Abstract
Metallothioneins (MTs) are low-molecular-weight proteins with high Cys content and high metal-chelating ability. CdMT and CuMT subfamilies present different characteristics in Tetrahymena. To explore the effect of the cysteine arrangement and sequence length of MTs for binding different metal ions, MTT1, truncated MTT1 (TM1), MTT2, and truncated MTT2 (TM2) were expressed in E. coli. The half-maximal inhibiting concentrations (IC50) of Cd2+ and Cu+ for the recombinant strains were different. Furthermore, E. coli cells expressing MTT1 and TM1 exhibited higher accumulating ability for Cd2+ than cells expressing MTT2 and TM2. However, the opposite is true for Cu+. The binding ability of the different recombinant proteins to Cd2+ and Cu+ were also different. MTT1 and truncated mutant TM1 were the preference for Cd2+, whereas MTT2 and truncated mutant TM2 were the preference for Cu+ coordination. These results showed that metal ion tolerance and accumulation ability not only depended on cysteine arrangement pattern but also on sequence length of MT in Tetrahymena.
Accumulation of metal ion Cd2+ and Cu+ in MT transformed cells.
Keywords:
Metallothioneins (MTs) constitute an extremely heterogeneous family of ubiquitous, low-molecular-weight, and cysteine-rich proteins [Citation1]. They are multifunctional proteins mainly involved in physiological metal homeostasis and toxic heavy metal chelation. MTs also play key roles in other cellular processes, such as free radical scavenging [Citation2], metalloenzyme and transcription factor regulation [Citation3,4], DNA damage repair [Citation5], cell growth and proliferation modulation [Citation6], and transportation of essential metal ions, such as Cu+ and Zn2+ [Citation7]. MT gene expression is regulated at the transcriptional level. In mammals, MTs are differentially expressed in various tissues and in several developmental stages. The in vivo stability of MT is enhanced by metal ions [Citation8]. In Neurospora crassa and Saccharomyces cerevisiae, MTs only contain single metal-binding β-domains [Citation9,10]. The MT from Callinectes sapidus binds six divalent metal ions in two separate metal-binding clusters [Citation11]. Vertebrate MT is composed of the N-terminal β-domain and the C-terminal α-domain that bind metals independently and separated by a special linker region [Citation12,13]. The α- and β-domains domain binds four and three bivalent ions, respectively [Citation14]. The Pacific oyster Crassostrea gigas MT (CgMT2) and snail Littorina littorea MTs comprise three metal-binding domains consisting of one α-domain and two β-domains, and two α-domain and one β-domain, respectively [Citation15,16]. From an evolutionary standpoint, the three-domain structure confers high functional efficiency on MT without greatly increasing the energy demand for its expression [Citation16].
The MTs from the Tetrahymena family exhibit unique features compared with standard MTs. Their lengths are longer and contain a higher number of Cys residues. The Cys residues constitute a hierarchical modular structural organization [Citation17]. The MTs fall into two subfamilies, 7a (CdMTs) and 7b (CuMTs) [Citation18], which differ mainly in their typical Cys residue clustering. In subfamilies 7a and 7b, the most abundant clusters are CCC and XCCX, and CXC, respectively [Citation18]. Intragenic repeat numbers provide functional diversity in proteins and allow rapid adaptation to the environment [Citation19]. Glutamines are important residues for MTs because they are involved in stabilizing the metal-protein complex. In general, Tetrahymena CdMTs have more Q residues than CuMTs [Citation1]. Limited knowledge on MT origin and differentiation patterns can mainly be attributed to the lack of comparative studies of MT isoforms [Citation20]. Recently, it has been proposed two complementary forces drive the evolution of MTs in eukaryotic cell: a qualitative one for metal specificity; and a quantitative one, to enlarge the metal-binding capacity of a basic peptide fragment [Citation21]. However, the function of different domain of different MT is less clear in the organisms. MTT1 and MTT2 have different protein sequence length and belong to different subfamily, functional comparision of similar length MT motify is important for discovering the different function of the MT isoforms.
In the present study, we focused on investigating how cysteine arrangement and sequence length of MT affect different metal ion binding. The full-length and truncated fragments of MTT1 and MTT2 from Tetrahymena thermophila were expressed in Escherichia coli, respectively. Moreover, the tolerance and accumulation of metal ions (Cd and Cu) were evaluated in the recombinant stains. Binding affinity between the metal ions and the MTs was also determined.
