aureus and Micrococcus luteus) with MIC values ranging from 0.78 to 3.1 μg/mL, whereas weak antibacterial activities were observed against gram-negative bacteria (Escherichia coli, Vibrio anguillarum and V. parahaemolyticus) with MIC values ranging from 13 to 25 μg/mL. This result suggests that ophiobolin K specifically inhibits the growth of gram-positive bacteria. In comparison, a common antibacterial agent, tetracycline, effectively inhibits the growth of both gram-positive and gram-negative bacteria including B. megaterium (MIC, 4 μg/mL), S. aureus, E. coli, and P. aeruginosa, with MICs of 8 μg/mL for all strains [Citation9]. Kaur et al. [Citation10] also reported that amoxicillin was effective against a variety of bacteria with MIC in the range of 0.06–4.0 μg/mL for most bacterial strains except S. epidermidis and S. aureus which required MICs up to 64 μg/mL and ≥256 μg/mL, respectively. Based on the comparison with standard agents, we found that ophiobolin K showed remarkable inhibitory activity, with a wide antibacterial spectrum against gram-positive bacteria.
Table 2. Antibacterial activity of ophiobolin K.
However, the antimicrobial mode of action of ophiobolin K is not fully understood [Citation11]. Ophiobolins have been reported to exhibit cytotoxicity against chronic lymphocytic leukemia cells and that the presence of the hydroxyl group at C-3, the aldehyde group at C-21 and the stereochemistry at C-6 is crucial for this activity [Citation5]. These findings are in agreement with previous reports that ophiobolins covalently bind to calmodulin [Citation12] and inhibit calmodulin activated cyclic nucleotide phosphodiesterase that is commonly overexpressed in cancer cells [Citation13]. In filamentous fungi, calcium signaling involving calmodulin plays a critical role in several processes of development and morphogenesis including cell cycle, formation and germination of spores, growth of hyphal tips as well as orientation and branching of the hyphae [Citation14].
According to various reports, most standard antimicrobial agents show antimicrobial activities specific to either fungi or bacteria [Citation15,16]. However, in the present study, we found that ophiobolin K was effective against both fungi and bacteria, especially A. repens, A. glaucus, and gram-positive bacteria. The results demonstrate that ophiobolin K exhibits a wide antimicrobial spectrum. This finding could serve as a basis for further research towards the design of potent antimicrobial agents with activity against a wide range of microbial pathogens.
Author contributions
H. Kanzaki and T. Nitoda designed the research. N. Sohsomboon performed the experiments.
N. Sohsomboon wrote the draft of manuscript and T. Nitoda supervised manuscript preparation. All authors reviewed the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed at https://doi.org/10.1080/09168451.2018.1429890.
Supplemental_materials_Sohsomboon_et_al.pdf
Download PDF (690 KB)Acknowledgments
We greatly appreciate to Marutomo Co., Ltd. for the provision of fungal strains, A. repens MA0310 and A. glaucus MA0196. We thank Prof. Duanporn Kantachote, Faculty of Science, Prince of Songkla University, Thailand for giving us the V. parahaemolyticus SR2 strain. We are also grateful to the SC-NMR Laboratory of Okayama University and the MS Laboratory of the Faculty of Agriculture, Okayama University, respectively, for assistance in NMR and MS experiments.
References
- Kanzaki H, Yanagisawa S, Kanoh K, et al. A novel potent cell cycle inhibitor dehydrophenylahistin: enzymatic synthesis and inhibitory activity toward sea urchin embryo. J Antibiot. 2002;55:1042–1047.10.7164/antibiotics.55.1042
- Yagi R, Doi M. Isolation of an antioxidative substance produce by Aspergillus repens. Biosci Biotechnol Biochem. 1999;63:932–933. 10.1271/bbb.63.932
- Kanoh K, Kohno S, Asari T, et al. (-)-Phenylahistin: a new mammalian cell cycle inhibitor produced by Aspergillus ustus. Bioorg Med Chem Lett. 1997;7:2847–2852.10.1016/S0960-894X(97)10104-4
- Wei H, Itoh T, Konoshita M, et al. Cytotoxic sesterterpenes, 6-epi-ophiobolin G and 6-epi-ophiobolin N, from marine derived fungus Emericella variecolor GF10. Tetrahedron. 2004;60:6015–6019.10.1016/j.tet.2004.05.021
- Bladt TT, Dürr C, Knudsen PB, et al. Bio-activity and dereplication-base discovery of ophiobolins and other fungal secondary metabolites targeting leukemia cells. Molecules. 2013;18:14629–14650.10.3390/molecules181214629
- Singh SB, Smith JL, Sabnis GS, et al. Structure and conformation of ophiobolin K and 6-epiobolin K from Aspergillus ustus as a nematocidal agent. Tetrahedron. 1991;47:6931–6938.10.1016/S0040-4020(01)96148-4
- Dannaoui E, Persat F, Monier MF, et al. In-vitro susceptibility of Aspergillus spp. isolates to amphotericin B and itraconazole. J Antimicrob Chemother. 1999;44:553–555.10.1093/jac/44.4.553
- Hsueh PR, Lau YJ, Chuang YC, et al. Antifungal susceptibilities of clinical isolates of Candida species, Cryptococcus neoformans and Aspergillus species from Taiwan: surveillance of multicentre antimicrobial resistance in Taiwan program data from 2003. Antimicrob Agents Chemother. 2005;49:512–517.10.1128/AAC.49.2.512-517.2005
- Hussin WA, El-Sayed WM. Synergistic interactions between selected botanical extracts and tetracycline against Gram positive and Gram negative bacteria. J Biol Sci. 2011;11:433–441.
- Kaur SP, Rao R, Nanda S. Amoxicillin: a broad spectrum antibiotic. Int J Pharm Pharm Sci. 2011;3:30–37.
- de Carvalho CR, Vieira MDLA, Cantrell CL. Biological activities of ophiobolin K and 6-epi-ophiobolin K produced by the endophytic fungus Aspergillus calidoustus. Nat Prod Res. 2016;30:478–481.10.1080/14786419.2015.1022777
- Evidente A, Andolfi A, Cimmino A. Herbicidal potential of ophiobolins produced by Drechslera gigantaa. J Agric Food Chem. 2006;54:1779–1783.10.1021/jf052843 l
- Arai M, Niikawa H, Kobayashi M. Marine-derived fungal sesterterpenes, ophiobolins, inhibit biofilm formation of Mycobacterium species. J Nat Med. 2013;67:271–275.10.1007/s11418-012-0676-5
- Kriśan K, Bencsik O, Nyilasi I. Effect of the sesterterpene-type metabolites, ophiobolins A and B on zygomycetes fungi. FEMS Microbiol Lett. 2010;313:135–140.
- Whiffen AJ. The production, assay, and antibiotic activity of acti-dione, an antibiotic from Streptomyces griseus. J Bacteriol. 1948;56:283–291.
- Logemann W, Almirante L, Galimberti S, et al. Influence of dichloroacetylation on the antimicrobial activity of chloramphenicol derivatives and of various amines. Br J Pharmacol. 1961;17:286–296.