Materials and methods
Cell culture and recombinant plasmid construction
Tetrahymena B2086 was grown axenically at 30 °C in SPP medium, which contanins 1% proteose peptone, 0.2% glucose, 0.1% yeast extract, and 0.003% Ethylene Diamine Tetraacetic Acid Ferric Sodium medium. Genomic DNA was extracted as previously described [Citation22]. MTT1 and MTT2 were amplified by PCR with primers MTT1-F/MTT1-R and MTT2-F/MTT2-R (Table ), respectively. PCR amplification was performed as follows: initial denaturation of 95 °C for 5 min; 30 cycles of 95 °C for 30 s, 54 °C for 60 s, and 72 °C for 1 min; and final extension at 72 °C for 5 min. The PCR products were inserted into the expression vector pGEX-4T-1. TM1 and TM2 were amplified with primers TM1-F/TM1-R and TM2-F/TM2-R, respectively (Table1). Amplification was conducted as follows: initial denaturation at 95 °C for 5 min; 30 cycles of 95 °C for 30 s, 54 °C for 30 s, and 72 °C for 1 min; and final extension at 72 °C for 5 min. The PCR products were inserted into the expression vector pGEX-4T-1. The resulting plasmids pGEX-MTT1, pGEX-MTT2, pGEX-TM1, and pGEX-TM2 were verified by sequencing and transformed into E. coli (BL21).
Table 1. PCR primers used in this study.
Expression and purification of MTs
E. coli BL21/pGEX-TM1, E. coli BL21/pGEX-TM2, E. coli BL21/pGEX-MTT1, and E. coli BL21/pGEX-MTT2 were inoculated in Luria-Bertani medium containing 50 μg/mL ampicillin. When the cultures reached an optical density of 0.6 (OD600), they were induced with 0.5 mM isopropyl-β-d-thiogalactoside (IPTG) for 4 h. The culture medium (5 mL) was harvested by centrifugation. The samples were resuspended in 100 uL 10 mM Tris–HCl and then lysed by ultrasonication (SONICS & MATERA, INC, UC455, USA). The lysates were centrifuged at 15,000 × g at 4 °C for 30 min (Hitachi CR22G III, Hitachi Inc, Japan). The supernatant was collected, mixed with Glutathione S-transferase (GST) agarose, and shaken at 4 °C for 1 h to capture GST-tagged proteins. Upon completion of capturing, the solution was washed with 10 mM Tris–HCl (pH 8.0) to remove residual uncaptured proteins and with 300 μL of 50 mmol/L GSH solution to disassociate the GST-tagged proteins. GST-tagged proteins were further purified by Superdex 75 gel filtration chromatography. Proteins were analyzed via 12.5% SDS-PAGE.
Tolerance of recombinant E. coli for Cd and Cu
E. coli BL21/pGEX-TM1, E. coli BL21/pGEX-TM2, E. coli BL21/pGEX-MTT1, and E. coli BL21/pGEX-MTT2 were inoculated and cultured in LB medium at 37 °C. Different concentrations Cd (0, 50, 100, 150, 200, 250, 300, and 400 μM) and Cu (0, 50, 100, 200, 300, 500, 800, and 1000 μM) were added to the culture medium. After 8 h, the cell concentration was measured at 600 nm using a spectrophotometer, and relative inhibition rates were calculated. Equations correlating inhibition rate and logarithms of metal concentrations were obtained using Graphpad 5.01 (Graphpad Prism Software, San Diego, CA). Half-maximal inhibiting concentration (IC50) was calculated.
Accumulation of Cd and Cu in recombinant E. coli
Overnight cultures of the E. coli BL21/pGEX-TM1, E. coli BL21/pGEX-TM2, E. coli BL21/pGEX-MTT1, and E. coli BL21/pGEX-MTT2 were diluted to 1:100 in 100 mL fresh LB medium supplemented with 50 μg/ml of ampicillin. Recombinant bacteria were induced with 0.5 mM IPTG. After 30 min, 287.1 μM CdCl2 and 429.5 μM CuSO4 were respectively added to the culture medium. After 8 h, cells were precipitated via centrifugation at 8,000 × g for 20 min. Bioaccumulation of heavy metal ions was analyzed using an atomic absorption spectrometer (SHIMDZU AA-6300, Japan) (n = 3). Data was analyzed using one way Analysis of Variance (ANOVA) followed by Tukey post hoc test using GraphPad Prism software,version 5.01.
Formation of metal MT complexes
The apo-proteins were prepared and reconstituted with metals as previously described with slight modifications [Citation23–25]. The GST-TM1, GST-TM2, GST-MTT1, and GST-MTT2 were incubated with 100 mM Dithiothreitol (DTT) overnight at 4 °C. The solution was adjusted to pH 2.0 [Citation14]. Metal ions in the solution were removed using a centrifugal ultrafiltration tube (Millipore) supplemented with 100 mM HEPES buffer (pH 2.0) for five times, and then 100 mM HEPES buffer (pH 2.0) was replaced with 100 mM HEPES buffer (pH 8.0). Reconstitution with metals was achieved by adding 10 equivalents of Cd2+ and Cu+. The metal/MT complexes were dialyzed with 10 mM Tris–HCl at 4 °C overnight.
Reaction between metal-MT complex and 5,5′-dithio-bis(2-nitrobenzoate) (DTNB)
Protein complex (1.5 nmol) was diluted in 300 μl of 10 mM Tris–HCl (pH 8.0) and placed in a quartz cuvette. The reaction was started by adding 75 nmol of DTNB. Absorbance at 412 nm was recorded on the spectrophotometer at 25 °C for 60 min, reflecting the formation of 2-nitro-5-thiobenzoic acid (TNB). Tris–HCl (300 μL, 10 mM, pH 8.0) solution containing 75 nmol of DTNB was used as blank. Initial velocity was obtained from each curve of absorbance vs. the reaction time.
Results and discussion
Expression and purification of MTT1, MTT2, TM1, and TM2
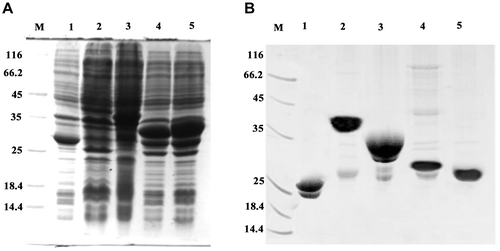
Tetrahymena MT protein sequences exhibit a remarkably regular and hierarchical modular organization [Citation17]. Cys motif and distribution pattern are significantly different between MTT1 and MTT2 (Figure (A)). MTT1 with 163 amino acids contains six, two, and one “C3X6C2”, “C2X6/8CXCX2CXC2X1/2”, and “CXCX2CXC2X1/2” motifs, respectively. MTT2 with 108 amino acids contains 15 “CXC” and 1 “CXXC” motifs (Figure (B)). To explore the function of different Cys arrangement, we expressed full-length MTT1, truncated MTT1 (59 amino acid TM1), full-length MTT2, and truncated MTT2 (60 amino acid, TM2) in E. coli. TM1 contained a total of 17 cysteins with 2 “C3X6C2” and 1 “C2X6CXCX2CXC2” motifs (Figure (C)). TM2 comprised 16 cysteines with 7 “CxC” and 1 “CxxC” motifs (Figure (D)). GST, GST-MTT1, GST-MTT2, GST-TM1, and GST-TM2 were expressed in recombinant E. coli, respectively (Figure (A)). The theoretical molecular weight of GST-TM1, GST-TM2, GST-MTT1, and GST-MTT2 are 32, 32, 42, and 37 kDa, respectively. The results showed that the different fusion proteins are soluble and exhibited less inclusion body relative to the previous expression of His6-MTT1 and His6-MTT2 [Citation25]. Previously, our results showed that one apoMTT1 and apoMTT2 molecule bind to 16 and 11 Cd2+ in vitro, respectively [Citation25]. The binding stability of Cd-MTT1 was also stronger than that of Cd-MTT2. Cu+ can not replace the Cd2+ from Cd16-MTT1 complex. By contrast, Cu+ can replace the Cd2+ from Cd16-MTT2 complex. MTT1 plays a more important role in the detoxification of heavy metal ions than MTT2, which is involved in the homeostasis of Cu+ [Citation25]. To explore the function of the different MT isoforms, the recombinant GST-TM1, GST-TM2, GST-MTT1, and GST-MTT2 were further purified by GST affinity chromatography and Superdex 75 gel filtration chromatography (Figure (B)). The results showed that GST tag promotes solubility and stability of the different MTs protein [Citation26].
Figure 1. Amino acid sequences of MTT1 and MTT2 from T. thermophila. The C3X6C2, C2X6/8CXCX2CXC2X1/2, and CXC represent different motifs. TM1 and TM2 indicate the truncated MTT1 and MTT2, respectively.
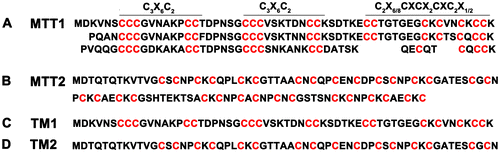
Figure 2. Expression and purification of GST, GST-MTT1, GST-MTT2, GST-TM1, and GST-TM2. (A) SDS-PAGE (12.5%) analysis of recombinant proteins. M: protein molecular weight markers; lane 1: total cell lysate of E. coli/pGEX-4T-1; lane 2: total cell lysate of E. coli/pGEX-MTT1; lane 3: total cell lysate of E. coli /pGEX-MTT2; lane 4: total cell lysate of E. coli/pGEX-TM1; lane 5: total cell lysate of E. coli /pGEX-TM2. (B) SDS-PAGE (12.5%) analysis of purified proteins. M: protein molecular weight markers; lane 1: GST; lane 2: GST-MTT1; lane 3: GST-MTT2; lane 4: GST-TM1; lane 5: GST-TM2. The proteins were purified using glutathione resins and Superdex 75 gel filtration chromatography.
Different proliferation of recombinant MT strains under Cd and Cu stress
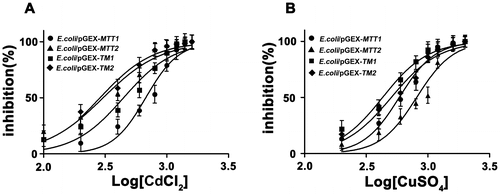
Gene duplication and subsequent diversification are considered to be important mechanisms involved in MT evolution [Citation1]. The marine common periwinkle Littorina littorea MT possesses a longer sequence with 27 cys residues than the 18 Cys commonly found in the other snails. This novel three-domain MT confers an evolutionary advantage to L. littorea by way of structural adaptation to high-risk metal exposure through a simple domain duplication event. Its Cd-binding capacity has been increased during evolution through the addition of a third metal-binding domain [Citation27]. The presence of an extra CXC motif in Colocasia esculenta MT (CeMT2b) contributes to enhanced Cd-tolerance [Citation28]. Furthermore, the arrangement of Cys residues is also crucial in determining the metal-binding properties and functions of MT proteins [Citation28]. To better understand the effect of MT cysteine length and arrangement for cellular heavy metal stress, we analyzed the IC50 values of the recombinant different MT strains for metal ion Cd2+ and Cu+. The IC50 values of the recombinant strains for Cd2+ were found in the following order: E. coli/pGEX-MTT1 (674.4 μM) > E. coli/pGEX-TM1 (445.6 μM) > E. coli/pGEX-MTT2 (304.2 μM) > E. coli/pGEX-TM2 (287.1 μM). These results indicated that increased MT length improves cellular tolerance to Cd. In addition, Cys arrangement pattern was more important for Cd tolerance because E. coli/pGEX-TM1 > E. coli/pGEX-MTT2. The IC50 values of the recombinant strains for Cu+ was also analyzed. We observed the following order: E. coli/pGEX-MTT2 (811.2 μM) > E. coli/pGEX-MTT1 (585.9 μM) > E. coli/pGEX-TM2 (505.1 μM) > E. coli/pGEX-TM1 (429.5 μM) (Figure ). Length of MTT2 is shorter than that of MTT1, but cellular tolerance for Cu is E. coli/pGEX-MTT2 > E. coli/pGEX-MTT1. These results also indicated that Cys arrangement is more important for Cu tolerance. However, for sequence similar MT, increasing length of MT also improved cellular tolerance for metal ion since E. coli/pGEX-MTT2 > E. coli/pGEX-TM2 and E. coli/pGEX-MTT1 > E. coli/pGEX-TM1.
Figure 3. Cd and Cu dose–response curve of recombinant MT E. coli. Recombinant E. coli/pGEX-MTT1, E. coli/pGEX-MTT2, E. coli/pGEX-TM1, and E. coli/pGEX-TM2 were exposed to CdCl2 (A) and CuSO4 (B). Cell concentration was measured at 600 nm using a spectrophotometer. All data are expressed as mean ± SD (n = 3).
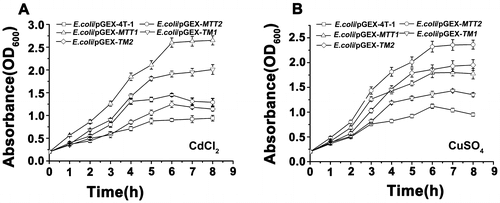
E. coli/pGEX-TM2 proliferates slowly and exhibits lower concentration than the E. coli/pGEX-TM1 with Cd2+ stress (Figure (A)), which is opposite with Cu+ stress (Figure (B)). Furthermore, E. coli/pGEX-TM1 and E. coli/pGEX-TM2 proliferate slowly and has lower concentration than E. coli/pGEX-MTT1 and E. coli/pGEX-MTT2 with Cd2+ and Cu+ stress, respectively. In Hyriopsis schlegeli, the transgenic bacteria of HsMT1 show higher tolerance than HsMT2 lacking the Cys-Cys motifs in Cd2+ environment [Citation29]. These results indicated that different cystein arrangements and sequence lengths in MTs affected cellular proliferation under heavy metal Cd2+ and Cu+ stress.
Figure 4. Proliferation of recombinant MT in E. coli. Recombinant E. coli/pGEX-MTT1, E. coli/pGEX-MTT2, E. coli/pGEX-TM1, and E. coli/pGEX-TM2 were exposed to 287.1 μM CdCl2 (A) and 429.5 μM CuSO4 (B). E. coli/pGEX-4T-1 as a control, cell concentration was measured at 600 nm using a spectrophotometer. All data are expressed as mean ± SD (n = 3).
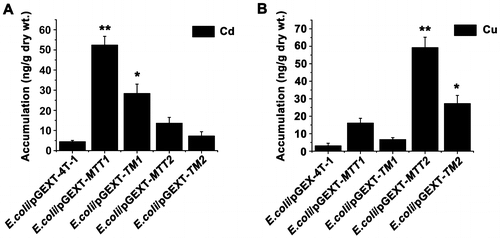
Different Cd and Cu accumulation in recombinant MTs E. coli
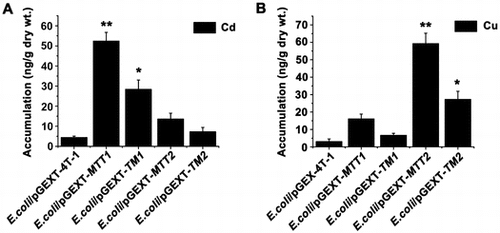
Cellular metal tolerance and cellular metal accumulation are related by MT metal-binding capability [Citation28]. Cd-MTT2 complexes can not be recovered from Cd-supplemented bacterial cells, indicating the incapacity of MTT2 to fold into stable Cd complexes in an intracellular environment [Citation21]. But, MTT2 formed homometallic Cu20-MTT2 complexes in Cu-supplemented hosts [Citation21]. To compare metal ion accumulation in different recombinant E. coli, we cultured four recombinant strains under Cd2+ and Cu+ stress. Cd2+ at 4.39, 28.35, 7.26, 52.38, and 13.6 ng/g dry weight and Cu+ at 3.05, 6.65, 27.24, 16.07, and 59.21 ng/g dry weight accumulated in the recombinant E. coli cells expressing GST, GST-TM1, GST-TM2, GST-MTT1, and GST-MTT2, respectively (Figure (A)). Cd2+ and Cu+ were effectively coordinated and sequestered in recombinant E. coli/pGEX-TM1 and E. coli/pGEX-MTT1, and recombinant E. coli/pGEX-TM2 and E. coli/pGEX-MTT2, respectively. In pulmonate mollusks, synthesized CdMT and CuMT isoforms are isolated from native sources as homometallic Cd2+ and Cu+ complexes, respectively. CdMT and CuMT are involved in different metal-specific physiological processes [Citation1]. In mammalian cells, the β- and α-domain of MT specifically bind Cu+, and Zn2+ or Cd2+, respectively [Citation30,31]. The Cd2+ bioaccumulation capacities of E. coli/pGEX-MTT1 was about 1.84 times higher than that of E. coli/pGEX-TM1 (Figure (A)), and the Cu+ bioaccumulation capacities of E. coli/pGEX-MTT2 were about 2.17 times higher than that of E. coli/pGEX-TM2 (Figure (A)). Increased tolerance and metal binding capacity can be achieved by increasing the number of integrated MT genes coding for oligomeric MTs [Citation25,32].
Figure 5. Accumulation of metal ion Cd2+ and Cu+ in MT transformed cells. Recombinant E. coli/pGEX-MTT1, E. coli/pGEX-MTT2, E. coli/pGEX-TM1, and E. coli/pGEX-TM2 were exposed to 287.1 μM CdCl2 (A) and 429.5 μM CuSO4 (B). E. coli/pGEX-4T-1 as a control. The metal ion was analyzed using an atomic absorption spectrometer. All data are expressed as mean ± SD (n = 3), *p < 0.05, **p < 0.001.
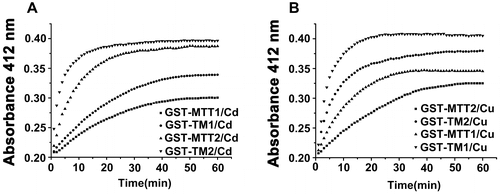
Stability of different Metal-MT Complexes in the presence of DTNB
Metal ion binding to the sulfhydryl groups of apo-metallothionein causes the formation of native metal–thiolate clusters and polypeptide chain folding [Citation33,34]. Sulfhydryl concentration was determined colorimetrically with DTNB. DTNB reduction yields the TNB chromophore whose appearance can be monitored by optical density measurements. DTNB and metal ions directly compete for the thiolate groups of MT. Metal complex stability can be evaluated via a competitive reaction with DTNB as previously described [Citation33,35,36]. The affinities between Cd2+ and MTs, and Cu+ and MTs, were as follows: GST-MTT1 > GST-TM1 > GST-MTT2 > GST-TM2 (Figure (A)) and GST-MTT2 > GST-TM2 > GST-MTT1 > GST-TM1 (Figure (B)), respectively. Although TM1 (59 aa) and TM2 (60 aa) possess similar amino acid length and similar cysteine number, they indeed display different binding properties. GST-TM1 exhibits a stronger ability to bind Cd2+ than GST-TM2. By contrast, GST-TM2 demonstrates a stronger affinity to bind Cu+ than GST-TM1. The rate of reaction with DTNB was lower for the purified α-fragment of human MT2 than for the purified wild-type MT2. Cd is much more tightly bound in the α-fragment than wild-type MT [Citation35]. However, Tetrahymena full-length MTT1 shows a stronger ability to bind Cd2+ than truncated TM1. Similarly, full-length MTT2 displays a stronger ability to bind Cu+ than truncated TM2. The results indicated that full-length MT has evolved stronger ion-binding ability and formed more stable spatial conformation in the Tetrahymena. The number of Cys residues and their distribution in their amino-acid sequence seems to be important for metal-chelating ability.
Figure 6. Reaction profiles of metal-binding complexs with DTNB. Each metal-bound protein sample at 1.5 nmol was dissolved in 300 μL of 10 mM PBS (pH 8.0), and reaction was started by adding 75 nmol of DTNB to solution. Absorbance at 412 nm at 25 °C was recorded for 60 min. A: Cd-bound protein sample; B: Cu-bound protein sample. Each value was the mean of two replicates.
Conclusions
Two different full-length Tetrahymena MTs and two corresponding truncated MTs were expressed and purified in E. coli, respectively. The recombinant E. coli expressing full-length MTs accumulate more metal ions than that expressing their truncated mutants. However, truncated TM1 effectively bound Cd2+ compared with full-length MTT2. By contrast, truncated TM2 effectively bound Cu+ compared with full-length MTT1. Different organisms have evolved various mechanisms to reduce metal toxicity. Evolutionary origin of Ciliates has been proposed for around 109 years ago [Citation37]. Tetrahymena MTs make longer by internal tandem repeats. The MT system constitutes an invaluable model for MT evolutionary studies [Citation21]. Our results showed that tandem repetition of basic building blocks of MT improved the sequestration of Cd2+ or Cu+. In summary, the sequence length and cysteine arrangement pattern of MT evolutionarily determine the metal ion-binding number and coordination type in Tetrahymena.
Author contributions
Conceived and designed the experiments: W. Wang, H. Zhou; Performed the experiments: J. Xu, H. Zhou; Analyzed the data: W. Wang, J. Xu; Wrote the paper: W. Wang, H. Zhou.
Funding
This work was supported by the National Natural Scientific Foundation of China [grant numbers 31471999, 31572253]; International cooperation project of Shanxi Province [grant number 2015081032]; Natural Science Foundation of Shanxi Province [grant numbers 2015011078]; and Shanxi Scholarship Council of China [grant numbers 2015-008].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Capdevila M, Atrian S. Metallothionein protein evolution: a miniassay. J Biol Inorg Chem. 2011;16:977–989.10.1007/s00775-011-0798-3
- Blindauer CA, Leszczyszyn OI. Metallothioneins: unparalleled diversity in structures and functions for metal ion homeostasis and more. Nat Prod Rep. 2010;27:720–741.10.1039/b906685n
- Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol. 2000;59:95–104.10.1016/S0006-2952(99)00301-9
- Bratic AM, Majic DB, Samardzic JT, et al. Functional analysis of the buckwheat metallothionein promoter: Tissue specificity pattern and up-regulation under complex stress stimuli. Journal of Plant Physiology. 2009;166:996–1000.10.1016/j.jplph.2008.12.002
- Drechsel V, Schauer K, Srut M, et al. Regulatory plasticity of earthworm wMT-2 gene expression. Int J Mol Sci. 2017;18(6):1113. DOI:10.3390/ijms18061113.
- Toh PPC, Li JJ, Yip GWC, et al. Modulation of metallothionein isoforms is associated with collagen deposition in proliferating keloid fibroblasts in vitro. Exp Dermatol. 2010;19:987–993.10.1111/exd.2010.19.issue-11
- Sutherland DE, Summers KL, Stillman MJ. Noncooperative metalation of metallothionein 1a and its isolated domains with zinc. Biochemistry. 2012;51:690–700.10.1021/bi3004523
- Zimeri AM, Dhankher OP, McCaig B, et al. The plant MT1 metallothioneins are stabilized by binding cadmiums and are required for cadmium tolerance and accumulation. Plant Mol Biol. 2005;58:839–855.10.1007/s11103-005-8268-3
- Beltramini M, Lerch K. Primary structure and spectroscopic studies of Neurospora copper metallothionein. Environ Health Perspect. 1986;65:21–27.
- Winge DR, Nielson KB, Gray WR, et al. Yeast metallothionein. Sequence and metal-binding properties. J Biol Chem. 1985;260:14464–11470.
- Narula SS, Brouwer M, Hua Y, et al. Three-dimensional solution structure of callinectes sapidus Metallothionein-1 determined by homonuclear and heteronuclear magnetic resonance spectroscopy. Biochemistry. 1995;34:620–631.10.1021/bi00002a029
- Braun W, Vasak M, Robbins AH, et al. Comparison of the NMR solution structure and the X-ray crystal structure of rat metallothionein-2. Proc Natl Acad Sci U S A. 1992;89:10124–10128.10.1073/pnas.89.21.10124
- Riek R, Precheur B, Wang Y, et al. NMR Structure of the Sea Urchin (Strongylocentrotus purpuratus) Metallothionein MTA. J Mol Biol. 1999;291:417–28.10.1006/jmbi.1999.2967
- Jiang LJ, Vasak M, Vallee BL, et al. Zinc transfer potentials of the alpha – and beta-clusters of metallothionein are affected by domain interactions in the whole molecule. Proc Natl Acad Sci U S A. 2000;97:2503–2508.10.1073/pnas.97.6.2503
- Tanguy A, Moraga D. Cloning and characterization of a gene coding for a novel metallothionein in the Pacific oyster Crassostrea gigas (CgMT2): a case of adaptive response to metal-induced stress? Gene. 2001;273:123–30.10.1016/S0378-1119(01)00577-7
- Baumann C, Beil A, Jurt S, et al. Structural adaptation of a protein to increased metal stress: NMR structure of a marine snail metallothionein with an additional domain. Angew Chem Int Ed Engl. 2017;56:4617–4622.10.1002/anie.201611873
- Chang Y, Liu G, Guo L, et al. Cd-Metallothioneins in three additional tetrahymena species: intragenic repeat patterns and induction by metal ions. J Eukaryot Microbiol. 2014;61:333–342.10.1111/jeu.2014.61.issue-4
- Diaz S, Amaro F, Rico D, et al. Tetrahymena metallothioneins fall into two discrete subfamilies. PLoS One. 2007;2:e291.10.1371/journal.pone.0000291
- Verstrepen kJ, Jansen A, Lewitter F, et al. Intragenic tandem repeats generate functional variability. Nat Genet. 2005;37:986–990.10.1038/ng1618
- Shuja RN, Taimuri SU, Shakoori FR, et al. Efficient expression of truncated recombinant cadmium-metallothionein gene of a ciliate, Tetrahymena tropicalis lahorensis in Escherichia coli. Mol Biol Rep. 2013;40:7061–7068.10.1007/s11033-013-2827-5
- Espart A, Marin M, Gil-Moreno S, et al. Hints for metal-preference protein sequence determinants: different metal binding features of the five tetrahymena thermophila metallothioneins. Int J Biol Sci. 2015;11:456–471.10.7150/ijbs.11060
- Gorovsky MA, Yao MC, Keevert JB, et al. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 1975;9:311–327.10.1016/S0091-679X(08)60080-1
- Dallinger R, Wang Y, Berger B, et al. Spectroscopic characterization of metallothionein from the terrestrial snail, Helix pomatia. Eur J Biochem. 2001;268:4126–4133.10.1046/j.1432-1327.2001.02318.x
- Toriumi S, Saito T, Hosokawa T, et al. Metal binding ability of metallothionein-3 expressed in Escherichia coli. Basic Clin Pharmacol Toxicol. 2005;96:295–301.10.1111/pto.2005.96.issue-4
- Wang Q, Xu J, Chai B, et al. Functional comparison of metallothioneins MTT1 and MTT2 from Tetrahymena thermophila. Arch Biochem Biophys. 2011;509:170–176.10.1016/j.abb.2011.02.015
- Esposito D, Chatterjee DK. Enhancement of soluble protein expression through the use of fusion tags. Curr Opin Biotechnol. 2006;17:353–358.10.1016/j.copbio.2006.06.003
- Niederwanger M, Calatayud S, Zerbe O, et al. Biomphalaria glabrata Metallothionein: Lacking Metal Specificity of the Protein and Missing Gene Upregulation Suggest Metal Sequestration by Exchange Instead of through Selective Binding. Int J Mol Sci. 2017;18(7):1457. DOI:10.3390/ijms18071457.
- Kim YO, Patel DH, Lee DS, et al. High cadmium-binding ability of a novel Colocasia esculenta metallothionein increases cadmium tolerance in Escherichia coli and tobacco. Biosci Biotechnol Biochem. 2011;75:1912–1920.10.1271/bbb.110289
- Wang C, Sheng J, Hong Y, et al. Molecular characterization and expression of metallothionein from freshwater pearl mussel. Hyriopsis schlegelii. Biosci Biotechnol Biochem. 2016;80:1327–1335.10.1080/09168451.2016.1153954
- Briggs RW, Armitage IM. Evidence for site-selective metal binding in calf liver metallothionein. J Biol Chem. 1982;257:1259–1262.
- Meloni G, Sonois V, Delaine T, et al. Metal swap between Zn7-metallothionein-3 and amyloid-beta-Cu protects against amyloid-beta toxicity. Nat Chem Biol. 2008;4:366–372.10.1038/nchembio.89
- Ma Y, Lin JQ, Zhang CJ, et al. Cd(II) and As(III) bioaccumulation by recombinant Escherichia coli expressing oligomeric human metallothioneins. J Hazardous Mater. 2011;185:1605–1608.10.1016/j.jhazmat.2010.10.051
- Ejnik J, Robinson J, Zhu J, et al. Folding pathway of apo-metallothionein induced by Zn2+, Cd2+ and Co2+. J Inorg Biochem. 2002;88:144–152.10.1016/S0162-0134(01)00393-2
- Zhou H, Xu J, Wang W. Functional Analysis of Metallothionein MTT5 From Tetrahymena thermophila. J Cell Biochem. 2017. DOI:10.1002/jcb.26482.
- Kurasaki M, Emoto T, Arias AR, et al. Independent self-assembly of cadmium-binding alpha-fragment of metallothionein in Escherichia coli without participation of beta-fragment. Protein Eng. 1996;9:1173–1180.10.1093/protein/9.12.1173
- Dauplais M, Lazard M, Blanquet S, et al. Neutralization by metal ions of the toxicity of sodium selenide. PLoS One. 2013;8:e54353.10.1371/journal.pone.0054353
- Parfrey LW, Lahr DJ, Knoll AH, et al. Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proc Natl Acad Sci U S A. 2011;108:13624–13629.10.1073/pnas.1110633108
